Exploring Growth Phase Effect on Polysaccharide Composition and Metal Binding Properties in Parachlorella hussii
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Site, Strain Isolation, and Identification
2.2. Batch Culture and Growth Measurement of the Microalgal Strain
- N: Average number of cells counted in one large square.
- W: Weight of the filter
- X: Dry weight of biomass (g L−1) at time tT and t0 (days)
- XT and X0: Dry biomass concentrations at time ‘T’ and time ‘0’ (days).
2.3. Polysaccharide Extraction and Quantification
2.4. Polysaccharide Characterisation
2.4.1. Biochemical Analysis
2.4.2. Monosaccharide Composition Determination
2.4.3. Molecular Weight Determination
2.4.4. Attenuated Total Reflection Spectroscopy
2.5. Metal Removal Activity
2.5.1. Metal Cell Contact
2.5.2. Quantification of the Removed Metal
- C0: initial concentration of the metal solution (mg L−1),
- Ct: concentration of the metal solution after‘t’ time (mg L−1),
- V: volume of the solution in the oscillated flasks (L).
- m: the mass of the biosorbent (g L−1).
2.6. Statistical Analysis
3. Results
3.1. Sampling Site
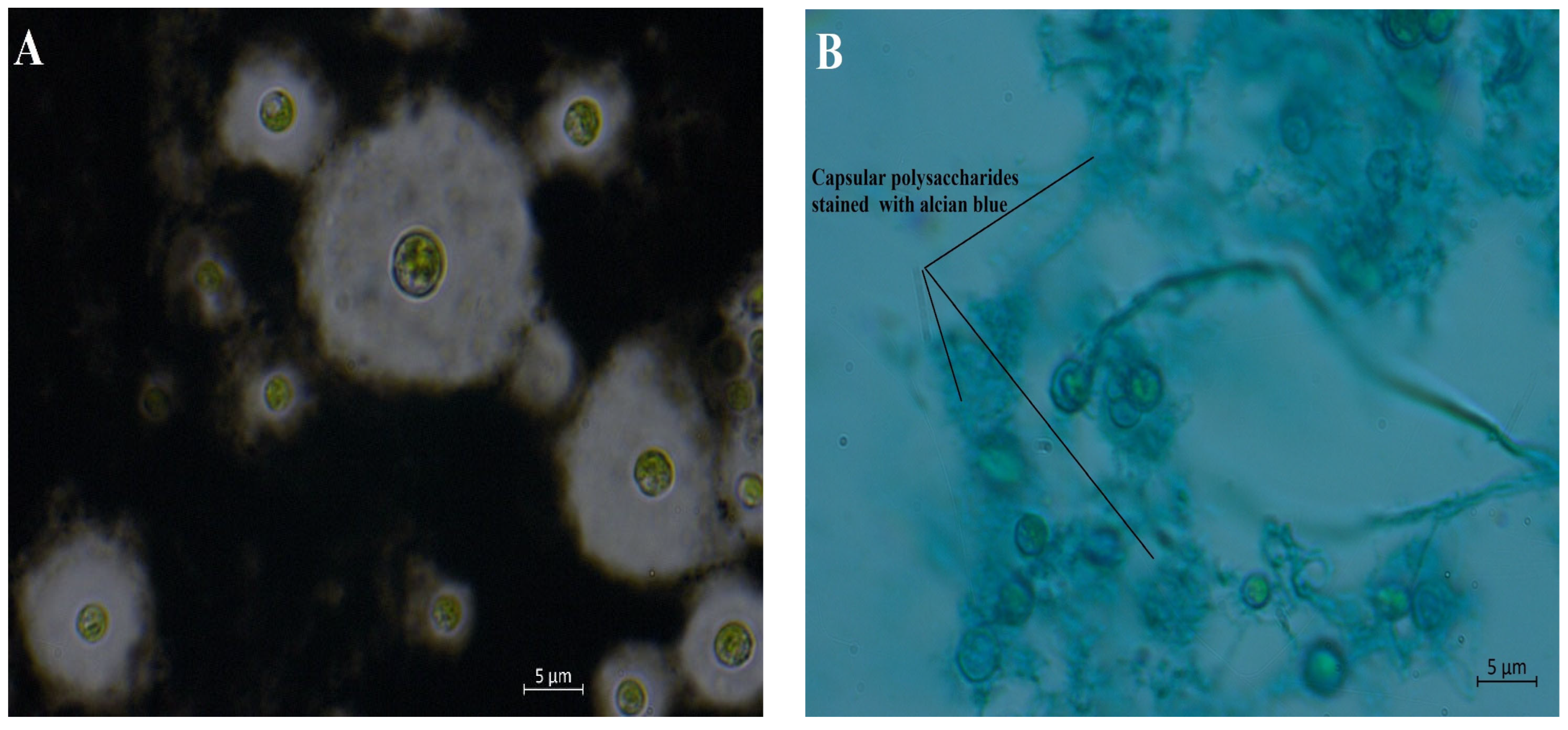
3.2. Microalga Isolation, Molecular Identification, and PSs Staining
3.3. Microalga Cultivation and Growth Rate
3.4. Polysaccharide Production and Yield
3.5. Biochemical Analysis of Extracts and PSs Molecular Weight Determination
3.6. Monosaccharide Composition
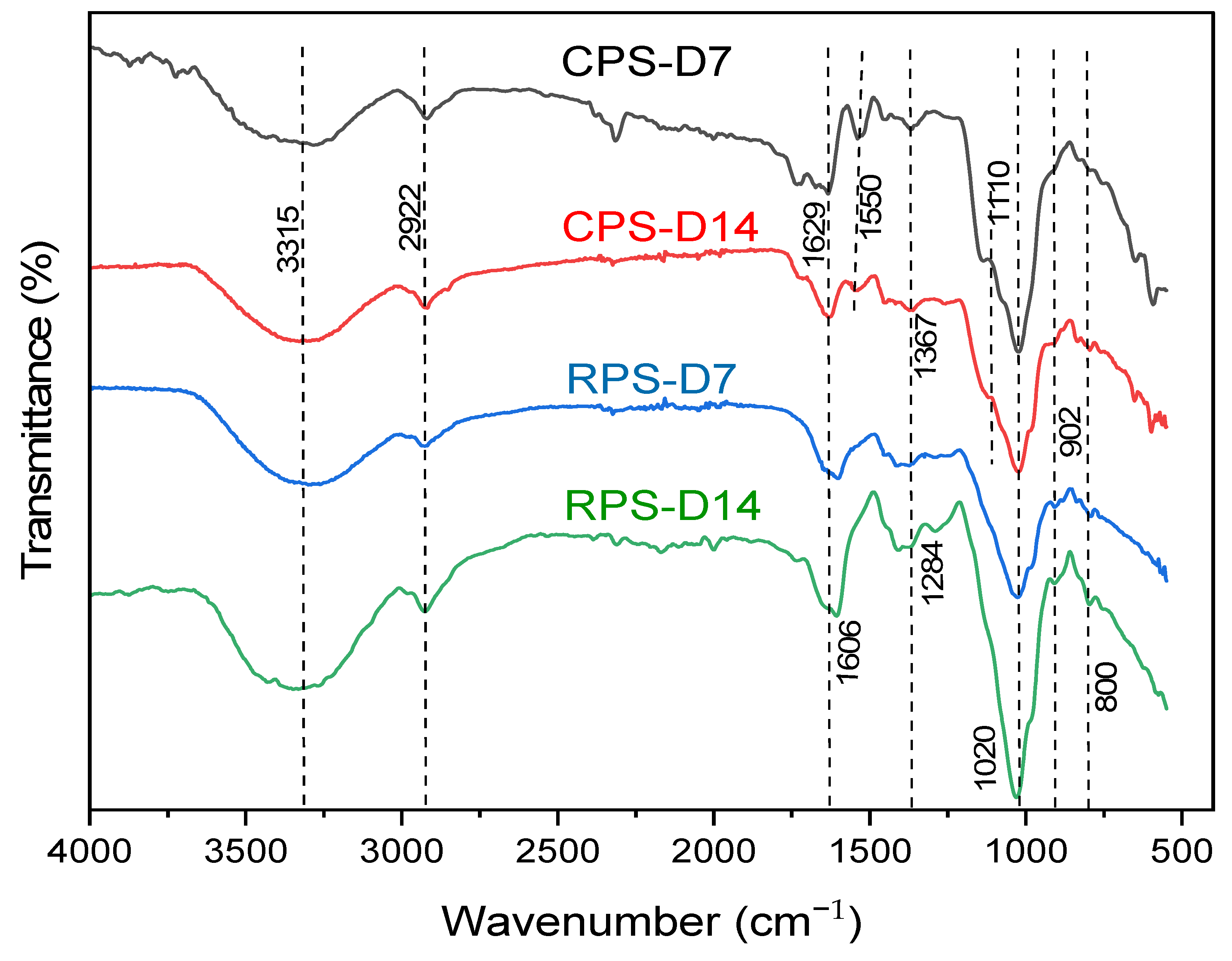
3.7. ATR Analysis of Polysaccharides
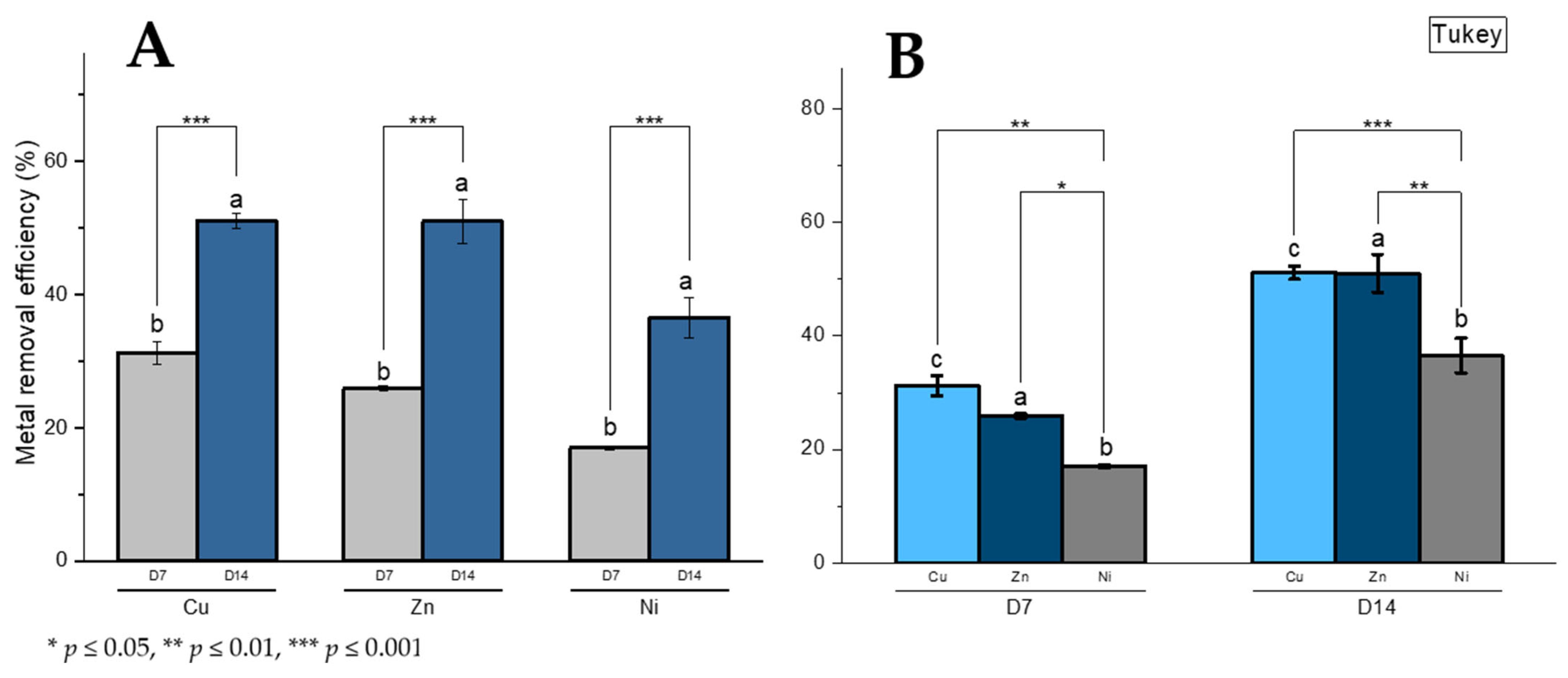
3.8. Metal Removal Capacity and Metal Removal Efficiency Determination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RPSs | Released polysaccharides |
| CPSs | Capsular polysaccharides |
| PSs | Polysaccharides |
| HPLC | high-performance liquid chromatography |
| ATR | Attenuated total reflection |
| BBM | Bold’s Basal medium |
| DW | Dry weight |
| DCC | Direct cell count |
| qe | Metal removal capacity |
| R% | Metal removal efficiency percentage |
| TFA | Trifluoroacetic Acid |
| BCHO | Biomass sugar content |
| Mw | Molecular weight |
| SEC | Size exclusion chromatography |
References
- De Philippis, R.; Paperi, R.; Sili, C. Heavy metal sorption by released polysaccharides and whole cultures of two exopolysaccharide-producing cyanobacteria. Biodegradation 2007, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Febrianto, J.; Kosasih, A.N.; Sunarso, J.; Ju, Y.-H.; Indraswati, N.; Ismadji, S. Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: A summary of recent studies. J. Hazard. Mater. 2009, 162, 616–645. [Google Scholar] [CrossRef] [PubMed]
- Barquilha, C.; Cossich, E.; Tavares, C.; Silva, E. Biosorption of nickel(II) and copper(II) ions in batch and fixed-bed columns by free and immobilized marine algae Sargassum sp. J. Clean. Prod. 2017, 150, 58–64. [Google Scholar] [CrossRef]
- Fawzy, M.A.; Hifney, A.F.; Adam, M.S.; Al-Badaani, A.A. Biosorption of cobalt and its effect on growth and metabolites of Synechocystis pevalekii and Scenedesmus bernardii: Isothermal analysis. Environ. Technol. Innov. 2020, 19, 100953. [Google Scholar] [CrossRef]
- Bauenova, M.O.; Sadvakasova, A.K.; Mustapayeva, Z.O.; Kokociński, M.; Zayadan, B.K.; Wojciechowicz, M.K.; Balouch, H.; Akmukhanova, N.R.; Alwasel, S.; Allakhverdiev, S.I. Potential of microalgae Parachlorella kessleri Bh-2 as bioremediation agent of heavy metals cadmium and chromium. Algal Res. 2021, 59, 102463. [Google Scholar] [CrossRef]
- Shrestha, R.; Ban, S.; Devkota, S.; Sharma, S.; Joshi, R.; Tiwari, A.P.; Kim, H.Y.; Joshi, M.K. Technological trends in heavy metals removal from industrial wastewater: A review. J. Environ. Chem. Eng. 2021, 9, 105688. [Google Scholar] [CrossRef]
- Ciempiel, W.; Czemierska, M.; Szymańska-Chargot, M.; Zdunek, A.; Wiącek, D.; Jarosz-Wilkołazka, A.; Krzemińska, I. Soluble Extracellular Polymeric Substances Produced by Parachlorella kessleri and Chlorella vulgaris: Biochemical Characterization and Assessment of Their Cadmium and Lead Sorption Abilities. Molecules 2022, 27, 7153. [Google Scholar] [CrossRef]
- Ciani, M.; Decorosi, F.; Ratti, C.; De Philippis, R.; Adessi, A. Semi-continuous cultivation of EPS-producing marine cyanobacteria: A green biotechnology to remove dissolved metals obtaining metal-organic materials. N. Biotechnol. 2024, 82, 33–42. [Google Scholar] [CrossRef]
- Xie, Y.; Li, H.; Wang, X.; Ng, I.-S.; Lu, Y.; Jing, K. Kinetic simulating of Cr(VI) removal by the waste Chlorella vulgaris biomass. J. Taiwan. Inst. Chem. Eng. 2014, 45, 1773–1782. [Google Scholar] [CrossRef]
- Goher, M.E.; El-Monem, A.M.A.; Abdel-Satar, A.M.; Ali, M.H.; Hussian, A.-E.M.; Napiórkowska-Krzebietke, A. Biosorption of some toxic metals from aqueous solution using non-living algal cells of Chlorella vulgaris. J. Elementol. 2016, 21, 703–714. [Google Scholar] [CrossRef]
- Abu Al-Rub, F.; El-Naas, M.; Ashour, I.; Al-Marzouqi, M. Biosorption of copper on Chlorella vulgaris from single, binary and ternary metal aqueous solutions. Process. Biochem. 2006, 41, 457–464. [Google Scholar] [CrossRef]
- Ahmad, A.; Bhat, A.; Buang, A. Biosorption of transition metals by freely suspended and Ca-alginate immobilised with Chlorella vulgaris: Kinetic and equilibrium modeling. J. Clean. Prod. 2018, 171, 1361–1375. [Google Scholar] [CrossRef]
- Akhtar, N.; Iqbal, J.; Iqbal, M. Removal and recovery of nickel(II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: Characterization studies. J. Hazard. Mater. 2004, 108, 85–94. [Google Scholar] [CrossRef]
- Mallick, N. Biotechnological potential of Chlorella vulgaris for accumulation of Cu and Ni from single and binary metal solutions. World J. Microbiol. Biotechnol. 2003, 19, 695–701. [Google Scholar] [CrossRef]
- Avinash, B.J.; Mishra. Microbial Exopolysaccharides. Prokaryotes Appl. Bacteriol. Biotechnol. 2013, 9783642313, 1–393. [Google Scholar] [CrossRef]
- Babiak, W.; Krzemińska, I. Extracellular polymeric substances (EPS) as microalgal bioproducts: A review of factors affecting EPS synthesis and application in flocculation processes. Energies 2021, 14, 4007. [Google Scholar] [CrossRef]
- Kronusová, O.; Kaštánek, P.; Koyun, G.; Kaštánek, F.; Brányik, T. Factors Influencing the Production of Extracellular Polysaccharides by the Green Algae Dictyosphaerium chlorelloides and Their Isolation, Purification, and Composition. Microorganisms 2022, 10, 1473. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- Guehaz, K.; Boual, Z.; Abdou, I.; Telli, A.; Belkhalfa, H. Microalgae’s polysaccharides, are they potent antioxidants? Critical review. Arch. Microbiol. 2024, 206, 14. [Google Scholar] [CrossRef]
- Guehaz, K.; Boual, Z.; Telli, A.; Meskher, H.; Belkhalfa, H.; Pierre, G.; Michaud, P.; Adessi, A. A sulfated exopolysaccharide derived from Chlorella sp. exhibiting in vitro anti-α-d-Glucosidase activity. Arch. Microbiol. 2024, 206, 218. [Google Scholar] [CrossRef]
- Corzo, J.; Pérez-Galdona, R.; León-Barrios, M.; Gutiérrez-Navarro, A.M. Alcian blue fixation allows silver staining of the isolated polysaccharide component of bacterial lipopolysaccharides in polyacrylamide gels. Electrophoresis 1991, 12, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Pini, F.; Frascella, A.; Santopolo, L.; Bazzicalupo, M.; Biondi, E.G.; Scotti, C.; Mengoni, A. Exploring the plant-associated bacterial communities in Medicago sativa L. BMC Microbiol. 2012, 12, 78. [Google Scholar] [CrossRef] [PubMed]
- Piampiano, E.; Pini, F.; Biondi, N.; Pastorelli, R.; Giovannetti, L.; Viti, C. Analysis of microbiota in cultures of the green microalga Tetraselmis suecica. Eur. J. Phycol. 2019, 54, 497–508. [Google Scholar] [CrossRef]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic l6S-iike rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef]
- Bruns, T.D.; Lee, S.B.; Taylor, J.W. Amplification and direct sequencing of fungal ribosomal RNA Genes for phylogenetics. PCR Protoc. Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Tamura, K.; Stecher, G.; Kumar, S.; Battistuzzi, F.U. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Zingone, A.; Escalera, L.; Aligizaki, K.; Fernández-Tejedor, M.; Ismael, A.; Montresor, M.; Mozetič, P.; Taş, S.; Totti, C. Toxic marine microalgae and noxious blooms in the Mediterranean Sea: A contribution to the Global HAB Status Report. Harmful Algae 2021, 102, 101843. [Google Scholar] [CrossRef]
- Nigam, H.; Malik, A.; Singh, V. A novel nanoemulsion-based microalgal growth medium for enhanced biomass production. Biotechnol. Biofuels 2021, 14, 111. [Google Scholar] [CrossRef]
- Santo, G.E.; Barros, A.; Costa, M.; Pereira, H.; Trovão, M.; Cardoso, H.; Carvalho, B.; Soares, M.; Correia, N.; Silva, J.T.; et al. Scenedesmus rubescens Heterotrophic Production Strategies for Added Value Biomass. Mar. Drugs 2023, 21, 411. [Google Scholar] [CrossRef]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Barranguet, C.; van Beusekom, S.; Veuger, B.; Neu, T.; Manders, E.; Sinke, J.; Admiraal, W. Studying undisturbed autotrophic biofilms: Still a technical challenge. Aquat. Microb. Ecol. 2004, 34, 1–9. [Google Scholar] [CrossRef]
- Di Pippo, F.; Ellwood, N.T.W.; Guzzon, A.; Siliato, L.; Micheletti, E.; De Philippis, R.; Albertano, P.B. Effect of light and temperature on biomass, photosynthesis and capsular polysaccharides in cultured phototrophic biofilms. J. Appl. Phycol. 2012, 24, 211–220. [Google Scholar] [CrossRef]
- Lowry, O.H.; Randall, R.J. Protein Measurement byt the Folin Reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Zanolla, V.; Biondi, N.; Niccolai, A.; Abiusi, F.; Adessi, A.; Rodolfi, L.; Tredici, M.R. Protein, phycocyanin, and polysaccharide production by Arthrospira platensis grown with LED light in annular photobioreactors. J. Appl. Phycol. 2022, 34, 1189–1199. [Google Scholar] [CrossRef]
- Caichiolo, M.; Zampieri, R.M.; Adessi, A.; Ciani, M.; Caldara, F.; Valle, L.D.; La Rocca, N. Microbial Polysaccharides Extracted from Different Mature Muds of the Euganean Thermal District Show Similar Anti-Inflammatory Activity In Vivo. Int. J. Mol. Sci. 2024, 25, 4999. [Google Scholar] [CrossRef]
- Mota, R.; Vidal, R.; Pandeirada, C.; Flores, C.; Adessi, A.; De Philippis, R.; Nunes, C.; Coimbra, M.A.; Tamagnini, P. Cyanoflan: A cyanobacterial sulfated carbohydrate polymer with emulsifying properties. Carbohydr. Polym. 2020, 229, 115525. [Google Scholar] [CrossRef]
- Flores, C.; Lima, R.T.; Adessi, A.; Sousa, A.; Pereira, S.B.; Granja, P.L.; De Philippis, R.; Soares, P.; Tamagnini, P. Characterization and antitumor activity of the extracellular carbohydrate polymer from the cyanobacterium Synechocystis ΔsigF mutant. Int. J. Biol. Macromol. 2019, 136, 1219–1227. [Google Scholar] [CrossRef]
- Guezoul, O.; Chenchouni, H.; Sekour, M.; Ababsa, L.; Souttou, K.; Doumandji, S. An avifaunal survey of mesic manmade ecosystems “Oases” in algerian hot-hyperarid lands. Saudi J. Biol. Sci. 2013, 20, 37–43. [Google Scholar] [CrossRef]
- Piasecka, A.; Krzemińska, I.; Tys, J. Enrichment of Parachlorella kessleri biomass with bioproducts: Oil and protein by utilization of beet molasses. J. Appl. Phycol. 2017, 29, 1735–1743. [Google Scholar] [CrossRef]
- Barboríková, J.; Šutovská, M.; Kazimierová, I.; Jošková, M.; Fraňová, S.; Kopecký, J.; Capek, P. Extracellular polysaccharide produced by Chlorella vulgaris—Chemical characterization and anti-asthmatic profile. Int. J. Biol. Macromol. 2019, 135, 1–11. [Google Scholar] [CrossRef]
- Markou, G.; Nerantzis, E. Microalgae for high-value compounds and biofuels production: A review with focus on cultivation under stress conditions. Biotechnol. Adv. 2013, 31, 1532–1542. [Google Scholar] [CrossRef] [PubMed]
- Naveed, S.; Li, C.; Lu, X.; Chen, S.; Yin, B.; Zhang, C.; Ge, Y. Microalgal extracellular polymeric substances and their interactions with metal(loid)s: A review. Crit. Rev. Environ. Sci. Technol. 2019, 49, 1769–1802. [Google Scholar] [CrossRef]
- Tounsi, L.; Hentati, F.; Ben Hlima, H.; Barkallah, M.; Smaoui, S.; Fendri, I.; Michaud, P.; Abdelkafi, S. Microalgae as feedstock for bioactive polysaccharides. Int. J. Biol. Macromol. 2022, 221, 1238–1250. [Google Scholar] [CrossRef]
- Soanen, N.; Da Silva, E.; Gardarin, C.; Michaud, P.; Laroche, C. Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour. Technol. 2016, 213, 231–238. [Google Scholar] [CrossRef]
- Halaj, M.; Chválová, B.; Cepák, V.; Lukavský, J.; Capek, P. Searching for microalgal species producing extracellular biopolymers. Chem. Pap. 2018, 72, 2673–2678. [Google Scholar] [CrossRef]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef]
- De Philippis, R. Exocellular polysaccharides from cyanobacteria and their possible applications. FEMS Microbiol. Rev. 1998, 22, 151–175. [Google Scholar] [CrossRef]
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef]
- Madsen, M.A.; Semerdzhiev, S.; Twigg, J.D.; Moss, C.; Bavington, C.D.; Amtmann, A. Environmental modulation of exopolysaccharide production in the cyanobacterium Synechocystis 6803. Appl. Microbiol. Biotechnol. 2023, 107, 6121–6134. [Google Scholar] [CrossRef]
- Barkia, I.; Saari, N.; Manning, S.R. Microalgae for high-value products towards human health and nutrition. Mar. Drugs 2019, 17, 304. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Zille, A.; Micheletti, E.; Moradas-Ferreira, P.; De Philippis, R.; Tamagnini, P. Complexity of cyanobacterial exopolysaccharides: Composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiol. Rev. 2009, 33, 917–941. [Google Scholar] [CrossRef] [PubMed]
- Sushytskyi, L.; Lukáč, P.; Synytsya, A.; Bleha, R.; Rajsiglová, L.; Capek, P.; Pohl, R.; Vannucci, L.; Čopíková, J.; Kaštánek, P. Immunoactive polysaccharides produced by heterotrophic mutant of green microalga Parachlorella kessleri HY1 (Chlorellaceae). Carbohydr. Polym. 2020, 246, 116588. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Takagi, A.; Sasaki, D.; Nakamura, A.; Asayama, M. Characteristics and function of an extracellular polysaccharide from a green alga Parachlorella. Carbohydr. Polym. 2020, 254, 117252. [Google Scholar] [CrossRef]
- Halaj, M.; Matulová, M.; Capek, P. Structural features of biologically active extracellular polysaccharide produced by green microalgae Dictyosphaerium chlorelloides. Int. J. Biol. Macromol. 2022, 214, 152–161. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef]
- Malinowska, E.; Klimaszewska, M.; Strączek, T.; Schneider, K.; Kapusta, C.; Podsadni, P.; Łapienis, G.; Dawidowski, M.; Kleps, J.; Górska, S.; et al. Selenized polysaccharides—Biosynthesis and structural analysis. Carbohydr. Polym. 2018, 198, 407–417. [Google Scholar] [CrossRef]
- Trabelsi, L.; Chaieb, O.; Mnari, A.; Abid-Essafi, S.; Aleya, L. Partial characterization and antioxidant and antiproliferative activities of the aqueous extracellular polysaccharides from the thermophilic microalgae Graesiella sp. BMC Complement. Altern. Med. 2016, 16, 210. [Google Scholar] [CrossRef]
- Li, H.; Su, L.; Chen, S.; Zhao, L.; Wang, H.; Ding, F.; Chen, H.; Shi, R.; Wang, Y.; Huang, Z. Physicochemical Characterization and Functional Analysis of the Polysaccharide from the Edible Microalga Nostoc sphaeroides. Molecules 2018, 23, 508. [Google Scholar] [CrossRef]
- Kamble, P.; Cheriyamundath, S.; Lopus, M.; Sirisha, V.L. Chemical characteristics, antioxidant and anticancer potential of sulfated polysaccharides from Chlamydomonas reinhardtii. J. Appl. Phycol. 2018, 30, 1641–1653. [Google Scholar] [CrossRef]
- Molaei, H.; Jahanbin, K. Structural features of a new water-soluble polysaccharide from the gum exudates of Amygdalus scoparia Spach (Zedo gum). Carbohydr. Polym. 2018, 182, 98–105. [Google Scholar] [CrossRef]
- Manzoor, F.; Karbassi, A.; Golzary, A. Removal of Heavy Metal Contaminants from Wastewater by Using Chlorella vulgaris Beijerinck: A Review. Curr. Environ. Manag. 2019, 6, 174–187. [Google Scholar] [CrossRef]
- Urrutia, C.; Yañez-Mansilla, E.; Jeison, D. Bioremoval of heavy metals from metal mine tailings water using microalgae biomass. Algal Res. 2019, 43, 101659. [Google Scholar] [CrossRef]
- Mehta, S.K.; Gaur, J.P. Characterization and optimization of Ni and Cu sorption from aqueous solution by Chlorella vulgaris. Ecol. Eng. 2001, 18, 1–13. [Google Scholar] [CrossRef]
- Mehta, S.; Gaur, J. Concurrent sorption of Ni2+ and Cu2+ by Chlorella vulgaris from a binary metal solution. Appl. Microbiol. Biotechnol. 2001, 55, 379–382. [Google Scholar] [CrossRef]
- Mubashar, M.; Naveed, M.; Mustafa, A.; Ashraf, S.; Baig, K.S.; Alamri, S.; Siddiqui, M.H.; Zabochnicka-Świątek, M.; Szota, M.; Kalaji, H.M. Experimental Investigation of Chlorella vulgaris and Enterobacter sp. MN17 for Decolorization and Removal of Heavy Metals from Textile Wastewater. Water 2020, 12, 3034. [Google Scholar] [CrossRef]
- Monteiro, C.M.; Castro, P.M.L.; Malcata, F.X. Metal uptake by microalgae: Underlying mechanisms and practical applications. Biotechnol. Prog. 2012, 28, 299–311. [Google Scholar] [CrossRef]
- Petrovič, A.; Simonič, M. Removal of heavy metal ions from drinking water by alginate-immobilised Chlorella sorokiniana. Int. J. Environ. Sci. Technol. 2016, 13, 1761–1780. [Google Scholar] [CrossRef]
- Abdel-ghani, N.T.; El-chaghaby, G.A. Biosorption for Metal Ions Removal From Aqueous Solutions: A Review of recent studies. Int. J. Latest Res. Sci. Technol. 2014, 3, 24–42. [Google Scholar]





| Parameter | Starting Culture ‘Day 0’ | ‘Day 7’ | ‘Day 14’ |
|---|---|---|---|
| Optical density | 1.59 ± 0.044 | 6.99 ± 0.172 | 9.70 ± 0.145 |
| Cell number (cell/mL) | (6.45 ± 0.24) × 106 | (7.15 ± 0.49) × 107 | (6.51 ± 0.185) × 107 |
| Dry weight (g L−1) | 0.5 ± 0.013 | 2.30 ± 0.036 | 4.33 ± 0.005 |
| Specific growth rate (µmax d−1) | - | 0.572 ± 0.098 | - |
| Biomass productivity (g L−1 d−1) | - | 0.257 ± 0.056 | 0.309 ± 0.042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guehaz, K.; Boual, Z.; Daly, G.; Ciani, M.; Belkhalfa, H.; Adessi, A. Exploring Growth Phase Effect on Polysaccharide Composition and Metal Binding Properties in Parachlorella hussii. Polysaccharides 2025, 6, 58. https://doi.org/10.3390/polysaccharides6030058
Guehaz K, Boual Z, Daly G, Ciani M, Belkhalfa H, Adessi A. Exploring Growth Phase Effect on Polysaccharide Composition and Metal Binding Properties in Parachlorella hussii. Polysaccharides. 2025; 6(3):58. https://doi.org/10.3390/polysaccharides6030058
Chicago/Turabian StyleGuehaz, Karima, Zakaria Boual, Giulia Daly, Matilde Ciani, Hakim Belkhalfa, and Alessandra Adessi. 2025. "Exploring Growth Phase Effect on Polysaccharide Composition and Metal Binding Properties in Parachlorella hussii" Polysaccharides 6, no. 3: 58. https://doi.org/10.3390/polysaccharides6030058
APA StyleGuehaz, K., Boual, Z., Daly, G., Ciani, M., Belkhalfa, H., & Adessi, A. (2025). Exploring Growth Phase Effect on Polysaccharide Composition and Metal Binding Properties in Parachlorella hussii. Polysaccharides, 6(3), 58. https://doi.org/10.3390/polysaccharides6030058







