Abstract
Background/Objectives: Type 2 diabetes mellitus (T2DM) is a major global public health challenge with a significant impact on human life. The current study aims to provide a comprehensive analysis of the magnitude of dyslipidemia and the factors associated with elevated LDL-C levels among Black South Africans with T2DM. Methods: This was a cross-sectional study conducted in a tertiary hospital. Blood samples for glycated hemoglobin (HbA1c) and lipid profile were collected from the study participants and analyzed using Siemens Atellica™ analyzer. The data was entered into Microsoft excel and analyzed using SPSS version 24. Bivariate and multivariate logistic regression was employed to identify variables significantly associated with the outcomes, with a p-value ≤ 0.05 and a 95% confidence interval. Results: A total of 194 study participants with T2DM were recruited in the study. The overall prevalence of dyslipidemia was 90.72%. Of those with dyslipidemia, 40.9% had an isolated dyslipidemia, 39.7% had a combined dyslipidemia and 19.3% had atherogenic dyslipidemia. Significant factors associated with elevated levels of LDL-C included age, non-adherence to treatment (NAT) and duration. However, after multivariate analysis, NAT was found to be an independent associated factor with elevated levels of LDL-C (AOR: 4.596; 95% CI: 0.177–2.874; p = 0.027). Conclusions: Our study found that dyslipidemia is highly prevalent among Black South African patients with T2DM at a tertiary hospital, despite the use of lipid-lowering therapy. NAT was significantly associated with elevated levels of LDL-C. However, it is important to note that the study employed a cross-sectional design, conducted at a single hospital, which may impair the generalizability of the findings.
1. Introduction
Type 2 diabetes mellitus (T2DM) is a major global public health challenge with a significant impact on quality of life [1]. Globally, T2DM accounts for over 90% of the 463 million adults living with diabetes, which is primarily driven by sedentary lifestyles, poor dietary habits, and rising obesity rates [2]. Sub-Saharan Africa has the lowest prevalence estimate of 5%; however, it is expected that the number of people living with diabetes will increase to 142% by 2050 [3]. Additionally, a study by Chikwati et al. has shown a high incidence of T2DM of 14.6 cases per 1000 person years in a middle-aged sub-Saharan African population, and with the highest incidence observed in South Africa [4]. Cardiovascular diseases (CVD), mainly ischemic heart disease and cerebrovascular accident, are the leading causes of morbidity and mortality globally, as well as in Sub-Saharan Africa [5]. The risk factors for CVD include diabetes mellitus, together with hypertension and dyslipidemia [6]. The presence of dyslipidemia drives the atherosclerosis process, and it is a modifiable risk factor in individuals with diabetes mellitus [5]. Additionally, dyslipidemia in diabetes is characterized by raised fasting and postprandial triglycerides, low HDL-cholesterol (HDL-C), raised LDL-cholesterol (LDL-C), and the predominance of small dense LDL particles [7,8]. Dyslipidemia is a modifiable risk factor in the prevention of cardiovascular events in diabetic individuals, and the primary goal of lipid-lowering treatment is to obtain optimal LDL-C levels in this population [5,9]. However, studies performed in South Africa have revealed that dyslipidemia was prevalent in diabetic patients, and many failed to meet the target fasting LDL-C concentration of 1.8 mmol/L [9,10]. These findings suggest that there are factors associated with elevated levels of LDL-C among T2DM patients. These findings suggest that there are factors associated with elevated levels of LDL-C among T2DM patients. However, to date, no study has reported on factors associated with elevated levels of LDL-C among Black South Africans with T2DM. Furthermore, the magnitude of diabetic dyslipidemia remains underemphasized, particularly in the context of the Black South African population with T2DM. Therefore, the current study aims to provide a comprehensive analysis of the magnitude of diabetic dyslipidemia and the factors associated with elevated LDL-C levels among Black South Africans with T2DM. Understanding the magnitude of lipid abnormalities and challenges to effective care will guide the development of targeted strategies for early screening, patient education, and integrated management of diabetic dyslipidemia in high-risk individuals. Such interventions are vital to reducing ACVD-related morbidity and advancing health equity in South Africa’s evolving epidemiological landscape.
2. Materials and Methods
2.1. Study Design and Participants
This was a cross-sectional study which investigated the prevalence of dyslipidemia and factors associated with elevated LDL-C among Black South African patients with T2DM. This study was conducted at Dr George Mukhari Academic Hospital (DMGAH), located in the north of Pretoria, South Africa and primarily serves the Black South African population. A random sample of one hundred and ninety-four (194) Black South African patients diagnosed with T2DM, attending the outpatient diabetic clinic of DGMAH, were included in the study. Study participants were recruited after the purpose and significance of the study were explained to them, along with their right to participate in the study. Only those who provided informed consent were included in the study. The study participants whose medical records had missing information and had an active SARS-CoV-19 infection and HIV infection, taking antiretroviral therapy, were excluded from the study. The study was approved by the Sefako Makgatho Health Sciences University ethics committee (SMUREC/S/346/2021:PG, 1 November 2021).
2.2. Definition of Dyslipidemia
The recommended optimal lipid target for individuals with T2DM has been outlined in the 2018 Dyslipidemia Guidelines by the South African Heart Association and Lipid and Atherosclerosis Society of South Africa, as well as in the 2017 guidelines from the Society for Endocrinology, Metabolism, and Diabetes of South Africa, which provide guidelines on the management of T2DM. Using these guidelines, dyslipidemia was defined as: elevated levels of TC ≥ 4.5 mmol/L, elevated levels of LDL-C ≥ 1.8 mmol/L, elevated levels of TG ≥ 1.7 mmol/L, and low HDL-C < 1 mmol/L for men and <1.2 mmol/L for women. Isolated dyslipidemia was defined by the presence of one of the lipid abnormalities (TG, HDL-C, or LDL-C). Combined dyslipidemia was defined by the presence of two lipid abnormalities (TG, HDL-C or LDL-C), while mixed dyslipidemia was defined by the presence of three lipid abnormalities (TG, HDL-C, and LDL-C).
2.3. Data Collection
A structured questionnaire form was administered to the study participants to collect sociodemographic information and cardiovascular disease risk factors data. The data collected included age, gender, smoking, alcohol consumption, physical activity, family history of CVD, and duration of diabetes.
2.4. Blood Sample Collection and Biochemical Assessment
Venus blood samples for HbA1c and lipid profile were collected in EDTA and SST tubes (BD Vacutainers®, Franklin Lakes, NJ, USA), respectively, and taken to the laboratory for centrifugation at 3000 rpm for 10 min. Glycated hemoglobin (HbA1c) and lipid profile (TC, TG, HDL-C and LDL-C) were analyzed using Siemens Atellica™ analyzer (Tarrytown, NY, USA). Non-HDL-C was calculated by subtracting the HDL-C value from the TC value [11].
2.5. Data Analysis
The statistical analysis was performed using the IBM Statistical Package for Social Sciences (SPSS) software version 24 (IBM Corp., Armonk, NY, USA). Categorical data is reported as numbers and percentages, and continuous data is reported as mean ± standard deviation. Differences between the groups were determined using Fisher’s exact test and Student’s t-test, or analysis of variance. A p-value of less than 0.05 was considered statistically significant. Bivariate logistic regression was performed to determine factors associated with elevated levels of LDL-C among the study participants with T2DM. Factors with a p-value of less than 0.05 in the bivariate logistic regression analysis were included in the multivariate logistic regression model to identify independent variables for the presence of dyslipidemia.
3. Results
3.1. Socio-Demographic and Clinical Characteristics of Patients with Type 2 Diabetes Mellitus
The socio-demographic and clinical characteristics of patients with T2DM, stratified by sex, are represented in Table 1. A total of one hundred and ninety-four (194) study participants who are T2DM black South Africans were recruited into the study, with a median age of 63 years (IQR: 48.0–67.0). The median age of male participants was 64 years (IQR: 45–70), while that of female participants was 63 years (IQR: 48–67). Physical inactivity was markedly higher among females (34.3%) than males (14.8%) (p = 0.012), while the difference in family history of cardiovascular disease was marginally significant (p = 0.061). Biochemical profiles (HbA1c, TC, TG, HDL-C, LDL-C, non-HDL-C, and ApoB-100) between males and females in the current cohort were similar.

Table 1.
Socio-demographic and clinical characteristics of patients with type 2 diabetes mellitus.
3.2. Prevalence and Patterns of Dyslipidemia Among Patients with Type 2 Diabetes Mellitus
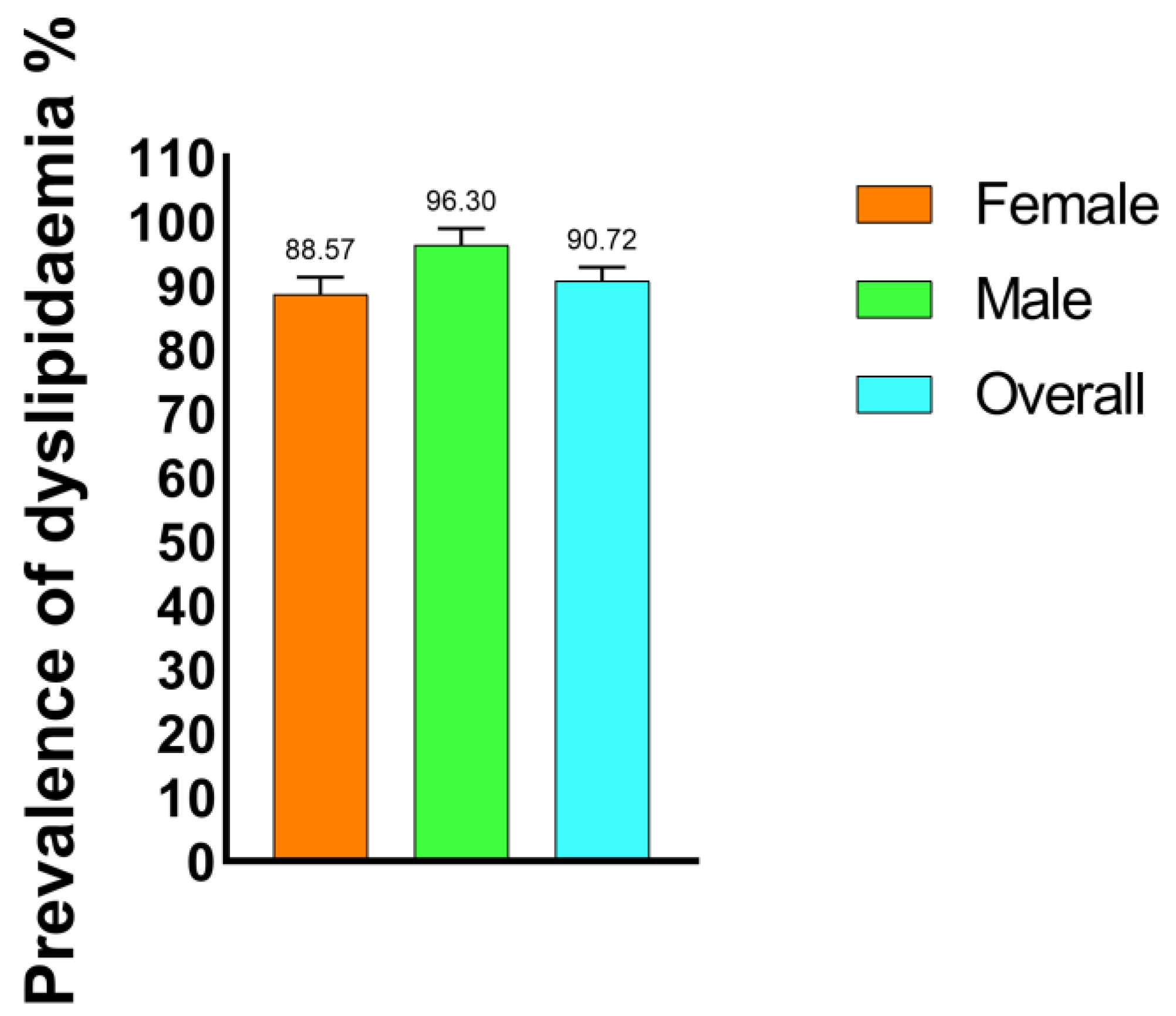
Out of a total of 194 study participants with T2DM, 176 (90.72%) were found to have at least one form of dyslipidemia. Male study participants exhibited the highest prevalence of dyslipidemia (96.29%), while female study participants showed a slightly lower prevalence of dyslipidemia (88.57%) (Figure 1).
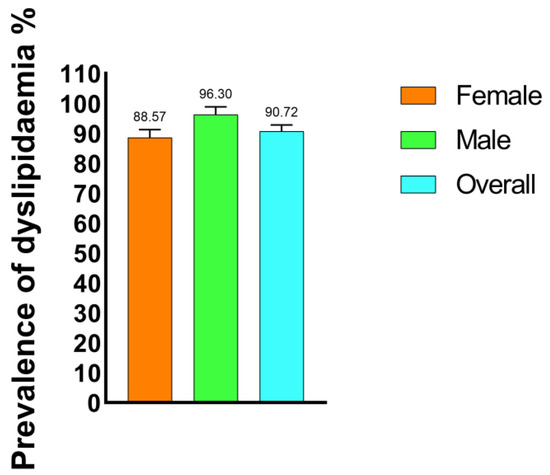
Figure 1.
Prevalence of dyslipidemia among the type 2 diabetic mellitus study participants.
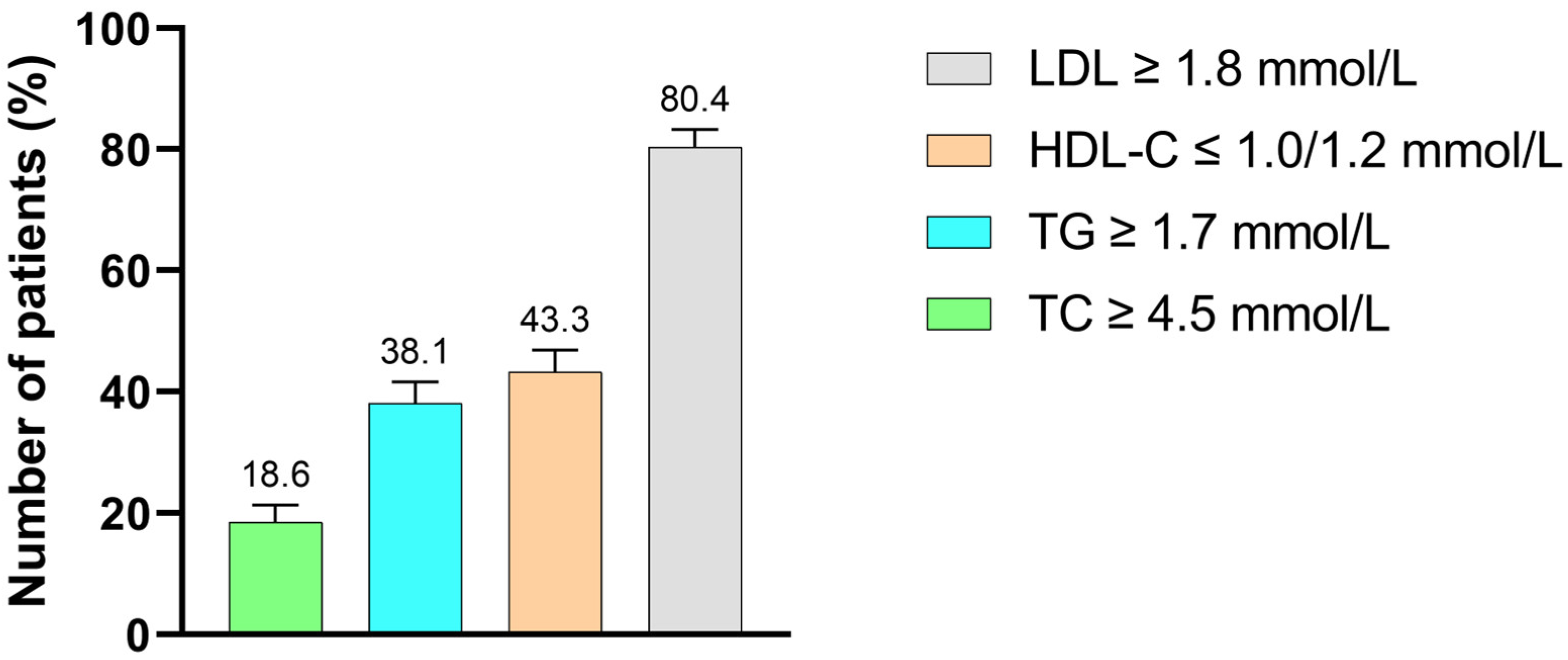
Out of the 176 T2DM patients with DD, elevated LDL-C was the most common lipid abnormality, observed in 156 patients (80.4%). This was followed by reduced HDL-C in 84 patients (43.3%), hypertriglyceridemia in 74 patients (38.1%) and hypercholesterolemia in 36 patients (18.6%) (Figure 2).
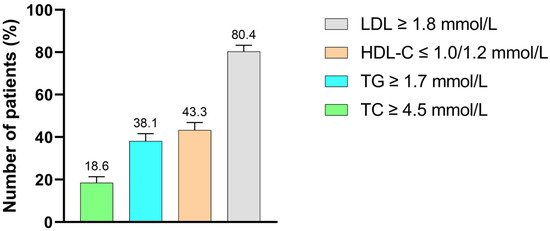
Figure 2.
Distribution of lipid abnormalities among T2DM study participants with dyslipidemia. TC: total cholesterol, TG: triglycerides, HDL-C: high-density lipoprotein cholesterol, LDL-C: low-density lipoprotein cholesterol.
3.3. Distribution of Lipid Abnormalities Among Study Participants, Stratified According to Gender, Age Differences and Duration of Diabetes Mellitus
Out of the 176 study participants with lipid abnormalities, the majority were female (n = 124; 70.5%) (Table 2). When stratified according to gender, the prevalence of lipid abnormalities varied across the study participants, with no statistical significance. Although there were no statistical differences, hypercholesterolemia was observed to be higher in females (24.2%) compared to males (11.5%). Females had a higher prevalence of both hypertriglyceridemia (46.7%) and reduced HDL-C (51.6%) compared to males. Elevated LDL-C was highly prevalent in both females (90.3%) and males (84.6%).

Table 2.
Distribution of lipid abnormalities according to gender among type 2 diabetic patients.
The prevalence of lipid abnormalities was stratified according to two age groups, and data were presented in Table 3. Hypercholesterolemia was more common among patients aged ≥ 50 years (22.2%) compared to those aged < 50 years (16%); the difference was not statistically significant (p = 0.413). In contrast, reduced HDL-C levels displayed a strong age-related pattern, with the highest prevalence in the ≥50 years group (55.5%), p = 0.001. Notably, elevated LDL-C was highly prevalent in the <50 years group, with a prevalence of 100% compared to the ≥50 years group (p = 0.001).

Table 3.
Age-stratification analysis of lipid abnormalities among type 2 diabetic mellitus patients.
The prevalence of lipid abnormalities was also stratified based on the duration of diabetes, categorized as ≤10 years and >10 years (Table 4). Study participants with a diabetes duration of >10 years had a significantly higher prevalence of hypercholesterolemia compared to those with a diabetes duration of ≤10 years, p < 0.001. Similarly, the prevalence of low HDL-C was significantly higher in participants with diabetes duration of >10 years (59.5%) than in those with diabetes duration of ≤10 years (37.0%), p = 0.004. Notably, elevated LDL-C was significantly higher in participants with a diabetes duration of ≤10 years compared to those with a diabetes duration of >10 years, p = 0.003.

Table 4.
Distribution of lipid abnormalities by duration of diabetes among type 2 diabetic mellitus patients.
3.4. Distribution of Dyslipidemia Phenotypes Among the Study Participants, Stratified by Gender
Table 5 displays the distribution of dyslipidemia phenotypes among the 176 study participants, stratified by gender. The most prevalent isolated dyslipidemia was elevated LDL-C, observed in 60 patients (34.1%), followed by reduced HDL-C, observed in 12 participants (6.8%). Notably, no participant had hypertriglyceridemia in isolation. Isolated dyslipidemia phenotypes showed a significant gender difference in reduced HDL-C levels, which were more prevalent among males (15.38%) as compared to females (3.2%), with a statistically significant p-value of 0.0066. The co-occurrence of hypertriglyceridemia and elevated LDL-C was the most prevalent combined dyslipidemia phenotype, observed in 32 participants (18.2%), with no significant gender difference (females: 17.7%, males: 19.23%, p = 0.832), while 30 participants (17.0%) had reduced HDL-C and elevated LDL-C co-occurrence. A smaller subset of 8 participants (4.5%) had hypertriglyceridemia and reduced HDL-C. Notably, a smaller subset of 8 participants (4.5%) had hypertriglyceridemia and reduced HDL-C co-occurrence, and this was observed only in females. (6.45%) and not in males.

Table 5.
Distribution of diabetic dyslipidemia phenotypes among the study participants, stratified by gender.
A significant proportion of study participants exhibited atherogenic dyslipidemia phenotype, with thirty-four patients (19.3%) displaying all three lipid abnormalities. The atherogenic dyslipidemia phenotype was observed more in female participants (22.6%) compared to male participants, with a p-value of 0.099. In this analysis, 104 out of the 176 participants with dyslipidemia had two or more overlapping lipid abnormalities.
3.5. Fisher’s Exact Test Analysis of Factors Associated with Elevated Levels of LDL-C Among the Study Participants
Fisher’s exact test was performed to identify factors associated with elevated levels of LDL-C (≥1.8 mmol/L) among the 194 study participants. Age, NAT, and duration of diabetes were significantly associated with the likelihood of elevated levels of LDL-C (≥1.8 mmol/L). In contrast, gender, smoking, alcohol consumption, family history of CVD, physical inactivity, poor glycemic control, and nitric oxide were not significantly associated with elevated LDL-C (Table 6).

Table 6.
Fisher’s exact test analysis of factors associated with elevated levels of LDL-C among the study participants.
3.6. Binary and Multivariate Logistic Regression Analysis of Risk Factors Associated with Elevated Levels of LDL-C Among Study Participants
In the multivariate logistic regression model, treatment non-adherence remained significantly associated with elevated LDL-C levels (AOR = 4.596, 95% CI: 1.19–17.71; p = 0.027). Participants who did not adhere to their treatment had more than four times the odds of elevated LDL-C compared to those who adhered. Although individuals older than 50 years showed lower odds of elevated LDL-C (AOR = 0.265, 95% CI: 0.05–1.35), this was not statistically significant (p = 0.110). Similarly, having diabetes for more than 10 years was not significantly associated with elevated LDL-C levels (AOR = 0.61, 95% CI: 0.19–1.96; p = 0.411) (Table 7).

Table 7.
Bivariate and multivariate analysis of factors associated with elevated levels of LDL-C among study participants.
4. Discussion
Dyslipidemia has emerged as a major global public health concern in developing countries, with its prevalence steadily rising due to economic growth and evolving lifestyle choices [12]. Dyslipidemia is prevalent in individuals with T2DM, and it contributes to the development of ACVD, with elevated LDL-C playing a major role [13]. Despite the global awareness of T2DM and its related complications, comprehensive data on the magnitude and patterns of dyslipidemia, particularly elevated LDL-C and its associated factors among T2DM patients in South Africa, remain limited. This study assessed the magnitude of dyslipidemia and factors associated with elevated levels of LDL-C among T2DM Black South Africans in a diabetic clinic at a tertiary hospital.
The overall prevalence of dyslipidemia in our study was 90.72%, with males (96%) showing higher rates of dyslipidemia compared to females (89%). Our findings were consistent with previous findings from the Western Cape province, South Africa, where they reported a significant prevalence of 89% dyslipidemia [14]. Similar findings were reported in studies conducted in Bangladesh, where Kamrul-Hasan et al. [15] observed a prevalence of 96.83%, in Jordan with Al Quran et al. [16] and Hyassat et al. [17] reporting 91.4% and 95.4%, respectively, in Bangalore, where Kolhar & P. [18] found 90%.
In contrast, the observed prevalence is much higher than that reported in studies conducted in Ethiopia and Nigeria, which reported a prevalence of 61.3% and 69.3% [19,20]. A study by Katundu et al. also found a lower prevalence of 58% in Malawians [5]. The observed disparity in the prevalence of dyslipidemia across studies may reflect heterogeneity in population characteristics, differences in study design, and settings. Additionally, the differences in prevalence may be attributed to the rising trend of urbanization and associated increased sedentary behavior, unhealthy dietary habits, and lifestyle factors that heighten the susceptibility of populations to lipid abnormalities. Moreover, the pathophysiology of dyslipidemia in T2DM patients is multifactorial, predominantly characterized by insulin resistance, which promotes adipocyte lipolysis and increased free fatty acid flux to the liver. This, in turn, stimulates the hepatic overproduction of TG-rich lipoproteins. Furthermore, impaired activity of endothelial-bound lipoprotein lipase hinders lipoprotein clearance, resulting in elevated levels of LDL-C and other lipid abnormalities [21,22].
Dyslipidemia is often characterized by phenotypic patterns involving single, combined, or mixed lipid abnormalities. In this study, we observed that elevated LDL-C was the most prevalent lipid abnormality, followed by reduced HDL-C, hypertriglyceridemia, and hypercholesterolemia, respectively. In our study, the frequencies of isolated, combined, and atherogenic dyslipidemia were 40.9%, 39.7%, and 19.3%, respectively (Table 5). These findings are similar to a study performed by Diawara et al. in a rural community in Ganadougou, Mali [13], as well as a study performed in a tertiary hospital in central South Africa by Pitso et al. [23]. Elevated LDL-C was the most prevalent isolated dyslipidemia, indicating that elevated LDL-C remains a predominant and clinically significant lipid abnormality within the current cohort. The isolated elevated LDL-C in our study may be due to chronic hyperglycemia, as evidenced by the high HbA1c concentration in our population and obesity. In a study done by Pitso et al., conducted at a tertiary hospital in central South Africa, a high prevalence of dyslipidemia in T2DM patients was reported despite being on lipid-lowering therapy, and two-thirds of the study participants were obese [23]. This highlights that obesity is strongly associated with dyslipidemia. Additionally, most of the study participants (60%) were not physically active, and 46% also reported being NAT. The current study setting caters to low-socioeconomic populations and many with low-education levels, which may also affect the understanding of the risk factors associated with diabetes mellitus as well as dyslipidemia. There is also minimal education on diabetes mellitus and dyslipidemia from the healthcare providers, which may also contribute to poor control of both diabetes mellitus and dyslipidemia.
Among study participants with isolated dyslipidemia, 6.8% exhibited reduced HDL-C, with a significant gender disparity (p = 0.0066). This finding aligns with a Nepalese study conducted at Hetauda Hospital, which reported a higher number of males with reduced HDL-C compared to females [24]. In contrast, a study conducted in Chongqing, China, observed that the prevalence of isolated low HDL-C was significantly higher in women than in men [25]. Interestingly, no study participant displayed hypertriglyceridemia in isolation, which warrants further investigation. Hypertriglyceridemia, together with elevated LDL-C, was the most prevalent combined dyslipidemia observed in our study. A similar observation was reported in a study conducted by Pitso et al. in South Africa, where the co-occurrence of hypertriglyceridemia with elevated LDL-C was the most prevalent combined dyslipidemia [23]. The co-occurrence of hypertriglyceridemia and reduced HDL-C only in female participants was unexpected, and this observation warrants further investigation. The prevalence of atherogenic dyslipidemia, characterized by the co-occurrence of the three lipid abnormalities, namely hypertriglyceridemia, reduced HDL-C, and elevated LDL-C, was found in 19.3% of the study participants. This observation was comparable to the findings of the research study conducted in South Africa that evaluated T2DM patients [23].
In the bivariate analysis, age, NAT, and duration of diabetes were found to be associated with elevated levels of LDL-C among T2DM patients. These findings were not unexpected, considering all these factors are known risk factors for dyslipidemia [26,27,28]. Multivariate logistic regression analysis was conducted to identify independent associated factors with elevated levels of LDL-C. NAT (adjusted odds ratio [AOR]: 4.596; 95% CI: 1.19–17.71; p = 0.027) was found to be associated with elevated levels of LDL-C. Consistent with the findings of our study, Chantzaras and Yfantopoulos [29], reported that NAT was associated with dyslipidemia, particularly elevated levels of LDL-C. The study further highlighted that factors associated with the likelihood of NAT were low-level education, shorter consultations, poor general health, self-perceived inadequacy of knowledge, presence of comorbidities, and negative views of medication. However, it is important to note that this is the first study to report on factors associated with elevated levels of LDL-C among black South Africans with T2DM.
Several limitations should be taken into consideration when interpreting the results of the study. Firstly, the study design was a cross-sectional study, and therefore, the cause-and-effect relationship could not be established in the study. Secondly, the sample size was small; therefore, large-scale epidemiological studies are needed to determine the overall prevalence and associated factors of dyslipidemia in the current cohort in South Africa. Thirdly, this study might be subject to self-reporting bias due to reliance on questionnaire-based responses. Finally, patients were recruited from a single hospital, rather than being a community-based sample. Thus, the findings could not be generalized beyond these study samples. However, despite these limitations, the findings of the current study offer insight into the magnitude of dyslipidemia and potential associated factors with elevated LDL-C among Black South Africans with T2DM.
5. Conclusions
Our study found that dyslipidemia is highly prevalent among Black South African patients with T2DM in our setting despite the use of lipid-lowering therapy. NAT was significantly associated with elevated levels of LDL-C. To address these issues effectively, contributing factors such as inadequate patient knowledge, negative perceptions toward treatment or medication, and insufficient healthcare access must be steadily targeted through comprehensive educational programs and improved communication between the patient and the healthcare provider to reduce the prevalence of dyslipidemia. There is also a need for collaborative effort to ensure better patient management and education. Moreover, studies should investigate the reasons behind NAT to develop personalized inventions. Improving medication adherence can significantly impact the lipid profile and reduce the risk of ACVD in T2DM patients. However, the findings of the current study should be interpreted with caution due to the nature of the study. Thus, we recommend multicenter and longitudinal studies with larger cohorts to support the findings of our study and better inform public health interventions.
Author Contributions
Conceptualization, M.N., S.M. and O.H.M.; methodology, M.N. and T.J.M.; software, M.N. and S.M.; validation, O.H.M., S.S.G. and N.S.B.; formal analysis, M.N., A.K.M. and S.M.; investigation, M.N., S.M. and T.J.M.; resources, S.S.G.; data curation, M.N. and S.M.; writing—original draft preparation, M.N.; writing—review and editing, O.H.M., S.S.G., T.J.M. and N.S.B.; visualization, A.K.M.; supervision, O.H.M., S.S.G. and N.S.B.; project administration, S.M. and M.N.; funding acquisition, S.S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the guidelines of the research and ethics committee of the Sefako Makgatho Health Sciences University (SMUREC/S/346/2021:PG, 1 November 2021).
Informed Consent Statement
Informed consent was obtained from all study participants involved in the study. Participants were assured that their responses would remain confidential to ensure compliance with the POPI Act.
Data Availability Statement
The data supporting the findings of this study are not publicly accessible but can be obtained upon reasonable request from the corresponding author.
Acknowledgments
We acknowledge the contributions of the nursing and medical personnel, as well as the phlebotomists at the outpatient diabetes clinic of Dr George Mukhari Academic Hospital. We are grateful for the research funding received from Sefako Makgatho Health Sciences University. We also acknowledge support from the South African Department of Science and Innovation and the International Centre for Genetic Engineering and Biotechnology, New Delhi, India.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| T2DM | Type 2 diabetes mellitus |
| ACVD | Atherosclerotic Cardiovascular Disease |
| Elevated TC | Hypercholesterolemia |
| Elevated TG | Hypertriglyceridemia |
| HDL-C | High-Density Lipoprotein Cholesterol |
| LDL-C | Low-Density Lipoproteins Cholesterol |
| HbA1c | Glycated Hemoglobin |
| DGMAH | Dr George Mukhari Academic Hospital |
| SPSS | Statistical Package for Social Sciences |
| COR | Crude Odd Ratio |
| AOR | Adjusted Odd Ratio |
| NAT | Non-Adherence to Treatment |
| CI | Confidence Interval |
References
- Goedecke, J.H.; Olsson, T. Pathogenesis of type 2 diabetes risk in black Africans: A South African perspective. J. Intern. Med. 2020, 288, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2025. [Google Scholar]
- Chikwati, R.P.; Crowther, N.J.; Ramsay, M.; Micklesfield, L.K.; Norris, S.A.; Seakamela, K.P.; Nonterah, E.A.; Agongo, G.; Mohamed, S.F.; Kisiangani, I.; et al. Incident type 2 diabetes and its risk factors in men and women aged 40–60 years from four sub-Saharan African countries: Results from the AWI-Gen study. Lancet Glob. Health 2025, 13, e459–e466. [Google Scholar] [CrossRef]
- Katundu, K.G.H.; Mukhula, V.; Phiri, T.; Phiri, C.; Filisa-Kaphamtengo, F.; Chipewa, P.; Chirambo, G.; Mipando, M.; Mwandumba, H.C.; Muula, A.S.; et al. High prevalence of dyslipidaemia among persons with diabetes mellitus and hypertension at a tertiary hospital in Blantyre, Malawi. BMC Cardiovasc. Disord. 2022, 22, 557. [Google Scholar] [CrossRef]
- Jellinger, P.S.; Handelsman, Y.; Rosenblit, P.D.; Bloomgarden, Z.T.; Fonseca, V.A.; Garber, A.J.; Grunberger, G.; Guerin, C.K.; Bell, D.S.H.; Mechanick, J.I.; et al. American Association of Clinical Endocrinologists and American College of Endocrinology Guidelines for Management of Dyslipidemia and Prevention of Cardiovascular Disease. Endocr. Pract. 2017, 23, 1–87. [Google Scholar] [CrossRef]
- Mooradian, A.D. Dyslipidemia in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2009, 5, 150–159. [Google Scholar] [CrossRef]
- Hirano, T. Pathophysiology of Diabetic Dyslipidemia. J. Atheroscler. Thromb. 2018, 25, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Daya, R.; Bayat, Z.; Raal, F. Prevalence and pattern of dyslipidaemia in type 2 diabetes mellitus patients at a tertiary care hospital. J. Endocrinol. Metab. Diabetes S. Afr. 2017, 22, 31–35. [Google Scholar] [CrossRef]
- Vezi, Z.B.; Naidoo, D.P. Dyslipidemia among Black patients with Type 2 Diabetes. Cardiovasc. J. S. Afr. 2005, 16, 194–198. [Google Scholar]
- Hermans, M.P.; Sacks, F.M.; Ahn, S.A.; Rousseau, M.F. Non-HDL-cholesterol as valid surrogate to apolipoprotein B100 measurement in diabetes: Discriminant Ratio and unbiased equivalence. Cardiovasc. Diabetol. 2011, 10, 20. [Google Scholar] [CrossRef]
- Obsa, M.S.; Ataro, G.; Awoke, N.; Jemal, B.; Tilahun, T.; Ayalew, N.; Woldegeorgis, B.Z.; Azeze, G.A.; Haji, Y. Determinants of Dyslipidemia in Africa: A Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2022, 8, 778891. [Google Scholar] [CrossRef]
- Diawara, A.; Coulibaly, D.M.; Kone, D.; Traore, M.A.; Konaté, D.; Bazi, D.S.; Kassogue, O.; Sylla, D.; Fofana, F.G.; Diabaté, O.; et al. Dyslipidemia in Adults with Type 2 Diabetes in a Rural Community in Ganadougou, Mali: A Cross-Sectional Study. J. Diabetes Mellit. 2024, 14, 133–152. [Google Scholar] [CrossRef]
- Omodanisi, E.I.; Tomose, Y.; Okeleye, B.I.; Ntwampe, S.K.O.; Aboua, Y.G. Prevalence of Dyslipidaemia among Type 2 Diabetes Mellitus Patients in the Western Cape, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 8735. [Google Scholar] [CrossRef]
- Kamrul-Hasan, A.B.M.; Alam, M.S.; Zarin, N.; Kabir, M.A.; Gaffar, A.J.; Hossain, M.F.; Talukder, S.K.; Bin Raunak, A.I.; Nabi, M.M.U.; Asaduzzaman, M.; et al. Prevalence and patterns of dyslipidemia among lipid-lowering drug-naïve patients with type 2 diabetes mellitus—A countrywide study in Bangladesh. Endocr. Metab. Sci. 2023, 13, 100152. [Google Scholar] [CrossRef]
- Al Quran, T.M.; Bataineh, Z.A.; Al-Mistarehi, A.-H.; Zein Alaabdin, A.M.; Allan, H.; Al Qura’an, A.; Weshah, S.M.; Alanazi, A.A.; Khader, Y.S. Prevalence and Pattern of Dyslipidemia and Its Associated Factors Among Patients with Type 2 Diabetes Mellitus in Jordan: A Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 7669–7683. [Google Scholar] [CrossRef] [PubMed]
- Hyassat, D.; Al-Saeksaek, S.; Naji, D.; Mahasneh, A.; Khader, Y.; Abujbara, M.; El-Khateeb, M.; Ajlouni, K. Dyslipidemia among patients with type 2 diabetes in Jordan: Prevalence, pattern, and associated factors. Front. Public Health 2022, 10, 1002466. [Google Scholar] [CrossRef]
- Kolhar, U.; P, P. Study of serum lipid profile in type 2 diabetes mellitus patients and its association with diabetic nephropathy. Int. J. Adv. Med. 2017, 4, 1513. [Google Scholar] [CrossRef]
- Addis, Z.; Nega, A.T.; Tebeje, R.D.; Asmare, E.; Tegegnie, A.B.; Tamir, W.; Alene, T. Dyslipidemia and associated factors among adult type two diabetes mellitus patients in Felege Hiywot Refral, Hospital, Bahir Dar, Ethiopia, 2023. Front. Cardiovasc. Med. 2024, 11, 1493447. [Google Scholar] [CrossRef] [PubMed]
- Bello-Ovosi, B.O.; Ovosi, J.O.; Ogunsina, M.A.; Asuke, S.; Ibrahim, M.S. Prevalence and pattern of dyslipidemia in patients with type 2 diabetes mellitus in Zaria, Northwestern Nigeria. Pan Afr. Med. J. 2019, 34, 123. [Google Scholar] [CrossRef]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef] [PubMed]
- Pitso, L.; Mofokeng, T.R.P.; Nel, R. Dyslipidaemia pattern and prevalence among type 2 diabetes mellitus patients on lipid-lowering therapy at a tertiary hospital in central South Africa. BMC Endocr. Disord. 2021, 21, 159. [Google Scholar] [CrossRef] [PubMed]
- Pokharel, D.R.; Khadka, D.; Sigdel, M.; Yadav, N.K.; Acharya, S.; Kafle, R.; Sapkota, R.M.; Sigdel, T. Prevalence and pattern of dyslipidemia in Nepalese individuals with type 2 diabetes. BMC Res. Notes 2017, 10, 146. [Google Scholar] [CrossRef]
- Qi, L.; Ding, X.; Tang, W.; Li, Q.; Mao, D.; Wang, Y. Prevalence and Risk Factors Associated with Dyslipidemia in Chongqing, China. Int. J. Environ. Res. Public Health 2015, 12, 13455–13465. [Google Scholar] [CrossRef]
- Osterberg, L.; Blaschke, T. Adherence to Medication. N. Engl. J. Med. 2005, 353, 487–497. [Google Scholar] [CrossRef]
- Mashele, T.S.; Mogale, M.A.; Towobola, O.A.; Moshesh, M.F. Central obesity is an independent risk factor of poor glycaemic control at Dr George Mukhari Academic Hospital. S. Afr. Fam. Pract. 2019, 61, 18–23. [Google Scholar] [CrossRef]
- Ahmmed, S.; Shuvo, S.D.; Paul, D.K.; Karim, M.R.; Kamruzzaman, M.; Mahmud, N.; Ferdaus, M.J.; Elahi, M.T. Prevalence of dyslipidemia and associated risk factors among newly diagnosed Type-2 Diabetes Mellitus (T2DM) patients in Kushtia, Bangladesh. PLoS Glob. Public Health 2021, 1, e0000003. [Google Scholar] [CrossRef]
- Chantzaras, A.; Yfantopoulos, J. Determinants of medication adherence in patients with diabetes, hypertension, and hyperlipidemia. Hormones 2025, 24, 443–459. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).