Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents
Abstract
1. Introduction
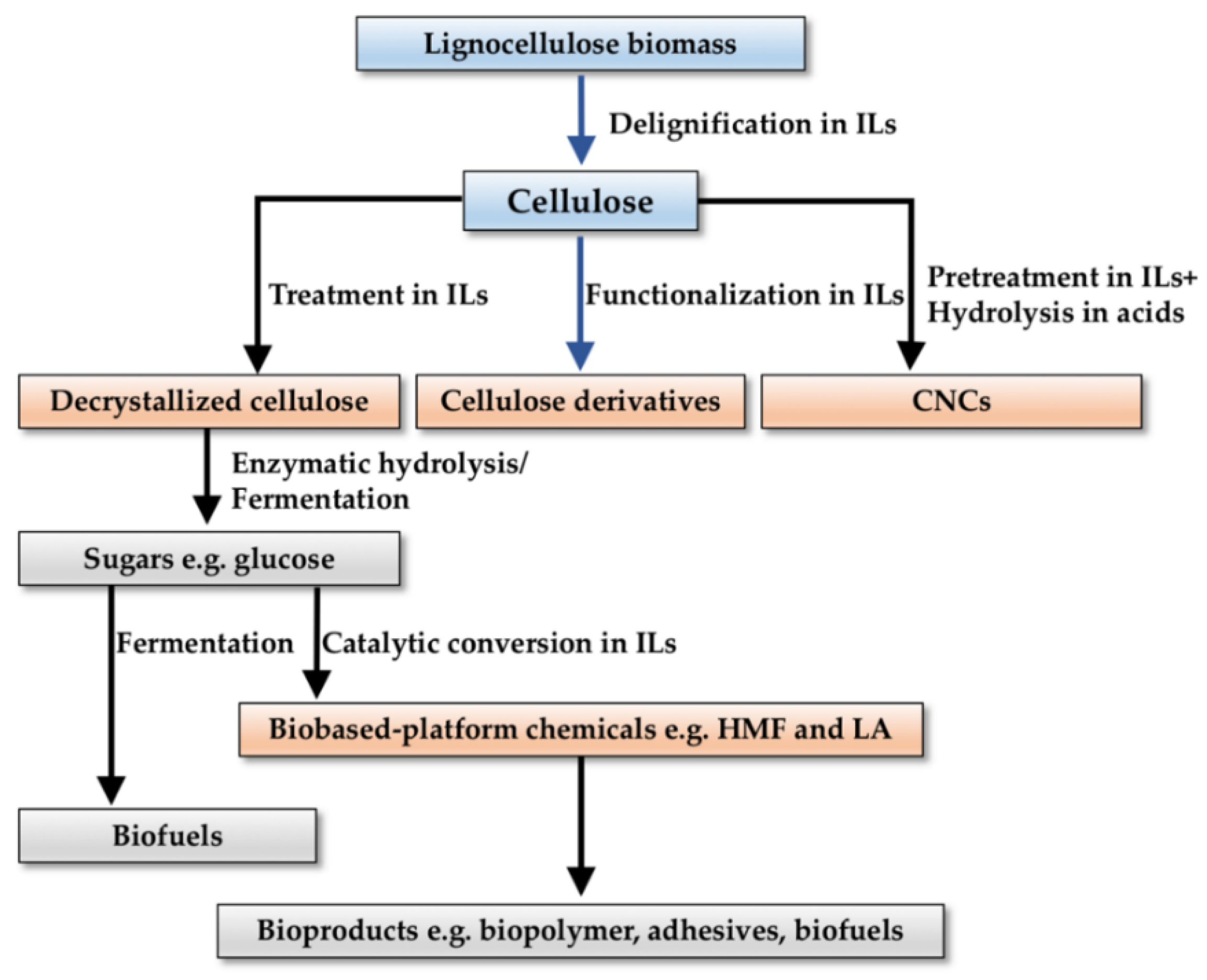
2. Pretreatment, Hydrolysis, and Regeneration of Cellulose in ILs
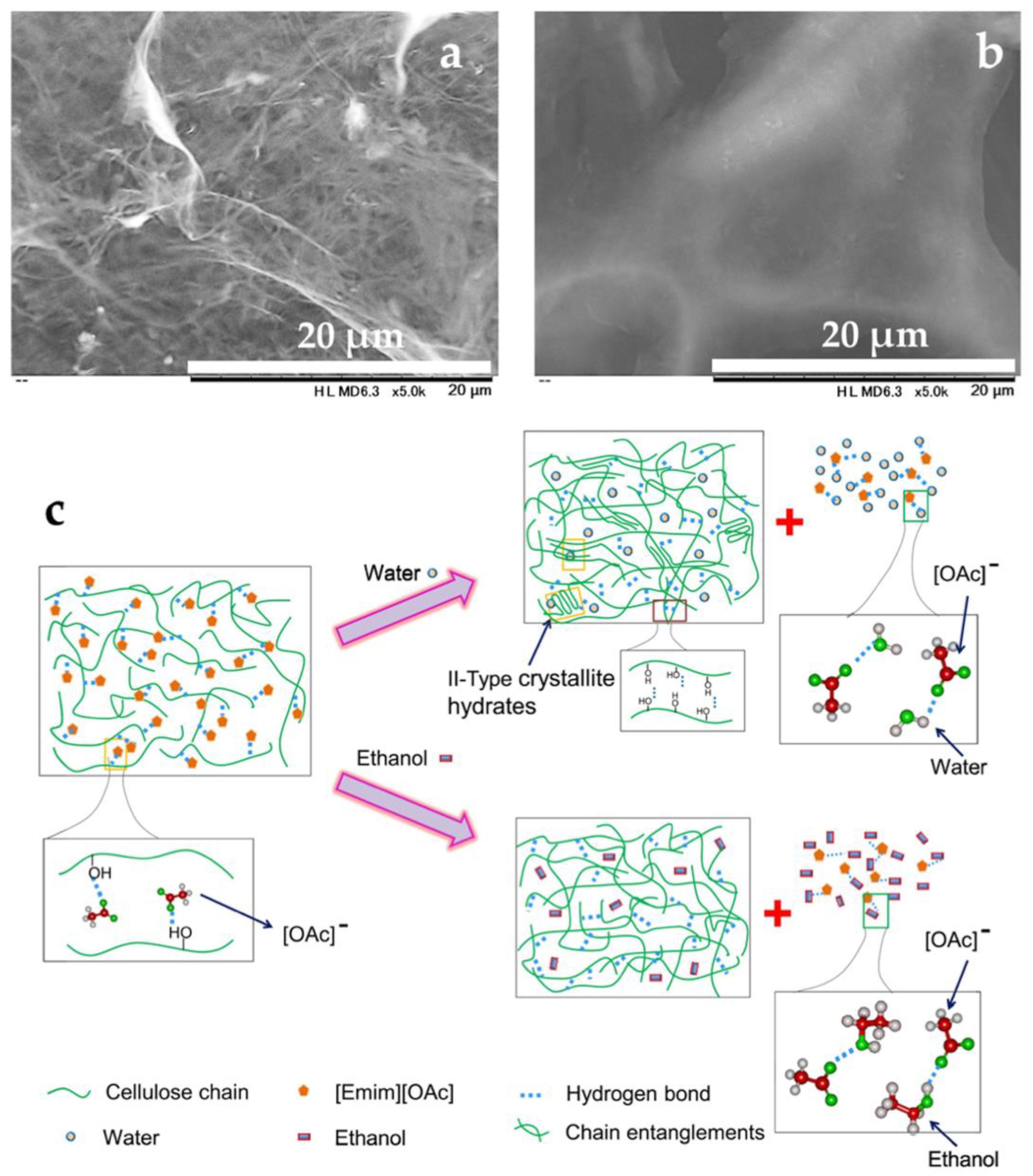
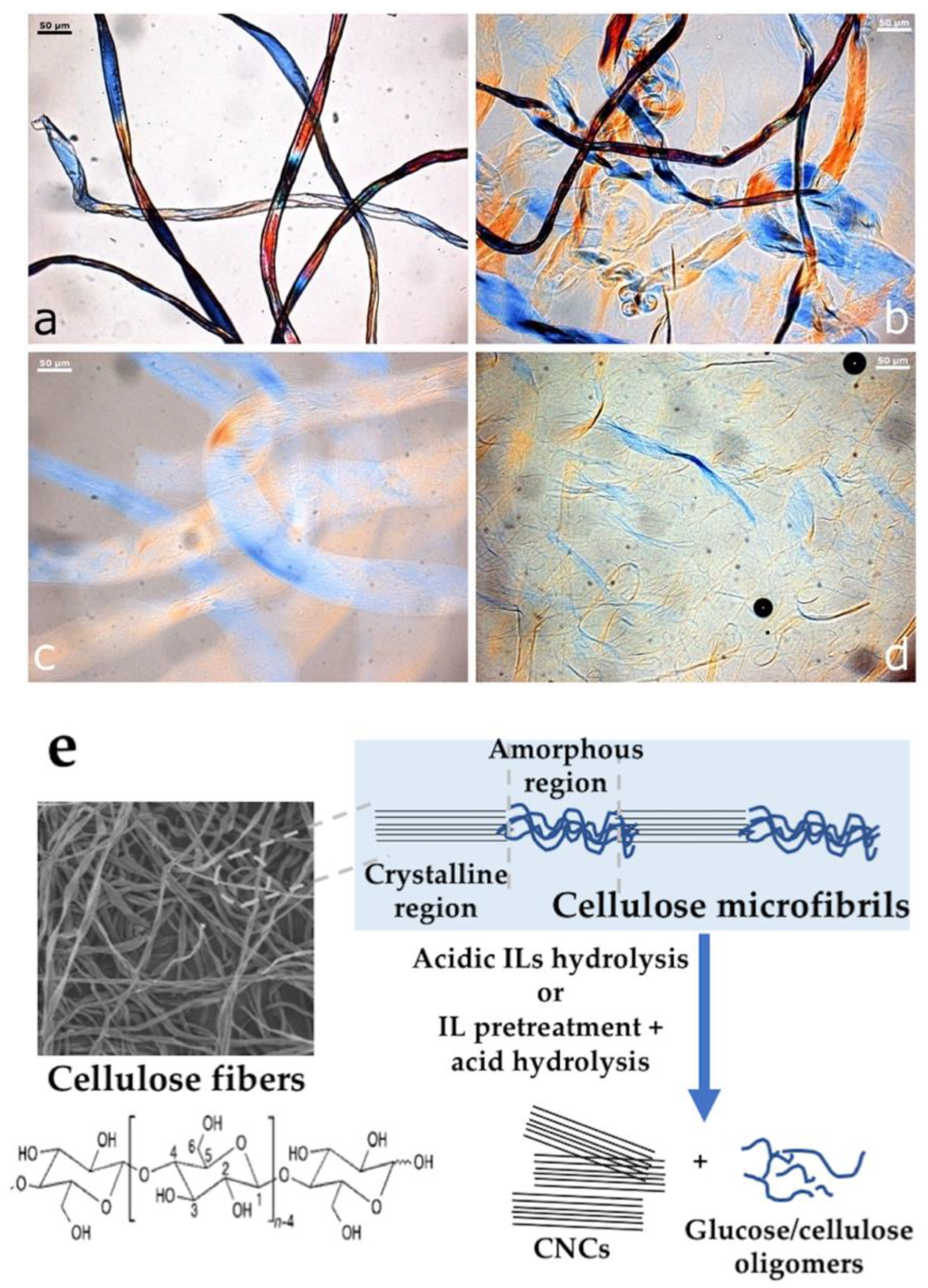
2.1. Decrystallized Cellulose
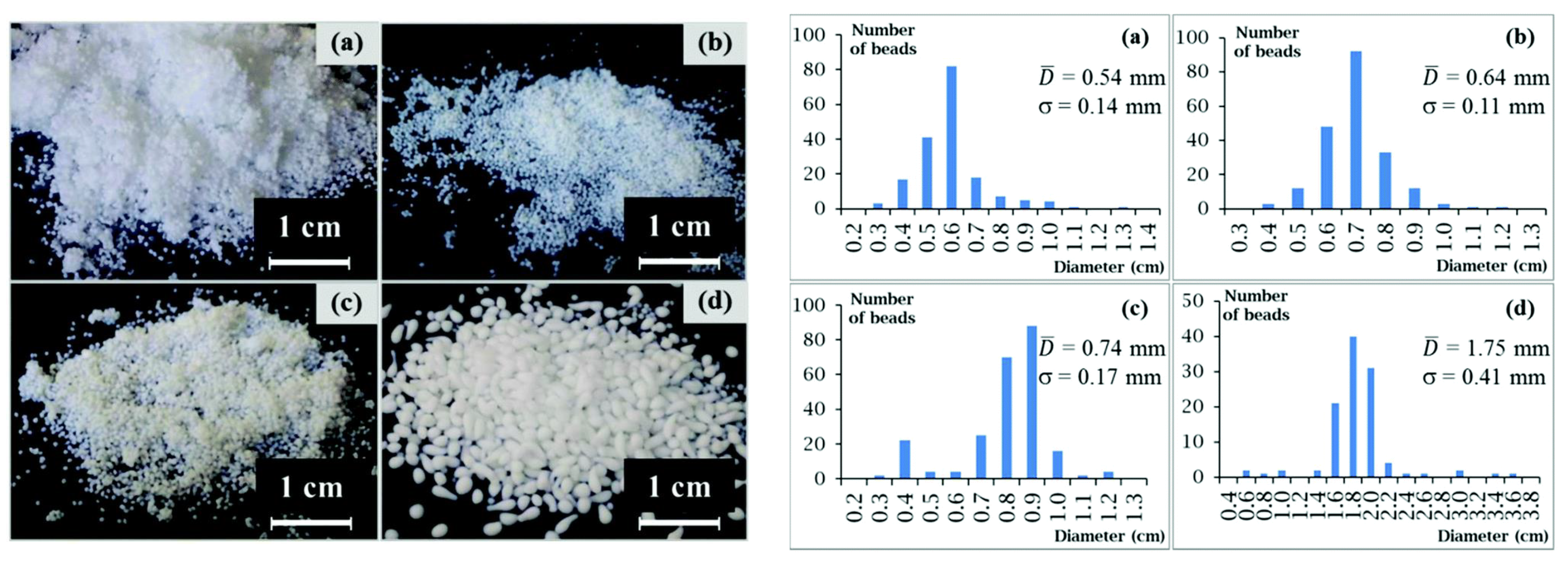
2.2. Cellulose Nanocrystals
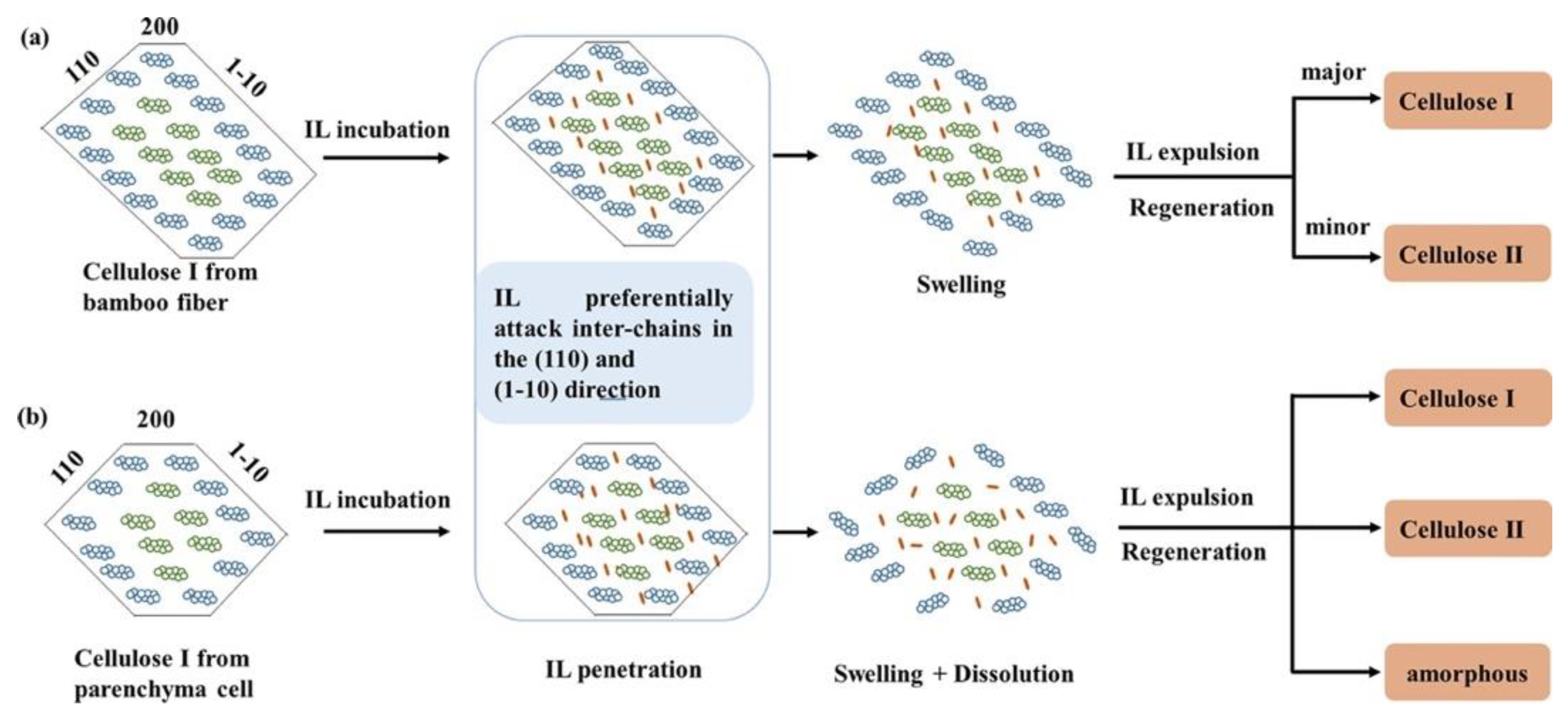
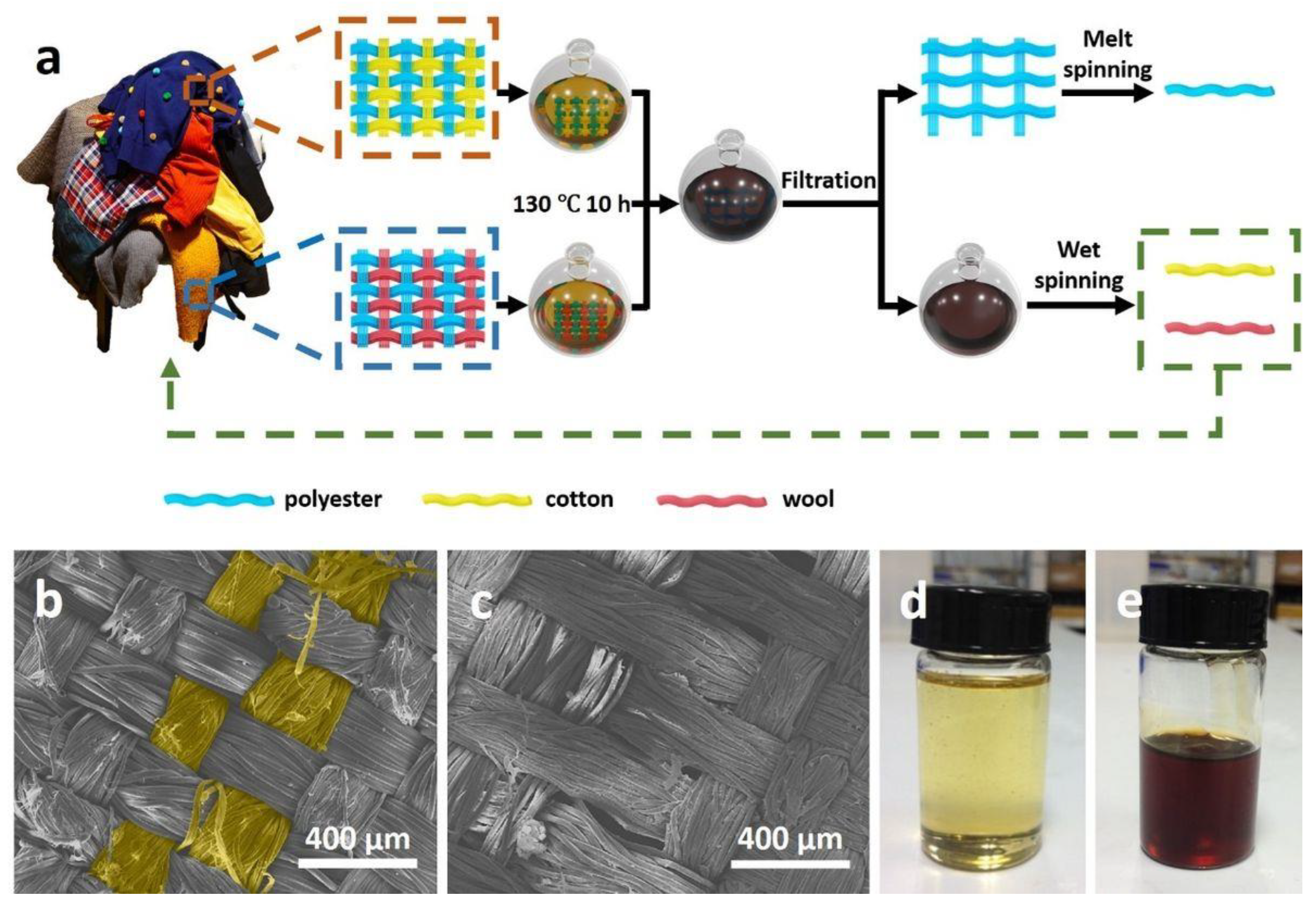
2.3. Shaped Regenerated Cellulose
| IL | Shape | Property | Ref. |
|---|---|---|---|
| [EMIM]OAc | Fiber | 8–50 MPa σ, 65% transmittance | [12,23] |
| 1-Ethyl-3-methylimidazolium diethyl phosphate ([EMIM]DEP) | Fiber | 200–900 MPa σ, 5–40% ε, 90% transmittance | [54,55,59,60] |
| 1-Ethyl-3-methylimidazolium Octanoate ([EMIM]Oc) | Fiber | 405 MPa σ, 33 GPa E | [50] |
| 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide ([EMIM]TFSI) | Ionogel (thick sheet) | High conductivity (7.8 mS/cm), 506 MPa E | [61] |
| [BMIM]Cl | Fiber | ~1 GPa σ, 30–40 GPa E | [48,49] |
| Film | 10–75 MPa σ, 3.4–3.6% ε, 76% transmittance | [56,62,63] | |
| [BMIM]OAc | Fiber | 6–14 MPa σ | [12] |
| [AMIM]Cl | Film | 5–152 MPa σ, 1–12 GPa E, 0.5–3% ε, 90% Transmittance | [64,65,66,67] |
| 1-Decyl-3-methylimidazolium chloride ([DMIM]Cl) | Fiber | 6–15 MPa σ | [12] |
| 1,5-Diazabicyclo[4.3.0]non-5-enium acetate ([DBNH]OAc) | Fiber | 552 MPa σ, 23 GPa E | [52] |
| 1,8-Diazabicyclo[5.4.0]undec-7-enium carboxylate (DBUH-SILs) and 1,5-Diazabicyclo[4.3.0]non-5-enium carboxylate (DBNH-SILs) | Film | 26–100 MPa σ, 1–3 GPa E, 2–6% ε | [60,68] |
| 1,5-Diazabicyclo[4.3.0]non-5-enium propionate ([DBNH]CO2Et) | Bead/aerogel | 0.5–0.7 mm Bead size, 240–340 m2/g specific surface area, 0.04–0.07 g/cm3 density | [69] |
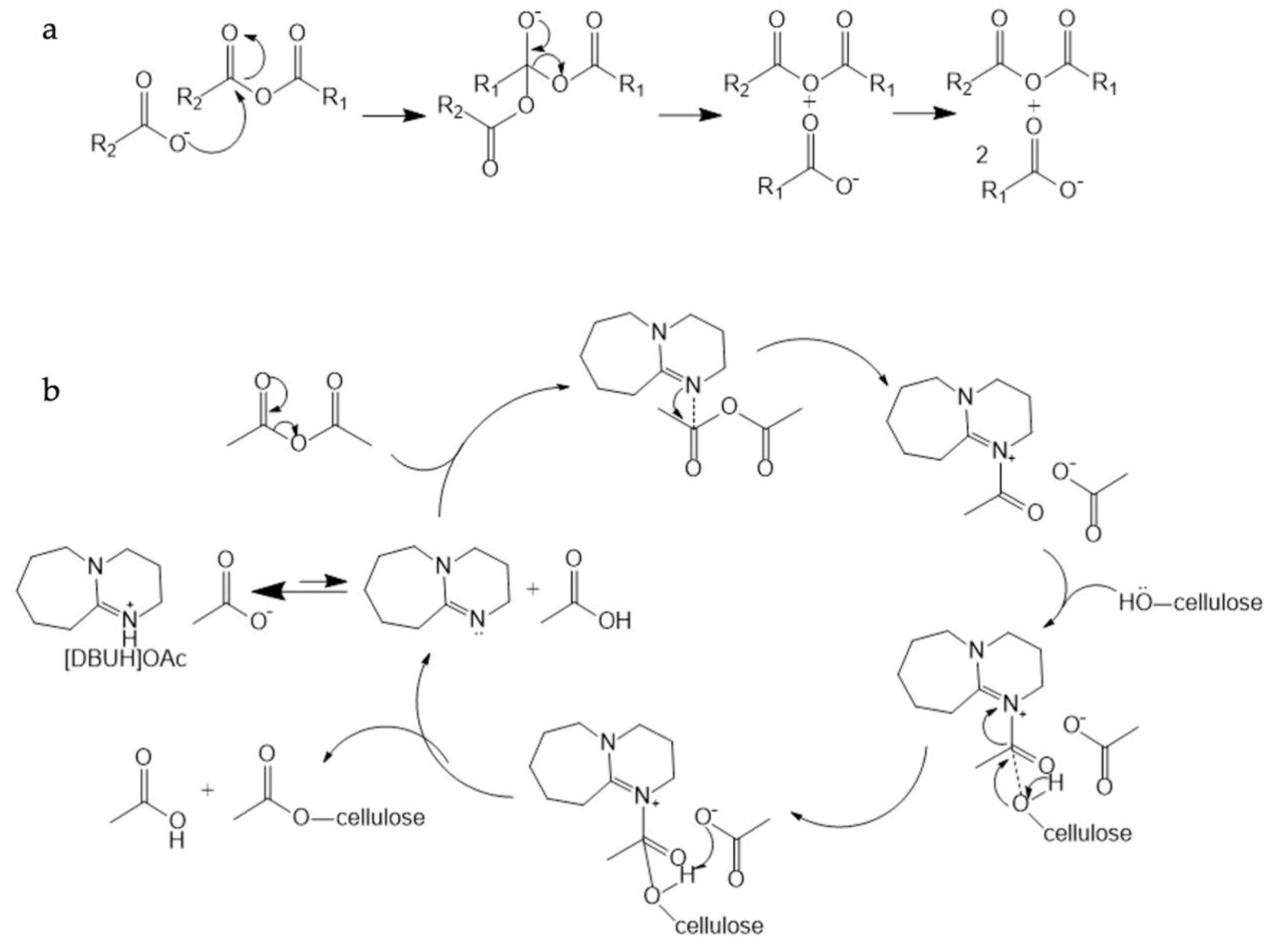
3. Functionalization
4. Conversion of Cellulose in ILs into Bio-Based Platform Chemicals
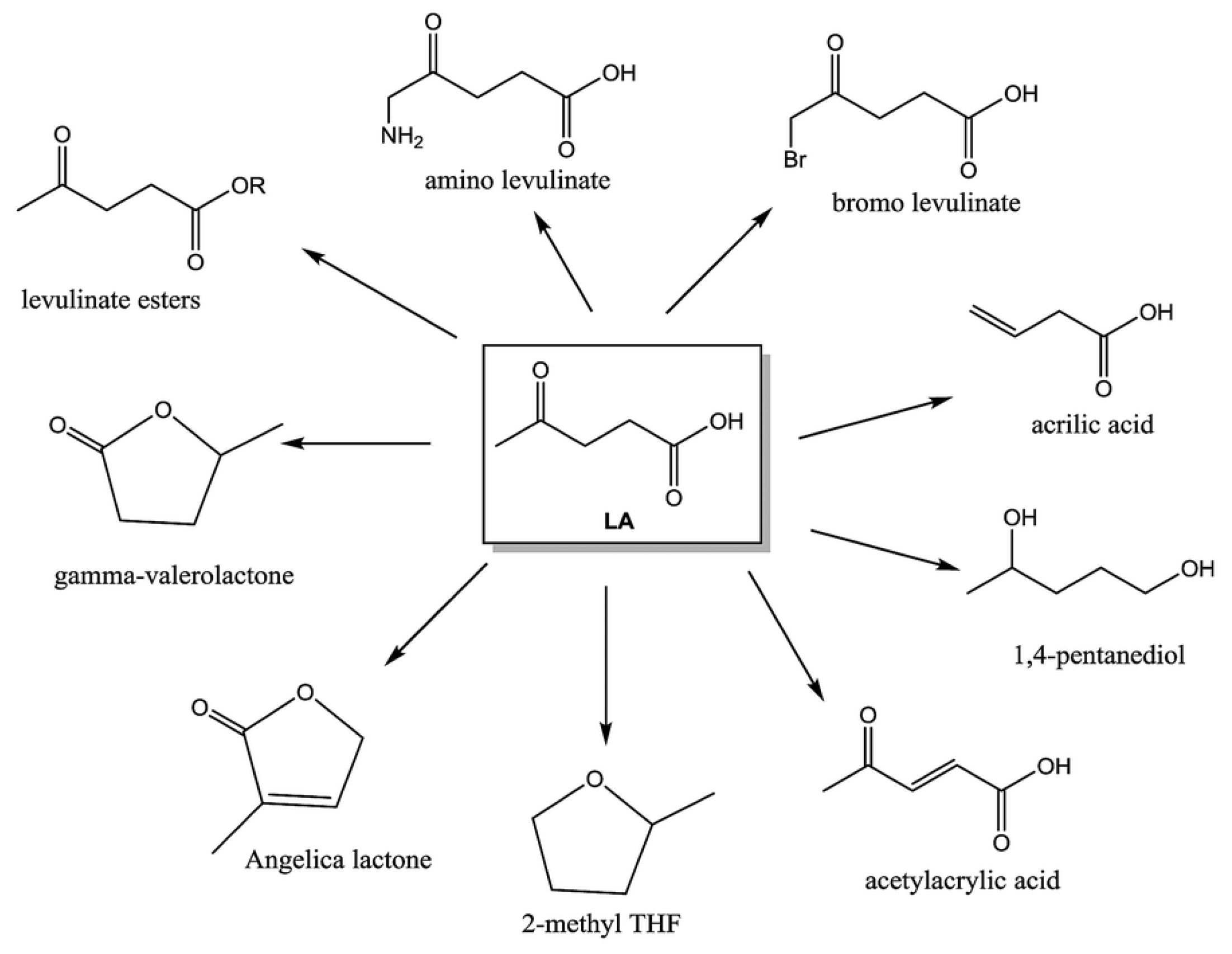
5. Commercial-Scale Processing of Cellulose Using ILs
6. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- How the EU Wants to Achieve a Circular Economy by 2050. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20210128STO96607/how-the-eu-wants-to-achieve-a-circular-economy-by-2050 (accessed on 24 August 2022).
- Costa, J.; Broega, A.C. New sustainable materials for the fashion industry: The button in the circular economy. In Proceedings of the Advances in Design, Music and Arts II, Cham, Switzerland, 7–9 July 2022; pp. 342–356. [Google Scholar]
- Provin, A.P.; Dutra, A.R.D.A.; de Sousa e Silva Gouveia, I.C.A.; Cubas, E.A.L.V. Circular economy for fashion industry: Use of waste from the food industry for the production of biotextiles. Technol. Forecast. Soc. Chang. 2021, 169, 120858. [Google Scholar] [CrossRef]
- Kostic, M.; Imani, M.; Ivanovska, A.; Radojevic, V.; Dimic-Misic, K.; Barac, N.; Stojanovic, D.; Janackovic, D.; Uskokovic, P.; Barcelo, E.; et al. Extending waste paper, cellulose and filler use beyond recycling by entering the circular economy creating cellulose-CaCO3 composites reconstituted from ionic liquid. Cellulose 2022, 29, 5037–5059. [Google Scholar] [CrossRef]
- Papadaki, A.; Manikas, A.C.; Papazoglou, E.; Kachrimanidou, V.; Lappa, I.; Galiotis, C.; Mandala, I.; Kopsahelis, N. Whey protein films reinforced with bacterial cellulose nanowhiskers: Improving edible film properties via a circular economy approach. Food Chem. 2022, 385, 132604. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wu, M.; Zhang, Q.; Tan, X.; Xu, F.; Zhang, X.; Sun, R. Comparison of physical properties of regenerated cellulose films fabricated with different cellulose feedstocks in ionic liquid. Carbohydr. Polym. 2015, 121, 71–78. [Google Scholar] [CrossRef]
- Taokaew, S.; Nakson, N.; Zhang, X.; Kongklieng, P.; Kobayashi, T. Biotransformation of okara extracted protein to nanocellulose and chitin by Gluconacetobacter xylinus and Bacillus pumilus. Bioresour. Technol. Rep. 2022, 17, 100904. [Google Scholar] [CrossRef]
- Raghuwanshi, V.S.; Cohen, Y.; Garnier, G.; Garvey, C.J.; Garnier, G. Deuterated bacterial cellulose dissolution in ionic liquids. Macromolecules 2021, 54, 6982–6989. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Ramezani, M.; Parthasarathy, R.; Shah, K. A comparison of ionic liquids and organic solvents on the separation of cellulose-rich material from river red gum. Bioenergy Res. 2019, 12, 275–291. [Google Scholar] [CrossRef]
- Karzarjeddi, M.; Ismail, M.Y.; Antti Sirviö, J.; Wang, S.; Mankinen, O.; Telkki, V.V.; Patanen, M.; Laitinen, O.; Liimatainen, H. Adjustable hydro-thermochromic green nanofoams and films obtained from shapable hybrids of cellulose nanofibrils and ionic liquids for smart packaging. Chem. Eng. J. 2022, 443, 136369. [Google Scholar] [CrossRef]
- Wei, J.; Gao, H.; Li, Y.; Nie, Y. Research on the degradation behaviors of wood pulp cellulose in ionic liquids. J. Mol. Liq. 2022, 356, 119071. [Google Scholar] [CrossRef]
- Krugly, E.; Pauliukaityte, I.; Ciuzas, D.; Bulota, M.; Peciulyte, L.; Martuzevicius, D. Cellulose electrospinning from ionic liquids: The effects of ionic liquid removal on the fiber morphology. Carbohydr. Polym. 2022, 285, 119260. [Google Scholar] [CrossRef]
- Ziembowicz, F.I.; Mattiazzi, L.M.; Bender, C.R.; Frizzo, C.P.; da Rosa, M.B.; Reichert, J.M.; Kloster, C.L.; Villetti, M.A. Thermodynamics of aggregation and modulation of Rheo-Thermal properties of hydroxypropyl cellulose by imidazolium ionic liquids. J. Mol. Liq. 2022, 359, 119314. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Samsudin, N.A.; Low, F.W.; Yusoff, Y.; Shakeri, M.; Tan, X.Y.; Lai, C.W.; Asim, N.; Oon, C.S.; Newaz, K.S.; Tiong, S.K.; et al. Effect of temperature on synthesis of cellulose nanoparticles via ionic liquid hydrolysis process. J. Mol. Liq. 2020, 308, 113030. [Google Scholar] [CrossRef]
- Sui, Y.; Cui, Y.; Wang, Y.; Zhao, Y.; Sun, G. An efficient strategy for enhancing glucose recovery of wheat straw by ionic liquid combined ball milling pretreatment. Bioenergy Res. 2022. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, Y.; Li, Y.; Lu, Z.; Cao, S.; Fan, M.; Huang, L.; Chen, L. Facile cellulose dissolution and characterization in the newly synthesized 1,3-diallyl-2-ethylimidazolium acetate ionic liquid. Polymers 2017, 9, 526. [Google Scholar] [CrossRef]
- Heinze, T.; Dorn, S.; Schöbitz, M.; Liebert, T.; Köhler, S.; Meister, F. Interactions of ionic liquids with polysaccharides—2: Cellulose. Macromol. Symp. 2008, 262, 8–22. [Google Scholar] [CrossRef]
- Javed, K.; Krumme, A.; Krasnou, I.; Mikli, V.; Viirsalu, M.; Plamus, T.; Vassiljeva, V.; Tarasova, E.; Savest, N.; Mendez, J.D. Impact of 1-butyl-3-methylimidazolium chloride on the electrospinning of cellulose acetate nanofibers. J. Macromol. Sci. Part A Pure Appl. Chem. 2018, 55, 142–147. [Google Scholar] [CrossRef]
- Erdmenger, T.; Haensch, C.; Hoogenboom, R.; Schubert, U.S. Homogeneous tritylation of cellulose in 1-butyl-3-methylimidazolium chloride. Macromol. Biosci. 2007, 7, 440–445. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, J.; Sun, S.; Bian, Y.; Hu, Y. Dissolution and recovery of cellulose from pine wood bits in ionic liquids and a co-solvent component mixed system. J. Eng. Fibers Fabrics 2019, 14, 1558925019838440. [Google Scholar] [CrossRef]
- Stolarska, O.; Pawlowska-Zygarowicz, A.; Soto, A.; Rodríguez, H.; Smiglak, M. Mixtures of ionic liquids as more efficient media for cellulose dissolution. Carbohydr. Polym. 2017, 178, 277–285. [Google Scholar] [CrossRef]
- Holding, A.J.; Parviainen, A.; Kilpeläinen, I.; Soto, A.; King, A.W.T.; Rodríguez, H. Efficiency of hydrophobic phosphonium ionic liquids and DMSO as recyclable cellulose dissolution and regeneration media. RSC Adv. 2017, 7, 17451–17461. [Google Scholar] [CrossRef]
- Tan, X.; Chen, L.; Li, X.; Xie, F. Effect of anti-solvents on the characteristics of regenerated cellulose from 1-ethyl-3-methylimidazolium acetate ionic liquid. Int. J. Biol. Macromol. 2019, 124, 314–320. [Google Scholar] [CrossRef]
- Cosby, T.; Aiello, A.; Durkin, D.P.; Trulove, P.C. Kinetics of ionic liquid-facilitated cellulose decrystallization by Raman spectral mapping. Cellulose 2021, 28, 1321–1330. [Google Scholar] [CrossRef]
- Aiello, A.; Cosby, T.; McFarland, J.; Durkin, D.P.; Trulove, P.C. Mesoporous xerogel cellulose composites from biorenewable natural cotton fibers. Carbohydr. Polym. 2022, 282, 119040. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Zhu, J.; Guo, F.; Guo, J.; Zhang, X.; Wang, H.; Yu, Y. Structural evolution of cellulose from bamboo fibers and parenchyma cells during ionic liquid pretreatment for enhanced hydrolysis. Biomacromolecules 2022, 23, 1938–1948. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Dai, L.; Gui, Y.; Yuan, L.; Zhang, C.; Lei, Y. Synergistic benefits from a lignin-first biorefinery of poplar via coupling acesulfamate ionic liquid followed by mild alkaline extraction. Bioresour. Technol. 2020, 303, 122888. [Google Scholar] [CrossRef]
- Raj, T.; Gaur, R.; Lamba, B.Y.; Singh, N.; Gupta, R.P.; Kumar, R.; Puri, S.K.; Ramakumar, S.S.V. Characterization of ionic liquid pretreated plant cell wall for improved enzymatic digestibility. Bioresour. Technol. 2018, 249, 139–145. [Google Scholar] [CrossRef]
- Xu, J.; Hou, H.; Hu, J.; Liu, B. Coupling of hydrothermal and ionic liquid pretreatments for sequential biorefinery of Tamarix austromongolica. Appl. Energy 2018, 229, 745–755. [Google Scholar] [CrossRef]
- Im, J.; Lee, S.; Jo, I.; Kang, J.W.; Kim, K.-S. Structural characteristics and thermal properties of regenerated cellulose, hemicellulose and lignin after being dissolved in ionic liquids. J. Ind. Eng. Chem. 2022, 107, 365–375. [Google Scholar] [CrossRef]
- Bernardo, J.R.; Gírio, F.M.; Łukasik, R.M. The effect of the chemical character of ionic liquids on biomass pre-treatment and posterior enzymatic hydrolysis. Molecules 2019, 24, 808. [Google Scholar] [CrossRef]
- Endo, T.; Fujii, S.; Aung, E.M.; Kuroda, K.; Tsukegi, T.; Ninomiya, K.; Takahashi, K. Cellulose structural change in various biomass species pretreated by ionic liquid at different biomass loadings. BioResources 2019, 13, 6663–6677. [Google Scholar] [CrossRef]
- Halder, P.; Kundu, S.; Patel, S.; Marzbali, M.H.; Parthasarathy, R.; Shah, K. Investigation of reaction mechanism and the effects of process parameters on ionic liquid–based delignification of sugarcane straw. Bioenergy Res. 2020, 13, 1144–1158. [Google Scholar] [CrossRef]
- Da Costa Lopes, A.M.; Lins, R.M.G.; Rebelo, R.A.; Łukasik, R.M. Biorefinery approach for lignocellulosic biomass valorisation with an acidic ionic liquid. Green Chem. 2018, 20, 4043–4057. [Google Scholar] [CrossRef]
- Chen, J.; Tang, C.; Yue, Y.; Qiao, W.; Hong, J.; Kitaoka, T.; Yang, Z. Highly translucent all wood plastics via heterogeneous esterification in ionic liquid/dimethyl sulfoxide. Ind. Crops Prod. 2017, 108, 286–294. [Google Scholar] [CrossRef]
- Liu, Y.; Jing, S.; Carvalho, D.; Fu, J.; Martins, M.; Cavaco-Paulo, A. Cellulose dissolved in ionic liquids for modification of the shape of keratin fibers. ACS Sustain. Chem. Eng. 2021, 9, 4102–4110. [Google Scholar] [CrossRef]
- Zheng, W.; Cui, Y.; Xu, Z.; Zhao, L.; Sun, W. Cellulose transformation into methyl glucosides catalyzed by H3PW12O40: Enhancement of ionic liquid pretreatment. Can. J. Chem. Eng. 2018, 96, 1250–1255. [Google Scholar] [CrossRef]
- Lazko, J.; Sénéchal, T.; Bouchut, A.; Paint, Y.; Dangreau, L.; Fradet, A.; Tessier, M.; Raquez, J.M.; Dubois, P. Acid-free extraction of cellulose type I nanocrystals using Brønsted acid-type ionic liquids. Nanocomposites 2016, 2, 65–75. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, H. Significant differences in the hydrolysis behavior of amorphous and crystalline portions within microcrystalline cellulose in hot-compressed water. Ind. Eng. Chem. Res. 2010, 49, 3902–3909. [Google Scholar] [CrossRef]
- Moulthrop, J.S.; Swatloski, R.P.; Moyna, G.; Rogers, R.D. High-resolution 13C NMR studies of cellulose and cellulose oligomers in ionic liquid solutions. Chem. Commun. 2005, 12, 1557–1559. [Google Scholar] [CrossRef]
- Tan, X.Y.; Abd Hamid, S.B.; Lai, C.W. Preparation of high crystallinity cellulose nanocrystals (CNCs) by ionic liquid solvolysis. Biomass Bioenergy 2015, 81, 584–591. [Google Scholar] [CrossRef]
- Iskak, N.A.M.; Julkapli, N.M.; Hamid, S.B.A. Understanding the effect of synthesis parameters on the catalytic ionic liquid hydrolysis process of cellulose nanocrystals. Cellulose 2017, 24, 2469–2481. [Google Scholar] [CrossRef]
- Fu, X.; Ji, H.; Wang, B.; Zhu, W.; Pang, Z.; Dong, C. Preparation of thermally stable and surface-functionalized cellulose nanocrystals by a fully recyclable organic acid and ionic liquid mediated technique under mild conditions. Cellulose 2020, 27, 1289–1299. [Google Scholar] [CrossRef]
- Jordan, J.H.; Easson, M.W.; Condon, B.D. Cellulose hydrolysis using ionic liquids and inorganic acids under dilute conditions: Morphological comparison of nanocellulose. RSC Adv. 2020, 10, 39413–39424. [Google Scholar] [CrossRef] [PubMed]
- Pang, Z.; Wang, P.; Dong, C. Ultrasonic pretreatment of cellulose in ionic liquid for efficient preparation of cellulose nanocrystals. Cellulose 2018, 25, 7053–7064. [Google Scholar] [CrossRef]
- Haron, G.A.S.; Mahmood, H.; Noh, H.B.; Goto, M.; Moniruzzaman, M. Cellulose nanocrystals preparation from microcrystalline cellulose using ionic liquid-DMSO binary mixture as a processing medium. J. Mol. Liq. 2022, 346, 118208. [Google Scholar] [CrossRef]
- Zhang, J.; Yamagishi, N.; Tominaga, K.; Gotoh, Y. High-strength regenerated cellulose fibers spun from 1-butyl-3-methylimidazolium chloride solutions. J. Appl. Polym. Sci. 2017, 134, 45551. [Google Scholar] [CrossRef]
- Zhang, J.; Yamagishi, N.; Gotoh, Y.; Potthast, A.; Rosenau, T. High performance cellulose fibers regenerated from 1-butyl-3-methylimidazolium chloride solution: Effects of viscosity and molecular weight. J. Appl. Polym. Sci. 2020, 137, 48681. [Google Scholar] [CrossRef]
- Vocht, M.P.; Beyer, R.; Thomasic, P.; Müller, A.; Ota, A.; Hermanutz, F.; Buchmeiser, M.R. High-performance cellulosic filament fibers prepared via dry-jet wet spinning from ionic liquids. Cellulose 2021, 28, 3055–3067. [Google Scholar] [CrossRef]
- Liu, Y.; Nie, Y.; Pan, F.; Zhou, L.; Ji, X.; Kang, Z.; Zhang, S. Study on ionic liquid/cellulose/coagulator phase diagram and its application in green spinning process. J. Mol. Liq. 2019, 289, 111127. [Google Scholar] [CrossRef]
- Hauru, L.K.J.; Hummel, M.; Michud, A.; Sixta, H. Dry jet-wet spinning of strong cellulose filaments from ionic liquid solution. Cellulose 2014, 21, 4471–4481. [Google Scholar] [CrossRef]
- Ciuzas, D.; Krugly, E.; Sriubaite, S.; Pauliukaityte, I.; Baniukaitiene, O.; Bulota, M.; Martuzevicius, D. Electrospun cellulose fibers from ionic liquid: Practical implications toward robust morphology. J. Appl. Polym. Sci. 2022, 139, 51525. [Google Scholar] [CrossRef]
- Zhang, J.; Kitayama, H.; Gotoh, Y.; Potthast, A.; Rosenau, T. Non-woven fabrics of fine regenerated cellulose fibers prepared from ionic-liquid solution via wet type solution blow spinning. Carbohydr. Polym. 2019, 226, 115258. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Kang, Z.; Nie, Y.; Li, L. Fabrication of regenerated cellulose fibers with good strength and biocompatibility from green spinning process of ionic liquid. Macromol. Mater. Eng. 2021, 306, 2000741. [Google Scholar] [CrossRef]
- Zheng, X.; Huang, F.; Chen, L.; Huang, L.; Cao, S.; Ma, X. Preparation of transparent film via cellulose regeneration: Correlations between ionic liquid and film properties. Carbohydr. Polym. 2019, 203, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hawkins, J.E.; Ries, M.E.; Hine, P.J. Dissolution of cotton by 1-ethyl-3-methylimidazolium acetate studied with time–temperature superposition for three different fibre arrangements. Cellulose 2021, 28, 715–727. [Google Scholar] [CrossRef]
- Liang, Y.; Ries, M.E.; Hine, P.J. Three methods to measure the dissolution activation energy of cellulosic fibres using time-temperature superposition. Carbohydr. Polym. 2022, 291, 119541. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, J.; Chang, P.R. Polycaprolactone grafting of cellulose nanocrystals in ionic liquid [BMIM]Cl. Wuhan Univ. J. Nat. Sci. 2014, 19, 117–122. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, H.; Zhang, P.; Qin, C.; Nie, Y.; Liu, X. Preparation of Degradable Wood Cellulose Films Using Ionic Liquids. ACS Appl. Polym. Mater. 2022, 4, 3598–3607. [Google Scholar] [CrossRef]
- Lee, H.; Erwin, A.; Buxton, M.L.; Kim, M.; Stryutsky, A.V.; Shevchenko, V.V.; Sokolov, A.P.; Tsukruk, V.V. Shape persistent, highly conductive ionogels from ionic liquids reinforced with cellulose nanocrystal network. Adv. Funct. Mater. 2021, 31, 2103083. [Google Scholar] [CrossRef]
- Haq, M.A.; Habu, Y.; Yamamoto, K.; Takada, A.; Kadokawa, J.I. Ionic liquid induces flexibility and thermoplasticity in cellulose film. Carbohydr. Polym. 2019, 223, 115058. [Google Scholar] [CrossRef]
- Nor Amalini, A.; Noor Haida, M.K.; Imran, K.; Haafiz, M.K.M. Relationship between dissolution temperature and properties of oil palm biomass based-regenerated cellulose films prepared via ionic liquid. Mater. Chem. Phys. 2019, 221, 382–389. [Google Scholar] [CrossRef]
- Ai, B.; Zheng, L.; Li, W.; Zheng, X.; Yang, Y.; Xiao, D.; Shi, J.; Sheng, Z. Biodegradable cellulose film prepared from banana pseudo-stem using an ionic liquid for mango preservation. Front. Plant Sci. 2021, 12, 625878. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Guo, X.; Nan, F.; Duan, Y.; Zhang, J. Modifying mechanical, optical properties and thermal Processability of iridescent cellulose nanocrystal films using ionic liquid. ACS Appl. Mater. Interfaces 2017, 9, 3085–3092. [Google Scholar] [CrossRef]
- Reddy, K.O.; Maheswari, C.U.; Dhlamini, M.S.; Mothudi, B.M.; Zhang, J.; Zhang, J.; Nagarajan, R.; Rajulu, A.V. Preparation and characterization of regenerated cellulose films using borassus fruit fibers and an ionic liquid. Carbohydr. Polym. 2017, 160, 203–211. [Google Scholar] [CrossRef]
- Olsson, C.; Westman, G. Co-solvent facilitated in situ esterification of cellulose in 1-ethyl-3-methylimidazolium acetate. BioResources 2017, 12, 1395–1402. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Li, H.; Ling, Z.; Xu, D.; You, T.; Wu, Y.-Y.; Xu, F. Room-temperature superbase-derived ionic liquids with facile synthesis and low viscosity: Powerful solvents for cellulose dissolution by destroying the cellulose aggregate structure. Macromolecules 2020, 53, 3284–3295. [Google Scholar] [CrossRef]
- Druel, L.; Niemeyer, P.; Milow, B.; Budtova, T. Rheology of cellulose-[DBNH][CO2Et] solutions and shaping into aerogel beads. Green Chem. 2018, 20, 3993–4002. [Google Scholar] [CrossRef]
- Aghmih, K.; Wakrim, H.; Boukhriss, A.; El Bouchti, M.; Majid, S.; Gmouh, S. Rheological study of microcrystalline cellulose/pyridinium-based ionic liquids solutions. Polym. Bull. 2021, 79, 8987–8999. [Google Scholar] [CrossRef]
- Ganske, K.; Heinze, T. Evaluation of the synthesis of soluble aromatic cellulose carbonates of low degree of substitution. Macromol. Chem. Phys. 2018, 219, 1800152. [Google Scholar] [CrossRef]
- Bui, C.V.; Rosenau, T.; Hettegger, H. Synthesis of polyanionic cellulose carbamates by homogeneous aminolysis in an ionic liquid/dmf medium. Molecules 2022, 27, 1384. [Google Scholar] [CrossRef]
- Kerche, E.F.; Neves, R.M.; Ornaghi, H.L.; Zattera, A.J.; Schrekker, H.S. The influence of ionic liquid concentration on microcrystalline cellulose modification. Carbohydr. Polym. Technol. Appl. 2022, 3, 100211. [Google Scholar] [CrossRef]
- Singh, R.K.; Gupta, P.; Sharma, O.P.; Ray, S.S. Homogeneous synthesis of cellulose fatty esters in ionic liquid (1-butyl-3-methylimidazolium chloride) and study of their comparative antifriction property. J. Ind. Eng. Chem. 2015, 24, 14–19. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, F.; Lang, X.; He, B.; Li, J.; Li, X. Facile one-pot fabrication of cellulose nanocrystals and enzymatic synthesis of its esterified derivative in mixed ionic liquids. RSC Adv. 2017, 7, 27017–27023. [Google Scholar] [CrossRef]
- Meenatchi, B.; Renuga, V.; Manikandan, A. Cellulose dissolution and regeneration using various imidazolium based protic ionic liquids. J. Mol. Liq. 2017, 238, 582–588. [Google Scholar] [CrossRef]
- Hanabusa, H.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-Fujita, M. Effect of alkyl chain length in anions on the physicochemical properties of cellulose-dissolving protic ionic liquids. Aust. J. Chem. 2019, 72, 55–60. [Google Scholar] [CrossRef]
- Kuzmina, O.; Bhardwaj, J.; Vincent, S.R.; Wanasekara, N.D.; Kalossaka, L.M.; Griffith, J.; Potthast, A.; Rahatekar, S.; Eichhorn, S.J.; Welton, T. Superbase ionic liquids for effective cellulose processing from dissolution to carbonisation. Green Chem. 2017, 19, 5949–5957. [Google Scholar] [CrossRef]
- Semerci, I.; Güler, F. Protic ionic liquids as effective agents for pretreatment of cotton stalks at high biomass loading. Ind. Crops Prod. 2018, 125, 588–595. [Google Scholar] [CrossRef]
- Hanabusa, H.; Izgorodina, E.I.; Suzuki, S.; Takeoka, Y.; Rikukawa, M.; Yoshizawa-Fujita, M. Cellulose-dissolving protic ionic liquids as low cost catalysts for direct transesterification reactions of cellulose. Green Chem. 2018, 20, 1412–1422. [Google Scholar] [CrossRef]
- Correia, D.M.; Lizundia, E.; Fernandes, L.C.; Costa, C.M.; Lanceros-Méndez, S. Influence of cellulose nanocrystal surface functionalization on the bending response of cellulose nanocrystal/ionic liquid soft actuators. Phys. Chem. Chem. Phys. 2021, 23, 6710–6716. [Google Scholar] [CrossRef]
- Correia, D.M.; Lizundia, E.; Meira, R.M.; Rincón-Iglesias, M.; Lanceros-Méndez, S. Cellulose nanocrystal and water-soluble cellulose derivative based electromechanical bending actuators. Materials 2020, 13, 2294. [Google Scholar] [CrossRef]
- Alam, M.I.; De, S.; Khan, T.S.; Haider, M.A.; Saha, B. Acid functionalized ionic liquid catalyzed transformation of non-food biomass into platform chemical and fuel additive. Ind. Crops Prod. 2018, 123, 629–637. [Google Scholar] [CrossRef]
- Kudo, S.; Goto, N.; Sperry, J.; Norinaga, K.; Hayashi, J.I. Production of levoglucosenone and dihydrolevoglucosenone by catalytic reforming of volatiles from cellulose pyrolysis using supported ionic liquid phase. ACS Sustain. Chem. Eng. 2017, 5, 1132–1140. [Google Scholar] [CrossRef]
- Galkin, K.I.; Ananikov, V.P. When will 5-hydroxymethylfurfural, the “sleeping giant” of sustainable chemistry, awaken? ChemSusChem 2019, 12, 2976–2982. [Google Scholar] [CrossRef]
- Ofrasio, B.I.G.; de Luna, M.D.G.; Chen, Y.-C.; Abarca, R.R.M.; Dong, C.-D.; Chang, K.-L. Catalytic conversion of sugars and biomass to furanic biofuel precursors by boron-doped biochar in ionic liquid. Bioresour. Technol. Rep. 2020, 11, 100515. [Google Scholar] [CrossRef]
- Zhang, C.; Cheng, Z.; Fu, Z.; Liu, Y.; Yi, X.; Zheng, A.; Kirk, S.R.; Yin, D. Effective transformation of cellulose to 5-hydroxymethylfurfural catalyzed by fluorine anion-containing ionic liquid modified biochar sulfonic acids in water. Cellulose 2017, 24, 95–106. [Google Scholar] [CrossRef]
- Li, M.; Zhang, Q.; Luo, B.; Chen, C.; Wang, S.; Min, D. Lignin-based carbon solid acid catalyst prepared for selectively converting fructose to 5-hydroxymethylfurfural. Ind. Crops Prod. 2020, 145, 111920. [Google Scholar] [CrossRef]
- Ramli, N.A.S.; Amin, N.A.S. Thermo-kinetic assessment of glucose decomposition to 5-hydroxymethyl furfural and levulinic acid over acidic functionalized ionic liquid. Chem. Eng. J. 2018, 335, 221–230. [Google Scholar] [CrossRef]
- Cheng, X.; Liu, Y.; Wang, K.; Yu, H.; Yu, S.; Liu, S. High-efficient conversion of cellulose to levulinic acid catalyzed via functional brønsted–lewis acidic ionic liquids. Catal. Lett. 2022, 152, 1064–1075. [Google Scholar] [CrossRef]
- Chiappe, C.; Rodriguez Douton, M.J.; Mezzetta, A.; Guazzelli, L.; Pomelli, C.S.; Assanelli, G.; de Angelis, A.R. Exploring and exploiting different catalytic systems for the direct conversion of cellulose into levulinic acid. New J. Chem. 2018, 42, 1845–1852. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, W.; Wang, J.; Jin, L.; Hu, G.; Zheng, Q.; Xie, H.; Chen, P. Unique gelation and rheological properties of the cellulose/CO2-based reversible ionic liquid/DMSO solutions. Carbohydr. Polym. 2019, 222, 115024. [Google Scholar] [CrossRef]
- Tyagi, U.; Anand, N.; Kumar, D. Simultaneous pretreatment and hydrolysis of hardwood biomass species catalyzed by combination of modified activated carbon and ionic liquid in biphasic system. Bioresour. Technol. 2019, 289, 121675. [Google Scholar] [CrossRef] [PubMed]
- Ghezali, W.; De Oliveira Vigier, K.; Kessas, R.; Jérôme, F. A choline chloride/DMSO solvent for the direct synthesis of diformylfuran from carbohydrates in the presence of heteropolyacids. Green Chem. 2015, 17, 4459–4464. [Google Scholar] [CrossRef]
- Chang, K.-L.; Muega, S.C.; Ofrasio, B.I.G.; Chen, W.-H.; Barte, E.G.; Abarca, R.R.M.; de Luna, M.D.G. Synthesis of 5-hydroxymethylfurfural from glucose, fructose, cellulose and agricultural wastes over sulfur-doped peanut shell catalysts in ionic liquid. Chemosphere 2022, 291, 132829. [Google Scholar] [CrossRef]
- Boissou, F.; Mühlbauer, A.; De Oliveira Vigier, K.; Leclercq, L.; Kunz, W.; Marinkovic, S.; Estrine, B.; Nardello-Rataj, V.; Jérôme, F. Transition of cellulose crystalline structure in biodegradable mixtures of renewably-sourced levulinate alkyl ammonium ionic liquids, γ-valerolactone and water. Green Chem. 2014, 16, 2463–2471. [Google Scholar] [CrossRef]
- Hu, Y.; Song, J.; Xie, C.; Wu, H.; Jiang, T.; Yang, G.; Han, B. Transformation of CO2 into α-alkylidene cyclic carbonates at room temperature cocatalyzed by CuI and ionic liquid with biomass-derived levulinate anion. ACS Sustain. Chem. Eng. 2019, 7, 5614–5619. [Google Scholar] [CrossRef]
- Mezzetta, A.; Becherini, S.; Pretti, C.; Monni, G.; Casu, V.; Chiappe, C.; Guazzelli, L. Insights into the levulinate-based ionic liquid class: Synthesis, cellulose dissolution evaluation and ecotoxicity assessment. New J. Chem. 2019, 43, 13010–13019. [Google Scholar] [CrossRef]
- He, F.; Chen, J.; Gong, Z.; Xu, Q.; Yue, W.; Xie, H. Dissolution pretreatment of cellulose by using levulinic acid-based protic ionic liquids towards enhanced enzymatic hydrolysis. Carbohydr. Polym. 2021, 269, 118271. [Google Scholar] [CrossRef]
- Morrissey, S. Self-Bonded Cellulosic Nonwoven Web and Method for Making. U.S. Patent 2017/0051443 A1, 16 April 2015. [Google Scholar]
- Charles, M.B.; Norma, L.B.; Michael, E.D.; Maryna, G.G.; Kuo, T.; Wang, B.I.N. Regioselectively Substituted Cellulose Esters Produced in a Halogenated Ionic Liquid Process and Products Produced Therefrom. U.S. Patent 9777074 B2, 18 September 2015. [Google Scholar]
- Charles, M.B.; Norma, L.B.; Robert, T.H.; Juanelle, L.L.; Michael, E.D.; Maryna, G.G.; Kuo, T.; Wang, B.I.N. Regioselectively Substituted Cellulose Esters Produced in a Carboxylated Ionic Liquid Process and Products Produced Therefrom. U.S. Patent 2017/0204201 A1, 6 February 2017. [Google Scholar]
- Aoki, T.; Kawashima, T.; Kusukame, H.; Taniike, Y. Ionic Liquid and Method for Dissolving Cellulose Using the Same. U.S. Patent 2020/0239647 A1, 20 February 2020. [Google Scholar]
- Aoki, T.; Kawashima, T.; Kusukame, H.; Taniike, Y. Ionic Liquid Composition and Method for Dissolving Cellulose Using the Same. U.S. Patent 2018/0215942 A1, 12 January 2018. [Google Scholar]
- Neto, G.C.O.; Teixeira, M.M.; Souza, G.L.V.; Arns, V.D.; Tucci, H.N.P.; Amorim, M. Assessment of the Eco-Efficiency of the Circular Economy in the Recovery of Cellulose from the Shredding of Textile Waste. Polymers 2022, 14, 1317. [Google Scholar] [CrossRef]
- Sun, X.; Wang, X.; Sun, F.; Tian, M.; Qu, L.; Perry, P.; Owens, H.; Liu, X. Textile waste fiber regeneration via a green chemistry approach: A molecular strategy for sustainable fashion. Adv. Mater. 2021, 33, 2105174. [Google Scholar] [CrossRef]
- Ioncell: Enter the New Era of Textile Production! Available online: https://ioncell.fi/ (accessed on 23 August 2022).
- GRETE—Tackling the Challenges of a Sustainable Growth through Technological Innovation. Available online: https://www.greteproject.eu/wood-to-textile-ionic-liquid/ (accessed on 23 August 2022).
- Harlin, A.L.I.; Määttänen, M.; Sivonen, E.; Vehviläinen, M.; Asikainen, S.; Valta, K.; SÄRkilahti, A. Treatment Process for Textile-Based Materials. U.S. Patent WO 2018/197756 A1, 27 April 2018. [Google Scholar]
- Sixta, H.; Ma, Y.; Hummel, M. A Method to Convert Mechanical Pulp Derived Waste Material into Value Added Cellulose Products. U.S. Patent WO 2018/142025 A1, 1 February 2018. [Google Scholar]
- Haslinger, S.; Hummel, M.; Sixta, H. Separation and Upcycling of Cellulose-Containing Blended Waste. U.S. Patent 2020/0079925 A1, 20 December 2020. [Google Scholar]
- Michud, A.; King Alistair, W.T.; Parviainen, A.; Sixta, H.; Hauru, L.; Hummel, M.; KilpelÄInen, I. Process for the Production of Shaped Cellulose Articles. U.S. Patent 10240259 B2, 4 April 2014. [Google Scholar]
- Sixta, H.; Hummel, M.; Le Boulch, K.; Kilpeläinen, A.I.; King Alistair, W.T.; Helminen, K.J.J.; Hellstén, S. A Process for Making a Cellulose Fibre or Film. U.S. Patent WO 2018/138416 A1, 30 January 2018. [Google Scholar]
- The Time is Now for Ionic Liquids. Available online: https://cen.acs.org/materials/ionic-liquids/time-ionic-liquids/98/i5 (accessed on 23 August 2022).
- We are Metsä Fibre. Available online: https://www.metsagroup.com/metsafibre/ (accessed on 23 August 2022).
- Material Portrait: Expand Fibre. Available online: https://herewear.eu/2021/12/08/material-portrait-expand-fibre/ (accessed on 23 August 2022).
- LIXEA Sustainable Solutions. Available online: https://www.lixea.co/ (accessed on 23 August 2022).
- Baral, N.R.; Shah, A. Techno-economic analysis of cellulose dissolving ionic liquid pretreatment of lignocellulosic biomass for fermentable sugars production. Biofuels Bioprod. Biorefining 2016, 10, 70–88. [Google Scholar] [CrossRef]
- Zhang, J.; Zou, D.; Zhai, S.; Yan, Y.; Yang, H.; He, C.; Ke, Y.; Singh, S.; Cheng, G. Enhancing the interaction between cellulose and dilute aqueous ionic liquid solutions and its implication to ionic liquid recycling and reuse. Carbohydr. Polym. 2022, 277, 118848. [Google Scholar] [CrossRef] [PubMed]
- Ovejero-Pérez, A.; Ayuso, M.; Rigual, V.; Domínguez, J.C.; García, J.; Alonso, M.V.; Oliet, M.; Rodriguez, F. Technoeconomic assessment of a biomass pretreatment + ionic liquid recovery process with aprotic and choline derived ionic liquids. ACS Sustain. Chem. Eng. 2021, 9, 8467–8476. [Google Scholar] [CrossRef]
- Brandt-Talbot, A.; Gschwend, F.J.V.; Fennell, P.S.; Lammens, T.M.; Tan, B.; Weale, J.; Hallett, J.P. An economically viable ionic liquid for the fractionation of lignocellulosic biomass. Green Chem. 2017, 19, 3078–3102. [Google Scholar] [CrossRef]
- Baaqel, H.; Díaz, I.; Tulus, V.; Chachuat, B.; Guillén-Gosálbez, G.; Hallett, J.P. Role of life-cycle externalities in the valuation of protic ionic liquids—A case study in biomass pretreatment solvents. Green Chem. 2020, 22, 3132–3140. [Google Scholar] [CrossRef]
- Bhatia, R.; Lad, J.B.; Bosch, M.; Bryant, D.N.; Leak, D.; Hallett, J.P.; Franco, T.T.; Gallagher, J.A. Production of oligosaccharides and biofuels from Miscanthus using combinatorial steam explosion and ionic liquid pretreatment. Bioresour. Technol. 2021, 323, 124625. [Google Scholar] [CrossRef] [PubMed]
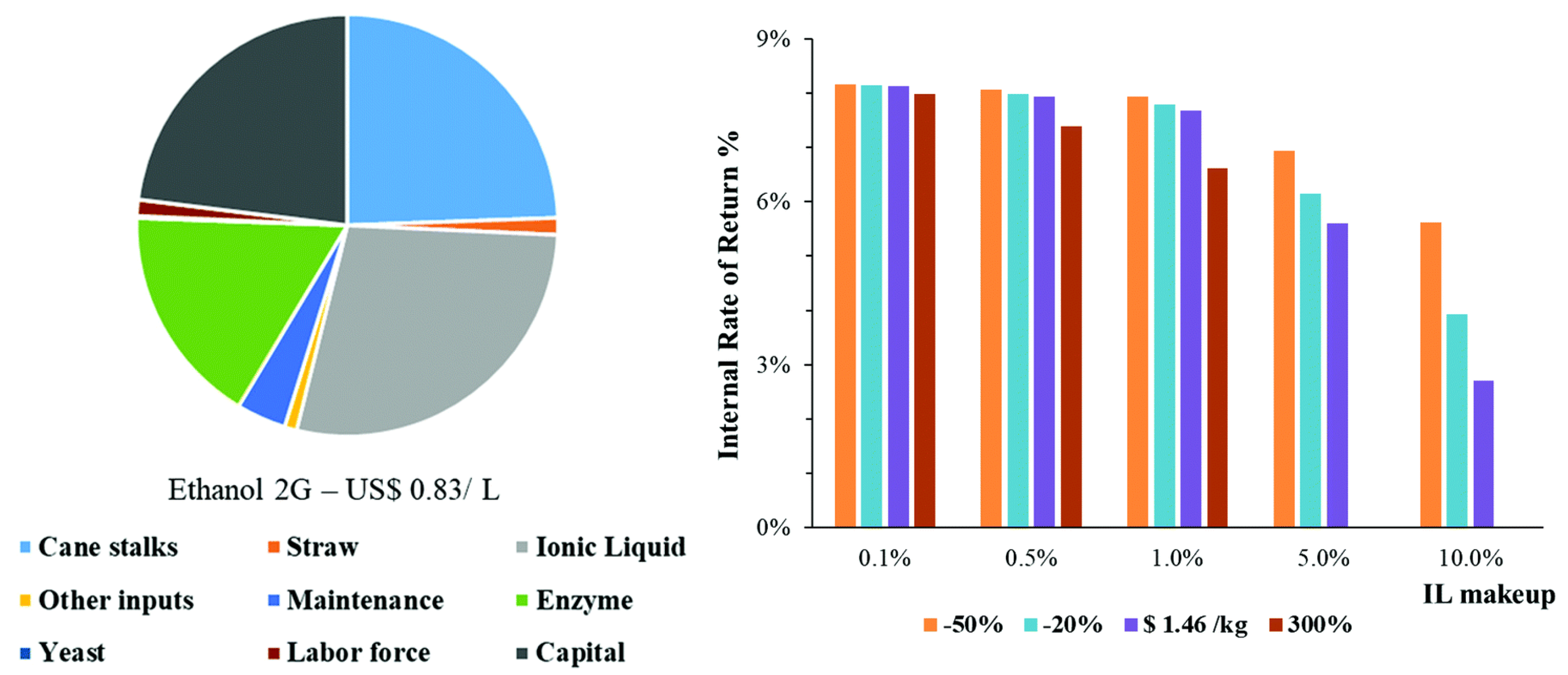
- Ferrari, F.A.; Nogueira, G.P.; Franco, T.T.; Dias, M.O.S.; Cavaliero, C.K.N.; Witkamp, G.J.; van der Wielen, L.A.M.; Forte, M.B.S. The role of ionic liquid pretreatment and recycling design in the sustainability of a biorefinery: A sugarcane to ethanol example. Green Chem. 2021, 23, 9126–9139. [Google Scholar] [CrossRef]
- Zhao, J.; Lee, J.; Wang, D. An integrated deep eutectic solvent-ionic liquid-metal catalyst system for lignin and 5-hydroxymethylfurfural production from lignocellulosic biomass: Technoeconomic analysis. Bioresour. Technol. 2022, 356, 127277. [Google Scholar] [CrossRef]



| IL | Condition | Result | Ref. |
|---|---|---|---|
| 1-Ethyl-3-methylimidazolium chloride ([EMIM]Cl) | 10–30% w/w Biomass, MCC 70–90% w/w IL, 50–100 °C, 2–5 h | ~12% Cellulose solubility, 40% glucan | [22,29] |
| 1-Ethyl-3-methylimidazolium acetate ([EMIM]OAc) | 5–50% w/w Cellulose from various plants, biomass, and MCC 50–95% w/w IL, 70–130 °C, 1–5 h | ~11–23% Cellulose solubility, 40–50% decrystallized cellulose, 10–37% glucose | [6,22,24,29,32,33,34] |
| 1-Ethyl-3-methylimidazolium hydrogen sulfate ([EMIM]HSO4) | 10% w/w Biomass 50–60% w/w IL, 100–160 °C, 2–4 h | 40–50% Decrystallized cellulose, 20–80% Arabinose and xylose, ~10% glucose | [32,35] |
| 1-Ethyl-3-methylimidazolium diethyl phosphate ([EMIM]DEP) | 3% w/w Pine cellulose, 28–70% w/w IL, 90–105 °C, 0.2–2 h | Decrystallized cellulose with lower DP of 117–320 | [21] |
| 1-Butyl-3-methylimidazolium chloride ([BMIM]Cl) | 5–50% w/w Cotton, cellulose acetate, MCC, biomass from mulberry and mustard stalk, 0–90% w/w IL, room temperature-130 °C, 2–5 h | 18–25% Cellulose solubility | [19,20,22,29,31,36,37] |
| 1-Butyl-3-methylimidazolium acetate ([BMIM]OAc) | 10% w/w Cellulose, 90%w/w IL 70–130 °C, 2–6 h | Decrystallized cellulose, 45–49% glucan | [29,27] |
| 1-Butyl-3-methylimidazolium tetrafluoroborate ([BMIM]BF4) | 10% w/w Biomass, 90% IL, 100–130 °C, 2–5 h | 39% Glucan | [29] |
| 1-Butyl-3-methylimidazolium acesulfamate ([BMIM]Ace) | 5% w/w Cellulose, 95% w/w IL, 110 °C, 3 h | 90% Glucan | [30] |
| 1-Allyl-3-methylimidazolium chloride ([AMIM]Cl) | 5% w/w MCC, 95% w/w IL, 95–110 °C, 1–5 h | Decrystallized cellulose, 10–20% methyl glucosides | [38] |
| N-Allylpyridinium chloride ([APy]Cl) | 3% w/w Pine cellulose, 97% w/w IL 120 °C, 2 h | Dissolved cellulose | [21] |
| Tetraoctylphosphonium acetate ([P8888]OAc) and trioctyl(tetradecyl)phosphonium acetate ([P14888]OAc) | 3–8% w/w MCC, 10–80% w/w IL, 120 °C, 2 h | Decrystallized cellulose | [23] |
| IL | Company | Ref. |
|---|---|---|
| [EMIM]OAc | 3M | [100] |
| [EMIM]Cl, [PMIM]Cl, [BMIM]Cl, [AMIM]Cl | Eastman Chemical | [101] |
| [EMIM]OAc, 1-Ethyl-3-methylimidazolium propionate ([EMIM]Pro), 1-Ethyl-3-methylimidazolium butyrate ([EMIM]But), [BMIM]OAc, 1-Butyl-3-methylimidazolium propionate ([BMIM]Pro), 1-Butyl-3-methylimidazolium butyrate ([BMIM]But) | Eastman Chemical | [102] |
| [(CH3)3N(CH2)2OH]+[NH2-L-CHNH2—COO]−, [(CH3)3N(CH2)2OH]+[NH2-L-COO]− | Panasonic | [103,104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taokaew, S.; Kriangkrai, W. Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides 2022, 3, 671-691. https://doi.org/10.3390/polysaccharides3040039
Taokaew S, Kriangkrai W. Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides. 2022; 3(4):671-691. https://doi.org/10.3390/polysaccharides3040039
Chicago/Turabian StyleTaokaew, Siriporn, and Worawut Kriangkrai. 2022. "Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents" Polysaccharides 3, no. 4: 671-691. https://doi.org/10.3390/polysaccharides3040039
APA StyleTaokaew, S., & Kriangkrai, W. (2022). Recent Progress in Processing Cellulose Using Ionic Liquids as Solvents. Polysaccharides, 3(4), 671-691. https://doi.org/10.3390/polysaccharides3040039






