Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites
Abstract
:1. Introduction
1.1. BC Coated on Fibers, Yarns, Fabrics
1.2. Regenerated BC Fibers
1.3. Development of BC Macrofibers
1.4. BC Purification, Bleaching and Dyeing
1.5. Improvement of BC Flexibility, Hydrophobicity and Mechanical Properties
2. Materials and Methods
2.1. Materials
2.2. Development of Composites with AESO Emulsion Polymerized before and after Exhaustion
2.2.1. Emulsion Polymerization and Development of the Composites
2.2.2. Characterization of the Composites
2.3. Finishing and Dyeing
2.3.1. Antimicrobial Finishing
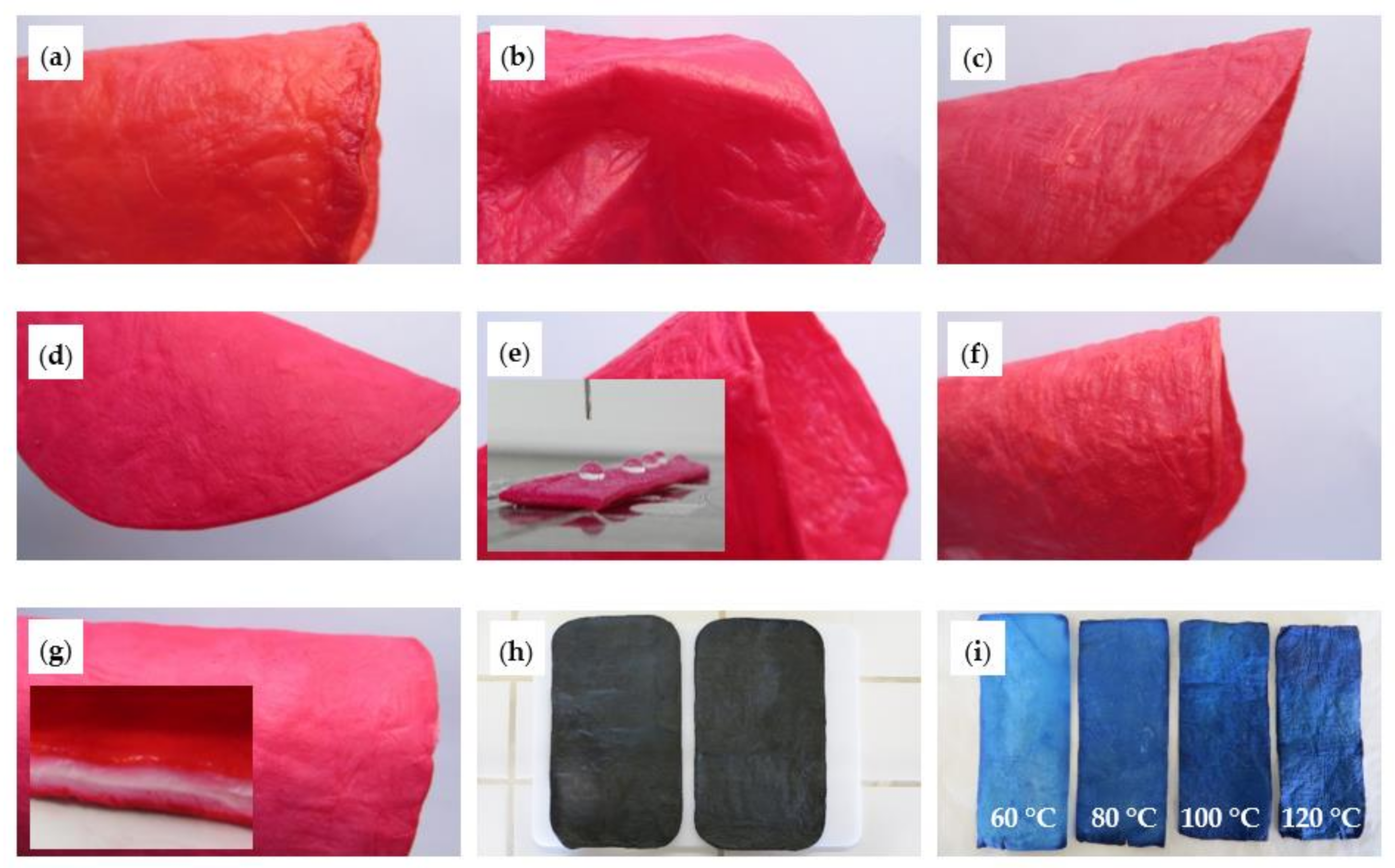
2.3.2. Dyeing of the Composites
- (a)
- Sirius Scarlet K-CF direct dye (0.1 g/100 g) was added to the mixture containing the AESO emulsion, PDMS and PEG 400 polymers, followed by the polymerization of the emulsion. The BC composites were then produced by the exhaustion process;
- (b)
- Same process used in (a) but with Procion Red H-E3N reactive dye;
- (c)
- Same process used in (a) but with Dianix Scarlet CC disperse dye;
- (d)
- Procion Red H-E3N reactive dye (0.1 g/100 g) was added to the mixture containing the AESO emulsion previously polymerized and the other polymers. The BC composites were then produced by the exhaustion process;
- (e)
- Procion Red H-E3N reactive dye (0.1 g/100 g) was added to the mixture containing the AESO emulsion and the other polymers. Then, the membranes were exhausted and finally polymerized;
- (f)
- Same process used in (e) but with Dianix Scarlet CC disperse dye.
- (g)
- Dyeing with the reactive dye Procion Red H-E3N (0.1 g/100 g) aqueous solution for 1.5 h at 30 °C;
- (h)
- Dyeing with the acid dye Solvaderm Black (0.1 g/100 g) aqueous solution for 1.5 h at 30 °C; and
- (i)
- Dyeing with the disperse dye Dianix Blue S-BG (0.1 g/100 g) aqueous dispersion for 1.5 h at different temperatures: 60, 80, 100 and 120 °C.
3. Results and Discussion
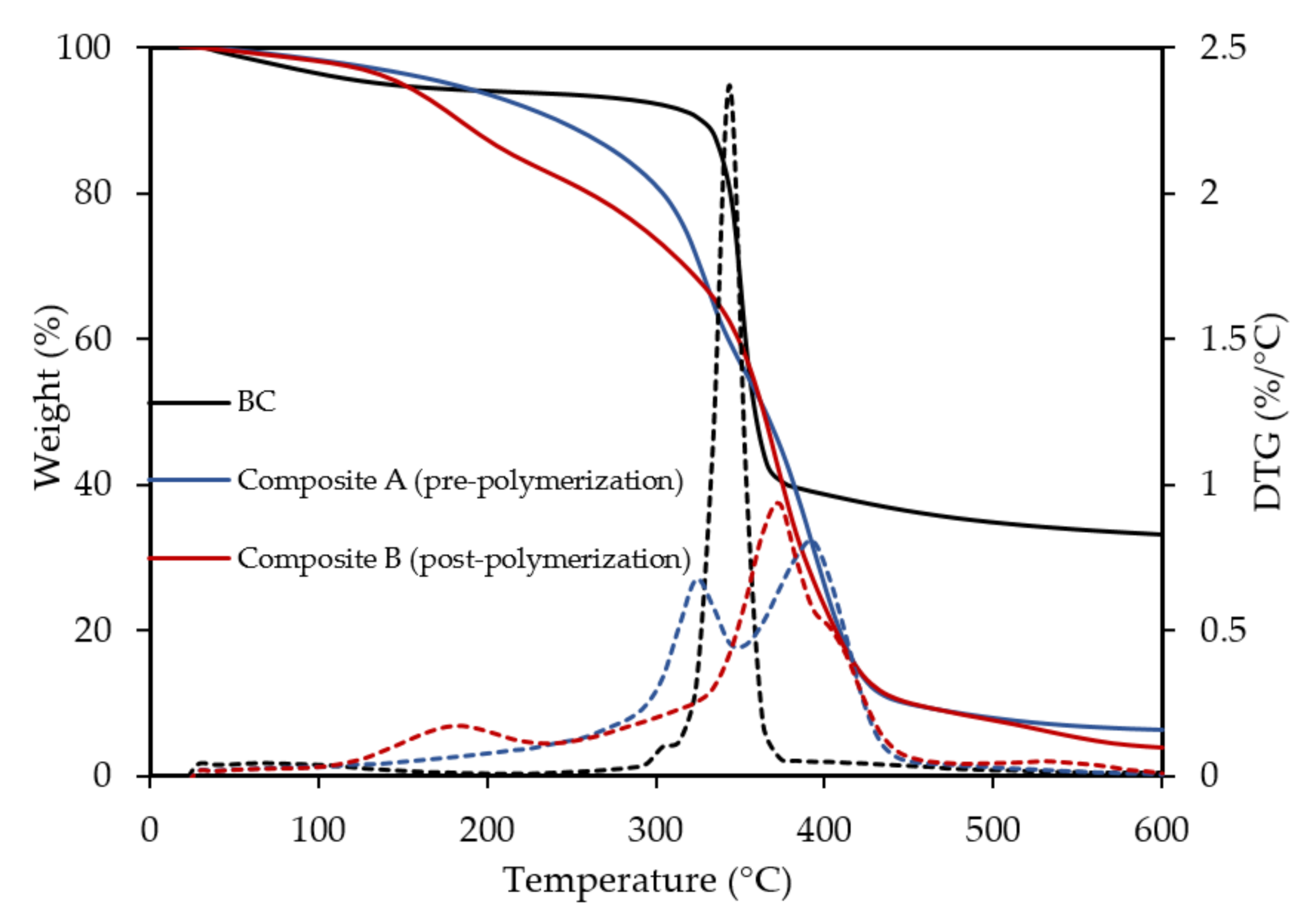
3.1. Properties of the Composites
3.2. Antimicrobial Activity
3.3. Dyeing
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gorgieva, S.; Trček, J. Bacterial Cellulose: Production, Modification and Perspectives in Biomedical Applications. Nanomaterials 2019, 9, 1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Liu, Z.; Shen, R.; Chen, S.; Yang, X. Bacterial Cellulose in Food Industry: Current Research and Future Prospects. Int. J. Biol. Macromol. 2020, 158, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Nishiyama, Y.; Kuga, S. Surface Acetylation of Bacterial Cellulose. Cellulose 2002, 9, 361–367. [Google Scholar] [CrossRef]
- Potivara, K.; Phisalaphong, M. Development and Characterization of Bacterial Cellulose Reinforced with Natural Rubber. Materials 2019, 12, 2323. [Google Scholar] [CrossRef] [Green Version]
- Cazón, P.; Velázquez, G.; Vázquez, M. Characterization of Bacterial Cellulose Fi Lms Combined with Chitosan and Polyvinyl Alcohol: Evaluation of Mechanical and Barrier Properties. Carbohydr. Polym. 2019, 216, 72–85. [Google Scholar] [CrossRef]
- Cazón, P.; Vázquez, M. Bacterial Cellulose as a Biodegradable Food Packaging Material: A Review. Food Hydrocoll. 2021, 113. [Google Scholar] [CrossRef]
- Silva, F.A.G.S.; Dourado, F.; Gama, M.; Poças, F. Nanocellulose Bio-Based Composites for Food Packaging. Nanomaterials 2020, 10, 2041. [Google Scholar] [CrossRef]
- Liu, W.; Du, H.; Zhang, M.; Liu, K.; Liu, H.; Xie, H.; Zhang, X.; Si, C. Bacterial Cellulose-Based Composite Scaffolds for Biomedical Applications: A Review. ACS Sustain. Chem. Eng. 2020, 8, 7536–7562. [Google Scholar] [CrossRef]
- Islam, S.U.; Ul-Islam, M.; Ahsan, H.; Ahmed, M.B.; Shehzad, A.; Fatima, A.; Sonn, J.K.; Lee, Y.S. Potential Applications of Bacterial Cellulose and Its Composites for Cancer Treatment. Int. J. Biol. Macromol. 2021, 168, 301–309. [Google Scholar] [CrossRef]
- Martins, D.; Rocha, C.; Dourado, F.; Gama, M. Bacterial Cellulose-Carboxymethyl Cellulose (BC:CMC) Dry Formulation as Stabilizer and Texturizing Agent for Surfactant-Free Cosmetic Formulations. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126380. [Google Scholar] [CrossRef]
- Alves, A.A.; Silva, W.E.; Belian, M.F.; Lins, L.S.G.; Galembeck, A. Bacterial Cellulose Membranes for Environmental Water Remediation and Industrial Wastewater Treatment. Int. J. Environ. Sci. Technol. 2020, 17, 3997–4008. [Google Scholar] [CrossRef]
- Wahid, F.; Zhao, X.J.; Duan, Y.X.; Zhao, X.Q.; Jia, S.R.; Zhong, C. Designing of Bacterial Cellulose-Based Superhydrophilic/Underwater Superoleophobic Membrane for Oil/Water Separation. Carbohydr. Polym. 2021, 257. [Google Scholar] [CrossRef]
- Poddar, M.K.; Dikshit, P.K. Recent Development in Bacterial Cellulose Production and Synthesis of Cellulose Based Conductive Polymer Nanocomposites. Nano Sel. 2021, 1–24. [Google Scholar] [CrossRef]
- Ma, L.; Bi, Z.; Xue, Y.; Zhang, W.; Huang, Q.; Zhang, L.; Huang, Y. Bacterial Cellulose: An Encouraging Eco-Friendly Nano-Candidate for Energy Storage and Energy Conversion. J. Mater. Chem. A 2020, 8, 5812–5842. [Google Scholar] [CrossRef]
- Material District. Leather-Free Handbag Made of Bacterial Cellulose. Available online: https://materialdistrict.com/article/handbag-bacterial-cellulose/ (accessed on 25 August 2020).
- Sick-Leitner, M. SOYA C(O)U(L)TURE—Useful Things Arise out of Waste. Available online: https://ars.electronica.art/aeblog/en/2015/09/30/soya-coulture/ (accessed on 14 September 2020).
- Raut, A. Malai: A Sustainable, Vegan Alternative to Leather. Available online: https://www.architecturaldigest.in/content/malai-a-sustainable-vegan-alternative-to-leather/ (accessed on 2 September 2020).
- Ng, F.M.C.; Wang, P.W. Natural Self-Grown Fashion from Bacterial Cellulose: A Paradigm Shift Design Approach in Fashion Creation. Des. J. 2016, 19, 837–855. [Google Scholar] [CrossRef]
- Ng, A. Grown Microbial 3D Fiber Art, Ava: Fusion of Traditional Art with Technology. In Proceedings of the International Symposium on Wearable Computers (ISWC), Maui, HI, USA, 5–8 May 2017; pp. 209–214. [Google Scholar]
- Chan, C.K.; Shin, J.; Jiang, S.X.K. Development of Tailor-Shaped Bacterial Cellulose Textile Cultivation Techniques for Zero-Waste Design. Cloth. Text. Res. J. 2018, 36, 33–44. [Google Scholar] [CrossRef]
- Tyurin, I.; Getmantseva, V.; Andreeva, E.; Kashcheev, O. The Study of the Molding Capabilities of Bacterial Cellulose. In Proceedings of the 19th World Textile Conference on Textiles at the Crossrorads (AUTEX 2019), Ghent, Belgium, 20–25 April 2019. [Google Scholar]
- Pommet, M.; Juntaro, J.; Heng, J.Y.Y.; Mantalaris, A.; Lee, A.F.; Wilson, K.; Kalinka, G.; Shaffer, M.S.P.; Bismarck, A. Surface Modification of Natural Fibers Using Bacteria: Depositing Bacterial Cellulose onto Natural Fibers to Create Hierarchical Fiber Reinforced Nanocomposites. Biomacromolecules 2008, 9, 1643–1651. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.; Fan, L.; Yuan, Y.; Wei, C.; Bai, Z.; Xu, J. All-Solid-State Yarn Supercapacitors Based on Hierarchically Structured Bacterial Cellulose Nanofiber-Coated Cotton Yarns. Cellulose 2016, 23, 3987–3997. [Google Scholar] [CrossRef]
- Mizuno, M.; Kamiya, Y.; Katsuta, T.; Oshima, N.; Nozaki, K.; Amano, Y. Creation of Bacterial Cellulose-Fabric Complexed Material. SEN I GAKKAISHI 2012, 68, 42–47. [Google Scholar] [CrossRef] [Green Version]
- Kamal, A.S.M.; Misnon, M.I.; Zakaria, M.N.; Kadir, M.I.A.; Ahmad, M.R. Characteristics of Cotton, Polyester and Rayon Fabrics Coated with Acetobacter Xylinum. Int. J. Eng. Technol. 2018, 7, 181–184. [Google Scholar]
- Naeem, M.A.; Alfred, M.; Saba, H.; Siddiqui, Q.; Naveed, T.; Shahbaz, U. A Preliminary Study on the Preparation of Seamless Tubular Bacterial Nanocomposite Fabrics. J. Compos. Mater. 2019, 53. [Google Scholar] [CrossRef]
- Felgueiras, C.; Azoia, N.G.; Gonçalves, C.; Gama, M.; Dourado, F. Trends on the Cellulose-Based Textiles: Raw Materials and Technologies. Front. Bioeng. Biotechnol. 2021, 9, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tokura, S.; Tamura, H.; Takai, M.; Higuchi, T.; Asano, H. Continuous Harvest of Cellulosic Filament during Cultivation of Acetobacter Xylinum. In Proceedings of the Cellulosic Pulps, Fibres and Materials Conference—Cellucon ’98, Bryan, TX, USA, 1–5 July 1998; pp. 3–12. [Google Scholar]
- Sakairi, N.; Asano, H.; Ogawa, M.; Nishi, N.; Tokura, S. A Method for Direct Harvest of Bacterial Cellulose Filaments during Continuous Cultivation of Acetobacter Xylinum. Carbohydr. Polym. 1998, 35, 233–237. [Google Scholar] [CrossRef]
- Tokura, S.; Asano, H.; Sakairi, N.; Nishi, N. Direct Filature of Bacterial Cellulose from Culture Medium. Macromol. Symp. 1998, 127, 23–30. [Google Scholar] [CrossRef]
- Chen, P.; Kim, H.S.; Kwon, S.M.; Yun, Y.S.; Jin, H.J. Regenerated Bacterial Cellulose/Multi-Walled Carbon Nanotubes Composite Fibers Prepared by Wet-Spinning. Curr. Appl. Phys. 2009, 9, e96–e99. [Google Scholar] [CrossRef]
- Chen, P.; Yun, Y.S.; Bak, H.; Cho, S.Y.; Jin, H.J. Multiwalled Carbon Nanotubes-Embedded Electrospun Bacterial Cellulose Nanofibers. Mol. Cryst. Liq. Cryst. 2010, 519, 169–178. [Google Scholar] [CrossRef]
- Gao, Q.; Shen, X.; Lu, X. Regenerated Bacterial Cellulose Fibers Prepared by the NmMo·H2O Process. Carbohydr. Polym. 2011, 83, 1253–1256. [Google Scholar] [CrossRef]
- Lu, X.; Shen, X. Solubility of Bacteria Cellulose in Zinc Chloride Aqueous Solutions. Carbohydr. Polym. 2011, 86, 239–244. [Google Scholar] [CrossRef]
- Zhang, S.; Luo, J. Preparation and Properties of Bacterial Cellulose/Alginate Blend Bio-Fibers. J. Eng. Fiber. Fabr. 2011, 6, 69–72. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Tang, S.; Huang, B.; Shen, X.; Hong, F. Preparation and Characterization of Bacterial Cellulose/Hydroxypropyl Chitosan Blend as-Spun Fibers. Fibers Polym. 2013, 14, 935–940. [Google Scholar] [CrossRef]
- Makarov, I.S.; Golova, L.K.; Vinogradov, M.I.; Levin, I.S.; Gromovykh, T.I.; Arkharova, N.A.; Kulichikhin, V.G. Cellulose Fibers from Solutions of Bacterial Cellulose in N-Methylmorpholine N-Oxide. Fibre Chem. 2019, 51, 175–181. [Google Scholar] [CrossRef]
- Yao, J.; Chen, S.; Chen, Y.; Wang, B.; Pei, Q.; Wang, H. Macrofibers with High Mechanical Performance Based on Aligned Bacterial Cellulose Nanofibers. ACS Appl. Mater. Interfaces 2017, 9, 20330–20339. [Google Scholar] [CrossRef]
- Wang, S.; Jiang, F.; Xu, X.; Kuang, Y.; Fu, K.; Hitz, E.; Hu, L. Super-Strong, Super-Stiff Macrofibers with Aligned, Long Bacterial Cellulose Nanofibers. Adv. Mater. 2017, 29, 1–8. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, S.; Wu, R.; Sheng, N.; Zhang, M.; Ji, P.; Wang, H. Top-down Peeling Bacterial Cellulose to High Strength Ultrathin Films and Multifunctional Fibers. Chem. Eng. J. 2020, 391, 123527. [Google Scholar] [CrossRef]
- Gao, H.L.; Zhao, R.; Cui, C.; Zhu, Y.B.; Chen, S.M.; Pan, Z.; Meng, Y.F.; Wen, S.M.; Liu, C.; Wu, H.A.; et al. Bioinspired Hierarchical Helical Nanocomposite Macrofibers Based on Bacterial Cellulose Nanofibers. Natl. Sci. Rev. 2020, 7, 73–83. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Shim, E.; Kim, H.R. Effects of Cultivation, Washing, and Bleaching Conditions on Bacterial Cellulose Fabric Production. Text. Res. J. 2019, 89, 1094–1104. [Google Scholar] [CrossRef]
- Kamal, A.S.M.; Misnon, M.I.; Fadil, F. The Effect of Sodium Hydroxide Concentration on Yield and Properties of Bacterial Cellulose Membranes. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Pattaya, Thailand, 13–15 March 2020; Volume 732, pp. 1–7. [Google Scholar]
- Miyamoto, H.; Tsuduki, M.; Ago, M.; Yamane, C.; Ueda, M.; Okajima, K. Influence of Dyestuffs on the Crystallinity of a Bacterial Cellulose and a Regenerated Cellulose. Text. Res. J. 2014, 84, 1147–1158. [Google Scholar] [CrossRef]
- Wood, D.; Hang, L.; Salusso, C.J. Production and Characterization of Bacterial Cellulose Fabrics. In Proceedings of the International Textile and Apparel Association (ITAA) Annual Conference Proceedings, Santa Fe, Mexico, 18–22 December 2015; pp. 11–13. [Google Scholar]
- Shim, E.; Kim, H.R. Coloration of Bacterial Cellulose Using in Situ and Ex Situ Methods. Text. Res. J. 2019, 89, 1297–1310. [Google Scholar] [CrossRef]
- Song, J.E.; Su, J.; Noro, J.; Cavaco-Paulo, A.; Silva, C.; Kim, H.R. Bio-Coloration of Bacterial Cellulose Assisted by Immobilized Laccase. AMB Express 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- de Costa, A.F.S.; de Amorim, J.D.P.; Almeida, F.C.G.; de Lima, I.D.; de Paiva, S.C.; Rocha, M.A.V.; Vinhas, G.M.; Sarubbo, L.A. Dyeing of Bacterial Cellulose Films Using Plant-Based Natural Dyes. Int. J. Biol. Macromol. 2019, 121, 580–587. [Google Scholar] [CrossRef]
- Lee, Y.-A.; Xiang, C.; Ghalachyan, A.; Ramasubramanian, G.; Li, R.; Madbouly, S.; Farr, C. Exploring Optimal Solutions for Sustainable Product Development Using Renewable Bacteria Cellulose Fiber and Biopolymer Composites. In Proceedings of the 71st Annual Conference of the International Textile and Apparel Association 2014 (ITAA #71), Charlotte, NC, USA, 12–16 November 2014; pp. 8–9. [Google Scholar]
- Lee, Y.A. Case study of renewable bacteria cellulose fiber and biopolymer composites in sustainable design practices. In Sustainable Fibres for Fashion Industry; Muthu, S.S., Gardetti, M.A., Eds.; Springer: Singapore, 2016; Volume 1, ISBN 978-981-10-0520-6. [Google Scholar]
- Araújo, S.; da Silva, F.M.; Gouveia, I.C. The Role of Technology towards a New Bacterial-Cellulose-Based Material for Fashion Design. J. Ind. Intell. Inf. 2015, 3, 168–172. [Google Scholar] [CrossRef] [Green Version]
- Song, J.E.; Cavaco-Paulo, A.; Silva, C.; Kim, H.R. Improvement of Bacterial Cellulose Nonwoven Fabrics by Physical Entrapment of Lauryl Gallate Oligomers. Text. Res. J. 2020, 90, 166–178. [Google Scholar] [CrossRef]
- Song, J.E.; Silva, C.; Cavaco-Paulo, A.M.; Kim, H.R. Functionalization of Bacterial Cellulose Nonwoven by Poly(Fluorophenol) to Improve Its Hydrophobicity and Durability. Front. Bioeng. Biotechnol. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Kamiński, K.; Jarosz, M.; Grudzień, J.; Pawlik, J.; Zastawnik, F.; Pandyra, P.; Kołodziejczyk, A.M. Hydrogel Bacterial Cellulose: A Path to Improved Materials for New Eco-Friendly Textiles. Cellulose 2020, 27, 5353–5365. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Song, J.E.; Kim, H.R. Comparative Study on the Physical Entrapment of Soy and Mushroom Proteins on the Durability of Bacterial Cellulose Bio-Leather. Cellulose 2021, 28, 3183–3200. [Google Scholar] [CrossRef]
- da Silva, C.J.G.; de Medeiros, A.D.M.; de Amorim, J.D.P.; do Nascimento, H.A.; Converti, A.; Costa, A.F.S.; Sarubbo, L.A. Bacterial Cellulose Biotextiles for the Future of Sustainable Fashion: A Review. Environ. Chem. Lett. 2021. [Google Scholar] [CrossRef]
- Rathinamoorthy, R.; Kiruba, T. Bacterial Cellulose-A Potential Material for Sustainable Eco-Friendly Fashion Products. J. Nat. Fibers 2020, 1–13. [Google Scholar] [CrossRef]
- da Silva, F.A.G.S.; Fernandes, M.; Souto, A.P.; Ferreira, E.C.; Dourado, F.; Gama, M. Optimization of Bacterial Nanocellulose Fermentation Using Recycled Paper Sludge and Development of Novel Composites. Appl. Microbiol. Biotechnol. 2019, 103, 9143–9154. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.; Souto, A.P.; Gama, M.; Dourado, F. Bacterial Cellulose and Emulsified AESO Biocomposites as an Ecological Alternative to Leather. Nanomaterials 2019, 9, 1710. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, M.; Gama, M.; Dourado, F.; Souto, A.P. Development of Novel Bacterial Cellulose Composites for the Textile and Shoe Industry. Microb. Biotechnol. 2019, 12, 650–661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S. Redox-Initiated Adiabatic Emulsion Polymerization. Theses and Dissertations. Ph.D. Thesis, Paper 1294. Lehigh University, Bethlehem, PA, USA, 2013. [Google Scholar]
- Kiatsimkul, P.P.; Sutterlin, W.R.; Suppes, G.J. Selective Hydrolysis of Epoxidized Soybean Oil by Commercially Available Lipases: Effects of Epoxy Group on the Enzymatic Hydrolysis. J. Mol. Catal. B Enzym. 2006, 41, 55–60. [Google Scholar] [CrossRef]
- Burkinshaw, S.M. Physico-Chemical Aspects of Textile Coloration; Society of Dyers and Colorists, Ed.; John Wiley & Sons, Inc.: West Sussex, UK, 2016; ISBN 978-1-118-72569-6. [Google Scholar]
- Cousinet, S.; Ghadban, A.; Fleury, E.; Lortie, F.; Pascault, J.P.; Portinha, D. Toward Replacement of Styrene by Bio-Based Methacrylates in Unsaturated Polyester Resins. Eur. Polym. J. 2015, 67, 539–550. [Google Scholar] [CrossRef]
- Wei, G.; Xu, H.; Chen, L.; Li, Z.; Liu, R. Isosorbide-Based High Performance UV-Curable Reactive Diluents. Prog. Org. Coatings 2019, 126, 162–167. [Google Scholar] [CrossRef]
- BS 7209:1990. Specification for Water Vapour Permeable Apparel Fabrics; British Standards Institute: London, UK, 1990. [Google Scholar]
- ISO. ISO 20645:2006 Textile Fabrics—Determination of Antibacterial Activity—Agar Diffusion Plate Test; International Organization for Standardization: Geneva, Switzerland, 2006. [Google Scholar]
- Xu, X.; Jagota, A.; Paretkar, D.; Hui, C. Surface Tension Measurement from the Indentation of Clamped Thin Films. Soft Matter 2016, 12, 5121–5126. [Google Scholar] [CrossRef]
- Tian, Y.; Ina, M.; Cao, Z.; Sheiko, S.S.; Dobrynin, A.V. How to Measure Work of Adhesion and Surface Tension of Soft Polymeric Materials. Macromolecules 2018, 51, 4059–4067. [Google Scholar] [CrossRef]
- Li, C.; Xiao, H.; Wang, X.; Zhao, T. Development of Green Waterborne UV-Curable Vegetable Oil-Based Urethane Acrylate Pigment Prints Adhesive: Preparation and Application. J. Clean. Prod. 2018, 180, 272–279. [Google Scholar] [CrossRef]
- Soykeabkaew, N.; Sian, C.; Gea, S.; Nishino, T.; Peijs, T. All-Cellulose Nanocomposites by Surface Selective Dissolution of Bacterial Cellulose. Cellulose 2009, 16, 435–444. [Google Scholar] [CrossRef]
- Asgher, M.; Ahmad, Z.; Iqbal, H.M.N. Bacterial Cellulose-Assisted de-Lignified Wheat Straw-PVA Based Bio-Composites with Novel Characteristics. Carbohydr. Polym. 2017, 161, 244–252. [Google Scholar] [CrossRef]
- Gea, S.; Bilotti, E.; Reynolds, C.T.; Soykeabkeaw, N.; Peijs, T. Bacterial Cellulose-Poly(Vinyl Alcohol) Nanocomposites Prepared by an in-Situ Process. Mater. Lett. 2010, 64, 901–904. [Google Scholar] [CrossRef]
- Salarbashi, D.; Bazeli, J.; Tafaghodi, M. Environment-Friendly Green Composites Based on Soluble Soybean Polysaccharide: A Review. Int. J. Biol. Macromol. 2019, 122, 216–223. [Google Scholar] [CrossRef]
- Boon, D.; Lim, K.; Gong, H. Highly Stretchable and Transparent Films Based on Cellulose. Carbohydr. Polym. 2018, 201, 446–453. [Google Scholar] [CrossRef]
- Sun, Y.; Meng, C.; Zheng, Y.; Wang, Y.; Qiao, K.; Yue, L.; Xie, Y.; He, W. The Effects of Two Biocompatible Plasticizers on the Performance of Dry Bacterial Cellulose Membrane: A Comparative Study. Cellulose 2018, 25, 5893–5908. [Google Scholar] [CrossRef]
- Frone, A.N.; Panaitescu, D.M.; Chiulan, I.; Nicolae, C.A.; Casarica, A.; Gabor, A.R.; Trusca, R.; Damian, C.M.; Purcar, V.; Alexandrescu, E.; et al. Surface Treatment of Bacterial Cellulose in Mild, Eco-Friendly Conditions. Coatings 2018, 8, 221. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Sheng, J.; Yan, Z.; Ke, Q. Completely Amorphous Cellulose Biosynthesized in Agitated Culture at Low Temperature. Int. J. Biol. Macromol. 2018, 117, 967–973. [Google Scholar] [CrossRef]
- Liu, C.; Liu, Z.; Tisserat, B.H.; Wang, R.; Schuman, T.P.; Zhou, Y.; Hu, L. Microwave-Assisted Maleation of Tung Oil for Bio-Based Products with Versatile Applications. Ind. Crop. Prod. 2015, 71, 185–196. [Google Scholar] [CrossRef]
- Liu, K.; Madbouly, S.A.; Kessler, M.R. Biorenewable Thermosetting Copolymer Based on Soybean Oil and Eugenol. Eur. Polym. J. 2015, 69, 16–28. [Google Scholar] [CrossRef]
- Liu, F.; Miao, L.; Wang, Y.; Xue, X.; Yang, H. Progress in Organic Coatings Green Fabrication of Ultraviolet Curable Epoxy Acrylate-Silica Hybrid Coatings. Prog. Org. Coatings 2017, 109, 38–44. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, M.; Cochran, E.W.; Kessler, M.R. Biorenewable Polymers Based on Acrylated Epoxidized Soybean Oil and Methacrylated Vanillin. Mater. Today Commun. 2015, 5, 18–22. [Google Scholar] [CrossRef]
- Sousa, A.F.; Ferreira, S.; Lopez, A.; Borges, I.; Pinto, R.J.B.; Silvestre, A.J.D.; Freire, C.S.R. Thermosetting AESO-Bacterial Cellulose Nanocomposite Foams with Tailored Mechanical Properties Obtained by Pickering Emulsion Templating. Polymer 2017, 118, 127–134. [Google Scholar] [CrossRef]
- Radhakrishnan, T.S. Thermal Degradation of Poly(Dimethylsilylene) and Poly(Tetramethyldisilylene-Co-Styrene). J. Appl. Polym. Sci. 2005, 99, 2679–2686. [Google Scholar] [CrossRef]
- Wei, B.; Yang, G.; Hong, F. Preparation and Evaluation of a Kind of Bacterial Cellulose Dry Films with Antibacterial Properties. Carbohydr. Polym. 2011, 84, 533–538. [Google Scholar] [CrossRef]


| AESO Mixture (%) | AESO Emulsion (%) | Polymers Mixture (Exhaustion) (%) | |||
|---|---|---|---|---|---|
| AESO | 50 | AESO mixture | 20 | AESO emulsion | 75 |
| Lauryl methacrylate | 40 | Triton-X-100/Butanol (2/1) | 2 | Persoftal MS Con.01 | 20 |
| 1,6-hexanodiol diacrylate | 5 | Water | 78 | PEG 400 | 5 |
| Tri(propylene glycol diacrylate | 5 |
| Sample | Si BAC (%) (w/w) | Smart Fix (%) (w/w) | Time (min) |
|---|---|---|---|
| Control | - | - | 30 |
| 1 BAC | 2 | 0.4 | 30 |
| 2 BAC | 2 | 0.4 | 60 |
| 3 BAC | 4 | 0.4 | 60 |
| Sample | Thickness (mm) | WCA (°) | WVP (g·m−2·24 h−1) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|---|---|
| BC | 0.48 ± 0.01 | 63.1 ± 4.7 | 289.6 | 37.5 ± 0.8 | 3.6 ± 0.6 |
| Composite A (pre-polymerization) | 1.27 ± 0.01 | 93.1 ± 5.7 | 65.1 ± 1.3 | 12.1 ± 1.8 | 15.5 ± 0.9 |
| Composite B (post-polymerization) | 1.22 ± 0.01 | 103.6 ± 3.2 | 28.8 ± 2.7 | 8.3 ± 0.4 | 19.1 ± 4.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, M.; Souto, A.P.; Dourado, F.; Gama, M. Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites. Polysaccharides 2021, 2, 566-581. https://doi.org/10.3390/polysaccharides2030034
Fernandes M, Souto AP, Dourado F, Gama M. Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites. Polysaccharides. 2021; 2(3):566-581. https://doi.org/10.3390/polysaccharides2030034
Chicago/Turabian StyleFernandes, Marta, António Pedro Souto, Fernando Dourado, and Miguel Gama. 2021. "Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites" Polysaccharides 2, no. 3: 566-581. https://doi.org/10.3390/polysaccharides2030034
APA StyleFernandes, M., Souto, A. P., Dourado, F., & Gama, M. (2021). Application of Bacterial Cellulose in the Textile and Shoe Industry: Development of Biocomposites. Polysaccharides, 2(3), 566-581. https://doi.org/10.3390/polysaccharides2030034







