A Polysaccharide-Based Integrated Nutrient Management System Enhances the Antioxidant Properties in Origanum dictamnus (Lamiaceae), a Valuable Local Endemic Plant of Crete
Abstract
1. Introduction
2. Materials and Methods
2.1. Focal Species and Origin of Plant Material
2.2. Field Experiment
2.3. Fertilization Regimes
2.4. Measurements of Plant Features
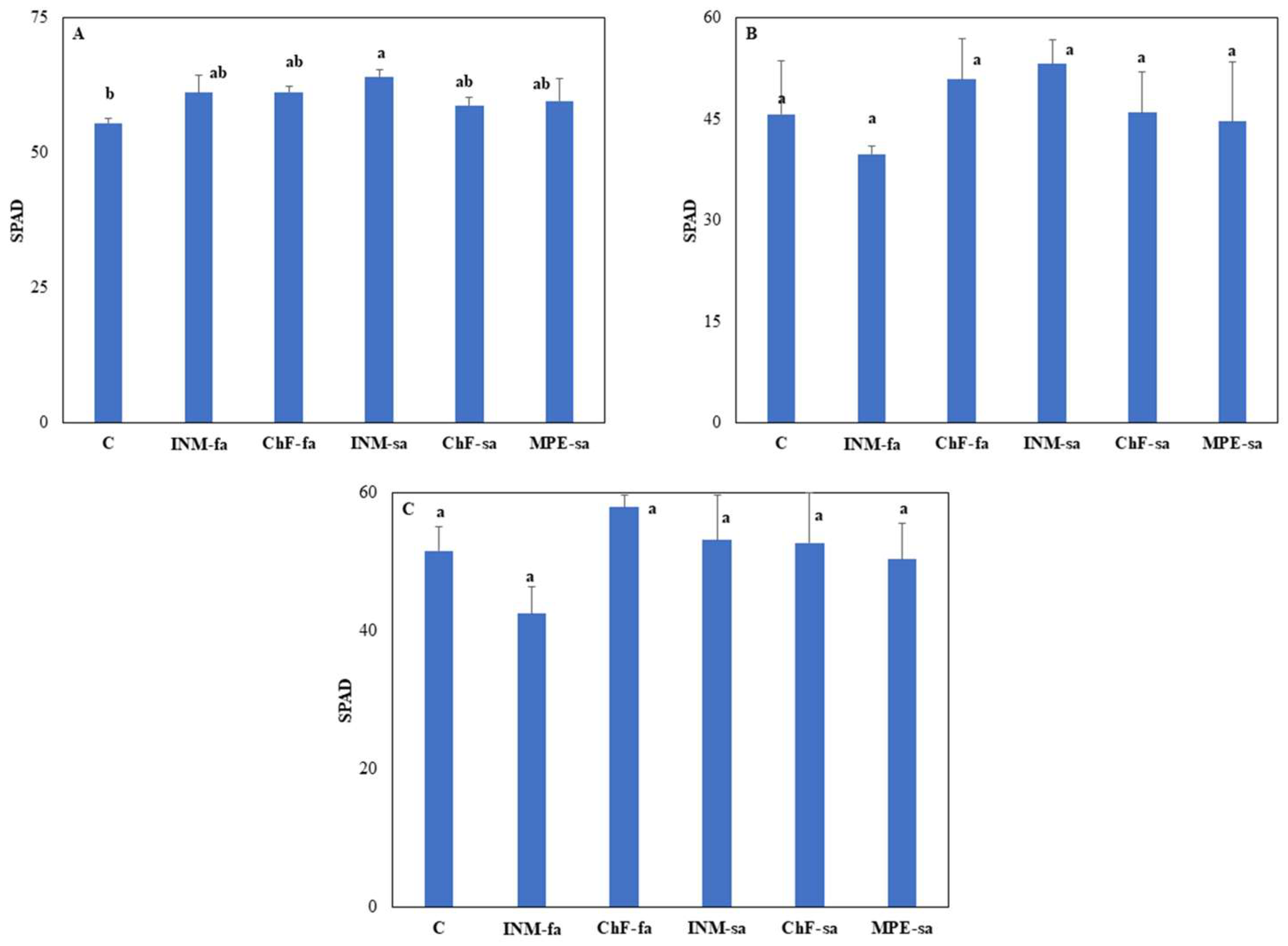
2.4.1. Non-Destructive Evaluation of Leaf Coloration at Three Growth Stages
- (i)
- Leaf SPAD value as a proxy of chlorophyll content was determined by using a SPAD-502 (Konica Minolta Corp., Solna, Sweden).
- (ii)
- The index of absorbance difference (IAD) accurately evaluating fruit ripeness, since it is closely associated with outer mesocarp chlorophyll content and IAD was computed as the difference between the absorbance values at 670 and 720 nm, near the chlorophyll absorbance peak [26]. The potential of IAD in reflecting respective differences in leaves has not been previously evaluated. In this investigation, IAD was determined in leaves by using the DA meter (tr DA Meter, T.R. Turoni, Italy) [53].
- (iii)
- Leaf color was quantified by using a Chroma Meter (Model CR-400, Minolta Corp., Tokyo, Japan). CIE L*a*b* coordinates were recorded using D65 illuminants and a 10° Standard Observer as a reference system. L* [a measure of lightness, ranging from 0 (black) to 100 (white)], a* (a measure of intensity in the green to red range, where negative values refer to green and positive to red), and b* (a measure of representing intensity in the blue to yellow range, where negative values refer to blue and positive to yellow) were obtained [28].
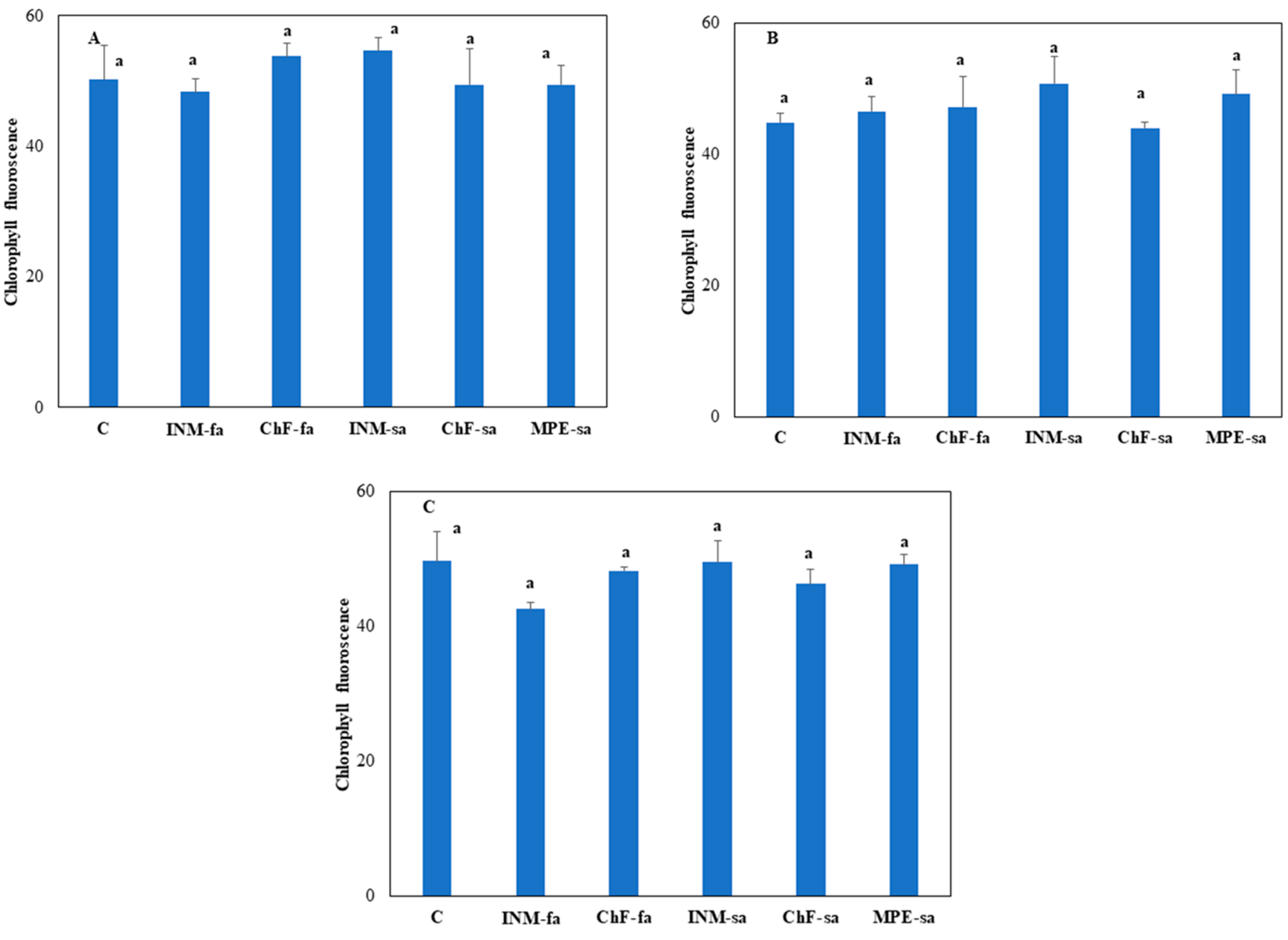
2.4.2. Non-Destructive Evaluation of Photosynthetic Performance at Three Growth Stages
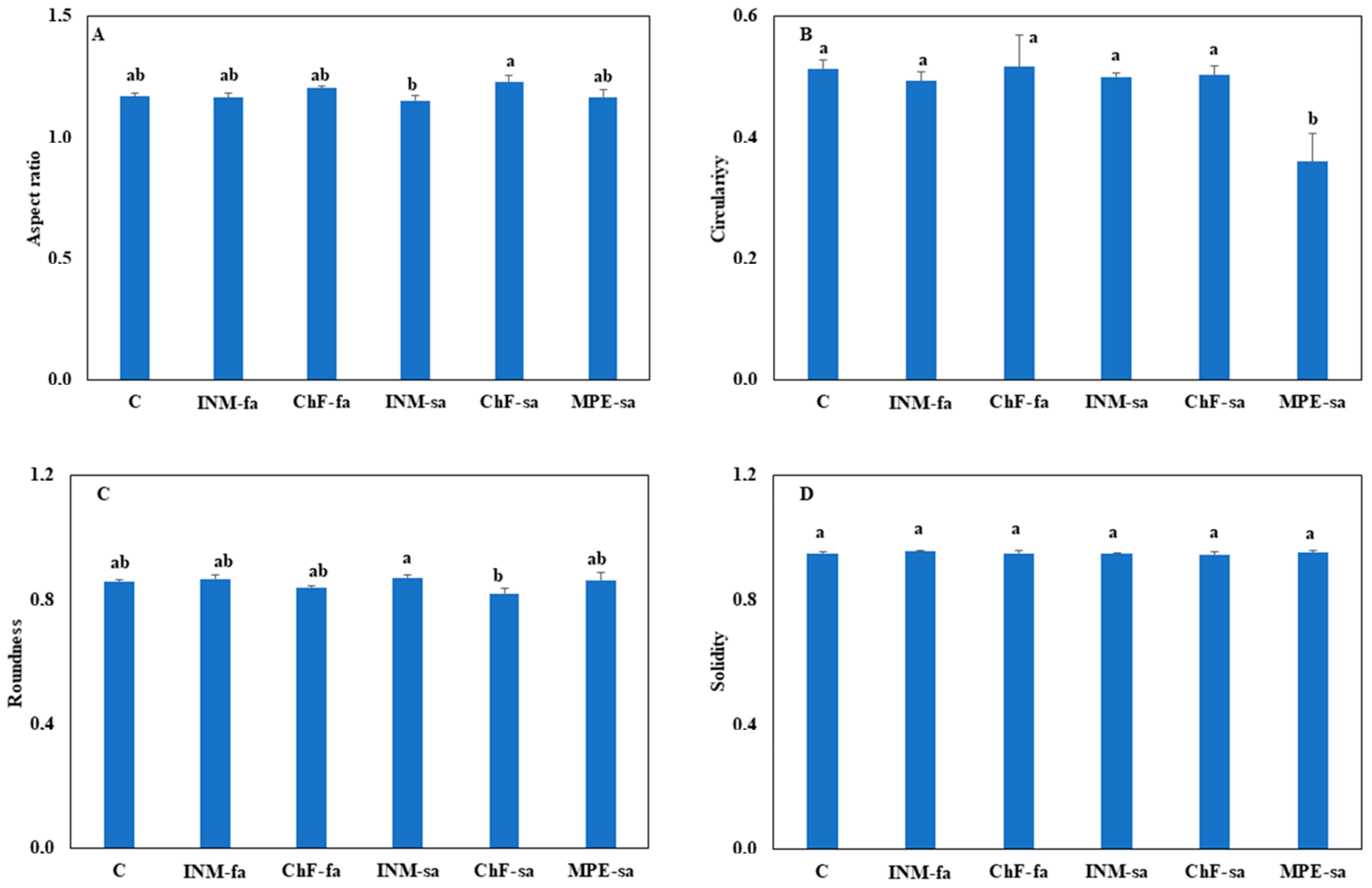
2.4.3. Leaf Shape Indicators
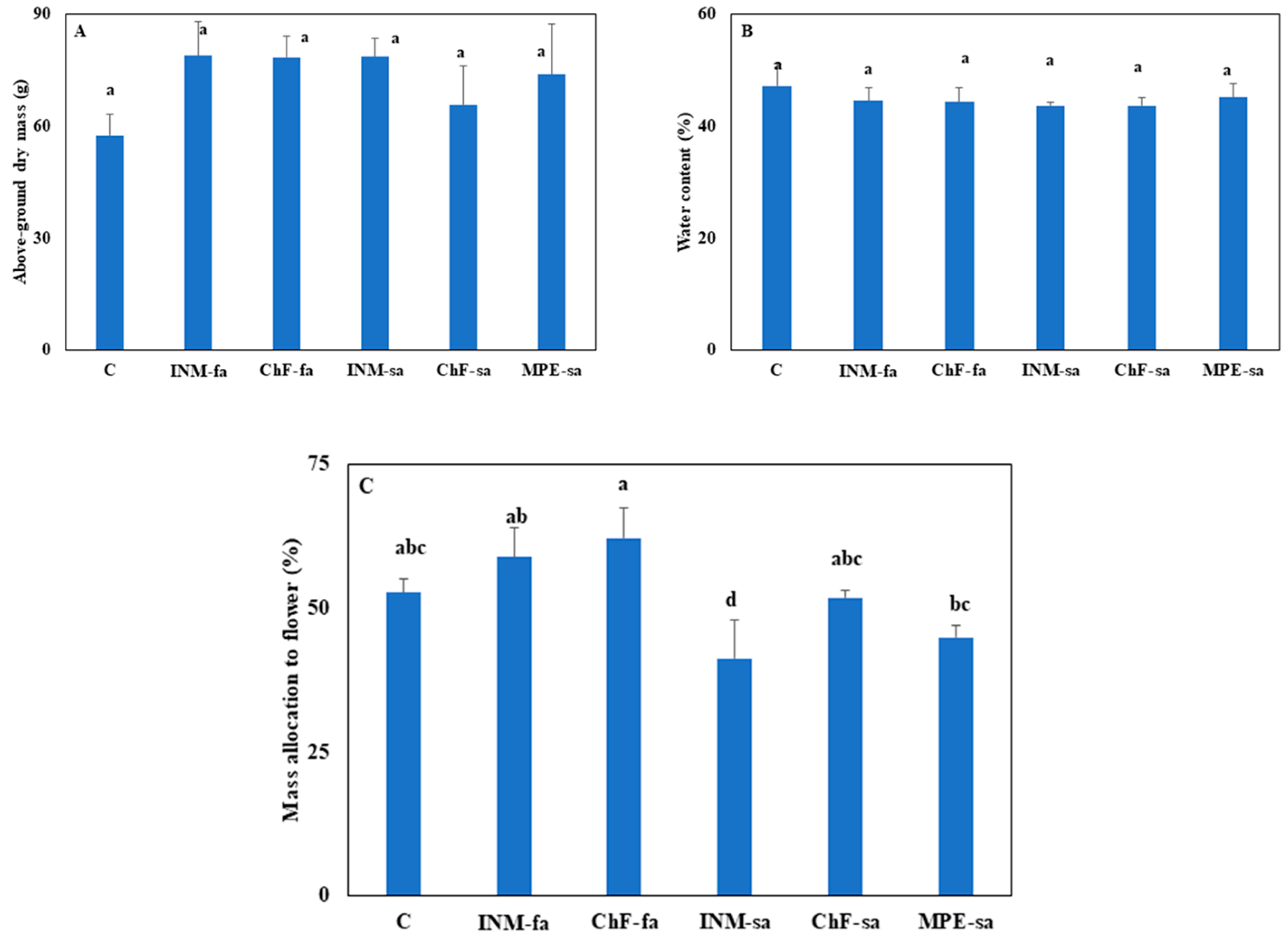
2.4.4. Plant Growth and Biomass Partitioning to Generative Organs
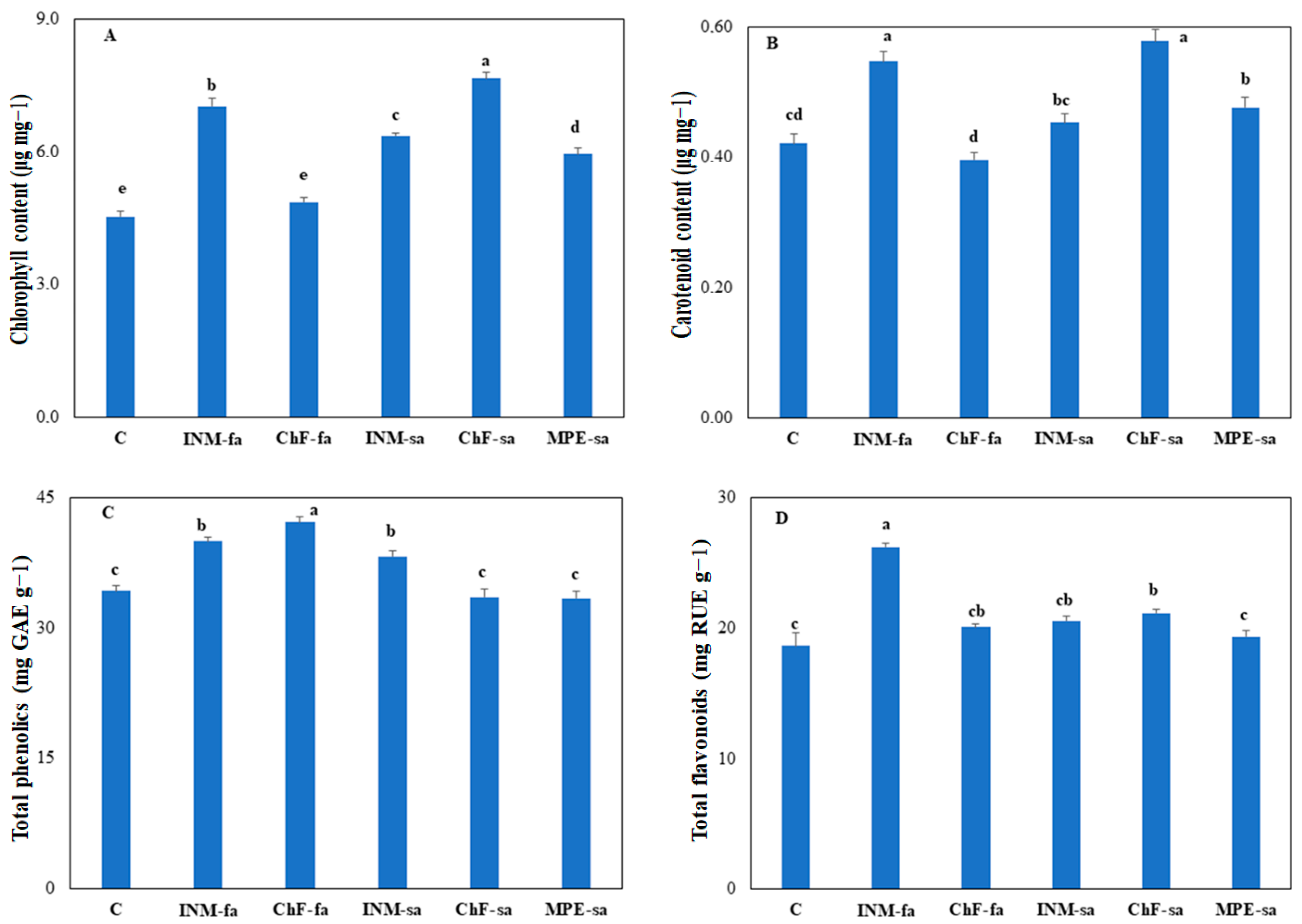
2.4.5. Leaf Chlorophyll and Carotenoid Contents
2.4.6. Leaf Total Phenolic and Total Flavonoid Contents
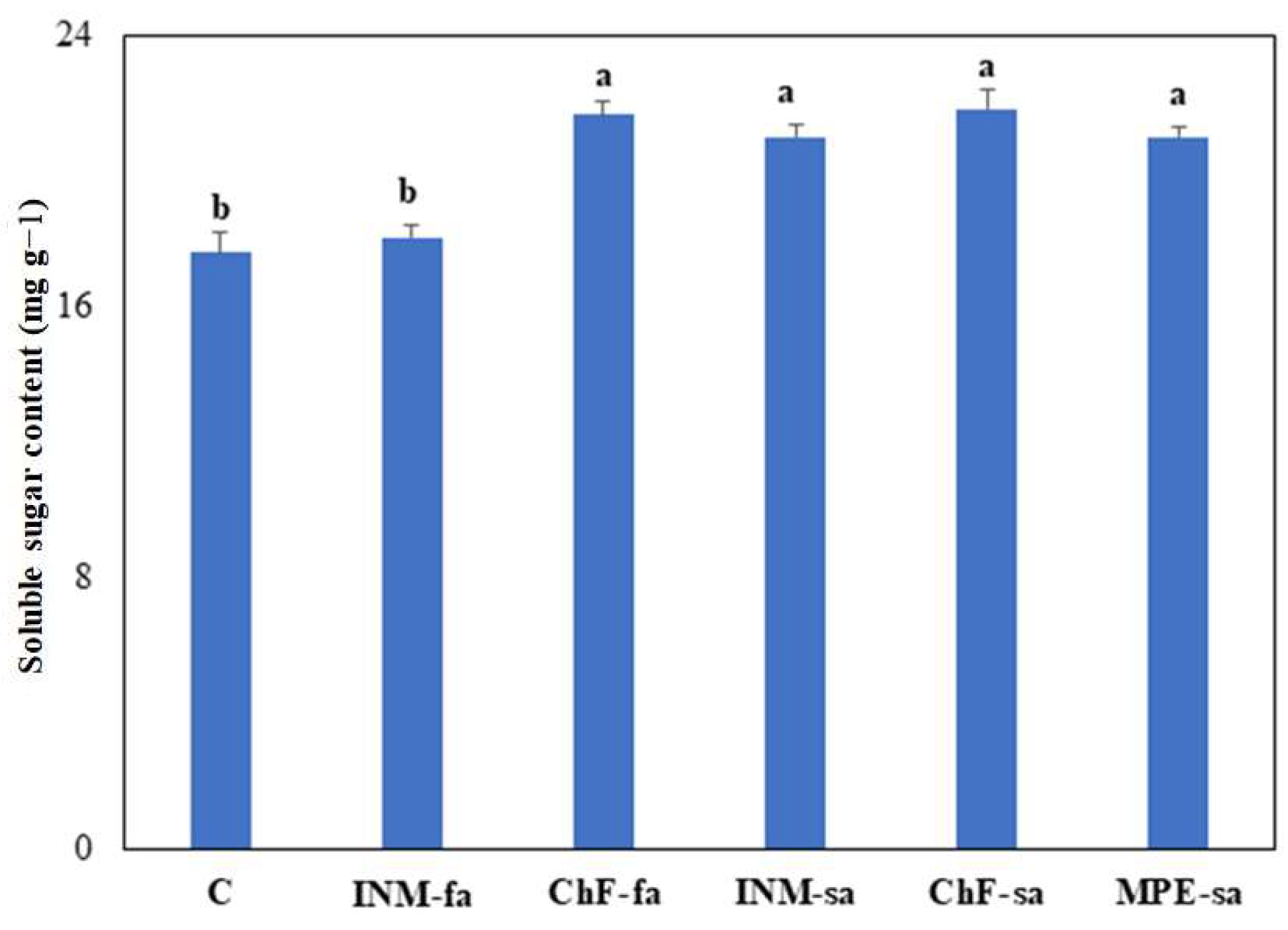
2.4.7. Leaf-Soluble Sugar Content
2.4.8. Leaf and Inflorescence Nutrient Analysis
2.5. Statistical Analysis
3. Results
3.1. The Fertilization Scheme Exerted Limited Effects on Leaf Color
3.2. The Fertilization Scheme Did Not Affect Leaf Photosynthetic Performance
3.3. Fertilization-Induced Minor Effects on Leaf Shape
3.4. Fertilization Did Not Stimulate Plant Growth but Affected Biomass Allocation
3.5. Fertilization Stimulated Leaf Chlorophyll Content and Affected Leaf Antioxidant Substances’ Content
3.6. Fertilization Treatments Generally Stimulated Leaf-Soluble Sugar Content
3.7. Fertilization Treatment Affected Plant Nutrient Content
4. Discussion
5. Conclusions and Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- United Nations 2019. World Population Prospects. Retrieved from Department of Economic and Social Affairs 2019. Available online: http://www.ncbi.nlm.nih.gov/pubmed/12283219 (accessed on 12 February 2024).
- Dhima, K.; Vasilakoglou, I.; Paschalidis, K.; Karagiannidis, N.; Ilias, I. Salinity tolerance evaluation of barley germplasm for marginal soil utilization. Ital. J. Agron. 2021, 16, 1830. [Google Scholar] [CrossRef]
- Lam, S.K.; Wille, U.; Hu, H.; Caruso, F.; Mumford, K.; Liang, X.; Pan, B.; Malcolm, B.; Roessner, U.; Suter, H.; et al. Next-generation enhanced-efficiency fertilizers for sustained food security. Nat. Food 2022, 3, 575–580. [Google Scholar] [CrossRef]
- Ninou, E.G.; Paschalidis, K.A.; Mylonas, I.G.; Vasilikiotis, C.; Mavromatis, A.G. The effect of genetic variation and nitrogen fertilization on productive characters of greek oregano. Acta Agric. Scand. Soil Plant Sci. 2017, 67, 372–379. [Google Scholar] [CrossRef]
- Chiaregato, C.G.; Franca, D.; Messa, L.L.; Dos Santos Pereira, T.; Faez, R. A review of advances over 20 years on polysaccharide-based polymers applied as enhanced efficiency fertilizers. Carbohydr. Polym. 2022, 279, 119014. [Google Scholar] [CrossRef]
- Malerba, M.; Cerana, R. Chitin- and chitosan-based derivatives in plant protection against biotic and abiotic stresses and in recovery of contaminated soil and water. Polysaccharides 2020, 1, 21–30. [Google Scholar] [CrossRef]
- Soni, A.T.; Rookes, J.E.; Arya, S.S. Chitosan nanoparticles as seed priming agents to alleviate salinity stress in rice (Oryza sativa L.) seedlings. Polysaccharides 2023, 4, 129–141. [Google Scholar] [CrossRef]
- Cruz-Monterrosa, R.G.; Rayas-Amor, A.A.; González-Reza, R.M.; Zambrano-Zaragoza, M.L.; Aguilar-Toalá, J.E.; Liceaga, A.M. Application of polysaccharide-based edible coatings on fruits and vegetables: Improvement of food quality and bioactivities. Polysaccharides 2023, 4, 99–115. [Google Scholar] [CrossRef]
- Gemin, L.G.; Bocchetti de Lara, G.; Mógor, Á.F.; Mazaro, S.M.; Sant‘Anna-Santos, B.F.; Mógor, G.; Oliveira Amatussi, J.; Negrelli Cordeiro, E.C.; Costa Marques, H.M. Polysaccharides combined to copper and magnesium improve tomato growth, yield, anti-oxidant and plant defense enzymes. Sci. Hortic. 2023, 310, 111758. [Google Scholar] [CrossRef]
- Hatzilazarou, S.; Pipinis, E.; Kostas, S.; Stagiopoulou, R.; Gitsa, K.; Dariotis, E.; Avramakis, M.; Samartza, I.; Plastiras, I.; Kriemadi, E.; et al. Influence of temperature on seed germination of five wild-growing Tulipa species of Greece associated with their ecological profiles: Implications for conservation and cultivation. Plants 2023, 12, 1574. [Google Scholar] [CrossRef] [PubMed]
- Anestis, I.; Pipinis, E.; Kostas, S.; Papaioannou, E.; Karapatzak, E.; Dariotis, E.; Tsoulpha, P.; Koundourakis, E.; Chatzileontari, E.; Tsoktouridis, G.; et al. Gis-facilitated germination of stored seeds from five wild-growing populations of Campanula pelviformis Lam. and fertilization effects on growth, nutrients, phenol content and antioxidant potential. Horticulturae 2023, 9, 877. [Google Scholar] [CrossRef]
- Bilias, F.; Ipsilantis, I.; Samara, E.; Tsoktouridis, G.; Glavakis, E.; Grigoriadou, K.; Krigas, N.; Matsi, T. From the wild to the field: Effect of foliar or soil application of inorganic or semi-organic fertilizers on various parameters of four local endemic plant species of Crete (Greece). Braz. J. Bot. 2023, 46, 319–336. [Google Scholar] [CrossRef]
- Papagrigoriou, T.; Iliadi, P.; Mitic, M.N.; Mrmosanin, J.M.; Papanastasi, K.; Karapatzak, E.; Maloupa, E.; Gkourogianni, A.V.; Badeka, A.V.; Krigas, N.; et al. Wild-growing and conventionally or organically cultivated Sambucus nigra germplasm: Fruit phytochemical profile, total phenolic content, antioxidant activity, and leaf elements. Plants 2023, 12, 1701. [Google Scholar] [CrossRef]
- Kakar, K.; Xuan, T.D.; Noori, Z.; Aryan, S.; Gulab, G. Effects of organic and inorganic fertilizer application on growth, yield, and grain quality of rice. Agriculture 2020, 10, 544. [Google Scholar] [CrossRef]
- Thamkaew, G.; Sjöholm, I.; Galindo, F. A review of drying methods for improving the quality of dried herbs. Crit. Rev. Food Sci. Nutr. 2021, 61, 1763–1786. [Google Scholar] [CrossRef] [PubMed]
- Taheri-Garavand, A.; Mumivand, H.; Fanourakis, D.; Fatahi, S.; Taghipour, S. An artificial neural network approach for non-invasive estimation of essential oil content and composition through considering drying processing factors: A case study in Mentha aquatica. Ind. Crops Prod. 2021, 171, 113985. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Karimi, E.; Ghasemzadeh, A. Impact of organic and inorganic fertilizers application on the phytochemical and antioxidant activity of kacip fatimah (Labisia pumila Benth). Molecules 2013, 18, 10973–10988. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Fanourakis, D.; Tsaniklidis, G.; Aliniaeifard, S.; Yang, Q.; Li, T. Low UVA intensity during cultivation improves the lettuce shelf-life, an effect that is not sustained at higher intensity. Postharvest Biol. Technol. 2021, 172, 111376. [Google Scholar] [CrossRef]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry, and corn using conventional, organic, and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Zhang, Y.; Ntagkas, N.; Fanourakis, D.; Tsaniklidis, G.; Zhang, Y.; Zou, J.; Cheng, R.; Yang, Q.; Li, T. The role of light intensity in mediating ascorbate content during postharvest tomato ripening: A transcriptomic analysis. Postharvest Biol. Technol. 2021, 180, 111622. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Hallmann, E.; Rusaczonek, A.; Rembiałkowska, E. Antioxidant Content in Black Currants from Organic and Conventional Cultivation. Food Sci. Technol. Res. 2008, 2, 57–61. Available online: http://www.ejpau.media.pl/volume11/issue2/art-28.html (accessed on 28 December 2023).
- Wang, S.Y.; Chen, C.T.; Sciarappa, W.; Wang, C.Y.; Camp, M.J. Fruit quality, antioxidant capacity, and flavonoid content of organically and conventionally grown blueberries. J. Agric. Food Chem. 2008, 56, 5788–5794. [Google Scholar] [CrossRef] [PubMed]
- Tõnutare, T.; Moor, U.; Mölder, K.; Põldma, P. Fruit Composition of Organically and Conventionally Cultivated Strawberry ‘polka’. Agron. Res. 2009, 7, 755–760. Available online: https://agronomy.emu.ee/vol07Spec2/p7sII27.pdf (accessed on 28 December 2023).
- Serri, F.; Souri, M.K.; Rezapanah, M. Growth, biochemical quality and antioxidant capacity of coriander leaves under organic and inorganic fertilization programs. Chem. Biol. Technol. Agric. 2021, 8, 33. [Google Scholar] [CrossRef]
- Mrid, R.B.; Benmrid, B.; Hafsa, J.; Boukcim, H.; Sobeh, M.; Yasri, A. Secondary metabolites as biostimulant and bioprotectant agents: A review. Sci. Total Environ. 2021, 777, 146204. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Kalogiannakis, K.; Debouba, F.J.; Ipsilantis, I.; Tsoktouridis, G.; et al. Pilot cultivation of the vulnerable cretan endemic Verbascum arcturus L. (Scrophulariaceae): Effect of fertilization on growth and quality features. Sustainability 2021, 13, 14030. [Google Scholar] [CrossRef]
- Fanourakis, D.; Paschalidis, K.; Tsaniklidis, G.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Liapaki, E.; Jouini, M.; Ipsilantis, I.; Maloupa, E.; et al. Pilot cultivation of the local endemic Cretan marjoram Origanum microphyllum (Benth.) Vogel (Lamiaceae): Effect of fertilizers on growth and herbal quality features. Agronomy 2022, 12, 94. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tsichlas, I.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Ipsilantis, I.; Grigoriadou, K.; Samartza, I.; et al. DNA Barcoding and fertilization strategies in Sideritis syriaca subsp. syriaca, a local endemic plant of Crete with high medicinal value. Int. J. Mol. Sci. 2024, 25, 1891. [Google Scholar] [CrossRef]
- Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tsichlas, I.; Tzanakakis, V.A.; Bilias, F.; Samara, E.; Ipsilantis, I.; Grigoriadou, K.; Matsi, T.; et al. Integrated nutrient management boosts inflorescence biomass and antioxidant profile of Carlina diae (Asteraceae)—An Endangered local endemic plant of Crete with medicinal and ornamental value. Agriculture 2024, 14, 259. [Google Scholar] [CrossRef]
- Amujoyegbe, B.J.; Opabode, J.T.; Olayinka, A. Effect of organic and inorganic fertilizer on yield and chlorophyll content of maize (Zea mays L.) and sorghum (Sorghum bicolor L.) Moench. Afr. J. Biotechnol. 2007, 6, 1869–1873. [Google Scholar] [CrossRef]
- Hoareau, L.; Da Silva, E.J. Medicinal plants: A re-emerging health aid. Electron. J. Biotechnol. 1999, 2, 3–4. [Google Scholar] [CrossRef]
- Bodeker, G.; Burford, G.; Volkov, A. Integrative, traditional and complementary medicine. In 325 International Encyclopedia of Public Health, 2nd ed.; Quah, S.R., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2017; pp. 288–295. [Google Scholar] [CrossRef]
- Jain, D.; Chaudhary, P.; Kotnala, A.; Hossain, R.; Bisht, K.; Hossain, M.N. Hepatoprotective activity of medicinal plants: A mini review. J. Med. Plants Stud. 2020, 8, 183–188. [Google Scholar] [CrossRef]
- Zuzarte, M.; Girao, H.; Salgueiro, L. Aromatic plant-based functional foods: A natural approach to manage cardiovascular diseases. Molecules 2023, 28, 5130. [Google Scholar] [CrossRef]
- Sattayakhom, A.; Wichit, S.; Koomhin, P. The effects of essential oils on the nervous system: A scoping review. Molecules 2023, 28, 3771. [Google Scholar] [CrossRef]
- Cheminal, A.; Kokkoris, I.P.; Strid, A.; Dimopoulos, P. Medicinal and aromatic Lamiaceae plants in Greece: Linking diversity and distribution patterns with ecosystem services. Forests 2020, 11, 661. [Google Scholar] [CrossRef]
- Kougioumoutzis, K.; Kotsakiozi, P.; Stathi, E.; Trigas, P.; Parmakelis, A. Conservation genetics of four Critically Endangered Greek endemic plants: A preliminary assessment. Diversity 2021, 13, 152. [Google Scholar] [CrossRef]
- Turland, N.J. Origanum dictamnus L. (Lamiaceae), Vulnerable (Vu). In The Red Data Book of Rare and Threatened Plants of Greece; Phitos, D., Strid, A., Snogerup, S., Greuter, W., Eds.; World Wide Fund for Nature (WWF): Athens, Greece, 1995; pp. 394–395. [Google Scholar]
- Krigas, N.; Lazari, D.; Maloupa, E.; Stikoudi, M. Introducing dittany of crete (Origanum dictamnus L.) to gastronomy: A new culinary concept for a traditionally used medicinal plant. Int. J. Gastron. Food Sci. 2015, 2, 112–118. [Google Scholar] [CrossRef]
- Bourgou, S.; Ben Haj Jilani, I.; Karous, O.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Libiad, M.; Khabbach, A.; El Haissoufi, M.; Lamchouri, F.; Greveniotis, V.; et al. Medicinal-cosmetic potential of the local endemic plants of Crete (Greece), Northern Morocco and Tunisia: Priorities for conservation and sustainable exploitation of neglected and underutilized phytogenetic resources. Biology 2021, 10, 1344. [Google Scholar] [CrossRef]
- EMA/HMPC. Final Assessment Report on Origanum dictamnus L., Herba (200431/2012); European Medicines Agency/Committee on Herbal Medicinal Products: London, UK, 2013. Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-origanum-dictamnus-l-herba-first-version_en.pdf (accessed on 12 February 2024).
- Letsiou, S.; Trapali, M.; Vougiouklaki, D.; Tsakni, A.; Antonopoulos, D.; Houhoula, D. An-tioxidant profile of Origanum dictamnus L. exhibits antiaging properties against UVA irradiation. Cosmetics 2023, 10, 124. [Google Scholar] [CrossRef]
- Tsakni, A.; Chatzilazarou, A.; Tsakali, E.; Tsantes, A.G.; Van Impe, J.; Houhoula, D. Identification of bioac-tive compounds in plant extracts of Greek flora and their antimicrobial and antioxidant activity. Separations 2023, 10, 373. [Google Scholar] [CrossRef]
- Skotti, E.; Kountouri, S.; Bouchagier, P.; Tsitsigiannis, D.I.; Polissiou, M.; Tarantilis, P.A. FTIT spectroscopic evaluation of changes in the cellular biochemical composition of the phytopathogenic fungus Alternaria alternata induced by extracts of some Greek medicinal and aromatic plants. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 127, 463–472. [Google Scholar] [CrossRef]
- Martini, A.N.; Papafotiou, M.; Massas, I.; Chorianopoulou, N. Growing of the Cretan therapeutic herb Origanum dictamnus in the urban fabric: The effect of substrate and cultivation site on plant growth and potential toxic element accumulation. Plants 2023, 12, 336. [Google Scholar] [CrossRef] [PubMed]
- Economakis, C.; Skaltsa, H.; Demetzos, C.; Sokovic, M.; Thanos, C.A. Effect of phosphorus concentration of the nutrient solution on the volatile constituents of leaves and bracts of Origanum dictamnus. J. Agric. Food Chem. 2002, 50, 6276–6280. [Google Scholar] [CrossRef] [PubMed]
- Sarropoulou, V.; Maloupa, E.; Grigoriadou, K. Cretan dittany (Origanum dictamnus L.), a valuable local endemic plant: In vitro regeneration potential of different type of explants for conservation and sustainable exploitation. Plants 2023, 12, 182. [Google Scholar] [CrossRef] [PubMed]
- Lionis, C.; Petelos, E.; Linardakis, M.; Diamantakis, A.; Symvoulakis, E.; Karkana, M.N.; Kampa, M.; Pirintsos, S.A.; Sourvinos, G.; Castanas, E. A mixture of essential oils from three cretan aromatic plants inhibits Sars-Cov-2 proliferation: A proof-of-concept intervention study in ambulatory patients. Diseases 2023, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Strid, A. Atlas of the Aegean flora. Part 1: Text & plates. Part 2: Maps. In Englera 33 (1 & 2); Botanic Garden and Botanical Museum Berlin, Freie Universität Berlin: Berlin, Germany, 2016. [Google Scholar] [CrossRef]
- Krigas, N.; Tsoktouridis, G.; Anestis, I.; Khabbach, A.; Libiad, M.; Megdiche-Ksouri, W.; Ghrabi-Gammar, Z.; Lamchouri, F.; Tsiripidis, I.; Tsiafouli, M.A.; et al. Exploring the potential of neglected local endemic plants of three Mediterranean regions in the ornamental sector: Value chain feasibility and readiness timescale for their sustainable exploitation. Sustainability 2021, 13, 2539. [Google Scholar] [CrossRef]
- Bosabalidis, A.M. Ultrastructure, development and histochemistry of the polysaccharide-containing subcuticular compartments in Origanum dictamnus L. peltate glandular hairs. Flavour Fragr. J. 2010, 25, 202–205. [Google Scholar] [CrossRef]
- Asayesh, E.J.; Aliniaeifard, S.; Askari, N.; Roozban, M.R.; Sobhani, M.; Tsaniklidis, G.; Woltering, E.J.; Fanourakis, D. Supplementary light with increased blue fraction accelerates emergence and improves development of the inflorescence in Aechmea, Guzmania and Vriesea. Horticulturae 2021, 7, 485. [Google Scholar] [CrossRef]
- Kapse, S.; Kausley, S.B.; Rai, B. Portable food diagnostic devices and methods: A review. J. Food Process Eng. 2022, 45, 14159. [Google Scholar] [CrossRef]
- Sørensen, H.K.; Fanourakis, D.; Tsaniklidis, G.; Bouranis, D.; Rezaei Nejad, A.; Ottosen, C.-O. Using artificial lighting based on electricity price without a negative impact on growth, visual quality or stomatal closing response in passiflora. Sci. Hortic. 2020, 267, 109354. [Google Scholar] [CrossRef]
- Koubouris, G.; Bouranis, D.; Vogiatzis, E.; Nejad, A.R.; Giday, H.; Tsaniklidis, G.; Ligoxigakis, E.K.; Blazakis, K.; Kalaitzis, P.; Fanourakis, D. Leaf area estimation by considering leaf dimensions in olive tree. Sci. Hortic. 2018, 240, 440–445. [Google Scholar] [CrossRef]
- Gupta, S.; Rosenthal, D.M.; Stinchcombe, J.R.; Baucom, R.S. The remarkable morphological diversity of leaf shape in sweet potato (Ipomoea batatas): The influence of genetics, environment, and g9e. New Phytol. 2020, 225, 2183–2195. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi-Majd, M.; Rezaei Nejad, A.; Mousavi-Fard, S.; Fanourakis, D. Postharvest application of single, multi-walled carbon nanotubes and graphene oxide stimulates rose keeping quality. J. Hortic. Sci. Biotechnol. 2021, 97, 346–360. [Google Scholar] [CrossRef]
- Ross, I.A. Medicinal Plants of the World (Volume 3): Chemical Constituents, Traditional and Modern Medicinal Uses; Humana Press Inc.: Totowa, NJ, USA, 2005; pp. 110–132. Available online: https://link.springer.com/book/10.1007/978-1-59259-887-8 (accessed on 28 December 2023).
- Bentley, R.E. Medicinal Plants; Domville-Fife Press: London, UK, 2010; pp. 23–46. ISBN 1445576627, 9781445576626. [Google Scholar]
- Pimm, S.; Russell, G.; Gittleman, J.; Brooks, T. The future of biodiversity. Science 1995, 269, 347. [Google Scholar] [CrossRef] [PubMed]
- Laird, S.A.; Pierce, A.R. Promoting Sustainable and Ethical Botanicals: Strategies to Improve Commercial Raw Material Sourcing, Results from the Sustainable Botanicals Pilot Project Industry Surveys, Case Studies and Standards Collection; Rainforest Alliance: New York, NY, USA, 2002; Available online: http://www.rainforest-alliance.org/news/archives/news/news44.html (accessed on 28 December 2023).
- Paschalidis, K.A.; Moschou, P.N.; Aziz, A.; Toumi, T.; Roubelakis-Angelakis, K.A. Polyamines in grapevine: An update. In Grapevine Molecular Physiology & Biotechnology; Roubelakis-Angelakis, K.A., Ed.; Springer: Dordrecht, The Netherlands, 2009; pp. 207–228. [Google Scholar] [CrossRef]
- Fernandes, P.A.R.; Coimbra, M.A. The antioxidant activity of polysaccharides: A structure-function relationship overview. Carbohydr. Polym. 2023, 314, 120965. [Google Scholar] [CrossRef]
- Zeng, F.-S.; Yao, Y.-F.; Wang, L.-F.; Li, W.-J. Polysaccharides as antioxidants and prooxidants in managing the double-edged sword of reactive oxygen species. Biomed. Pharmacother. 2023, 159, 114221. [Google Scholar] [CrossRef]


| Treatment | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (g kg–1) | |||||
| C | 15.7± 0.4 bc * | 1.7 ± 0.1 c | 15.9 ± 1.1 bc | 38.9 ± 0.5 ab | 3.4 ± 0.0 a |
| INM-fa | 18.7 ± 0.1 ab | 4.9 ± 0.2 a | 17.7 ± 0.4 abc | 32.8 ± 1.1 bc | 2.9 ± 0.0 bc |
| ChF-fa | 18.8 ± 0.4 ab | 4.9 ± 0.1 a | 20.2 ± 0.8 ab | 29.6 ± 1.2 c | 3.4 ± 0.1 a |
| INM-sa | 14.7 ± 0.7 c | 3.2 ± 0.1 b | 15.4 ± 0.3 cd | 41.1 ± 1.0 a | 3.3 ± 0.0 ab |
| ChF-sa | 20.8 ± 0.5 a | 3.9 ± 0.1 b | 20.3 ± 0.5 a | 37.0 ± 0.8 ab | 3.4 ± 0.1 a |
| MPE-sa | 14.0 ± 0.3 c | 1.6 ± 0.0 c | 11.1 ± 0.3 d | 36.7 ± 1.0 ab | 2.7 ± 0.1 c |
| p F-test | <0.001 | <0.001 | 0.001 | 0.020 | 0.013 |
| Treatment | Cu | Zn | Fe | Mn | B |
|---|---|---|---|---|---|
| (mg kg–1) | |||||
| C | 14.5 ± 0.5 cd * | 33.5 ± 0.6 cd | 1732 ± 48 b | 79 ± 5 de | 36.3 ± 0.4 c |
| INM-fa | 20.4 ± 0.4 a | 53.1 ± 2.2 a | 1916 ± 73 a | 210 ± 12 a | 51.4 ± 1.0 a |
| ChF-fa | 18.7 ± 0.5 ab | 47.6 ± 1.6 ab | 2009 ± 94 a | 174 ± 7 ab | 42.6 ± 0.8 b |
| INM-sa | 15.6 ± 0.3 bcd | 33.0 ± 0.3 d | 1691 ± 49 b | 118 ± 4 cd | 38.6 ±0.7 bc |
| ChF-sa | 17.7 ± 0.6 abc | 42.4 ± 1.4 bc | 1528 ± 44 c | 132 ± 6 bc | 42.5 ± 0.7 b |
| MPE-sa | 12.5 ± 0.5 d | 33.1 ± 0.7 d | 1236 ± 62 d | 60 ± 2 e | 28.8 ± 0.6 d |
| p F-test | <0.001 | <0.001 | 0.022 | <0.001 | <0.001 |
| Treatment | N | P | K | Ca | Mg |
|---|---|---|---|---|---|
| (g kg–1) | |||||
| C | 10.54 ± 0.4 a * | 1.70 ± 0.1 b | 13.74 ± 0.6 a | 16.31 ± 0.4 a | 1.92 ± 0.1 a |
| INM-fa | 11.85 ± 0.7 a | 1.83 ± 0.1 b | 15.49 ± 0.5 a | 17.00 ± 0.9 a | 1.70 ± 0.2 a |
| ChF-fa | 12.84 ± 0.3 a | 2.24 ± 0.2 a | 15.45 ± 1.5 a | 15.91 ± 0.1 a | 1.90 ± 0.1 a |
| INM-sa | 12.58 ± 0.8 a | 1.35 ± 0.1 c | 13.07 ± 0.9 a | 16.39 ± 1.6 a | 1.50 ± 0.1 a |
| ChF-sa | 10.53 ± 1.6 a | 1.69 ± 0.2 b | 17.31 ± 1.5 a | 15.98 ± 1.5 a | 1.46 ± 0.2 a |
| MPE-sa | 9.78 ± 0.6 a | 1.48 ± 0.1 b | 13.38 ± 1.6 a | 16.00 ± 0.7 a | 1.40 ± 0.1 a |
| p F-test | # NS | 0.003 | NS | NS | NS |
| Treatment | Zn | Fe | B |
|---|---|---|---|
| (mg kg−1) | |||
| C | 12.97 ± 1.4 a * | 21.88 ± 1.4 a | 222.79 ± 5.1 a |
| INM-fa | 14.68 ± 2.8 a | 20.25 ± 1.1 a | 322.93 ± 18.8 a |
| ChF-fa | 15.57 ± 0.5 a | 21.95 ± 0.6 a | 270.11 ± 19.9 a |
| INM-sa | 14.31 ± 0.4 a | 12.61 ± 1.2 b | 241.95 ± 40.8 a |
| ChF-sa | 15.42 ± 1.0 a | 15.03 ± 2.2 b | 204.19 ± 35.3 a |
| MPE-sa | 15.73 ± 0.4 a | 14.49 ± 0.6 b | 225.95 ± 37.4 a |
| p F-test | # NS | 0.001 | NS |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paschalidis, K.; Fanourakis, D.; Tsaniklidis, G.; Tzanakakis, V.A.; Kardamaki, I.; Bilias, F.; Samara, E.; Ipsilantis, I.; Grigoriadou, K.; Matsi, T.; et al. A Polysaccharide-Based Integrated Nutrient Management System Enhances the Antioxidant Properties in Origanum dictamnus (Lamiaceae), a Valuable Local Endemic Plant of Crete. Polysaccharides 2024, 5, 28-48. https://doi.org/10.3390/polysaccharides5010003
Paschalidis K, Fanourakis D, Tsaniklidis G, Tzanakakis VA, Kardamaki I, Bilias F, Samara E, Ipsilantis I, Grigoriadou K, Matsi T, et al. A Polysaccharide-Based Integrated Nutrient Management System Enhances the Antioxidant Properties in Origanum dictamnus (Lamiaceae), a Valuable Local Endemic Plant of Crete. Polysaccharides. 2024; 5(1):28-48. https://doi.org/10.3390/polysaccharides5010003
Chicago/Turabian StylePaschalidis, Konstantinos, Dimitrios Fanourakis, Georgios Tsaniklidis, Vasileios A. Tzanakakis, Ioanna Kardamaki, Fotis Bilias, Eftihia Samara, Ioannis Ipsilantis, Katerina Grigoriadou, Theodora Matsi, and et al. 2024. "A Polysaccharide-Based Integrated Nutrient Management System Enhances the Antioxidant Properties in Origanum dictamnus (Lamiaceae), a Valuable Local Endemic Plant of Crete" Polysaccharides 5, no. 1: 28-48. https://doi.org/10.3390/polysaccharides5010003
APA StylePaschalidis, K., Fanourakis, D., Tsaniklidis, G., Tzanakakis, V. A., Kardamaki, I., Bilias, F., Samara, E., Ipsilantis, I., Grigoriadou, K., Matsi, T., Tsoktouridis, G., & Krigas, N. (2024). A Polysaccharide-Based Integrated Nutrient Management System Enhances the Antioxidant Properties in Origanum dictamnus (Lamiaceae), a Valuable Local Endemic Plant of Crete. Polysaccharides, 5(1), 28-48. https://doi.org/10.3390/polysaccharides5010003











