Carbohydrate Polymer-Based Targeted Pharmaceutical Formulations for Colorectal Cancer: Systematic Review of the Literature
Abstract
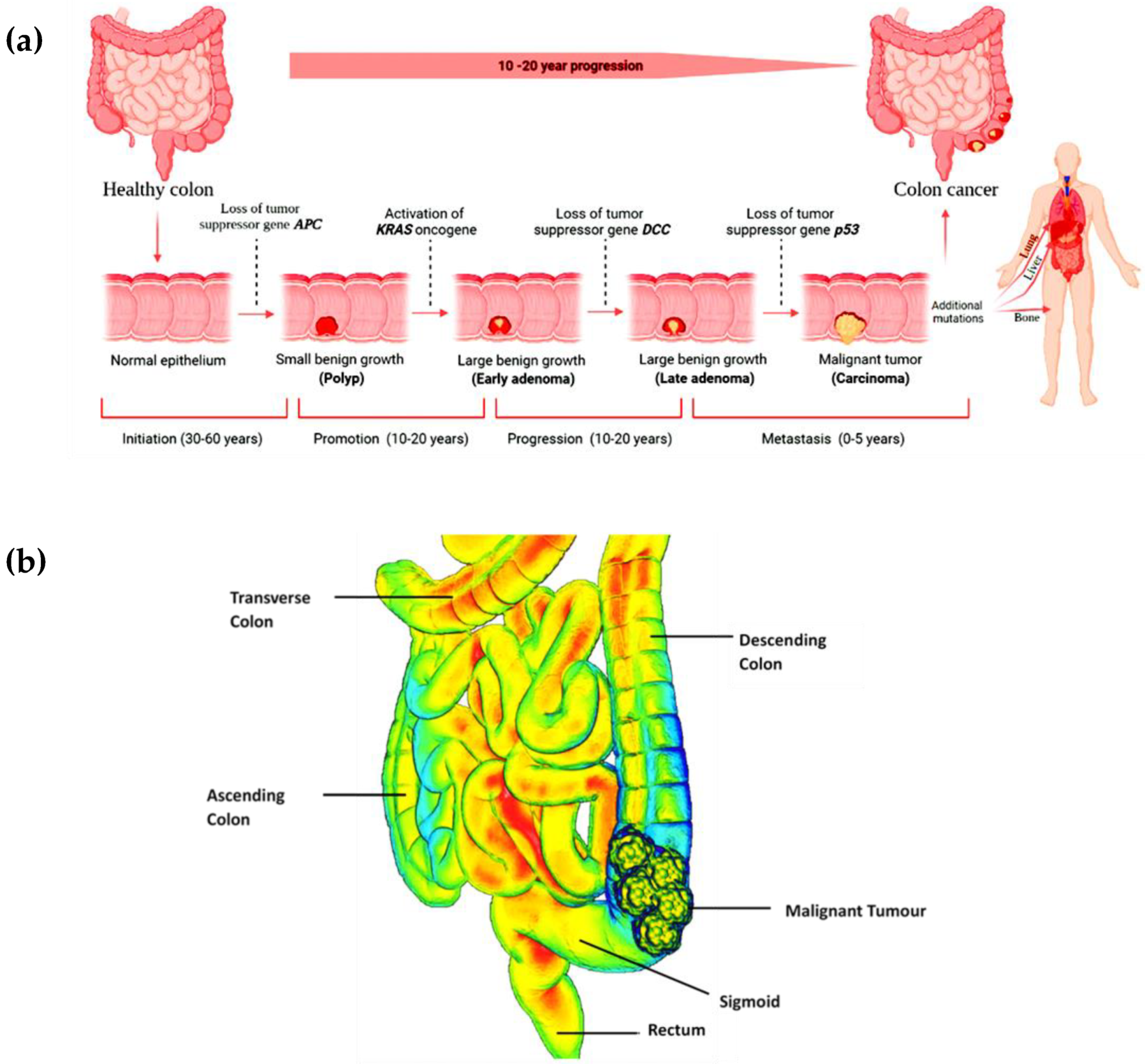
1. Introduction
2. Methodology
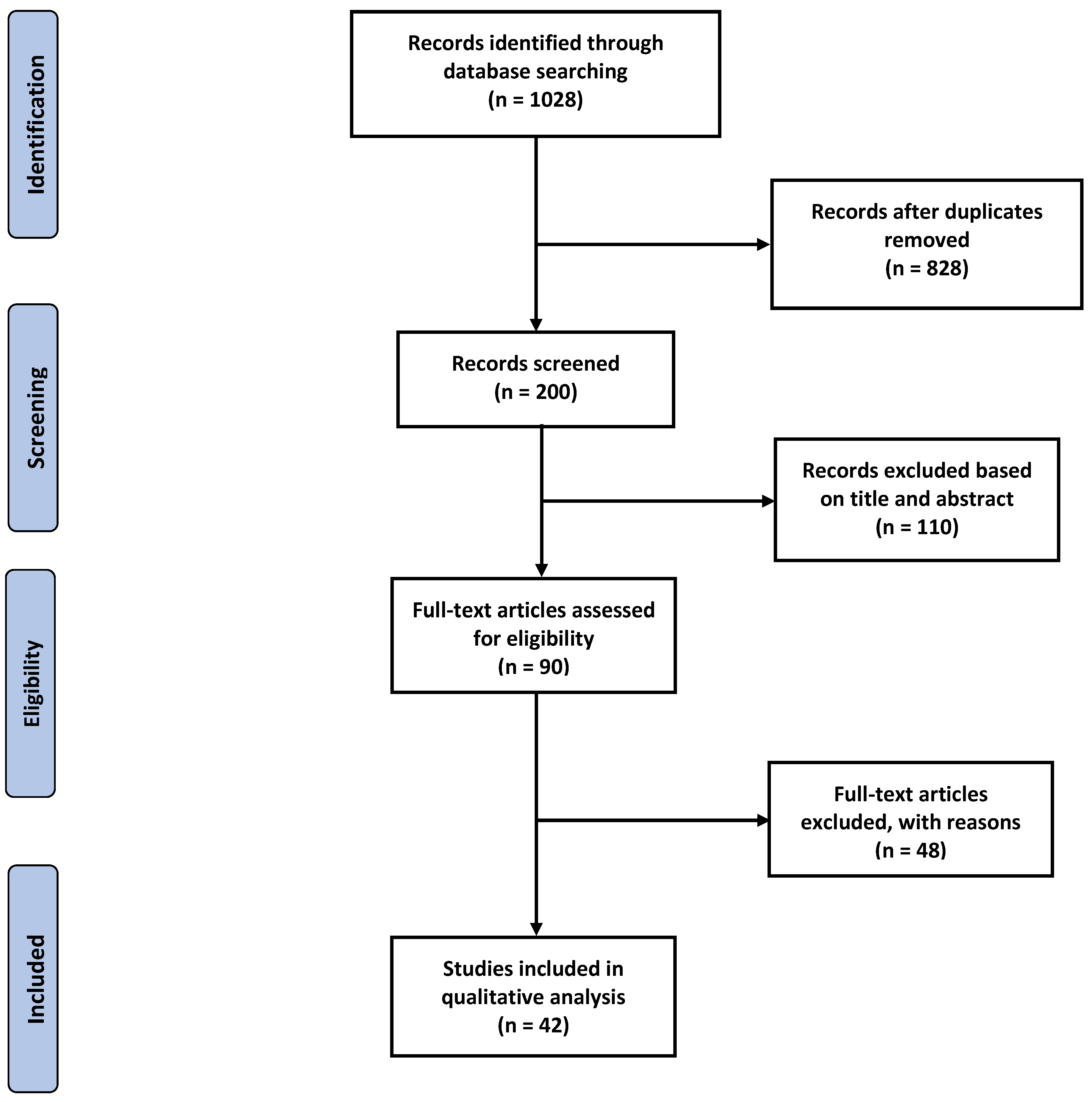
2.1. Search Plot, Information Sources, and Screening Process
2.2. Study Selection
2.3. Data Extraction and Collection
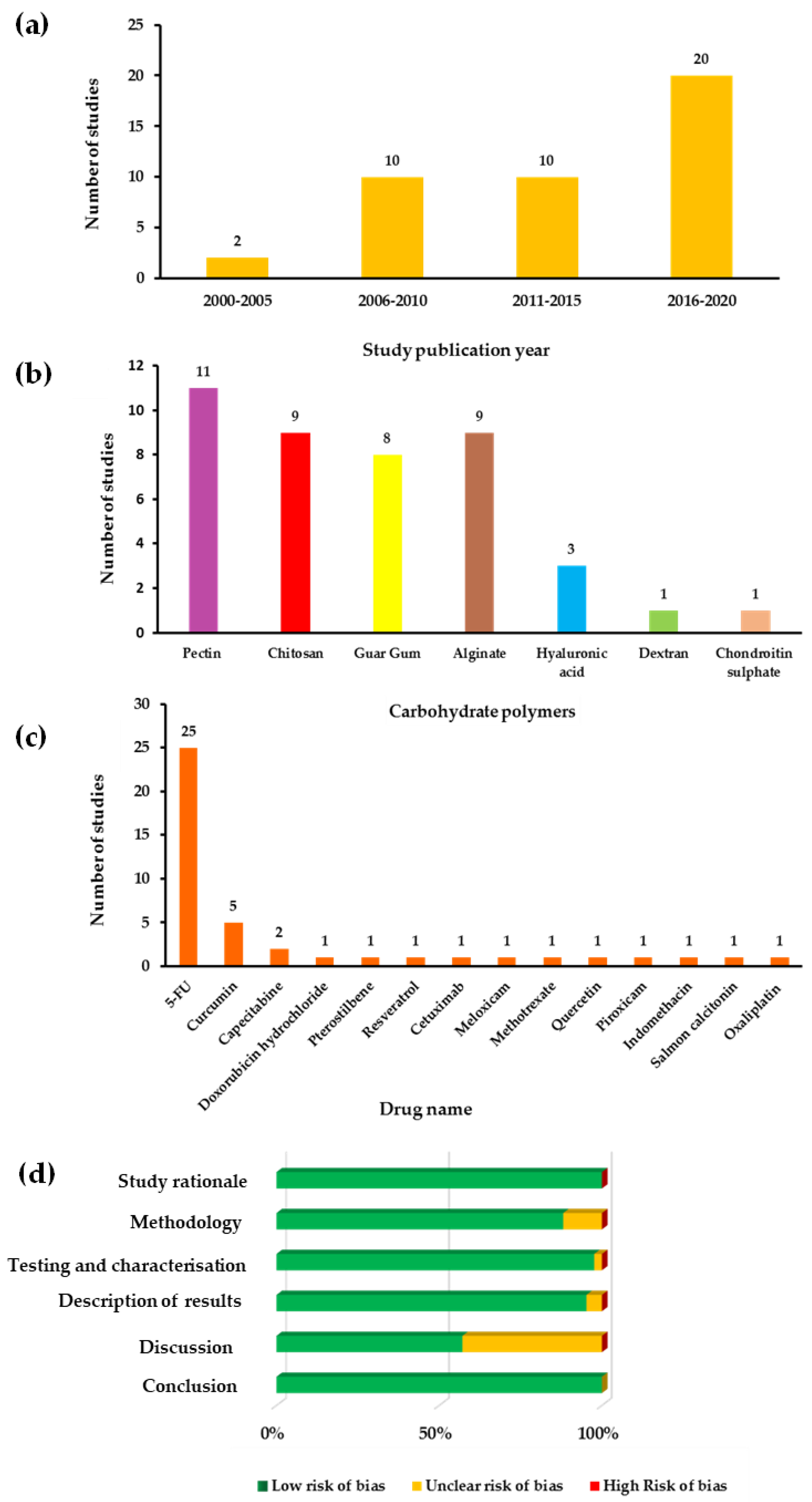
2.4. Risk of Bias Assessment
3. Results and Discussion
3.1. Pectin
3.2. Chitosan
3.3. Guar Gum
3.4. Alginate
3.5. Hyaluronic Acid
3.6. Dextran
3.7. Chondroitin Sulphate
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Dyba, T.; Randi, G.; Bettio, M.; Gavin, A.; Visser, O.; Bray, F. Cancer incidence and mortality patterns in Europe: Estimates for 40 countries and 25 major cancers in 2018. Eur. J. Cancer 2018, 103, 356–387. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Marshall, J.R. Prevention of colorectal cancer: Diet, chemoprevention, and lifestyle. Gastrointest. Endosc. Clin. N. Am. 2008, 37, 73–82. [Google Scholar] [CrossRef]
- Grieu, F.; Malaney, S.; Ward, R.; Joseph, D.; Iacopetta, B. Lack of association between CCND1 G870A polymorphism and the risk of breast and colorectal cancers. Anticancer Res. 2003, 23, 4257–4259. [Google Scholar] [PubMed]
- Hossain, S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Dawson, D.M.; Willis, J.; Willson, J.K.V. Focus on colon cancer. Cancer Cell. 2002, 1, 233–236. [Google Scholar] [CrossRef]
- Markowitz, S.D.; Bertagnolli, M.M. Molecular origins of cancer: Molecular basis of colorectal cancer. N. Engl. J. Med. 2009, 361, 2449–2460. [Google Scholar] [CrossRef]
- Derakhshankhah, H.; Izadi, Z.; Alaei, L.; Lotfabadi, A.; Saboury, A.A.; Dinarvand, R.; Divsalar, A.; Seyedarabi, A.; Barzegari, E.; Evini, M. Colon cancer and specific ways to deliver drugs to the large intestine. Curr. Med. Chem. Anticancer Agents. 2017, 17, 1317–1327. [Google Scholar] [CrossRef]
- Amersi, F.; Agustin, M.; Ko, C.Y. Colorectal cancer: Epidemiology, risk factors, and health services. Clin. Colon Rectal Surg. 2005, 18, 133–140. [Google Scholar] [CrossRef]
- Haggar, F.A.; Boushey, R.P. Colorectal cancer epidemiology: Incidence, mortality, survival, and risk factors. Clin. Colon Rectal Surg. 2009, 22, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Weissman, S.; Sebrow, J.; Gonzalez, H.H.; Weingarten, M.J.; Rosenblatt, S.; Mehta, T.I.; Thaker, R.; Krzyzak, M.; Saleem, S. Diagnosis of Primary Colorectal Carcinoma with Primary Breast Cancer: Associations or Connections? Cureus 2019, 11, e4287. [Google Scholar] [CrossRef] [PubMed]
- Fedirko, V.; Tramacere, I.; Bagnardi, V.; Rota, M.; Scotti, L.; Islami, F.; Negri, E.; Straif, K.; Romieu, I.; La Vecchia, C. Alcohol drinking and colorectal cancer risk: An overall and dose–response meta-analysis of published studies. Ann. Oncol. 2011, 22, 1958–1972. [Google Scholar] [CrossRef] [PubMed]
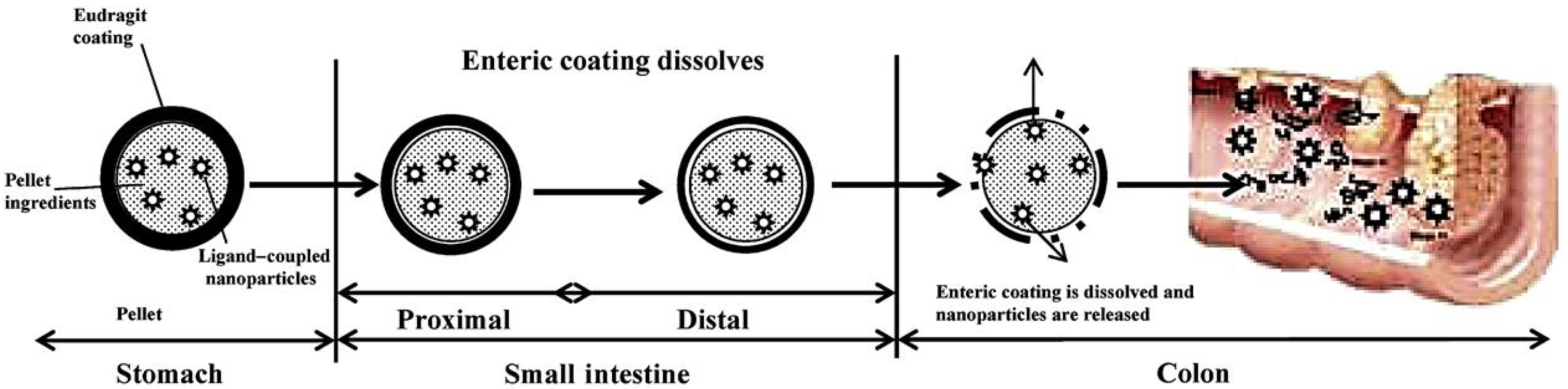
- Krishnaiah, Y.S.; Khan, M.A. Strategies of targeting oral drug delivery systems to the colon and their potential use for the treatment of colorectal cancer. Pharm. Dev. Technol. 2012, 17, 521–540. [Google Scholar] [CrossRef]
- Watson, A.J. An overview of apoptosis and the prevention of colorectal cancer. Crit. Rev. Oncol. Hematol. 2006, 57, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Gulbake, A.; Jain, A.; Jain, A.; Jain, A.; Jain, S.K. Insight to drug delivery aspects for colorectal cancer. World J. Gastroenterol. 2016, 22, 582–599. [Google Scholar] [CrossRef]
- Marshall, J.L. Managing potentially resectable metastatic colon cancer. Gastrointest. Cancer. Res. 2008, 2, S23–S26. [Google Scholar]
- Michor, F.; Iwasa, Y.; Lengauer, C.; Nowak, M.A. Dynamics of colorectal cancer. Semin. Cancer Biol. 2005, 15, 484–493. [Google Scholar] [CrossRef]
- Meany, D.L.; Sokoll, L.J.; Chan, D.W. Early Detection of Cancer: Immunoassays for Plasma Tumor Markers. Expert Opin. Med. Diagn. 2009, 3, 597–605. [Google Scholar]
- Ravi, V.; Pramod Kumar, T.M.; Siddaramaiah. Novel colon targeted drug delivery system using natural polymers. Indian J. Pharm. Sci. 2008, 70, 111–113. [Google Scholar]
- Naeem, M.; Awan, U.A.; Subhan, F.; Cao, J.; Hlaing, S.P.; Lee, J.; Im, E.; Jung, Y.; Yoo, J.W. Advances in colon-targeted nano-drug delivery systems: Challenges and solutions. Arch. Pharmacal. Res. 2020, 43, 153–169. [Google Scholar] [CrossRef]
- Rubinstein, A. Colonic drug delivery. Drug Discov. Today Technol. 2005, 2, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Philip, A.K.; Philip, B. Colon targeted drug delivery systems: A review on primary and novel approaches. Oman Med. J. 2010, 25, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Amidon, S.; Brown, J.E.; Dave, V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS PharmSciTech 2015, 16, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Gulati, M.; Singh, S.K.; Shivapooja, A. Facts, fallacies and future of dissolution testing of polysaccharide based colon-specific drug delivery. J. Control. Release 2014, 178, 55–62. [Google Scholar] [CrossRef]
- Shah, N.N.; Casella, E.; Capozzi, D.; McGettigan, S.; Gangadhar, T.C.; Schuchter, L.; Myers, J.S. Improving the safety of oral chemotherapy at an academic medical center. J. Oncol. Pract. 2016, 12, 71–76. [Google Scholar] [CrossRef]
- Faisal, W.; Farag, F.; Abdellatif, A.A.; Abbas, A. Taste masking approaches for medicines. Curr. Drug Deliv. 2018, 15, 167–185. [Google Scholar] [CrossRef]
- Tawfeek, H.M.; Abdellatif, A.A.; Dennison, T.J.; Mohammed, A.R.; Sadiq, Y.; Saleem, I.Y. Colonic delivery of indometacin loaded PGA-co-PDL microparticles coated with Eudragit L100-55 from fast disintegrating tablets. Int. J. Pharm. 2017, 531, 80–89. [Google Scholar] [CrossRef]
- Leuva, V.; Patel, B.; Chaudhary, D.; Patel, J.; Modasiya, M. Oral colon-specific drug delivery system. J. Pharm. Res. 2012, 5, 2293–2297. [Google Scholar]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef]
- Rao, K.M.; Nagappan, S.; Seo, D.J.; Ha, C.S. pH sensitive halloysite-sodium hyaluronate/poly (hydroxyethyl methacrylate) nanocomposites for colon cancer drug delivery. Appl. Clay Sci. 2014, 97, 33–42. [Google Scholar] [CrossRef]
- Ashwanikumar, N.; Kumar, N.A.; Nair, S.A.; Kumar, G.V. Methacrylic-based nanogels for the pH-sensitive delivery of 5-fluorouracil in the colon. Int. J. Nanomed. 2012, 7, 5769. [Google Scholar]
- Tran, P.H.; Tran, T.T. Mucoadhesive formulation designs for oral controlled drug release at the colon. Curr. Pharm. Des. 2021, 27, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.I.; Singh, J.; Sharma, D.; Sharma, A. Colon specific drug delivery system: Review on novel approaches. Int. J. Pharm. Sci. Res. 2012, 3, 637. [Google Scholar]
- Sinha, V.; Kumria, R. Microbially triggered drug delivery to the colon. Eur. J. Pharm. Sci. 2003, 18, 3. [Google Scholar] [CrossRef]
- Ji, C.; Xu, H.; Wu, W. In vitro evaluation and pharmacokinetics in dogs of guar gum and Eudragit FS30D-coated colon-targeted pellets of indomethacin. J. Drug Target. 2007, 15, 123–131. [Google Scholar] [CrossRef]
- Kumar, M.; Ali, A.; Kaldhone, P.; Shirode, A.; Kadam, V.J. Report on pharmaceutical approaches to colon targeted drug delivery systems. J. Pharm. Res. 2010, 3, 470–473. [Google Scholar]
- Zhang, L.; Sang, Y.; Feng, J.; Li, Z.; Zhao, A. Polysaccharide-based micro/nanocarriers for oral colon-targeted drug delivery. J. Drug Target. 2016, 24, 579–589. [Google Scholar] [CrossRef]
- Godge, G.; Hiremath, S. Development and evaluation of colon targeted drug delivery system by using natural polysaccharides/polymers. Dhaka Univ. J. Pharm. Sci. 2014, 13, 105–113. [Google Scholar] [CrossRef]
- Shukla, R.K.; Tiwari, A. Carbohydrate polymers: Applications and recent advances in delivering drugs to the colon. Carbohydr. Polym. 2012, 88, 399–416. [Google Scholar] [CrossRef]
- Kotla, N.G.; Rana, S.; Sivaraman, G.; Sunnapu, O.; Vemula, P.K.; Pandit, A.; Rochev, Y. Bioresponsive drug delivery systems in intestinal inflammation: State-of-the-art and future perspectives. Adv. Drug Deliv. Rev. 2019, 146, 248–266. [Google Scholar] [CrossRef] [PubMed]
- Barclay, T.G.; Day, C.M.; Petrovsky, N.; Garg, S. Review of polysaccharide particle-based functional drug delivery. Carbohydr. Polym. 2019, 221, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Grewal, J.; Jyoti, K.; Jain, U.K.; Chandra, R.; Madan, J. Oral controlled and sustained drug delivery systems: Concepts, advances, preclinical, and clinical status. In Drug Targeting and Stimuli Sensitive Drug Delivery Systems; Elsevier: Amsterdam, The Netherlands, 2018; pp. 567–626. [Google Scholar]
- Ranjbari, J.; Mokhtarzadeh, A.; Alibakhshi, A.; Tabarzad, M.; Hejazi, M.; Ramezani, M. Anti-cancer drug delivery using carbohydrate-based polymers. Curr. Pharm. Des. 2017, 23, 6019–6032. [Google Scholar] [CrossRef]
- Chourasia, M.; Jain, S. Polysaccharides for colon targeted drug delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef]
- Das, S.; Deshmukh, R.; Jha, A. Role of Natural Polymers in the Development of Multiparticulate Systems for Colon Drug Targeting. Syst. Rev. Pharm. 2010, 1, 79. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Khizer, Z.; Sadia, A.; Sharma, R.; Farhaj, S.; Nirwan, J.S.; Kakadia, P.G.; Hussain, T.; Yousaf, A.M.; Shahzad, Y.; Conway, B.R.; et al. Drug Delivery Approaches for Managing Overactive Bladder (OAB): A Systematic Review. Pharmaceuticals 2021, 14, 409. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Conway, B.R.; Ghori, M.U. Investigating the association between body mass index and gastroesophageal reflux disease: A systematic review and meta-analysis. Turk. J. Gastroenterol. 2019, 30 (Suppl. 3), S592–S593. [Google Scholar]
- Yousaf, M.; Nirwan, J.S.; Smith, A.M.; Timmins, P.; Conway, B.R.; Ghori, M. Raft-forming polysaccharides for the treatment of gastroesophageal reflux disease (GORD): Systematic review. J. Appl. Polym. Sci. 2019, 136, 48012. [Google Scholar] [CrossRef]
- Hasan, S.S.; Zaidi, S.T.R.; Nirwan, J.S.; Ghori, M.U.; Javid, F.; Ahmadi, K.; Babar, Z.U.D. Use of central nervous system (CNS) medicines in aged care homes: A systematic review and meta-analysis. J. Clin. Med. 2019, 8, 1292. [Google Scholar] [CrossRef]
- Nirwan, J.S.; Hasan, S.S.; Babar, Z.U.D.; Conway, B.R.; Ghori, M.U. Global prevalence and risk factors of gastro-oesophageal reflux disease (GORD): Systematic review with meta-analysis. Sci. Rep. 2020, 10, 5814. [Google Scholar] [CrossRef] [PubMed]
- Nirwan, J.S.; Hasan, S.S.; Conway, B.R.; Ghori, M.U. Investigating the association between education level and gastroesophageal reflux disease (GERD): A systematic review and meta-analysis. Turk. J. Gastroenterol. 2019, 30 (Suppl. 3), S892–S893. [Google Scholar]
- Nirwan, J.S.; Hasan, S.S.; Conway, B.R.; Ghori, M.U. Investigating the association between diet and gastroesophageal reflux disease (GERD): A systematic review and meta-analysis. Turk. J. Gastroenterol. 2019, 30 (Suppl. 3), S616–S618. [Google Scholar]
- Wang, Q.-W.; Liu, X.-Y.; Liu, L.; Feng, J.; Li, Y.-H.; Guo, Z.J.; Mei, Q.B. Synthesis and evaluation of the 5-fluorouracil-pectin conjugate targeted at the colon. Med. Chem. Res. 2007, 16, 370–379. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, Y.; Jain, S.K. Potential of calcium pectinate beads for target specific drug release to colon. J. Drug Target. 2007, 15, 285–294. [Google Scholar] [CrossRef]
- Paharia, A.; Yadav, A.K.; Rai, G.; Jain, S.K.; Pancholi, S.S.; Agrawal, G.P. Eudragit-coated pectin microspheres of 5-fluorouracil for colon targeting. AAPS PharmSciTech 2007, 8, 12. [Google Scholar] [CrossRef]
- Wei, H.; Qing, D.; De-Ying, C.; Bai, X.; Li-Fang, F. In-vitro and in-vivo studies of pectin/ethylcellulose-film-coated pellets of 5-fluorouracil for colonic targeting. J. Pharm. Pharmacol. 2008, 60, 35–44. [Google Scholar] [CrossRef]
- Elyagoby, A.; Layas, N.; Wong, T. Colon-specific delivery of 5-fluorouracil from zinc pectinate pellets through In Situ intracapsular ethylcellulose–pectin plug formation. J. Pharm. Sci. 2013, 102, 604–616. [Google Scholar] [CrossRef]
- Subudhi, M.B.; Jain, A.; Jain, A.; Hurkat, P.; Shilpi, S.; Gulbake, A.; Jain, S.K. Eudragit S100 coated citrus pectin nanoparticles for colon targeting of 5-fluorouracil. Materials 2015, 8, 832–849. [Google Scholar] [CrossRef]
- Andishmand, H.; Tabibiazar, M.; Mohammadifar, M.A.; Hamishehkar, H. Pectin-zinc-chitosan-polyethylene glycol colloidal nano-suspension as a food grade carrier for colon targeted delivery of resveratrol. Int. J. Biol. Macromol. 2017, 97, 16–22. [Google Scholar] [CrossRef]
- Zhu, J.; Zhong, L.; Chen, W.; Song, Y.; Qian, Z.; Cao, X.; Huang, Q.; Zhang, B.; Chen, H.; Chen, W. Preparation and characterization of pectin/chitosan beads containing porous starch embedded with doxorubicin hydrochloride: A novel and simple colon targeted drug delivery system. Food Hydrocoll. 2019, 95, 562–570. [Google Scholar] [CrossRef]
- Ansari, M.; Sadarani, B.; Majumdar, A. Colon targeted beads loaded with pterostilbene: Formulation, optimization, characterization and in vivo evaluation. Saudi Pharm. J. 2019, 27, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Sabra, R.; Roberts, C.J.; Billa, N. Courier properties of modified citrus pectinate-chitosan nanoparticles in colon delivery of curcumin. Colloids Interface Sci. Commun. 2019, 32, 100192. [Google Scholar] [CrossRef]
- Sabra, R.; Billa, N.; Roberts, C.J. Cetuximab-conjugated chitosan-pectinate (modified) composite nanoparticles for targeting colon cancer. Int. J. Pharm. 2019, 572, 118775. [Google Scholar] [CrossRef]
- Tığlı Aydın, R.S.; Pulat, M. 5-Fluorouracil encapsulated chitosan nanoparticles for pH-stimulated drug delivery: Evaluation of controlled release kinetics. J. Nanomater. 2012, 2012, 42. [Google Scholar] [CrossRef]
- Dilip, N.T. Formulation and development of colon specific multiparticulate system of capecitabine. Asian J. Pharm. 2016, 10, 401–407. [Google Scholar]
- Liu, W.; Zhu, Y.; Wang, F.; Li, X.; Liu, X.; Pang, J.; Pan, W. Galactosylated chitosan-functionalized mesoporous silica nanoparticles for efficient colon cancer cell-targeted drug delivery. R. Soc. Open Sci. 2018, 5, 181027. [Google Scholar] [CrossRef]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T.; Ngawhirunpat, T.; Tonglairoum, P.; Opanasopit, P. Development of chitosan-based pH-sensitive polymeric micelles containing curcumin for colon-targeted drug delivery. AAPS PharmSciTech 2018, 19, 991–1000. [Google Scholar] [CrossRef]
- Dodov, M.G.; Calis, S.; Crcarevska, M.; Geskovski, N.; Petrovska, V.; Goracinova, K. Wheat germ agglutinin-conjugated chitosan–Ca–alginate microparticles for local colon delivery of 5-FU: Development and in vitro characterization. Int. J. Pharm. 2009, 381, 166–175. [Google Scholar] [CrossRef]
- Sinha, P.; Udhumansha, U.; Rathnam, G.; Ganesh, M.; Jang, H.T. Capecitabine encapsulated chitosan succinate-sodium alginate macromolecular complex beads for colon cancer targeted delivery: In vitro evaluation. Int. J. Biol. Macromol. 2018, 117, 840–850. [Google Scholar] [CrossRef]
- Tummala, S.; Kumar, M.S.; Prakash, A. Formulation and characterization of 5-Fluorouracil enteric coated nanoparticles for sustained and localized release in treating colorectal cancer. Saudi Pharm. J. 2015, 3, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K. Optimization of chitosan nanoparticles for colon tumors using experimental design methodology. Artif. Cells Nanomed. Biotechnol. 2016, 44, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.M. Formulation and development of di-dependent microparticulate system for colon-specific drug delivery. Drug Deliv. Transl. Res. 2017, 7, 312–324. [Google Scholar] [CrossRef] [PubMed]
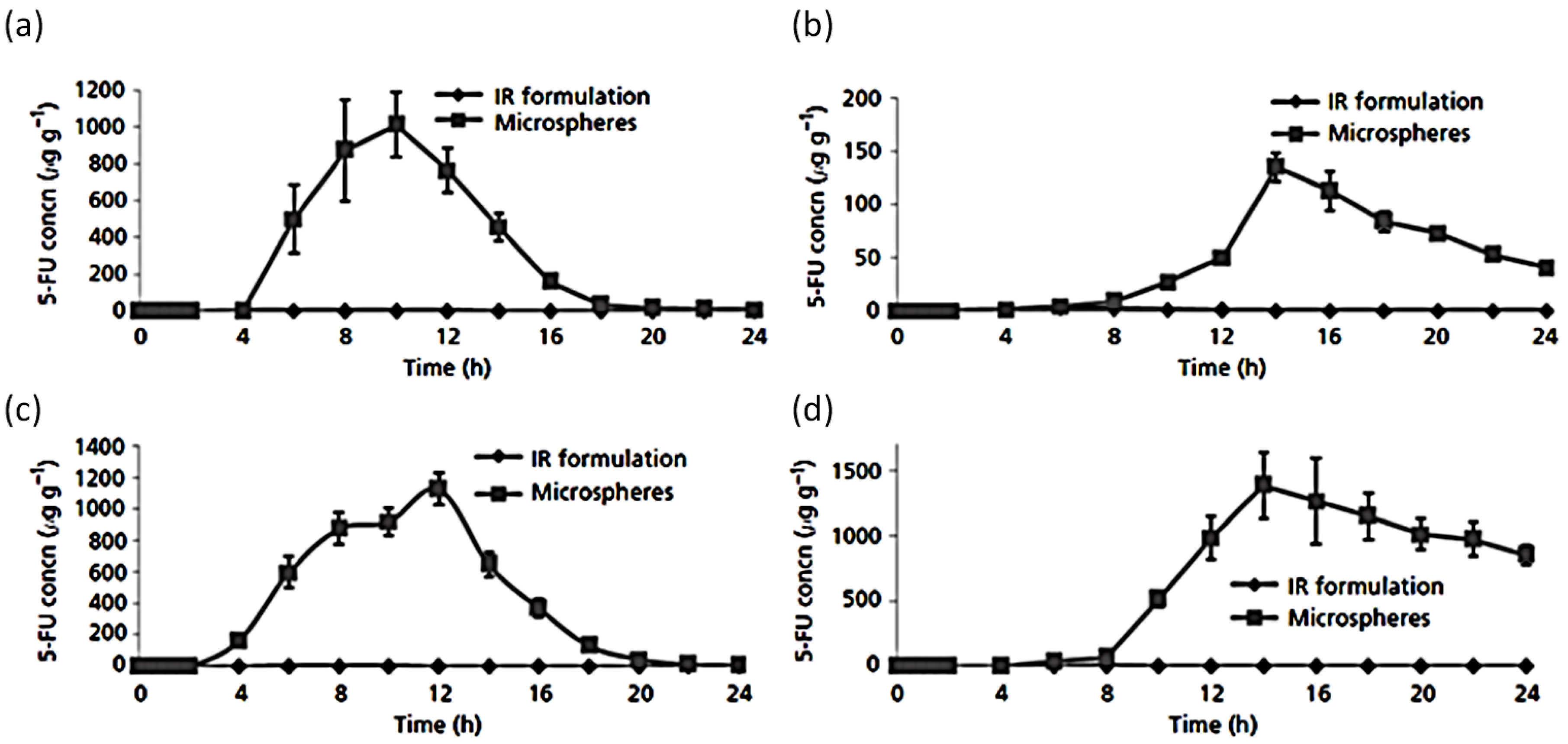
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Dinesh Kumar, B.; Karthikeyan, R.S. In vitro drug release studies on guar gum-based colon targeted oral drug delivery systems of 5-fluorouracil. Eur. J. Pharm. Sci. 2002, 16, 185–192. [Google Scholar] [CrossRef]
- Krishnaiah, Y.S.R.; Satyanarayana, V.; Dinesh Kumar, B.; Karthikeyan, R.S.; Bhaskar, P. In vivo pharmacokinetics in human volunteers: Oral administered guar gum-based colon-targeted 5-fluorouracil tablets. Eur. J. Pharm. Sci. 2003, 19, 355–362. [Google Scholar] [CrossRef]
- Chaurasia, M.; Chourasia, M.K.; Jain, N.K.; Jain, A.; Soni, V.; Gupta, Y.; Jain, S.K. Cross-linked guar gum microspheres: A viable approach for improved delivery of anticancer drugs for the treatment of colorectal cancer. AAPS PharmSciTech 2006, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Singhal, A.; Jain, H.; Singhal, V.; Elias, E.J.; Showkat, A. Colon-targeted quercetin delivery using natural polymer to enhance its bioavailability. Pharmacogn. Res. 2011, 3, 35–39. [Google Scholar] [CrossRef] [PubMed]
- Vats, A.; Pathak, K. Tabletted guar gum microspheres of piroxicam for targeted adjuvant therapy for colonic adenocarcinomas. Ther. Deliv. 2012, 3, 1281–1295. [Google Scholar] [CrossRef]
- Singh, S.; Kotla, N.G.; Tomar, S.; Maddiboyina, B.; Webster, T.J.; Sharma, D.; Sunnapu, O. A nanomedicine-promising approach to provide an appropriate colon-targeted drug delivery system for 5-fluorouracil. Int. J. Nanomed. 2015, 10, 7175. [Google Scholar]
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B 2017, 150, 352–361. [Google Scholar] [CrossRef]
- Kamal, T.; Sarfraz, M.; Arafat, M.; Mikov, M. Cross-linked guar gum and sodium borate based microspheres as colon-targeted anticancer drug delivery systems for 5-fluorouracil. Pak. J. Pharm. Sci. 2017, 30, 2329–2336. [Google Scholar] [PubMed]
- Zhang, B.; Yan, Y.; Shen, Q.; Ma, D.; Huang, L.; Cai, X.; Tan, S. A colon targeted drug delivery system based on alginate modificated graphene oxide for colorectal liver metastasis. Mater. Sci. Eng. C 2017, 79, 185–190. [Google Scholar] [CrossRef]
- Rahman, Z.; Kohli, K.; Khar, R.K.; Ali, M.; Charoo, N.A.; Shamsher, A.A. Characterization of 5-fluorouracil microspheres for colonic delivery. AAPS PharmSciTech 2006, 7, 113–121. [Google Scholar]
- Rahman, Z.; Kohli, K.; Zhang, S.Q.; Khar, R.K.; Ali, M.; Charoo, N.A.; Tauseef, M.; Shamsher, A.A.; Mohammed, N.N.; Repka, M.A. In-vivo evaluation in rats of colon-specific microspheres containing 5-fluorouracil. J. Pharm. Pharmacol. 2008, 60, 615–623. [Google Scholar] [PubMed]
- Ma, Y.; Coombes, A.G. Designing colon-specific delivery systems for anticancer drug-loaded nanoparticles: An evaluation of alginate carriers. J. Biomed. Mater. Res. A 2014, 102, 3167–3176. [Google Scholar]
- Agarwal, T.; Narayana, S.G.H.; Pal, K.; Pramanik, K.; Giri, S.; Banerjee, I. Calcium alginate-carboxymethyl cellulose beads for colon-targeted drug delivery. Int. J. Biol. Macromol. 2015, 75, 409–417. [Google Scholar] [PubMed]
- Sookkasem, A.; Chatpun, S.; Yuenyongsawad, S.; Wiwattanapatapee, R. Alginate beads for colon specific delivery of self-emulsifying curcumin. J. Drug Deliv. Sci. Technol. 2015, 29, 159–166. [Google Scholar] [CrossRef]
- Asnani, G.P.; Kokare, C.R. In vitro and in vivo evaluation of colon cancer targeted epichlorohydrin crosslinked Portulaca-alginate beads. Biomol. Concepts 2018, 9, 190–199. [Google Scholar] [CrossRef]
- Feng, K.; Li, C.; Wei, Y.-S.; Zong, M.-H.; Wu, H.; Han, S.-Y. Development of a polysaccharide based multi-unit nanofiber mat for colon-targeted sustained release of salmon calcitonin. J. Colloid Interface Sci. 2019, 552, 186–195. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.; Yang, L.-Y.; Ouyang, X.-k. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Int. J. Biol. Macromol. 2019, 132, 487–494. [Google Scholar] [CrossRef]
- Jain, A.; Jain, S.K. In vitro and cell uptake studies for targeting of ligand anchored nanoparticles for colon tumors. Eur. J. Pharm. Sci. 2008, 35, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Jain, S.K.; Ganesh, N.; Barve, J.; Beg, A.M. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomedicine 2010, 6, 179–190. [Google Scholar] [CrossRef] [PubMed]
- Kotla, N.G.; Burke, O.; Pandit, A.; Rochev, Y. An Orally Administrated Hyaluronan Functionalized Polymeric Hybrid Nanoparticle System for Colon-Specific Drug Delivery. Nanomaterials 2019, 9, 1246. [Google Scholar] [CrossRef] [PubMed]
- Rai, G.; Yadav, A.K.; Jain, N.K.; Agrawal, G.P. Eudragit-coated dextran microspheres of 5-fluorouracil for site-specific delivery to colon. Drug Deliv. 2016, 23, 328–337. [Google Scholar] [CrossRef]
- Raza, H.; Ranjha, N.M.; Mahmood, A.; Azam, F.; Sarfraz, R.M.; Rashid, Z. Development and in vitro characterization of 5-flurouracilloaded, colon-targeted drug delivery system. Trop. J. Pharm. Res. 2018, 17, 195–204. [Google Scholar] [CrossRef]
- Pranati, S.; Rishabha, M. Sources of pectin, extraction and its applications in pharmaceutical industry-an overview. Indian J. Nat. Prod. Res. 2011, 2, 10–18. [Google Scholar]
- Sriamornsak, P. Chemistry of pectin and its pharmaceutical uses: A review. SUST J. 2003, 3, 206. [Google Scholar]
- Aburto, J.; Moran, M.; Galano, A.; Torres-García, E. Non-isothermal pyrolysis of pectin: A thermochemical and kinetic approach. J. Anal. Appl. Pyrolysis 2015, 112, 94–104. [Google Scholar] [CrossRef]
- Itoh, K.; Hirayama, T.; Takahashi, A.; Kubo, W.; Miyazaki, S.; Dairaku, M.; Togashi, M.; Mikami, R.; Attwood, D. In situ gelling pectin formulations for oral drug delivery at high gastric pH. Int. J. Pharm. 2007, 335, 90–96. [Google Scholar] [CrossRef]
- Shariatinia, Z. Pharmaceutical applications of chitosan. Adv. Colloid Interface Sci. 2019, 263, 131–194. [Google Scholar] [CrossRef] [PubMed]
- Nazar, H. Developing biomaterials for tissue engineering and regenerative medicine. Pharm. J. 2013, 291, 223. [Google Scholar]
- Morin-Crini, N.; Lichtfouse, E.; Torri, G.; Crini, G. Applications of chitosan in food, pharmaceuticals, medicine, cosmetics, agriculture, textiles, pulp and paper, biotechnology, and environmental chemistry. Environ. Chem. Lett. 2019, 17, 1667–1692. [Google Scholar] [CrossRef]
- Silva, A.J.C., Jr.; Alves, S.; Tonholo, J.; Ribeiro, A.S. Dansyl-based fluorescent films prepared by chemical and electrochemical methods: Cyclic voltammetry, afm and spectrofluorimetry characterization. J. Braz. Chem. Soc. 2011, 22, 1808–1815. [Google Scholar] [CrossRef]
- Manjunath, M.; Anjali; Gowda, D.V.; Kumar, P.; Srivastava, A.; Osmani, R.A.; Shinde, C.G.; Hatna, S. Guar gum and its pharmaceutical and biomedical applications. Adv. Sci. Eng. Med. 2016, 8, 589–602. [Google Scholar] [CrossRef]
- Patel, J.J.; Karve, M.; Patel, N.K. Guar gum: A versatile material for pharmaceutical industries. Int. J. Pharm. Pharm. Sci. 2014, 6, 13–19. [Google Scholar]
- Oprea, S. Effects of guar gum content on structure and properties of multi-crosslinked polyurethane composite films. Compos. B. Eng. 2013, 44, 76–83. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 7697031. [Google Scholar] [CrossRef]
- Kothale, D.; Verma, U.; Dewangan, N.; Jana, P.; Jain, A.; Jain, D. Alginate as promising natural polymer for pharmaceutical, food, and biomedical applications. Curr. Drug Deliv. 2020, 17, 755–775. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 11867. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Huang, H. Application of hyaluronic acid as carriers in drug delivery. Drug Deliv. 2018, 25, 766–772. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic acid in the third millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef]
- Sayed Aly, M.N. Intra-articular drug delivery: A fast growing approach. Recent Pat. Drug Deliv Formul. 2008, 2, 231–237. [Google Scholar] [CrossRef]
- Bhavani, A.L.; Nisha, J. Dextran—The polysaccharide with versatile uses. Int. J. Pharm. Biol. Sci. 2010, 1, 569–573. [Google Scholar]
- Huang, S.; Huang, G. Preparation and drug delivery of dextran-drug complex. Drug Deliv. 2019, 26, 252–261. [Google Scholar] [CrossRef]
- Atamanov, M.K.; Noboru, I.; Shotaro, T.; Amrousse, R.; Tulepov, M.Y.; Kerimkulova, A.R.; Hobosyan, M.A.; Hori, K.; Martirosyan, K.S.; Mansurov, Z.A. Investigation of Combustion and Thermal Analysis of Ammonium Nitrate with Carbonaceous Materials. Combust. Sci. Technol. 2016, 188, 2003–2011. [Google Scholar] [CrossRef]
- Amhare, A.F.; Lei, J.; Deng, H.; Lv, Y.; Han, J.; Zhang, L. Biomedical application of chondroitin sulfate with nanoparticles in drug delivery systems: Systematic review. J. Drug Target. 2020, 29, 259–268. [Google Scholar] [CrossRef]
- Oprea, A.M.; Neamtu, A.; Stoica, B.; Vasile, C. Cellulose/chondroitin sulphate hydrogels as carr-iers for drug delivery applications. An. Ştiinţifice Ale Univ. Alexandru Ioan Cuza Din Iași Sect. II A Genet. Si Biol. Mol. 2009, 10, 85–92. [Google Scholar]
- Liu, Y.; Wang, J.-Y.; Jiang, W. An increasing prominent disease of Klebsiella pneumoniae liver abscess: Etiology, diagnosis, and treatment. Gastroenterol. Res. Prac. 2013, 2013, 258514. [Google Scholar]
- Xi, M.M.; Zhang, S.Q.; Wang, X.Y.; Fang, K.Q.; Gu, Y. Study on the characteristics of pectin–ketoprofen for colon targeting in rats. Int. J. Pharm. 2005, 298, 91–97. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin structure and biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef]
- Wong, T.W.; Colombo, G.; Sonvico, F. Pectin Matrix as Oral Drug Delivery Vehicle for Colon Cancer Treatment. AAPS PharmSciTech 2011, 12, 201–214. [Google Scholar] [CrossRef]
- Perera, G.; Barthelmes, J.; Bernkop-Schnürch, A. Novel pectin–4-aminothiophenole conjugate microparticles for colon-specific drug delivery. J. Control. Release 2010, 145, 240–246. [Google Scholar] [CrossRef]
- Tiwari, A.; Ramteke, S.; Dahima, R.; Shukla, R. Preparation and characterization of satranidazole loaded calcium pectinate microbeads for colon specific delivery; Application of response surface methodology. Curr. Nanosci. 2011, 7, 608–615. [Google Scholar] [CrossRef]
- Shukla, S.; Jain, D.; Verma, K.; Verma, S. Pectin-based colon-specific drug delivery. Chron. Young Sci. 2011, 2, 83–89. [Google Scholar] [CrossRef]
- Sundarraj, A.A.; Ranganathan, T.V. A review-Pectin from Agro and industrial waste. Int. J. Appl. Enviro. Sci. 2017, 12, 1777–1801. [Google Scholar]
- Pandey, S.; Mishra, A.; Raval, P.; Patel, H.; Gupta, A.; Shah, D. Chitosan–pectin polyelectrolyte complex as a carrier for colon targeted drug delivery. J. Young. Pharm. 2013, 5, 160–166. [Google Scholar] [CrossRef]
- Vaidya, A.; Jain, S.; Agrawal, R.K.; Jain, S.K. Pectin–metronidazole prodrug bearing microspheres for colon targeting. J. Saudi Chem. Soc. 2015, 19, 257–264. [Google Scholar] [CrossRef]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Park, J.H.; Saravanakumar, G.; Kim, K.; Kwon, I.C. Targeted delivery of low molecular drugs using chitosan and its derivatives. Adv. Drug Deliv. Rev. 2010, 62, 28–41. [Google Scholar] [CrossRef]
- Agnihotri, S.A.; Mallikarjuna, N.N.; Aminabhavi, T.M. Recent advances on chitosan-based micro-and nanoparticles in drug delivery. J. Control. Release 2004, 100, 5–28. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.A.; Aminabhavi, T.M. Development of novel interpenetrating network gellan gum-poly (vinyl alcohol) hydrogel microspheres for the controlled release of carvedilol. Drug Dev. Ind. Pharm. 2005, 31, 491–503. [Google Scholar] [CrossRef] [PubMed]
- Sinha, V.R.; Kumria, R. Polysaccharides in colon-specific drug delivery. Int. J. Pharm. 2001, 224, 19–38. [Google Scholar] [CrossRef]
- Vemula, S.K.; Bontha, V.K. Colon Targeted Guar Gum Compression Coated Tablets of Flurbiprofen: Formulation, Development, and Pharmacokinetics. Biomed. Res. Int. 2013, 2013, 287919. [Google Scholar] [CrossRef]
- Aminabhavi, T.M.; Nadagouda, M.N.; Joshi, S.D.; More, U.A. Guar gum as platform for the oral controlled release of therapeutics. Expert Opin. Drug Deliv. 2014, 11, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Subuddhi, U. pH-Responsive guar gum hydrogels for controlled delivery of dexamethasone to the intestine. Int. J. Biol. Macromol. 2015, 79, 856–863. [Google Scholar] [CrossRef]
- Srivastava, M.; Kapoor, V.P. Seed Galactomannans: An Overview. Chem. Biodivers. 2005, 2, 295–317. [Google Scholar] [CrossRef]
- Sinha, V.; Singh, A.; Singh, S.; Bhinge, J. Compression coated systems for colonic delivery of 5-fluorouracil. J. Pharm. Pharmacol. 2007, 59, 359–365. [Google Scholar] [CrossRef]
- Singhal, A.; Nalwaya, N.; Jarald, E.E.; Ahmed, S. Colon targeted curcumin delivery using guar gum. Pharmacogn. Res. 2010, 2, 82. [Google Scholar] [CrossRef]
- Mudgil, D.; Barak, S.; Khatkar, B.S. Guar gum: Processing, properties and food applications—A review. J. Food Sci. Technol. 2014, 51, 409–418. [Google Scholar] [CrossRef]
- Thombare, N.; Jha, U.; Mishra, S.; Siddiqui, M.Z. Guar gum as a promising starting material for diverse applications: A review. Int. J. Biol. Macromol. 2016, 88, 361–372. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Gupta, Y.; Jain, S.K. Perspectives of biodegradable natural polysaccharides for site-specific drug delivery to the colon. J. Pharm. Pharm. Sci. 2007, 10, 86–128. [Google Scholar] [PubMed]
- Ji, C.; Xu, H.; Wu, W. Guar gum as potential film coating material for colon-specific delivery of fluorouracil. J. Biomater. Appl. 2009, 23, 311–329. [Google Scholar] [CrossRef]
- Narayanan, R.P.; Melman, G.; Letourneau, N.J.; Mendelson, N.L.; Melman, A. Photodegradable iron (III) cross-linked alginate gels. Biomacromolecules 2012, 13, 2465–2471. [Google Scholar] [CrossRef]
- Wang, Q.-S.; Wang, G.-F.; Zhou, J.; Gao, L.N.; Cui, Y.L. Colon targeted oral drug delivery system based on alginate-chitosan microspheres loaded with icariin in the treatment of ulcerative colitis. Int. J. Pharm. 2016, 515, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M.L. Alginate microparticles as oral colon drug delivery device: A review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef]
- Shah, N.; Shah, T.; Amin, A. Polysaccharides: A targeting strategy for colonic drug delivery. Expert Opin. Drug Deliv. 2011, 8, 779–796. [Google Scholar] [CrossRef]
- Dheer, D.; Arora, D.; Jaglan, S.; Rawal, R.K.; Shankar, R. Polysaccharides based nanomaterials for targeted anti-cancer drug delivery. J. Drug Target. 2017, 25, 779–796. [Google Scholar] [CrossRef]
- Geetha, P.; Latha, M.; Pillai, S.S.; Deepa, B.; Kumar, K.S.; Koshy, M. Green synthesis and characterization of alginate nanoparticles and its role as a biosorbent for Cr (VI) ions. J. Mol. Struct. 2016, 1105, 54–60. [Google Scholar] [CrossRef]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; ÖBek, C.; Pham, H.T.; Wei, D.; Young, M.J.; Duncan, R.C.; Soloway, M.S.; Block, N.L. Urinary hyaluronic acid and hyaluronidase: Markers for bladder cancer detection and evaluation of grade. J. Urol. 2000, 163, 348–356. [Google Scholar] [CrossRef]
- Brown, M.B.; Jones, S.A. Hyaluronic acid: A unique topical vehicle for the localized delivery of drugs to the skin. J. Eur. Acad. Dermatol. Venereol. 2005, 19, 308–318. [Google Scholar] [CrossRef]
- Pitarresi, G.; Casadei, M.A.; Mandracchia, D.; Paolicelli, P.; Palumbo, F.S.; Giammona, G. Photocrosslinking of dextran and polyaspartamide derivatives: A combination suitable for colon-specific drug delivery. J. Control. Release 2007, 119, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Posocco, B.; Dreussi, E.; De Santa, J.; Toffoli, G.; Abrami, M.; Musiani, F.; Grassi, M.; Farra, R.; Tonon, F.; Grassi, G. Polysaccharides for the delivery of antitumor drugs. Materials 2015, 8, 2569–2615. [Google Scholar] [CrossRef]
- Harboe, E.; Larsen, C.; Johansen, M.; Olesen, H.P. Macromolecular prodrugs. XV. Colon-targeted delivery—Bioavailability of naproxen from orally administered dextran–naproxen ester prodrugs varying in molecular size in the pig. Pharm. Res. 1989, 6, 919–923. [Google Scholar] [CrossRef]
- Mansur, H.S.; Sadahira, C.M.; Souza, A.N.; Mansur, A.A. FTIR spectroscopy characterization of poly (vinyl alcohol) hydrogel with different hydrolysis degree and chemically crosslinked with glutaraldehyde. Mater. Sci. Eng. C 2008, 28, 539–548. [Google Scholar] [CrossRef]


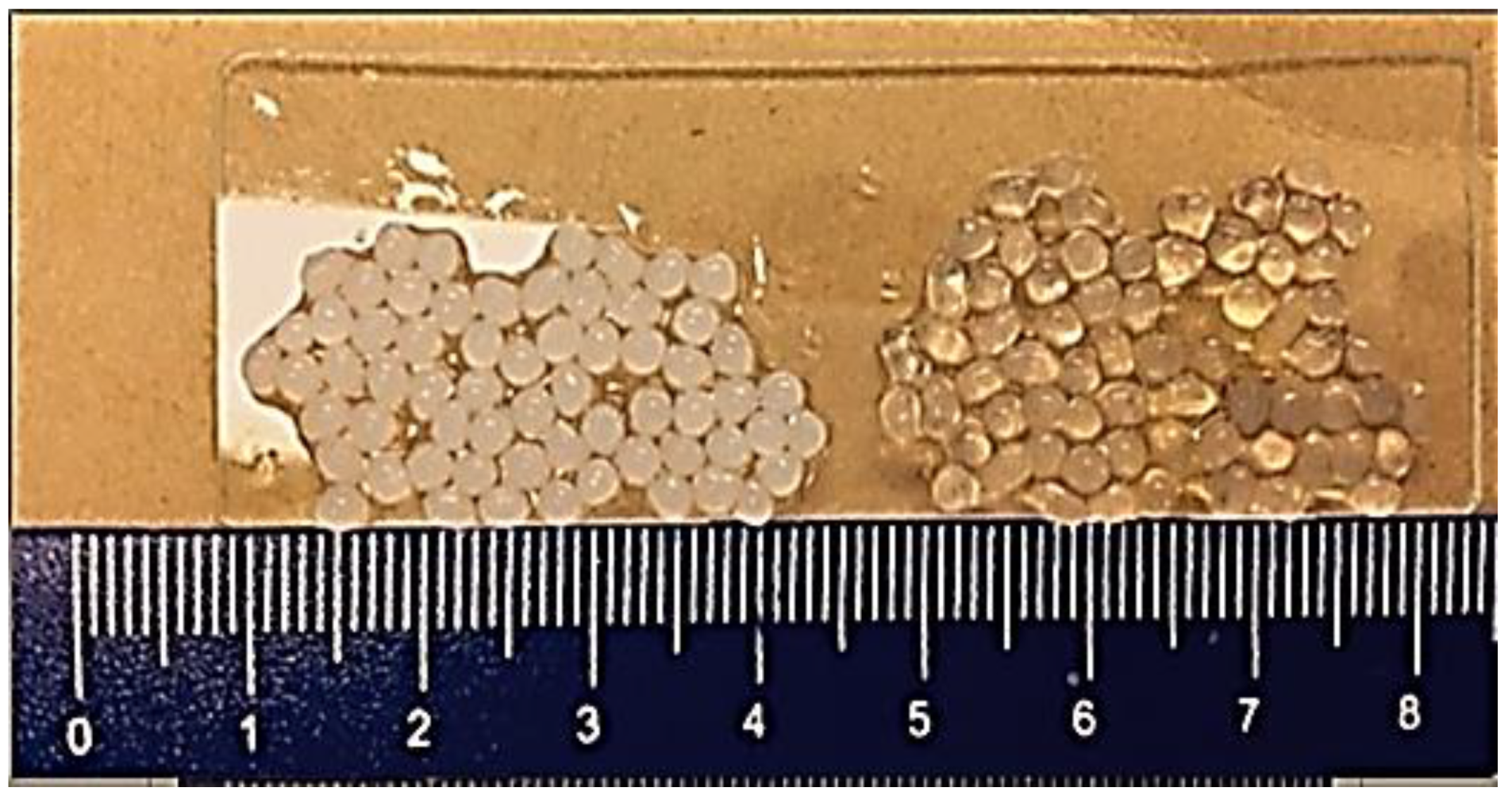
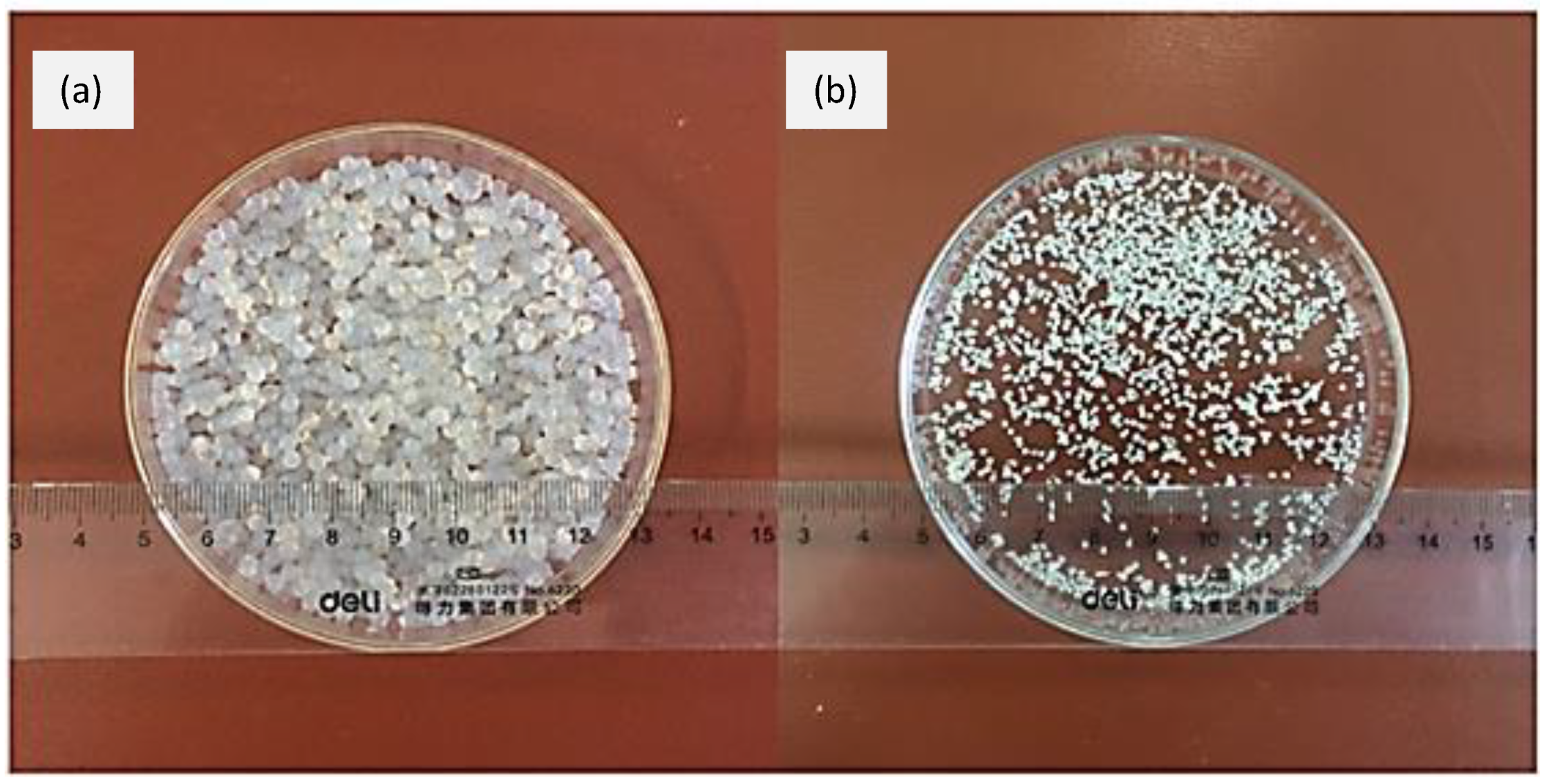

| Polymer | Structural Units | Other Applications | Chemical Structure | References |
|---|---|---|---|---|
| Pectin | (1→4)-linked α-D-galacturonic acid residues. |
| 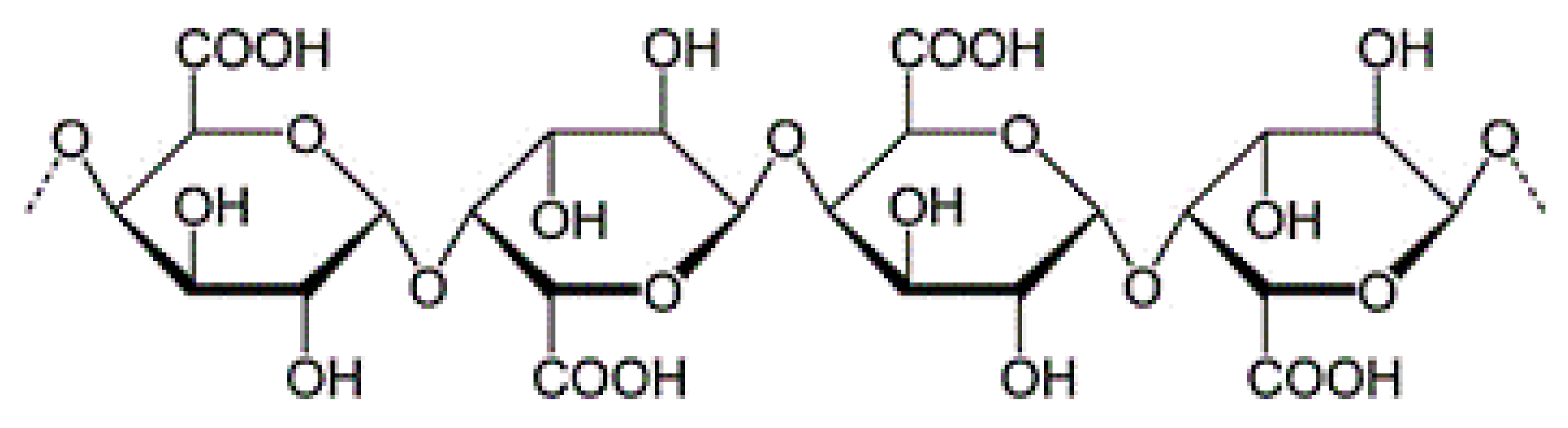 | [97,98,99,100] |
| Chitosan | β (1→ 4) linked glucosamine units together with some proportion of N-acetylglucosamine units |
| 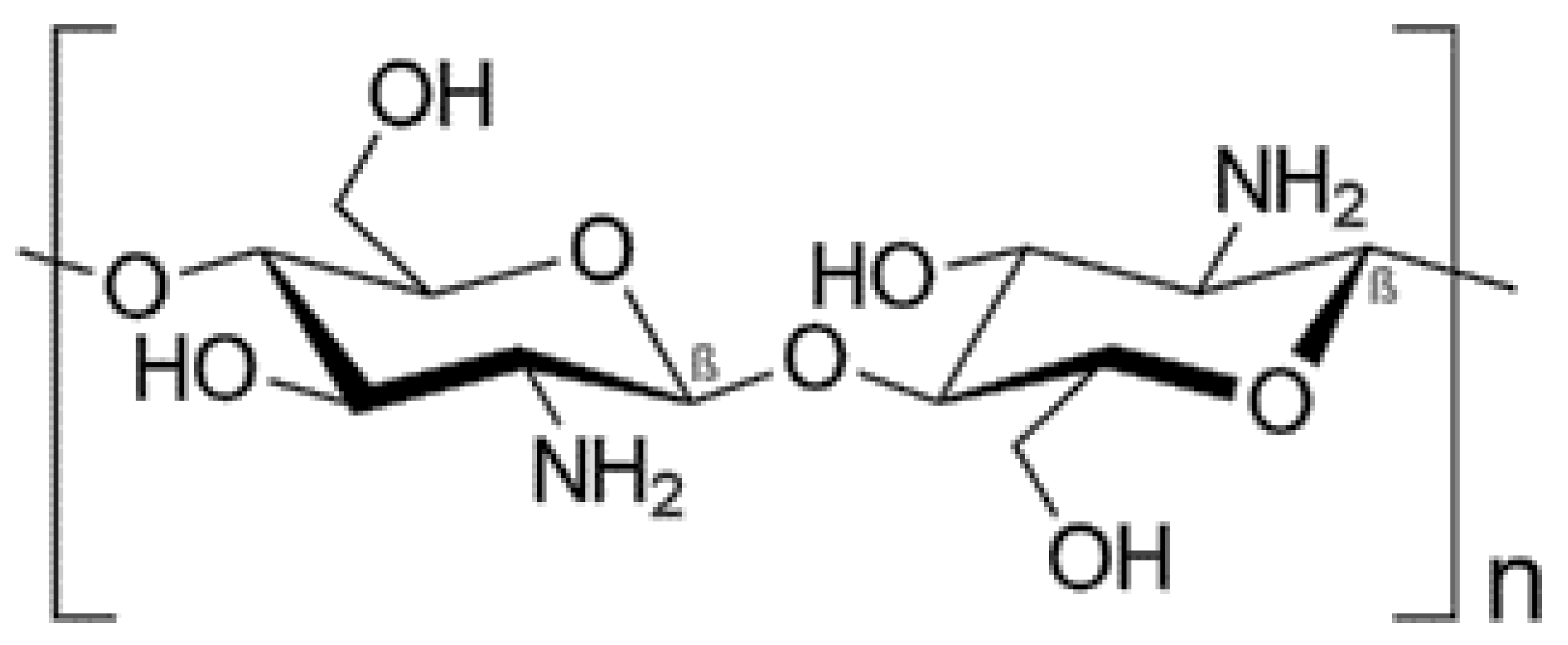 | [101,102,103,104] |
| Guar gum | (1→4)- β-D-mannopyranosyl units with (1 → 6)-linked α-D-galactopyranosyl |
| 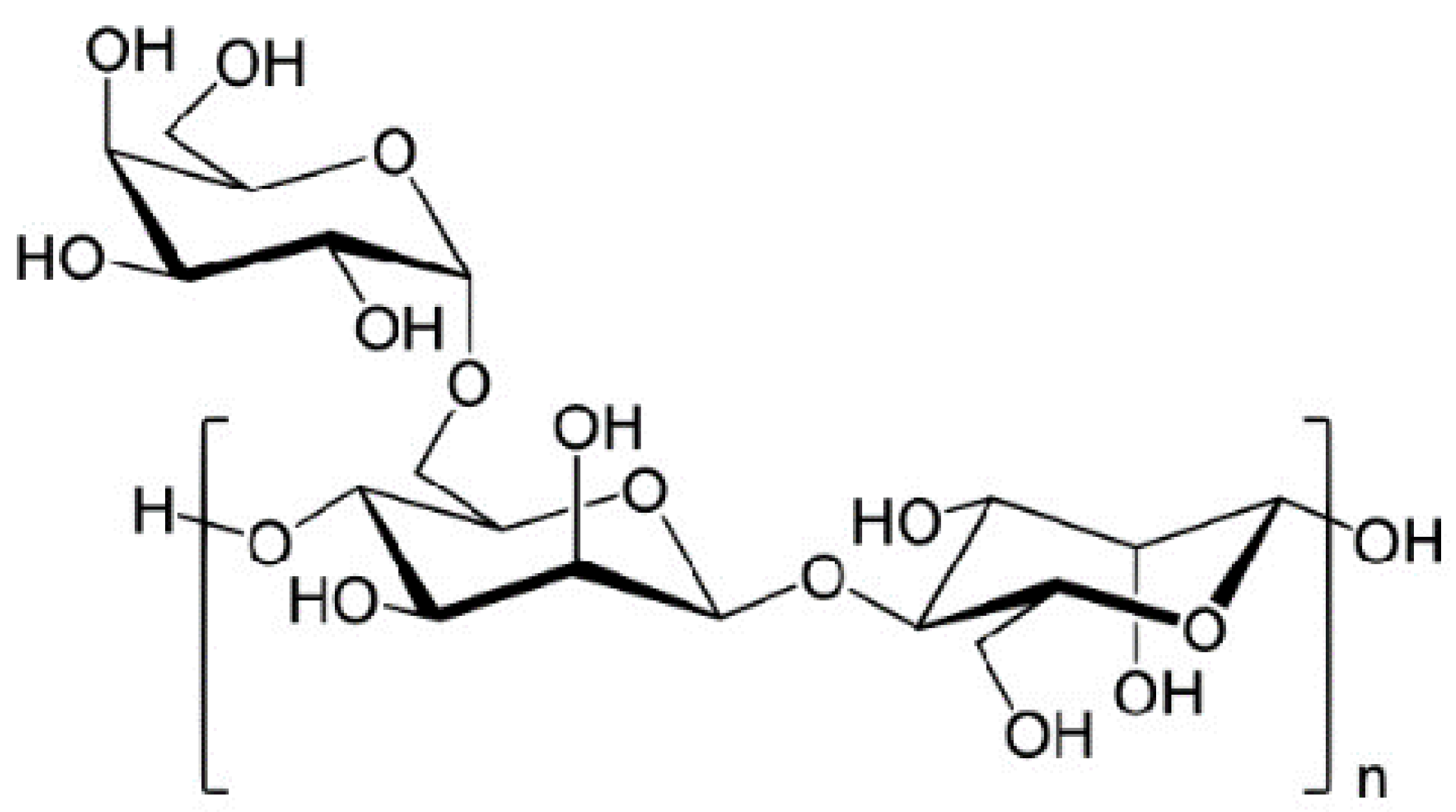 | [105,106,107] |
| Alginate | (1→4)-linked β-D-mannuronate (M) and α-L-guluronate (G) residues |
|  | [108,109,110,111] |
| Hyaluronic acid | N-acetyl-D-glucosamine (GlcNAc) and D-glucuronic acid linked by β-(1/3) bond |
| 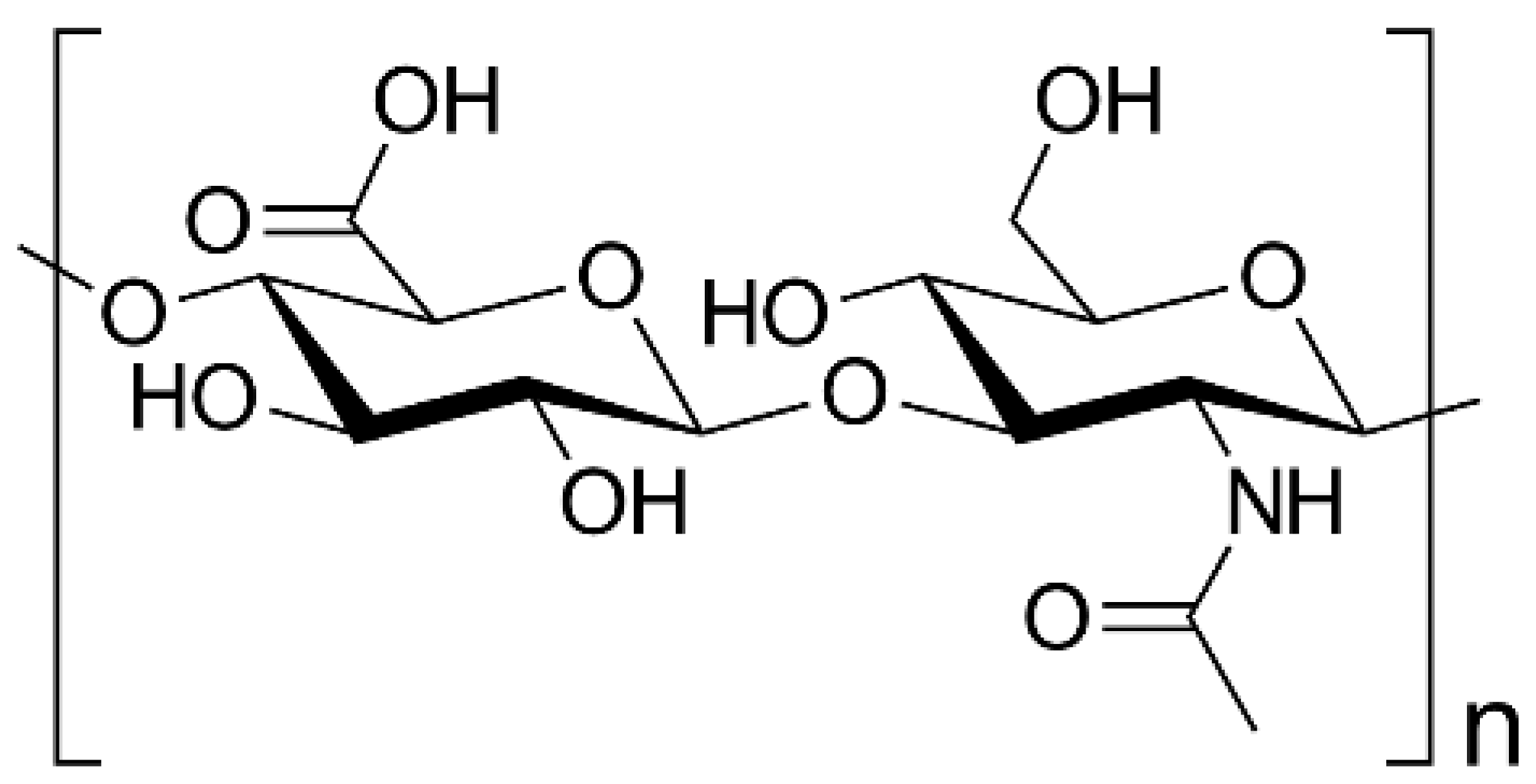 | [112,113,114] |
| Dextran | 1:6-α-glucose units with some degree of branching via 1:3-α-linkages |
| 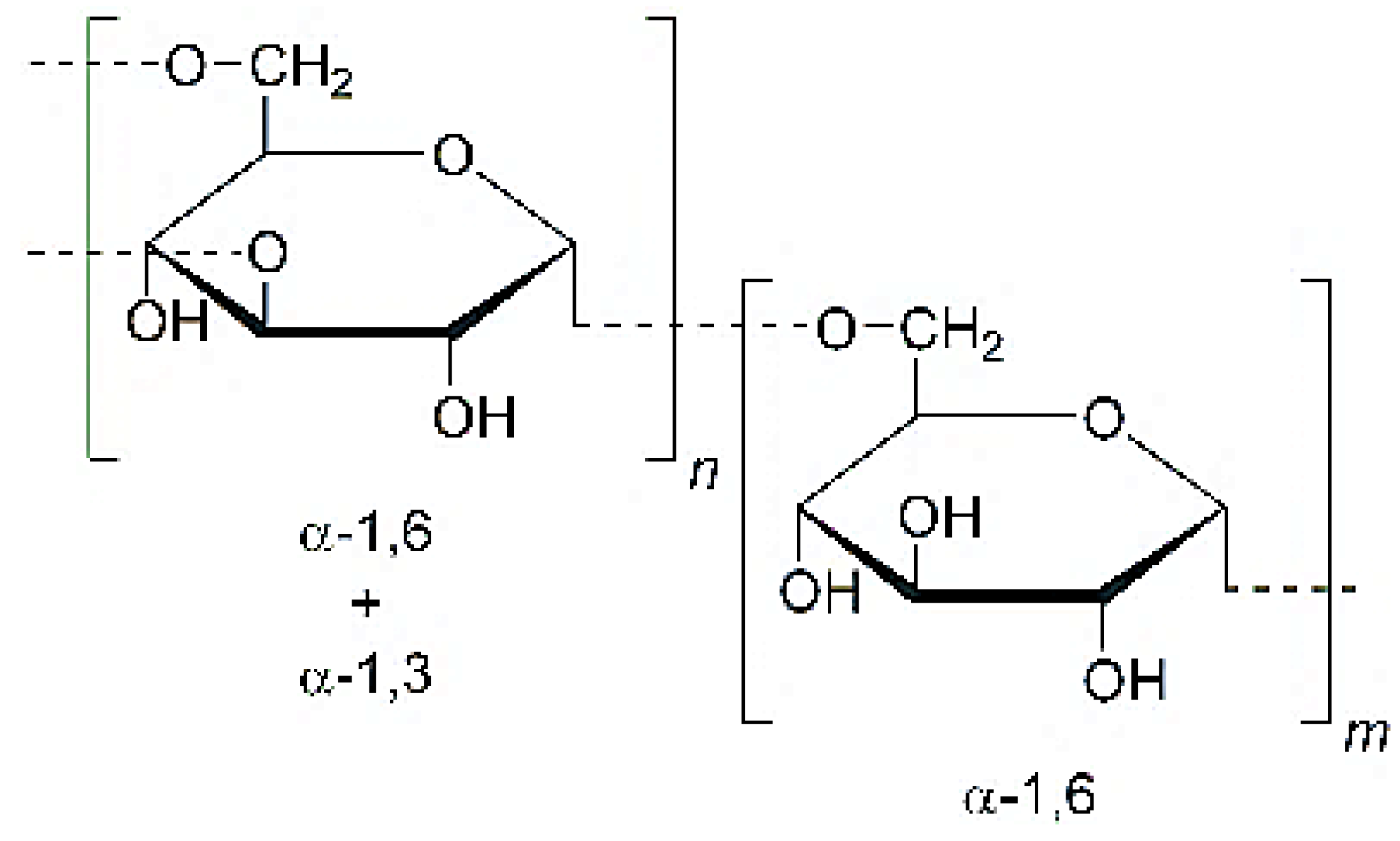 | [115,116,117] |
| Chondroitin sulphate | (1–3)-β-N-acetyl-d-galactosamine and (1–4)-β-glucuronic acid |
|  | [118,119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farhaj, S.; Agbotui, T.L.; Nirwan, J.S.; Mahmood, Q.; Yousaf, A.M.; Hussain, T.; Shahzad, Y.; Khan, N.; Conway, B.R.; Ghori, M.U. Carbohydrate Polymer-Based Targeted Pharmaceutical Formulations for Colorectal Cancer: Systematic Review of the Literature. Polysaccharides 2022, 3, 692-714. https://doi.org/10.3390/polysaccharides3040040
Farhaj S, Agbotui TL, Nirwan JS, Mahmood Q, Yousaf AM, Hussain T, Shahzad Y, Khan N, Conway BR, Ghori MU. Carbohydrate Polymer-Based Targeted Pharmaceutical Formulations for Colorectal Cancer: Systematic Review of the Literature. Polysaccharides. 2022; 3(4):692-714. https://doi.org/10.3390/polysaccharides3040040
Chicago/Turabian StyleFarhaj, Samia, Theodora L. Agbotui, Jorabar Singh Nirwan, Qaisar Mahmood, Abid Mehmood Yousaf, Talib Hussain, Yasser Shahzad, Nemat Khan, Barbara R. Conway, and Muhammad Usman Ghori. 2022. "Carbohydrate Polymer-Based Targeted Pharmaceutical Formulations for Colorectal Cancer: Systematic Review of the Literature" Polysaccharides 3, no. 4: 692-714. https://doi.org/10.3390/polysaccharides3040040
APA StyleFarhaj, S., Agbotui, T. L., Nirwan, J. S., Mahmood, Q., Yousaf, A. M., Hussain, T., Shahzad, Y., Khan, N., Conway, B. R., & Ghori, M. U. (2022). Carbohydrate Polymer-Based Targeted Pharmaceutical Formulations for Colorectal Cancer: Systematic Review of the Literature. Polysaccharides, 3(4), 692-714. https://doi.org/10.3390/polysaccharides3040040








