Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry
Abstract
1. Introduction
2. Skin Health Promoting Effects
2.1. Wound Healing
2.2. Moisturizing
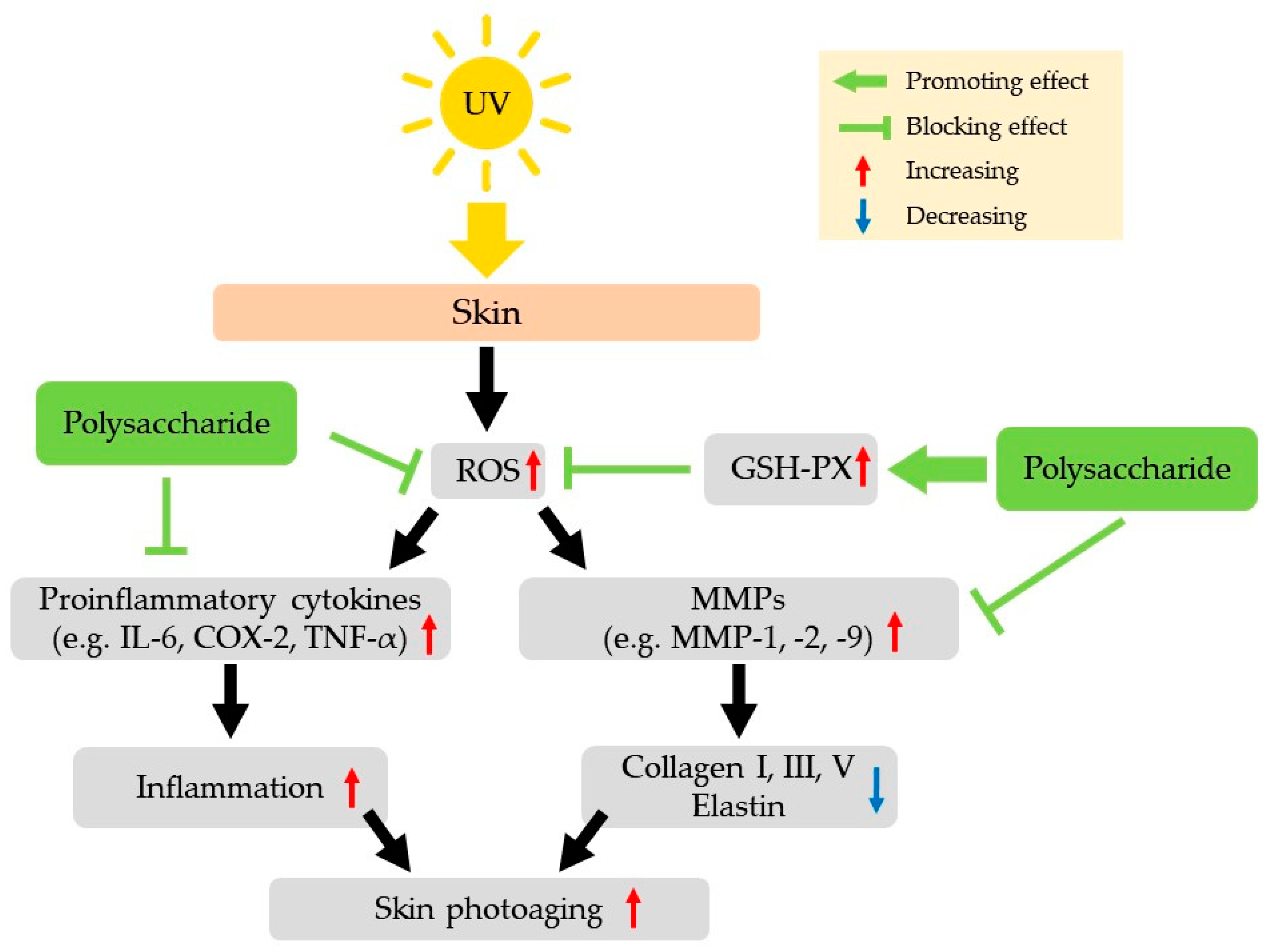
2.3. Anti-Aging
2.4. Whitening
3. Natural Polysaccharides
3.1. Polysaccharides Derived from Herbaceous Plants
3.2. Polysaccharides Derived from Algae
3.2.1. Red Algae
3.2.2. Brown Algae
3.2.3. Blue-Green Algae
3.3. Polysaccharides Derived from Fungi
4. Challenges and Prospects
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-E.; Lee, K.W. Molecular targets of phytochemicals for skin inflammation. Curr. Pharm. Des. 2018, 24, 1533. [Google Scholar] [CrossRef] [PubMed]
- Oli, A.N.; Eze, D.E.; Gugu, T.H.; Ezeobi, I.; Maduagwu, U.N.; Ihekwereme, C.P. Multi-antibiotic resistant extended-spectrum beta-lactamase producing bacteria pose a challenge to the effective treatment of wound and skin infections. Pan Afr. Med. J. 2017, 27, 66. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.B.S.; de Oliveira, W.F.; dos Santos Silva, P.M.; dos Santos Correia, M.T.; Kennedy, J.F.; Coelho, L.C.B.B. Skincare application of medicinal plant polysaccharides—A review. Carbohydr. Polym. 2022, 277, 118824. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Lourith, N. Biopolysaccharides for Skin Hydrating Cosmetics. Polysaccharides: Bioactivity and Biotechnology; Springer International Publishing: New York, NY, USA, 2015; p. 1867. [Google Scholar]
- Liu, Z.-h.; Niu, F.-j.; Xie, Y.-x.; Xie, S.-m.; Liu, Y.-n.; Yang, Y.-y.; Zhou, C.-z.; Wan, X.-h. A review: Natural polysaccharides from medicinal plants and microorganisms and their anti-herpetic mechanism. Biomed. Pharmacother. 2020, 129, 110469. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Li, H.; Wei, Z.; Lv, K.; Gao, C.; Liu, Y.; Zhao, L. Isolation, structures and biological activities of polysaccharides from Chlorella: A review. Int. J. Biol. Macromol. 2020, 163, 2199. [Google Scholar] [CrossRef] [PubMed]
- Bedoux, G.; Hardouin, K.; Burlot, A.S.; Bourgougnon, N. Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 71. [Google Scholar]
- Hu, Y.; Zeng, H.; Huang, J.; Jiang, L.; Chen, J.; Zeng, Q. Traditional Asian herbs in skin whitening: The current development and limitations. Front. Pharmacol. 2020, 11, 982. [Google Scholar] [CrossRef] [PubMed]
- Tseng, C.-C.; Yeh, H.-Y.; Liao, Z.-H.; Hung, S.-W.; Chen, B.; Lee, P.-T.; Nan, F.-H.; Shih, W.-L.; Chang, C.-C.; Lee, M.-C. An in vitro study shows the potential of Nostoc commune (Cyanobacteria) polysaccharides extract for wound-healing and anti-allergic use in the cosmetics industry. J. Funct. Foods 2021, 87, 104754. [Google Scholar] [CrossRef]
- Belvedere, R.; Pessolano, E.; Porta, A.; Tosco, A.; Parente, L.; Petrella, F.; Perretti, M.; Petrella, A. Mesoglycan induces the secretion of microvesicles by keratinocytes able to activate human fibroblasts and endothelial cells: A novel mechanism in skin wound healing. Eur. J. Pharmacol. 2020, 869, 172894. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.M.L.; Carvalho Júnior, A.R.; Vale de Macedo, G.H.R.; Chagas, V.L.; Silva, L.d.S.; Cutrim, B.d.S.; Santos, D.M.; Soares, B.L.L.; Zagmignan, A.; de Miranda, R.d.C.M. Polysaccharide-based formulations for healing of skin-related wound infections: Lessons from animal models and clinical trials. Biomolecules 2019, 10, 63. [Google Scholar] [CrossRef]
- Summa, M.; Russo, D.; Penna, I.; Margaroli, N.; Bayer, I.S.; Bandiera, T.; Athanassiou, A.; Bertorelli, R. A biocompatible sodium alginate/povidone iodine film enhances wound healing. Eur. J. Pharm. Biopharm. 2018, 122, 17. [Google Scholar] [CrossRef]
- Veeraperumal, S.; Qiu, H.-M.; Zeng, S.-S.; Yao, W.-Z.; Wang, B.-P.; Liu, Y.; Cheong, K.-L. Polysaccharides from Gracilaria lemaneiformis promote the HaCaT keratinocytes wound healing by polarised and directional cell migration. Carbohydr. Polym. 2020, 241, 116310. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lei, M.; Xu, H.; He, H.; Suvorov, A.; Wang, J.; Qiu, J.; Zhou, Q.; Yang, J.; Chen, L. The normal cell proliferation and wound healing effect of polysaccharides from Ganoderma amboinense. Food Sci. Hum. Wellness 2021, 10, 508. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Zhang, S.; Guo, L.; Jia, G.; Lin, W.; Gao, Z.; Gao, Y.; Sun, T. Preparation and characterization of selenium-rich polysaccharide from Phellinus igniarius and its effects on wound healing. Carbohydr. Polym. 2021, 264, 117982. [Google Scholar] [CrossRef] [PubMed]
- Eleroui, M.; Feki, A.; Hamzaoui, A.; Kammoun, I.; Bouhamed, M.; Boudawara, O.; Ayed, I.B.; Amara, I.B. Preparation and characterization of a novel hamada scoparia polysaccharide composite films and evaluation of their effect on cutaneous wound healing in rat. Int. J. Pharm. 2021, 608, 121056. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-S.; Song, K.B. Noni (Morinda citrifolia) fruit polysaccharide films containing blueberry (Vaccinium corymbosum) leaf extract as an antioxidant packaging material. Food Hydrocoll. 2021, 112, 106372. [Google Scholar] [CrossRef]
- Feki, A.; Bardaa, S.; Hajji, S.; Ktari, N.; Hamdi, M.; Chabchoub, N.; Kallel, R.; Boudawara, T.; Nasri, M.; Amara, I.B. Falkenbergia rufolanosa polysaccharide–Poly (vinyl alcohol) composite films: A promising wound healing agent against dermal laser burns in rats. Int. J. Biol. Macromol. 2020, 144, 954. [Google Scholar] [CrossRef]
- Feki, A.; Amara, I.B.; Bardaa, S.; Hajji, S.; Chabchoub, N.; Kallel, R.; Boudawara, T.; Zghal, S.; Salah, R.B.; Nasri, M. Preparation and characterization of polysaccharide based films and evaluation of their healing effects on dermal laser burns in rats. Eur. Polym. J. 2019, 115, 147. [Google Scholar] [CrossRef]
- Ahn, S.; Gil, S.; Kwon, O.; Chang, Y.; Jin, M.H. Skin hydration effect of Brasenia schreberi mucilage polysaccharide extract. J. Soc. Cosmet. Sci. Korea 2017, 43, 223. [Google Scholar]
- Yang, M.; Zhang, Z.; He, Y.; Li, C.; Wang, J.; Ma, X. Study on the structure characterization and moisturizing effect of Tremella polysaccharide fermented from GCMCC5. 39. Food Sci. Hum. Wellness 2021, 10, 471. [Google Scholar] [CrossRef]
- Katekawa, E.; Caverzan, J.; Mussi, L.; Camargo-Junior, F.B.; Sufi, B.; Padovani, G.; Nazato, L.; Nogueira, C.; Magalhães, W.V.; Di Stasi, L.C. Novel topical skin hydration agent containing Anadenanthera colubrina polysaccharide-standardized herbal preparation. J. Cosmet. Dermatol. 2020, 19, 1691. [Google Scholar] [CrossRef]
- Camargo, F.B.d., Jr.; Gaspar, L.R.; Campos, P.M.B.G.M. Immediate and long-term effects of polysaccharides-based formulations on human skin. Braz. J. Pharm. Sci. 2012, 48, 547. [Google Scholar] [CrossRef][Green Version]
- Zhang, T.; Guo, Q.; Xin, Y.; Liu, Y. Comprehensive review in moisture retention mechanism of polysaccharides from algae, plants, bacteria and fungus. Arab. J. Chem. 2022, 15, 104163. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, W.; Wang, Q.; Wang, D.; Dong, D.; Mu, H.; Ye, X.S.; Duan, J. Purification, antioxidant and immunological activities of polysaccharides from Actinidia chinensis roots. Int. J. Biol. Macromol. 2015, 72, 975–983. [Google Scholar] [CrossRef] [PubMed]
- Koohgoli, R.; Hudson, L.; Naidoo, K.; Wilkinson, S.; Chavan, B.; Birch-Machin, M.A. Bad air gets under your skin. Exp. Dermatol. 2017, 26, 384. [Google Scholar] [CrossRef]
- Durai, P.C.; Thappa, D.M.; Kumari, R.; Malathi, M. Aging in elderly: Chronological versus photoaging. Indian J. Dermatol. 2012, 57, 343. [Google Scholar]
- Wang, A.S.; Dreesen, O. Biomarkers of cellular senescence and skin aging. Front. Genet. 2018, 9, 247. [Google Scholar] [CrossRef]
- Lephart, E.D. Skin aging and oxidative stress: Equol’s anti-aging effects via biochemical and molecular mechanisms. Ageing Res. Rev. 2016, 31, 36. [Google Scholar] [CrossRef]
- Sajo, M.; Joy, E.; Kim, C.-S.; Kim, S.-K.; Shim, K.Y.; Kang, T.-Y.; Lee, K.-J. Antioxidant and anti-inflammatory effects of shungite against ultraviolet B irradiation-induced skin damage in hairless mice. Oxidative Med. Cell. Longev. 2017, 2017, 7340143. [Google Scholar] [CrossRef]
- Petruk, G.; Del Giudice, R.; Rigano, M.M.; Monti, D.M. Antioxidants from plants protect against skin photoaging. Oxidative Med. Cell. Longev. 2018, 2018, 1454936. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16. [Google Scholar] [CrossRef]
- Barbosa, J.R.; Freitas, M.M.S.; Oliveira, L.C.; Martins, L.H.S.; Almada-Vilhena, A.O.; Oliveira, R.M.; Pieczarka, J.C.; Davi do Socorro, B.; Junior, R.N.C. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chem. 2020, 330, 127173. [Google Scholar] [CrossRef] [PubMed]
- Mao, G.; Yu, P.; Zhao, T.; Chen, Y.; Feng, W.; Zhang, Q.; Yang, L.; Wu, X. Aqueous two-phase simultaneous extraction and purification of a polysaccharide from Grifola frondosa: Process optimization, structural characteristics and antioxidant activity. Ind. Crops Prod. 2022, 184, 114962. [Google Scholar] [CrossRef]
- Kim, J.E.; Jang, S.G.; Lee, C.H.; Lee, J.Y.; Park, H.; Kim, J.H.; Lee, S.; Kim, S.H.; Park, E.Y.; Lee, K.W. Beneficial effects on skin health using polysaccharides from red ginseng by-product. J. Food Biochem. 2019, 43, e12961. [Google Scholar] [CrossRef] [PubMed]
- Ruksiriwanich, W.; Sirithunyalug, J.; Boonpisuttinant, K.; Jantrawut, P. Potent in vitro collagen biosynthesis stimulating and antioxidant activities of edible mushroom Volvariella volvacea aqueous extract. Int. J. Pharm. Pharm. Sci. 2014, 6, 406–412. [Google Scholar]
- Meng, T.-X.; Zhang, C.-F.; Miyamoto, T.; Ishikawa, H.; Shimizu, K.; Ohga, S.; Kondo, R. The melanin biosynthesis stimulating compounds isolated from the fruiting bodies of Pleurotus citrinopileatus. J. Cosmet. Dermatol. Sci. Appl. 2012, 2, 151. [Google Scholar]
- Liu, Z.-J.; Wang, Y.-L.; Li, Q.-L.; Yang, L. Improved antimelanogenesis and antioxidant effects of polysaccharide from Cuscuta chinensis Lam seeds after enzymatic hydrolysis. Braz. J. Med. Biol. Res. 2018, 51, e7256. [Google Scholar] [CrossRef]
- Pavan, W.J.; Sturm, R.A. The genetics of human skin and hair pigmentation. Annu. Rev. Genom. Hum. Genet. 2019, 20, 41. [Google Scholar] [CrossRef]
- Schalka, S. New data on hyperpigmentation disorders. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 18. [Google Scholar] [CrossRef]
- Yun, C.-Y.; Ko, S.M.; Choi, Y.P.; Kim, B.J.; Lee, J.; Kim, J.M.; Kim, J.Y.; Song, J.Y.; Kim, S.-H.; Hwang, B.Y. α-Viniferin improves facial hyperpigmentation via accelerating feedback termination of cAMP/PKA-signaled phosphorylation circuit in facultative melanogenesis. Theranostics 2018, 8, 2031. [Google Scholar] [CrossRef]
- Xu, Z.; Chen, L.; Jiang, M.; Wang, Q.; Zhang, C.; Xiang, L.F. CCN1/Cyr61 stimulates melanogenesis through integrin α6β1, p38 MAPK, and ERK1/2 signaling pathways in human epidermal melanocytes. J. Investig. Dermatol. 2018, 138, 1825. [Google Scholar] [CrossRef]
- Hu, S.; Huang, J.; Pei, S.; Ouyang, Y.; Ding, Y.; Jiang, L.; Lu, J.; Kang, L.; Huang, L.; Xiang, H. Ganoderma lucidum polysaccharide inhibits UVB-induced melanogenesis by antagonizing cAMP/PKA and ROS/MAPK signaling pathways. J. Cell. Physiol. 2019, 234, 7330. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Huang, J.; Lu, J.; Hu, S.; Pei, S.; Ouyang, Y.; Ding, Y.; Hu, Y.; Kang, L.; Huang, L. Ganoderma lucidum polysaccharide reduces melanogenesis by inhibiting the paracrine effects of keratinocytes and fibroblasts via IL-6/STAT3/FGF2 pathway. J. Cell. Physiol. 2019, 234, 22799. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yang, X.; Zhu, C.; Liu, G.; Han, W.; Sun, Y.; Qian, L. Valorization of polysaccharides from Benincasa hispida: Physicochemical, moisturizing, and antioxidant skincare properties. Front. Pharmacol. 2022, 13, 912382. [Google Scholar] [CrossRef] [PubMed]
- Kanlayavattanakul, M.; Pawakongbun, T.; Lourith, N. Dendrobium orchid polysaccharide extract: Preparation, characterization and in vivo skin hydrating efficacy. Chin. Herb. Med. 2019, 11, 400. [Google Scholar] [CrossRef]
- Jesumani, V.; Du, H.; Pei, P.; Zheng, C.; Cheong, K.-L.; Huang, N. Unravelling property of polysaccharides from Sargassum sp. as an anti-wrinkle and skin whitening property. Int. J. Biol. Macromol. 2019, 140, 216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kou, L.; Wang, F.; Wang, Y. Size-dependent whitening activity of enzyme-degraded fucoidan from Laminaria japonica. Carbohydr. Polym. 2019, 225, 115211. [Google Scholar] [CrossRef]
- Del Carmen Velazquez Pereda, M.; de Campos Dieamant, G.; Eberlin, S.; Werka, R.M.; Colombi, D.; de Souza Queiroz, M.L.; Di Stasi, L.C. Expression of differential genes involved in the maintenance of water balance in human skin by Piptadenia colubrina extract. J. Cosmet. Dermatol. 2010, 9, 35. [Google Scholar] [CrossRef]
- Pan, L.-H.; Li, X.-F.; Wang, M.-N.; Zha, X.-Q.; Yang, X.-F.; Liu, Z.-J.; Luo, Y.-B.; Luo, J.-P. Comparison of hypoglycemic and antioxidative effects of polysaccharides from four different Dendrobium species. Int. J. Biol. Macromol. 2014, 64, 420. [Google Scholar] [CrossRef]
- Kang, H.K.; Park, S.B.; Kim, C.H. Effect of dietary supplementation of red ginseng by-product on laying performance, blood biochemistry, serum immunoglobulin and microbial population in laying hens. Asian-Australas J. Anim. Sci. 2016, 29, 1464. [Google Scholar] [CrossRef]
- Poulose, N.; Sajayan, A.; Ravindran, A.; Sreechithra, T.V.; Vardhan, V.; Selvin, J.; Kiran, G.S. Photoprotective effect of nanomelanin-seaweed concentrate in formulated cosmetic cream: With improved antioxidant and wound healing properties. J. Photochem. Photobiol. B Biol. 2020, 205, 111816. [Google Scholar] [CrossRef]
- Priyan Shanura Fernando, I.; Kim, K.-N.; Kim, D.; Jeon, Y.-J. Algal polysaccharides: Potential bioactive substances for cosmeceutical applications. Crit. Rev. Biotechnol. 2019, 39, 99. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.-T.; Zhang, X.; Liu, Y.; Chen, X.-Q.; Cheong, K.-L. Quantification of 3,6-anhydro-galactose in red seaweed polysaccharides and their potential skin-whitening activity. 3 Biotech. 2020, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Majee, S.; Avlani, D.; Roy Biswas, G. Pharmacological, pharmaceutical, cosmetic and diagnostic applications of sulfated polysaccharides from marine algae and bacteria. Afr. J. Pharm. Pharmacol. 2017, 11, 68. [Google Scholar]
- D’Mello, S.A.N.; Finlay, G.J.; Baguley, B.C.; Askarian-Amiri, M.E. Signaling pathways in melanogenesis. Int. J. Mol. Sci. 2016, 17, 1144. [Google Scholar] [CrossRef]
- Morales, D.; Smiderle, F.R.; Villalva, M.; Abreu, H.; Rico, C.; Santoyo, S.; Iacomini, M.; Soler-Rivas, C. Testing the effect of combining innovative extraction technologies on the biological activities of obtained β-glucan-enriched fractions from Lentinula edodes. J. Funct. Foods 2019, 60, 103446. [Google Scholar] [CrossRef]
- Pina-Pérez, M.C.; Rivas, A.; Martínez, A.; Rodrigo, D. Antimicrobial potential of macro and microalgae against pathogenic and spoilage microorganisms in food. Food Chem. 2017, 235, 34. [Google Scholar] [CrossRef]
- Morone, J.; Alfeus, A.; Vasconcelos, V.; Martins, R. Revealing the potential of cyanobacteria in cosmetics and cosmeceuticals—A new bioactive approach. Algal Res. 2019, 41, 101541. [Google Scholar] [CrossRef]
- Khondkar, P. Composition and partial structure characterization of Tremella polysaccharides. Mycobiology 2009, 37, 286. [Google Scholar] [CrossRef]
- Shen, T.; Duan, C.; Chen, B.; Li, M.; Ruan, Y.; Xu, D.; Shi, D.; Yu, D.; Li, J.; Wang, C. Tremella fuciformis polysaccharide suppresses hydrogen peroxide-triggered injury of human skin fibroblasts via upregulation of SIRT1. Mol. Med. Rep. 2017, 16, 1340. [Google Scholar] [CrossRef]
- Wang, B.-H.; Cao, J.-J.; Zhang, B.; Chen, H.-Q. Structural characterization, physicochemical properties and α-glucosidase inhibitory activity of polysaccharide from the fruits of wax apple. Carbohydr. Polym. 2019, 211, 227. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, X.; Wang, W.; Yang, Q.; Dong, Y.; Xu, N.; Zhang, C.; Song, X.; Ren, Z.; Zhao, F.; et al. Characteristic anti-inflammatory and antioxidative effects of enzymatic- and acidic-hydrolysed mycelium polysaccharides by Oudemansiella radicata on LPS-induced lung injury. Carbohydr. Polym. 2019, 204, 142. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Zhao, Y.; Yang, Y.; Zhang, H.; Ding, C.; Hu, C.; Zhou, L.; Zhang, Z.; Yuan, S.; Chen, Y.; et al. Microwave-assisted extraction, physicochemical characterization and bioactivity of polysaccharides from Camptotheca acuminata fruits. Int. J. Biol. Macromol. 2019, 133, 127. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Li, H.-y.; Wang, N.-n.; Ma, X.-f.; Yu, Z.-m.; Li, C.-y. Optimizing artificial cultivated conditions of Phellinus igniarius by response surface methodology. Zhong Yao Cai 2015, 38, 2459. [Google Scholar] [PubMed]
- Chen, L.; Pan, J.; Li, X.; Zhou, Y.; Meng, Q.; Wang, Q. Endo-polysaccharide of Phellinus igniarius exhibited anti-tumor effect through enhancement of cell mediated immunity. Int. Immunopharmacol. 2011, 11, 255. [Google Scholar] [CrossRef]
- Wang, Y.-q.; Mao, J.-b.; Zhou, M.-q.; Jin, Y.-w.; Lou, C.-h.; Dong, Y.; Shou, D.; Hu, Y.; Yang, B.; Jin, C.-y.; et al. Polysaccharide from Phellinus igniarius activates TLR4-mediated signaling pathways in macrophages and shows immune adjuvant activity in mice. Int. J. Biol. Macromol. 2019, 123, 157. [Google Scholar] [CrossRef]
- Bewick, M.; Coutie, W.; Tudhope, G.R. Superoxide dismutase, glutathione peroxidase and catalase in the red cells of patients with malignant lymphoma. Br. J. Haematol. 1987, 65, 347. [Google Scholar] [CrossRef]
| Polysaccharides | Actions | Mechanism | Type of Study | Ref. |
|---|---|---|---|---|
| Polysaccharides derived from herbaceous plants | ||||
| Anadenanthera colubrina polysaccharide-rich dermocosmetic preparation (ACP) | Moisturizing | Boost the AQP3 gene expression and induce the formation and cohesion of involucrin and FLG | In vivo and clinical trial | [22] |
| Purified Benincasa hispida (Thunb.) Cogn. (Cucurbitaceae) polysaccharides (BPS) preparation | Moisturizing and Antiaging | Boost the AQP3 gene expression and reduce the generation of intracellular ROS | In vitro and in vivo | [45] |
| Polysaccharides from low-quality Dendrobium flowers | Moisturizing | Not mentioned | In vivo and clinical trail | [46] |
| Polysaccharides from red-ginseng (Panax ginseng C.A Meyer) by-product | Antiaging | Inhibit solar ultraviolet-induced MMP-1 protein through activator protein-1 (AP-1) | In vitro and in vivo | [35] |
| Polysaccharides derived from algae | ||||
| Polysaccharide fraction GLP-2 from G. lemaneiformis | Wound healing | Promote cell proliferation by activating the PI3 K/aPKC signaling pathway during human keratinocytes wound healing | In vitro and in vivo | [13] |
| Crude polysaccharides isolated from Sargassum vachellianum, Sargassum horneri, and Sargassum hemiphyllum | Whitening and Antiaging and Moisturizing | Not mentioned | In vitro | [47] |
| Enzyme-degraded fucoidan from Laminaria japonica | Whitening | Not mentioned | In vitro | [48] |
| Sulfated polysaccharides extracted from Nostoc commune | Wound healing and Anti-allergic abilities | Inhibit the production of IL-6, down-regulate the degranulation of RBL-2H3 basophilic leukemia cells, and promote the collagen I secretion | In vitro | [9] |
| Polysaccharides derived from fungi | ||||
| Polysaccharide from mycelium of Ganoderma amboinense (GAMPS) | Wound healing | Not mentioned | In vitro | [14] |
| Fermented Tremella fuciformis polysaccharides (FTPS) purified components | Moisturizing | Promote the gene expression level of various moisturizing genes such as AQP3, TGM1, CASP14, HYAL2, and FLG. | In vitro and clinical trail | [21] |
| P. igniarius Selenium-rich mycelium Polysaccharides (PSeP) | Wound healing | Remove ROS and further enhance the ROS clearance ability by boosting the activity of GSH-PX | In vitro and in vivo | [15] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, Y.; Xu, B. Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry. Polysaccharides 2022, 3, 818-830. https://doi.org/10.3390/polysaccharides3040048
Yao Y, Xu B. Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry. Polysaccharides. 2022; 3(4):818-830. https://doi.org/10.3390/polysaccharides3040048
Chicago/Turabian StyleYao, Yueying, and Baojun Xu. 2022. "Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry" Polysaccharides 3, no. 4: 818-830. https://doi.org/10.3390/polysaccharides3040048
APA StyleYao, Y., & Xu, B. (2022). Skin Health Promoting Effects of Natural Polysaccharides and Their Potential Application in the Cosmetic Industry. Polysaccharides, 3(4), 818-830. https://doi.org/10.3390/polysaccharides3040048







