Exploring the Potential of 3D-Printable Agar–Urea Hydrogels as an Efficient Method of Delivering Nitrogen in Agricultural Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Evaluation of Natural Hydrogels for Initial Screening
2.3. Solvent Casting of the Agar-Based Hydrogel Formulations
2.4. Characterizations of Solvent Cast Agar-Based Hydrogel Formulations
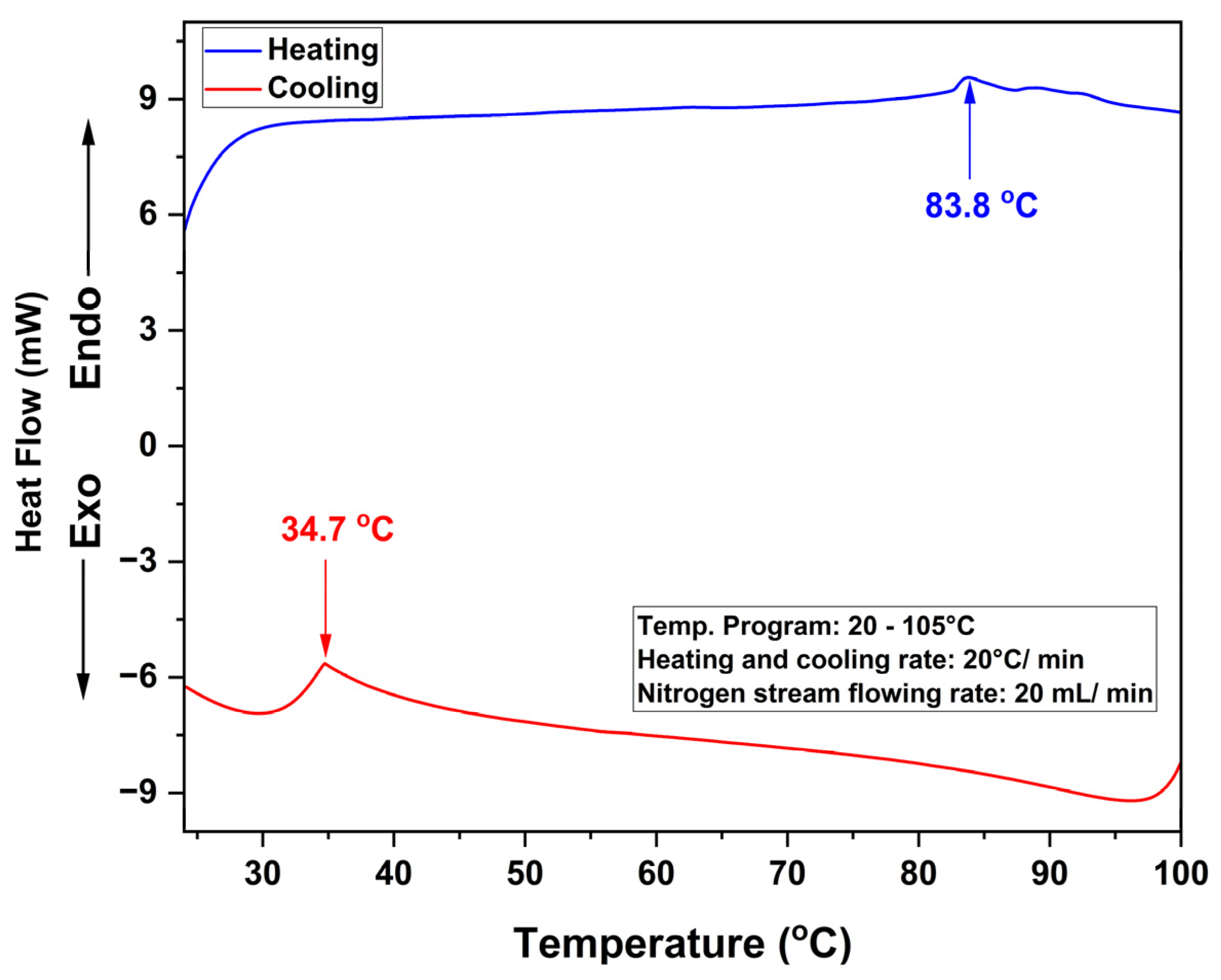
2.4.1. Differential Scanning Calorimetry
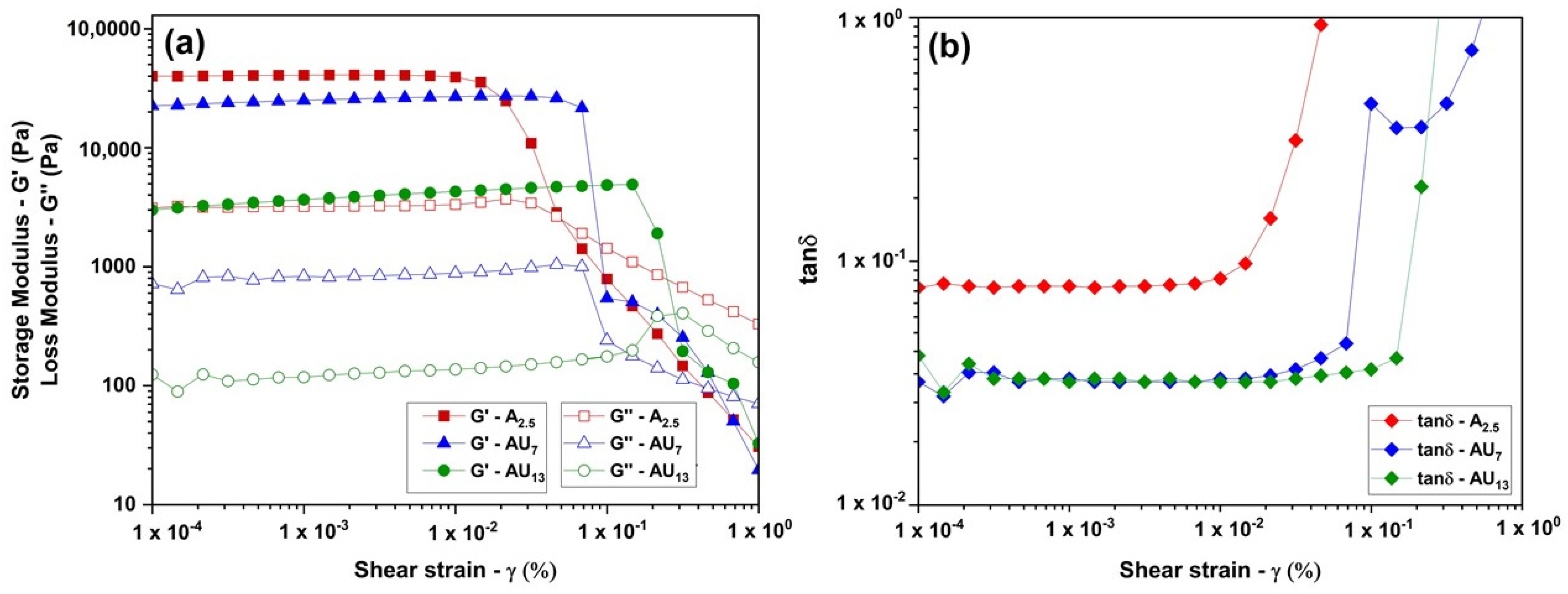
2.4.2. Rheological Analysis
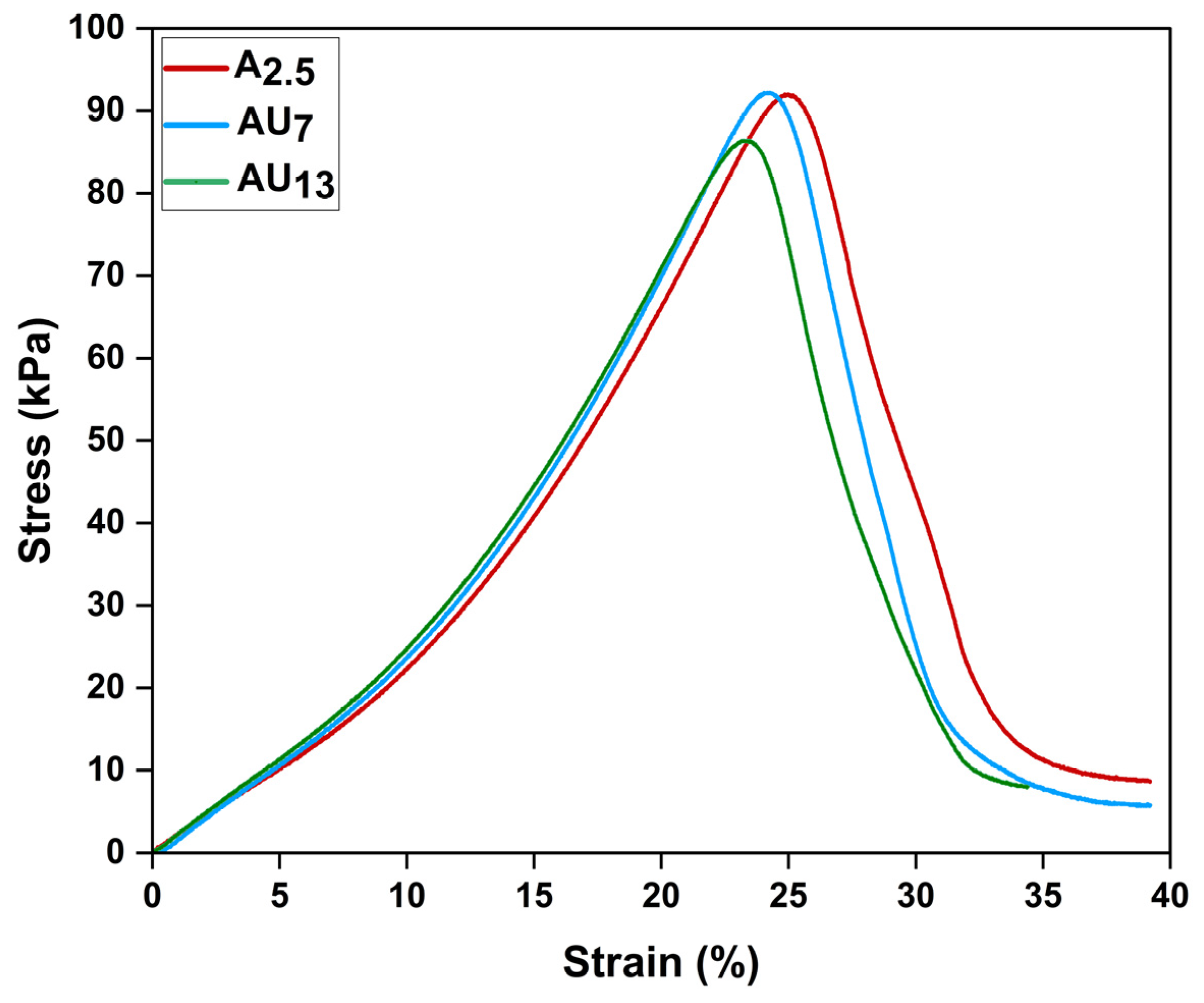
2.4.3. Mechanical Properties
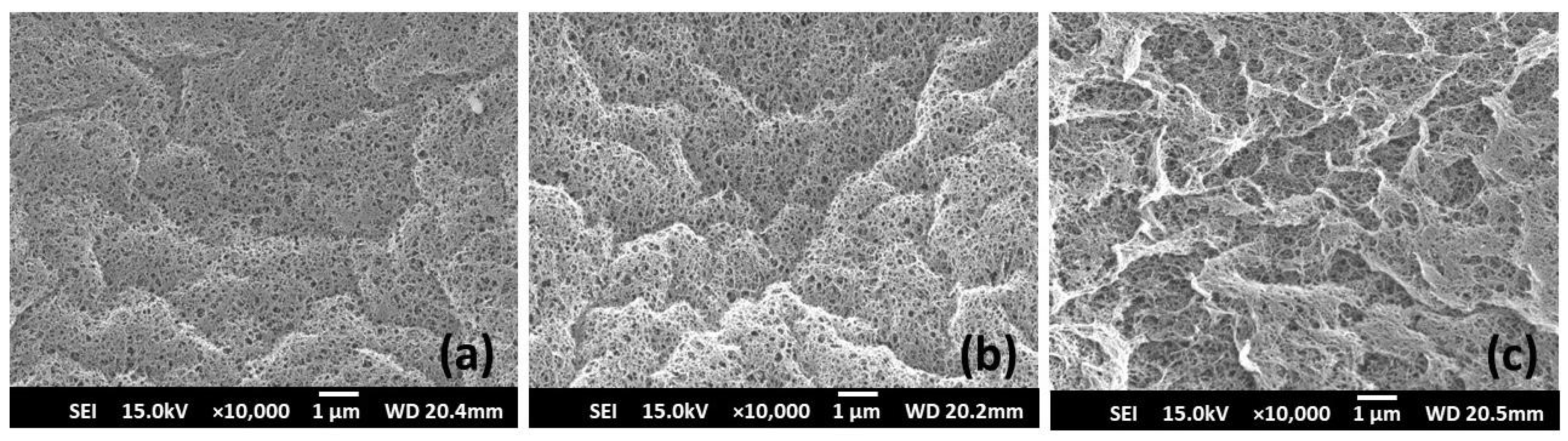
2.4.4. Scanning Electron Microscopy (SEM)
2.4.5. Water Sorption Capacity
2.5. Preparation of Agar-Based Hydrogel Formulations for 3D Printing
2.6. Determination of Ideal 3D Printing Parameters
2.7. 3D Printing
2.8. Nitrogen Release Studies of 3D Printed Agar-Based Hydrogel Formulations
3. Results and Discussion
3.1. Initial Screening for the Selection of Most Suitable Natural Hydrogel
3.2. Characterizations of Solvent Cast Agar-Based Hydrogel Formulations
3.2.1. Differential Scanning Calorimetry
3.2.2. Rheological Analysis
3.2.3. Mechanical Properties
3.2.4. SEM Morphology
3.2.5. Water Sorption Capacity
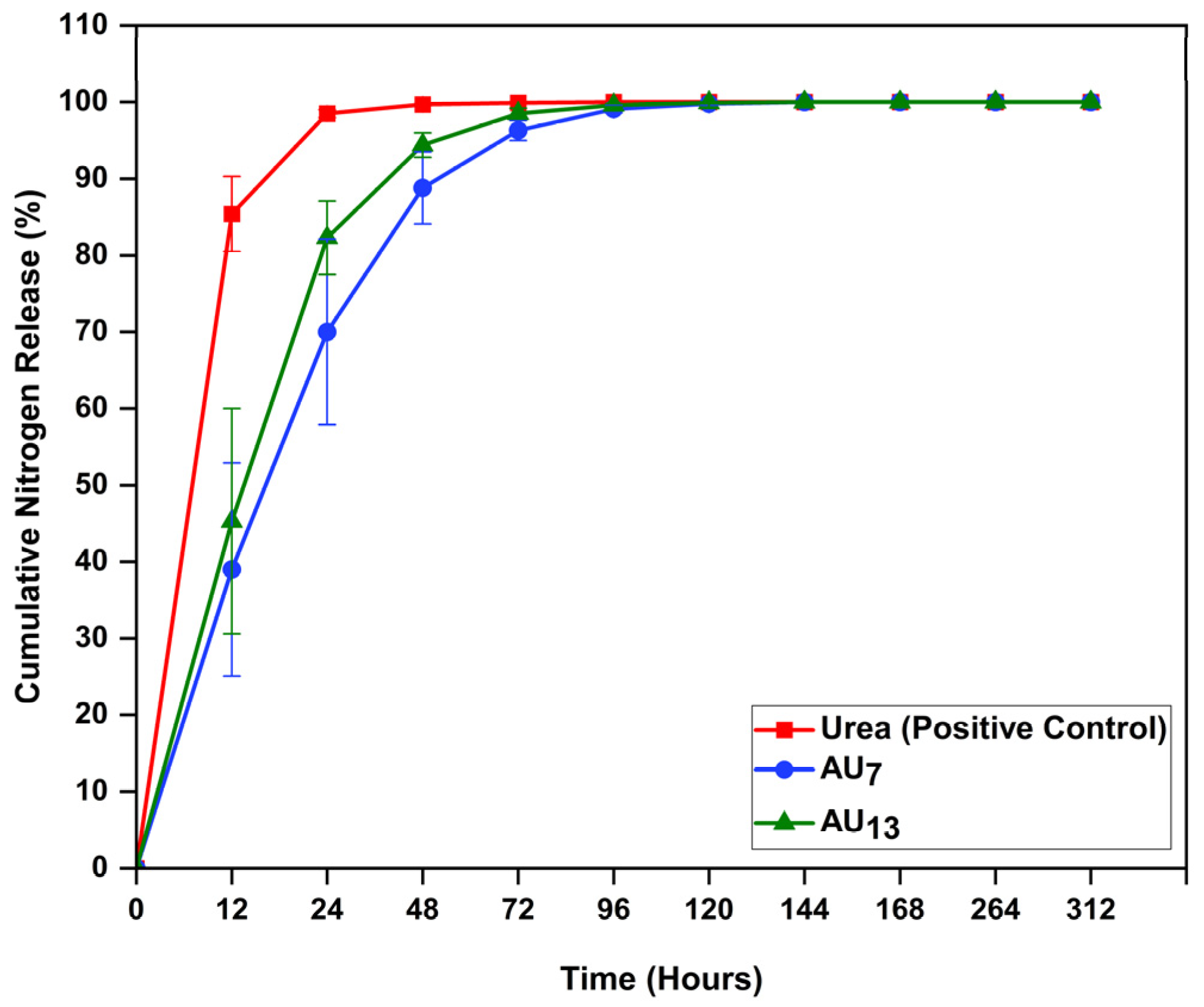
3.3. Nitrogen Release Studies of 3D Printed Agar-Based Hydrogel Formulations in Soil Medium
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Okolie, C.C.; Danso-Abbeam, G.; Groupson-Paul, O.; Ogundeji, A.A. Climate-Smart Agriculture Amidst Climate Change to Enhance Agricultural Production: A Bibliometric Analysis. Land 2023, 12, 50. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A. Urea-Hydroxyapatite Nanohybrids for Slow Release of Nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, L.; Xie, F.; Bao, X.; Liu, H.; Ji, Z.; Chen, L. One-step method to prepare starch-based superabsorbent polymer for slow release of fertilizer. Chem. Eng. J. 2017, 309, 607–616. [Google Scholar] [CrossRef]
- Tapia-Hernández, J.A.; Madera-Santana, T.J.; Rodríguez-Félix, F.; Barreras-Urbina, C.G. Controlled and Prolonged Release Systems of Urea from Micro-and Nanomaterials as an Alternative for Developing a Sustainable Agriculture: A Review. J. Nanomater. 2022, 2022, 5697803. [Google Scholar] [CrossRef]
- Vejan, P.; Khadiran, T.; Abdullah, R.; Ahmad, N. Controlled release fertilizer: A review on developments, applications and potential in agriculture. J. Control. Release 2021, 339, 168–3659. [Google Scholar] [CrossRef]
- Wang, X. Managing Land Carrying Capacity: Key to Achieving Sustainable Production Systems for Food Security. Land 2022, 11, 484. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Navarro, M.; Ashraf, M.; Akram, A.; Munné-Bosch, S. Nanofertilizer use for sustainable agriculture: Advantages and limitations. Plant Sci. 2019, 289, 110270. [Google Scholar] [CrossRef]
- Latha, M.; Subramanian, K.S.; Sharmila, D.J.S.; Raja, K.; Rajkishore, S.K.; Chitdeshwari, T. Urea-Lignin/Chitosan Nanocomposite as Slow-Release Nanofertilizer. ACS Agric. Sci. Technol. 2023, 3, 463–476. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.; Yao, H. Urea-based nitrogen fertilization in agriculture: A key source of N2O emissions and recent development in mitigating strategies. Arch. Agron. Soil Sci. 2022, 69, 663–678. [Google Scholar] [CrossRef]
- Hayashi, K.; Itsubo, N. Damage factors of stratospheric ozone depletion on human health impact with the addition of nitrous oxide as the largest contributor in the 2000s. Int. J. Life Cycle. 2023, 28, 990–1002. [Google Scholar] [CrossRef]
- Dhiman, S.; Yadav, A.; Debnath, N.; Das, S. Application of Core/Shell Nanoparticles in Smart Farming: A Paradigm Shift for Making the Agriculture Sector More Sustainable. J. Agric. Food Chem. 2021, 69, 3267–3283. [Google Scholar] [CrossRef]
- Chen, F.; Miao, C.; Duan, Q.; Jiang, S.; Liu, H.; Ma, L.; Li, Z.; Bao, X.; Lan, B.; Chen, L.; et al. Developing slow release fertilizer through in-situ radiation-synthesis of urea-embedded starch-based hydrogels. Ind. Crops Prod. 1159, 2023, 19171. [Google Scholar] [CrossRef]
- Kavitha, R.; Latifah, O.; Ahmed, O.H.; Charles, P.W.; Susilawati, K. Potential of Rejected Sago Starch as a Coating Material for Urea Encapsulation. Polymers 2023, 15, 1863. [Google Scholar] [CrossRef] [PubMed]
- Pushpamalar, J.; Langford, S.J.; Ahmad, M.B.; Lim, Y.Y.; Hashim, K. Eco-friendly smart hydrogels for soil conditioning and sustain release fertilizer. Int. J. Environ. Sci. Technol. 2018, 15, 2059–2074. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Di Francesco, D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef] [PubMed]
- Kaur, P.; Agrawal, R.; Pfeffer, F.M.; Williams, R.; Bohidar, H.B. Hydrogels in Agriculture: Prospects and Challenges. J. Polym. Environ. 2023, 31, 3701–3718. [Google Scholar] [CrossRef]
- Qu, B.; Luo, Y. Chitosan-based hydrogel beads: Preparations, modifications and applications in food and agriculture sectors—A review. Int. J. Biol. Macromol. 2020, 152, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Chakraborty, E. Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni. Suef. Univ. J. Basic. Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef]
- Sun, Z.; Song, C.; Wang, C.; Hu, Y.; Wu, J. Hydrogel-Based Controlled Drug Delivery for Cancer Treatment: A Review. Mol. Pharm. 2020, 17, 373–391. [Google Scholar] [CrossRef]
- Rudzinski, W.E.; Dave, A.M.; Vaishnav, U.H.; Kumbar, S.G.; Kulkarni, A.R.; Aminabhavi, T.M. Hydrogels as controlled release devices in agriculture. Des. Monomers Polym. 2002, 5, 39–65. [Google Scholar] [CrossRef]
- Xiang, Z.; Tang, N.; Jin, X.; Gao, W. Fabrications and applications of hemicellulose-based bio-adsorbents. Carbohydr. Polym. 1189, 2022, 27845. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; He, J.; Guo, B. Functional Hydrogels as Wound Dressing to Enhance Wound Healing. ACS Nano 2021, 15, 12687–12722. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Lv, Y.; Xi, X.; Dai, L.; Tong, S.; Chen, Z. Hydrogel as a Superwetting Surface Design Material for Oil/Water Separation: A Review. Adv. Mater. Interfaces 2002, 2021, 8030. [Google Scholar] [CrossRef]
- Singh, N.; Agarwal, S.; Jain, A.; Khan, S. 3-Dimensional cross linked hydrophilic polymeric network ‘hydrogels’: An agriculture boom. Agric. Water Manag. 2021, 253, 106939. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef]
- Sahmat, S.S.; Rafii, M.Y.; Oladosu, Y.; Jusoh, M.; Hakiman, M.; Mohidin, H. A Systematic Review of the Potential of a Dynamic Hydrogel as a Substrate for Sustainable Agriculture. Horticulturae 2022, 8, 1026. [Google Scholar] [CrossRef]
- Patra, S.K.; Poddar, R.; Brestic, M.; Acharjee, P.U.; Bhattacharya, P.; Sengupta, S.; Pal, P.; Bam, N.; Biswas, B.; Barek, V.; et al. Prospects of Hydrogels in Agriculture for Enhancing Crop and Water Productivity under Water Deficit Condition. Int. J. Polym. Sci. 2022, 2022, 4914836. [Google Scholar] [CrossRef]
- Vo, P.T.; Nguyen, H.T.; Trinh, H.T.; Nguyen, V.M.; Le, A.-T.; Tran, H.Q.; Nguyen, T.T.T. The nitrogen slow-release fertilizer based on urea incorporating chitosan and poly(vinyl alcohol) blend. Environ. Technol. Innov. 2021, 22, 101528. [Google Scholar] [CrossRef]
- Pang, L.; Gao, Z.; Feng, H.; Wang, S.; Wang, Q. Cellulose based materials for controlled release formulations of agrochemicals: A review of modifications and applications. J. Control. Release 2019, 316, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Li, S.; Guan, H.; Zhang, S.; Zhang, Y.; Zhang, M.; Yu, Y.; Chen, X. Preparation and Properties of a Novel Biodegradable Composite Hydrogel Derived from Gelatin/Chitosan and Polylactic Acid as Slow-Release N Fertilizer. Polymers 2023, 15, 997. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, H.; Li, W.; Liu, Z.; Liu, Y.; Wei, H.; Li, J. Synthesis and characterization of double-network hydrogels based on sodium alginate and halloysite for slow release fertilizers. Int. J. Biol. Macromol. 2020, 164, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhu, Y.; Liu, M.; Liu, Z.; Zhou, T.; Liu, Y.; Cheng, D. Network interpenetrating slow-release nitrogen fertilizer based on carrageenan and urea: A new low-cost water and fertilizer regulation carrier. Int. J. Biol. Macromol. 2023, 242, 124858. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.; Ogugbue, C.; Okpokwasili, G. Production and Application of Agar-based Slow-release Fertilizers, in the Bioremediation of Petroleum Hydrocarbon-impacted Soil. Br. Biotechnol. J. 2016, 13, 1–13. [Google Scholar] [CrossRef]
- Lin, J.; Jiao, G.; Kermanshahi-Pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef] [PubMed]
- Pandya, Y.; Bakshi, M.; Sharma, A.; Pandya, Y.H.; Pandya, H. Agar-agar extraction, structural properties and applications: A review. Pharma Innov. J. 2022, 11, 1151–1157. [Google Scholar]
- Cebrián-Lloret, V.; Martínez-Abad, A.; López-Rubio, A.; Martínez-Sanz, M. Exploring alternative red seaweed species for the production of agar-based hydrogels for food applications. Food Hydrocoll. 2024, 146, 109177. [Google Scholar] [CrossRef]
- Yin, Z.C.; Wang, Y.L.; Wang, K. A pH-responsive composite hydrogel beads based on agar and alginate for oral drug delivery. J. Drug Deliv. Sci. Technol. 2018, 43, 12–18. [Google Scholar] [CrossRef]
- Smułek, W.; Grząbka-Zasadzińska, A.; Kilian, A.; Ciesielczyk, F.; Borysiak, S.; Baranowska, H.M.; Walkowiak, K.; Kaczorek, E.; Jarzębski, M. Design of vitamin-loaded emulsions in agar hydrogel matrix dispersed with plant surfactants. Food Biosci. 2023, 53, 102559. [Google Scholar] [CrossRef]
- Baek, J.S.; Carlomagno, C.; Muthukumar, T.; Kim, D.; Park, J.H.; Song, J.E.; Migliaresi, C.; Motta, A.; Reis, R.L.; Khang, G. Evaluation of Cartilage Regeneration in Gellan Gum/agar Blended Hydrogel with Improved Injectability. Macromol. Res. 2019, 27, 558–564. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Ho, C.L. Gracilaria as the major source of agar for food, health and biotechnology applications. In Sustainable Global Resources of Seaweeds Volume 2: Food, Pharmaceutical and Health Applications; Springer International Publishing: Cham, Switzerland, 2022; Volume 2, pp. 145–161. [Google Scholar] [CrossRef]
- Lee, W.K.; Lim, Y.Y.; Leow, A.T.C.; Namasivayam, P.; Abdullah, J.O.; Ho, C.L. Biosynthesis of agar in red seaweeds: A review. Carbohydr. Polym. 2017, 164, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Global Agar-Agar Industry Research Report 2023, Competitive Landscape, Market Size, Regional Status and Prospect. Available online: https://www.researchreportsworld.com/global-agar-agar-industry-research-report-2023-competitive-landscape-market-22378959 (accessed on 25 January 2024).
- Khan, M.J.; Guo, Q.; Varley, R. Facile one pot synthesis of strong epoxy/agar hybrid hydrogels. J. Polym. Res. 2019, 26, 291. [Google Scholar] [CrossRef]
- Duman, O.; Polat, T.G.; Diker, C.Ö.; Tunç, S. Agar/κ-carrageenan composite hydrogel adsorbent for the removal of Methylene Blue from water. Int. J. Biol. Macromol. 2020, 160, 823–835. [Google Scholar] [CrossRef]
- Sansonetti, A.; Bertasa, M.; Canevali, C.; Rabbolini, A.; Anzani, M.; Scalarone, D. A review in using agar gels for cleaning art surfaces. J. Cult. Herit. 2020, 44, 285–296. [Google Scholar] [CrossRef]
- Li, W.; Wu, Z.; Zhao, J.; Jiang, M.; Yuan, L.; Guo, Y.; Li, S.; Hu, L.; Xie, X.; Zhang, Y.; et al. Fabrication of dual physically cross-linked polyvinyl alcohol/agar hydrogels with mechanical stability and antibacterial activity for wound healing. Int. J. Biol. Macromol. 2023, 247, 125652. [Google Scholar] [CrossRef]
- Sadrearhami, Z.; Morshed, M.; Varshosaz, J. Production and evaluation of polyblend of agar and polyacrylonitrile nanofibers for in vitro release of methotrexate in cancer therapy. Fibers Polym. 2015, 16, 254–262. [Google Scholar] [CrossRef]
- Date, P.; Ottoor, D. pH Dependent Controlled Release of CTAB Incorporated Dipyridamole Drug from Agar-Based Hydrogel. Polym. Plast. Technol. Eng. 2016, 55, 403–413. [Google Scholar] [CrossRef]
- Olak, T.; Turan, A.; Alpaslan, D.; Dudu, T.E.; Aktaş, N. Developing poly(Agar-co-Glycerol-co-Thyme Oil) based organo-hydrogels for the controlled drug release applications. J. Drug Deliv. Sci. Technol. 2020, 60, 102088. [Google Scholar] [CrossRef]
- Singh, B.; Sharma, D.K.; Negi, S.; Dhiman, A. Synthesis and characterization of agar-starch based hydrogels for slow herbicide delivery applications. Int. J. Plast. Technol. 2015, 19, 263–274. [Google Scholar] [CrossRef]
- Pérez-Pérez, M.P.; Gómez, E.; Sebastián, M.A. Delphi prospection on additive manufacturing in 2030: Implications for education and employment in Spain. Materials 2018, 11, 1500. [Google Scholar] [CrossRef]
- Javaid, M.; Haleem, A.; Singh, R.P.; Suman, R.; Rab, S. Role of additive manufacturing applications towards environmental sustainability. Adv. Ind. Eng. Polym. Res. 2021, 4, 312–322. [Google Scholar] [CrossRef]
- Kaliaraj, G.S.; Shanmugam, D.K.; Dasan, A.; Mosas, K.K.A. Hydrogels—A Promising Materials for 3D Printing Technology. Gels 2023, 9, 260. [Google Scholar] [CrossRef]
- Distler, T.; Boccaccini, A.R. 3D printing of electrically conductive hydrogels for tissue engineering and biosensors–A review. Acta Biomater. 2020, 101, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, X.; Zheng, Y.; Chen, K.; Zeng, W.; Wu, X. Recent advances in the 3D printing of electrically conductive hydrogels for flexible electronics. J. Mater. Chem. C Mater. 2022, 10, 5380–5399. [Google Scholar] [CrossRef]
- Rajabi, M.; McConnell, M.; Cabral, J.; Ali, M.A. Chitosan hydrogels in 3D printing for biomedical applications. Carbohydr. Polym. 2021, 260, 117768. [Google Scholar] [CrossRef]
- Liu, S.; Chen, X.; Zhang, Y. Hydrogels and hydrogel composites for 3D and 4D printing applications. In 3D and 4D Printing of Polymer Nanocomposite Materials: Processes, Applications, and Challenges; Elsevier: Amsterdam, The Netherlands, 2020; pp. 427–465. [Google Scholar] [CrossRef]
- Gomez-Florit, M.; Pardo, A.; Domingues, R.M.A.; Graça, A.L.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Natural-Based Hydrogels for Tissue Engineering Applications. Molecules 2020, 25, 5858. [Google Scholar] [CrossRef] [PubMed]
- Bergonzi, C.; Bianchera, A.; Remaggi, G.; Ossiprandi, M.C.; Bettini, R.; Elviri, L. 3D Printed Chitosan/Alginate Hydrogels for the Controlled Release of Silver Sulfadiazine in Wound Healing Applications: Design, Characterization and Antimicrobial Activity. Micromachines 2023, 14, 137. [Google Scholar] [CrossRef]
- Mandal, S.; Nagi, G.K.; Corcoran, A.A.; Agrawal, R.; Dubey, M.; Hunt, R.W. Algal polysaccharides for 3D printing: A review. Carbohydr. Polym. 2023, 300, 120267. [Google Scholar] [CrossRef]
- Serizawa, R.; Shitara, M.; Gong, J.; Makino, M.; Kabir, M.H.; Furukawa, H. 3D jet printer of edible gels for food creation. In Proceedings of the Behavior and Mechanics of Multifunctional Materials and Composites, San Diego, CA, USA, 9–13 March 2014; 2014; 9058, pp. 80–85. [Google Scholar] [CrossRef]
- Rahman, J.M.H.; Shiblee, M.N.I.; Ahmed, K.; Khosla, A.; Kawakami, M.; Furukawa, H. Rheological and mechanical properties of edible gel materials for 3D food printing technology. Heliyon 2020, 6, e05859. [Google Scholar] [CrossRef]
- Crisostomo, J.L.B.; Dizon, J.R.C. 3D Printing Applications in Agriculture, Food Processing, and Environmental Protection and Monitoring. Adv. Sustain. Sci. Eng. Technol. 2021, 3, 0210201. [Google Scholar] [CrossRef]
- Gungula, D.T.; Andrew, F.P.; Joseph, J.; Kareem, S.A.; Barminas, J.T.; Adebayo, E.F.; Saddiq, A.M.; Tame, V.T.; Dere, I.; Ahinda, W.J.; et al. Formulation and characterization of water retention and slow-release urea fertilizer based on Borassus aethiopum starch and Maesopsis eminii hydrogels. Results Mater. 2021, 12, 100223. [Google Scholar] [CrossRef]
- Wang, K.; Hou, J.; Zhang, S.; Hu, W.; Yi, G.; Chen, W.; Cheng, L.; Zhang, Q. Preparation of a new biochar-based microbial fertilizer: Nutrient release patterns and synergistic mechanisms to improve soil fertility. Sci. Total Environ. 2023, 860, 160478. [Google Scholar] [CrossRef]
- Arafa, E.G.; Sabaa, M.W.; Mohamed, R.R.; Kamel, E.M.; Elzanaty, A.M.; Mahmoud, A.M.; Abdel-Gawad, O.F. Eco-friendly and biodegradable sodium alginate/quaternized chitosan hydrogel for controlled release of urea and its antimicrobial activity. Carbohydr. Polym. 2022, 291, 119555. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Guillen, M.C.; Gimenez, B.; Lopez-Caballero, M.E.; Montero, M.P. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Atef, M.; Rezaei, M.; Behrooz, R. Preparation and characterization agar-based nanocomposite film reinforced by nanocrystalline cellulose. Int. J. Biol. Macromol. 2014, 70, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Siddhanta, A.K.; Rakshit, A.K.; Bhattacharya, A.; Ghosh, P.K. On the properties of agar gel containing ionic and non-ionic surfactants. Int. J. Biol. Macromol. 2005, 35, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Sawai, Y.; Takada, M. The Effect of Apparent Molecular Weight and Components of Agar on Gel Formation. Food Sci. Technol. Res. 2001, 7, 280–284. [Google Scholar] [CrossRef]
- Graham, S.; Marina, P.F.; Blencowe, A. Thermoresponsive polysaccharides and their thermoreversible physical hydrogel networks. Carbohydr. Polym. 2019, 207, 143–159. [Google Scholar] [CrossRef]
- Thompson, B.R.; Horozov, T.S.; Stoyanov, S.D.; Paunov, V.N. An ultra melt-resistant hydrogel from food grade carbohydrates. RSC Adv. 2017, 7, 45535–45544. [Google Scholar] [CrossRef]
- Pino-Ramos, V.H.; Duarte-Peña, L.; Bucio, E. Highly crosslinked agar/acrylic acid hydrogels with antimicrobial properties. Gels 2021, 7, 183. [Google Scholar] [CrossRef]
- Fernández, E.; López, D.; Mijangos, C.; Duskova-Smrckova, M.; Ilavsky, M.; Dusek, K. Rheological and thermal properties of agarose aqueous solutions and hydrogels. J. Polym. Sci. B Polym. Phys. 2008, 46, 322–328. [Google Scholar] [CrossRef]
- Cofelice, M.; Messia, M.C.; Marconi, E.; Cuomo, F.; Lopez, F. Effect of the xanthan gum on the rheological properties of alginate hydrogels. Food Hydrocoll. 2023, 142, 108768. [Google Scholar] [CrossRef]
- Ramin, M.A.; Latxague, L.; Sindhu, K.R.; Chassande, O.; Barthélémy, P. Low molecular weight hydrogels derived from urea based-bolaamphiphiles as new injectable biomaterials. Biomaterials 2017, 145, 72–80. [Google Scholar] [CrossRef]
- Norziah, M.H.; Foo, S.L.; Karim, A.A. Rheological studies on mixtures of agar (Gracilaria changii) and κ-carrageenan. Food Hydrocoll. 2006, 20, 204–217. [Google Scholar] [CrossRef]
- Wei, X.; Bao, X.; Yu, L.; Liu, H.; Lu, K.; Chen, L.; Bai, L.; Zhou, X.; Li, Z.; Li, W. Correlation Between Gel Strength of Starch-Based Hydrogel and Slow Release Behavior of Its Embedded Urea. J. Polym. Env. 2020, 28, 863–870. [Google Scholar] [CrossRef]
- Yu, H.; Yu, J.; Zhan, M. Study on mechanical behaviour of agar gel in compression mode. Bulg. Chem. Commun. 2018, 1, 225. [Google Scholar]
- Shamsuri, A.A.; Daik, R. Utilization of an ionic liquid/urea mixture as a physical coupling agent for agarose/talc composite films. Materials 2013, 6, 682–698. [Google Scholar] [CrossRef] [PubMed]
- Nayar, V.T.; Weiland, J.D.; Nelson, C.S.; Hodge, A.M. Elastic and viscoelastic characterization of agar. J. Mech. Behav. Biomed. Mater. 2012, 7, 60–68. [Google Scholar] [CrossRef]
- Giordano, A.; Caruso, M.R.; Lazzara, G. New tool for sustainable treatments: Agar spray—Research and practice. Herit. Sci. 2022, 10, 123. [Google Scholar] [CrossRef]
- Zhang, L.; Xiao, Q.; Xiao, Z.; Zhang, Y.; Weng, H.; Chen, F.; Xiao, A. Hydrophobic modified agar: Structural characterization and application in encapsulation and release of curcumin. Carbohydr. Polym. 2023, 308, 120644. [Google Scholar] [CrossRef]
- Bao, X.; Hayashi, K.; Li, Y.; Teramoto, A.; Abe, K. Novel agarose and agar fibers: Fabrication and characterization. Mater. Lett. 2010, 64, 2435–2437. [Google Scholar] [CrossRef]
- Jumaidin, R.; Sapuan, S.M.; Jawaid, M.; Ishak, M.R. Effect of agar on physical properties of thermoplastic starch derived from sugar palm tree. Pertanika J. Sci. Technol. 2017, 25, 1235–1248. Available online: https://www.researchgate.net/publication/326253661 (accessed on 11 September 2023).
- Song, J.; Chen, L.; Niu, Y.; Ruan, H. Sustained urea release performance of humic acid hydrogel for green vegetable growth environment evaluation. J. Porous Mater. 2022, 29, 1747–1758. [Google Scholar] [CrossRef]
- Dong, G.; Mu, Z.; Liu, D.; Shang, L.; Zhang, W.; Gao, Y.; Zhao, M.; Zhang, X.; Chen, S.; Wei, M. Starch phosphate carbamate hydrogel based slow-release urea formulation with good water retentivity. Int. J. Biol. Macromol. 2021, 190, 189–197. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Average Compressive Strength (kPa) | Average Young’s Modulus (kPa) |
|---|---|---|
| A2.5 | 92.0 ± 6.2 | 238 ± 10.1 |
| AU7 | 92.2 ± 1.5 | 256 ± 8.4 |
| AU13 | 86.4 ± 1.3 | 260 ± 2.5 |
| Formulation | Average Capacity (Δg/ginitial) | |||
|---|---|---|---|---|
| 30 min | 60 min | 90 min | 120 min | |
| A2.5 | 17.82 ± 2.10 | 18.30 ± 1.68 | 18.28 ± 1.55 | 18.77 ± 1.62 |
| AU7 | 6.52 ± 0.04 | 6.61 ± 0.10 | 6.58 ± 0.33 | 6.71 ± 0.13 |
| AU13 | 3.61 ± 0.05 | 3.58 ± 0.08 | 3.77 ± 0.02 | 3.76 ± 0.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dissanayake, W.; Najaf Zadeh, H.; Nazmi, A.R.; Stevens, C.; Huber, T.; Abhayawardhana, P.L. Exploring the Potential of 3D-Printable Agar–Urea Hydrogels as an Efficient Method of Delivering Nitrogen in Agricultural Applications. Polysaccharides 2024, 5, 49-66. https://doi.org/10.3390/polysaccharides5010004
Dissanayake W, Najaf Zadeh H, Nazmi AR, Stevens C, Huber T, Abhayawardhana PL. Exploring the Potential of 3D-Printable Agar–Urea Hydrogels as an Efficient Method of Delivering Nitrogen in Agricultural Applications. Polysaccharides. 2024; 5(1):49-66. https://doi.org/10.3390/polysaccharides5010004
Chicago/Turabian StyleDissanayake, Wathsala, Hossein Najaf Zadeh, Ali Reza Nazmi, Campbell Stevens, Tim Huber, and Pramuditha L. Abhayawardhana. 2024. "Exploring the Potential of 3D-Printable Agar–Urea Hydrogels as an Efficient Method of Delivering Nitrogen in Agricultural Applications" Polysaccharides 5, no. 1: 49-66. https://doi.org/10.3390/polysaccharides5010004
APA StyleDissanayake, W., Najaf Zadeh, H., Nazmi, A. R., Stevens, C., Huber, T., & Abhayawardhana, P. L. (2024). Exploring the Potential of 3D-Printable Agar–Urea Hydrogels as an Efficient Method of Delivering Nitrogen in Agricultural Applications. Polysaccharides, 5(1), 49-66. https://doi.org/10.3390/polysaccharides5010004









