Abstract
Circadian rhythms are intrinsic 24 h cycles that regulate metabolic processes across multiple tissues, with skeletal muscle emerging as a central node in this temporal network. Muscle clocks govern gene expression, fuel utilisation, mitochondrial function, and insulin sensitivity, thereby maintaining systemic energy homeostasis. However, circadian misalignment, whether due to behavioural disruption, nutrient excess, or metabolic disease, impairs these rhythms and contributes to insulin resistance, and the development of obesity, and type 2 diabetes mellitus. Notably, the muscle clock remains responsive to non-photic cues, particularly exercise, which can reset and amplify circadian rhythms even in metabolically impaired states. This work synthesises multi-level evidence from rodent models, human trials, and in vitro studies to elucidate the role of skeletal muscle clocks in circadian metabolic health. It explores how exercise entrains the muscle clock via molecular pathways involving AMPK, SIRT1, and PGC-1α, and highlights the time-of-day dependency of these effects. Emerging data demonstrate that optimally timed exercise enhances glucose uptake, mitochondrial biogenesis, and circadian gene expression more effectively than time-agnostic training, especially in individuals with metabolic dysfunction. Finally, findings are integrated from multi-omic approaches that have uncovered dynamic, time-dependent molecular signatures that underpin circadian regulation and its disruption in obesity. These technologies are uncovering biomarkers and signalling nodes that may inform personalised, temporally targeted interventions. By combining mechanistic insights with translational implications, this review positions skeletal muscle clocks as both regulators and therapeutic targets in metabolic disease. It offers a conceptual framework for chrono-exercise strategies and highlights the promise of multi-omics in developing precision chrono-medicine approaches aimed at restoring circadian alignment and improving metabolic health outcomes.
1. Introduction
Circadian rhythms are endogenous ~24 h cycles that regulate a wide array of physiological and behavioural processes, including sleep–wake timing, feeding behaviour, hormone secretion, and energy metabolism [1]. These rhythms are orchestrated by a hierarchical system of clocks, with the suprachiasmatic nucleus (SCN) in the hypothalamus acting as the master pacemaker. The SCN synchronises peripheral clocks located in nearly every tissue, including skeletal muscle, through neural and hormonal signals [2]. While the SCN is entrained primarily by light, peripheral oscillators are also responsive to non-photic cues such as feeding schedules, temperature fluctuations, and physical activity [3], Figure 1.
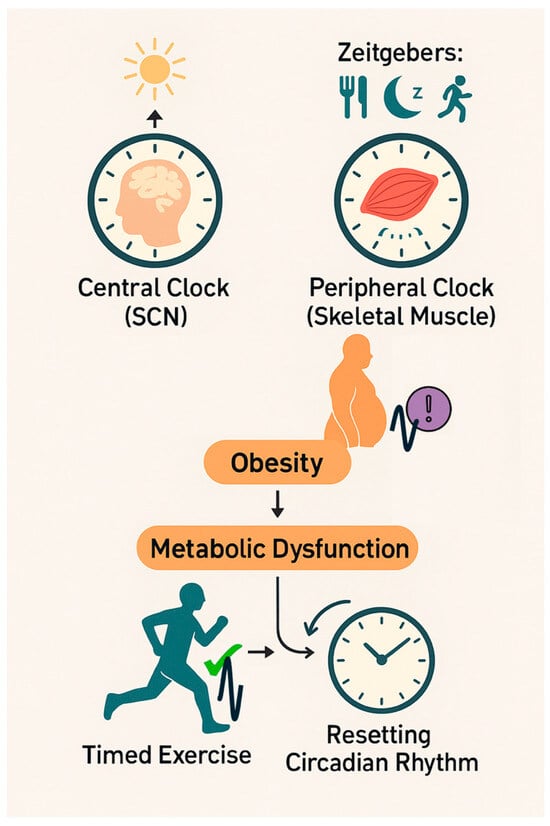
Figure 1.
The role of timed exercise in circadian reprogramming and metabolic health. This schematic highlights the potential of chronotherapeutic exercise interventions as a strategy to counteract circadian misalignment and mitigate obesity-related metabolic disorders, illustrating the interaction between the central and peripheral clocks in the regulation of metabolic homeostasis. The central clock located in the suprachiasmatic nucleus (SCN) is primarily entrained by light, whereas peripheral clocks, particularly in skeletal muscle, are responsive to non-photic zeitgebers such as feeding patterns, sleep–wake cycles, and exercise. In obesity and type 2 diabetes mellitus (T2DM), skeletal muscle clocks can become misaligned, leading to disrupted rhythmic gene expression, metabolic inflexibility, and systemic dysfunction. However, timed exercise can promote metabolic health by re-aligning muscle clocks to restore circadian rhythmicity.
Skeletal muscle accounts for ~40% of body mass and serves as a key site of glucose uptake, lipid oxidation, and thermogenesis, making it a pivotal tissue in metabolic homeostasis [4]. Importantly, skeletal muscle exhibits robust circadian rhythms in gene expression, mitochondrial activity, substrate utilisation, and insulin sensitivity [5,6]. These oscillations coordinate metabolic processes with behavioural cycles such as feeding and exercise, ensuring temporal synchrony between internal physiology and external environmental demands. The muscle clock consists of a transcriptional–translational feedback loop involving core clock genes such as BMAL1, CLOCK, PERs, and CRYs, which regulate downstream clock-controlled genes that govern local and systemic metabolism [7].
Disruption or misalignment of these intrinsic muscle clocks can lead to impaired substrate metabolism, altered gene expression, and metabolic dysfunction [8,9]. For instance, skeletal muscle-specific Bmal1 knockout in mice displays glucose intolerance, reduced insulin-stimulated glucose uptake, and defective mitochondrial respiration [10]. In humans, individuals with obesity or type 2 diabetes (T2DM) exhibit blunted circadian gene expression in skeletal muscle, including reduced rhythmicity of core clock factors [11,12].
Chrono-disruption is increasingly recognised as a contributing factor in the pathogenesis of obesity and T2DM [13,14], and temporal misalignment between behavioural cycles and endogenous clocks has been shown to impair glucose tolerance and rewire metabolic gene networks [15]. For example, forced circadian misalignment in healthy adults reduces skeletal muscle insulin sensitivity and alters core clock gene expression [13]. These effects are exacerbated by nutrient excess, particularly saturated fatty acid exposure, which further blunts circadian amplitude and impairs metabolic flexibility, which can lead to obesity or metabolic disorders [16].
However, skeletal muscle clocks remain remarkably responsive to physiologically relevant stimuli. Exercise, in particular, has emerged as a potent non-photic zeitgeber capable of resetting and amplifying muscle circadian rhythms [17]. Preclinical and clinical evidence demonstrate that both acute and chronic exercise can re-establish rhythmicity in metabolic gene expression, enhance mitochondrial function, and improve insulin sensitivity, even in metabolically impaired states [18,19]. These effects appear to be highly time-of-day dependent, with late-active phase training producing superior outcomes in both rodents and humans [20,21].
This review provides a timely synthesis of emerging evidence linking skeletal muscle circadian rhythms to metabolic health, with a particular emphasis on how exercise serves as a potent zeitgeber capable of restoring rhythmicity in metabolically compromised states such as obesity and T2DM. By integrating data from rodent models, human trials, and in vitro studies, it explores the mechanisms by which muscle clocks influence systemic metabolism and highlights the time-of-day specificity of exercise interventions. Critically, it outlines how cutting-edge multi-omic approaches have begun to unravel the temporal complexity of circadian–metabolic interactions. Structured into five core sections, this work progresses from the fundamental role of skeletal muscle in circadian regulation, through the consequences of misalignment, to therapeutic avenues such as exercise and omics-driven discovery. By the end, readers will be equipped with a comprehensive understanding of current research and insight to inform future investigations or develop chronotherapeutic strategies aimed at restoring and improving metabolic health.
2. Skeletal Muscle Is a Central Regulator of Circadian Metabolic Health
Skeletal muscle comprises almost 40% of body mass in healthy adults and is a primary site for glucose disposal, fatty acid oxidation, and circadian entrainment [6,22], positioning it as an important tissue for metabolic regulation. Skeletal muscle exhibits robust circadian rhythms across multiple domains, including gene expression, mitochondrial function, substrate metabolism, and contractile activity [23,24]. These rhythms are essential for aligning metabolic processes with behavioural cycles such as feeding and activity [25,26], thereby ensuring energetic efficiency and homeostasis. The intrinsic molecular clock in muscle, reviewed in detail in [1], is a transcriptional–translational feedback loop comprising core oscillators, such as BMAL1, CLOCK, PERs, and CRYs, which in turn drive tissue-specific rhythmic transcription of clock-controlled genes. The coordination of these rhythms and their associated specific output is what contributes to optimal muscle function and systemic metabolic homeostasis.
In healthy physiology, circadian gene expression in skeletal muscle orchestrates time-of-day-specific variation in fuel utilisation, insulin sensitivity, and mitochondrial efficiency [27,28]. High-resolution transcriptomic studies have revealed that approximately 2300 genes in skeletal muscle cycle diurnally in both rodents and humans, many of which are directly implicated in pathways governing insulin signalling, mitochondrial respiration, lipid metabolism, and protein turnover [5,8]. For example, Ppargc1a, Glut4, and Cpt1b demonstrate circadian variation in expression, with peaks generally coinciding with the active phase of the diurnal cycle [5]. These molecular oscillations not only affect muscle function locally but are also synchronised with systemic rhythms in nutrient availability, hormonal signalling, and energy expenditure [6,24].
Functional disruption of the muscle clock, as demonstrated in skeletal muscle-specific Bmal1 knockout mice, leads to profound impairments in metabolic regulation. These animals fail to anticipate daily cycles of activity, exhibit glucose intolerance, and show mislocalisation of GLUT4, impairing insulin-stimulated glucose uptake [9]. Mice lacking Bmal1 specifically in skeletal muscle also exhibit mitochondrial dysfunction, increased oxidative stress, and disrupted amino acid and lipid metabolism [10]. Moreover, the muscle-specific Bmal1 knockout impairs the rhythmic expression of genes involved in calcium handling and sarcomeric integrity, potentially linking circadian disruption to reduced fatigue resistance and altered excitation–contraction coupling [29]. Collectively, these data underscore the importance of circadian regulation in muscle physiology and highlight its critical role in maintaining metabolic health.
In the context of obesity and metabolic disorders, muscle clocks are often dampened or phase-shifted, which may contribute to the systemic metabolic inflexibility observed in these conditions. Human studies show that individuals with obesity or insulin resistance exhibit reduced rhythmicity and amplitude of muscle clock gene expression, particularly PER2, NR1D1 (Rev-erbα), and BMAL1, alongside diminished temporal variation in mitochondrial oxidative capacity and glucose oxidation [7]. For example, forced circadian misalignment protocols in healthy volunteers reveal that desynchronisation between behavioural cycles and internal muscle clocks acutely impairs insulin-stimulated glucose uptake and reduces phosphocreatine recovery following exertion, highlighting the sensitivity of muscle energetics to temporal regulation [30,31].
Recent integrative approaches have identified key clock-regulated metabolic nodes in muscle, including NAD+/SIRT1 signalling, mTORC1 activity, and AMPK responsiveness, that oscillate across the circadian cycle and modulate nutrient sensing and energy metabolism [23,24,32]. These pathways serve as molecular links between circadian control and cellular bioenergetics, and their disruption may partially underlie the metabolic dysfunction seen in circadian misalignment and obesity. For example, SIRT1-mediated deacetylation of BMAL1 and PGC1α integrates metabolic stress with clock regulation, allowing circadian systems to adapt to cellular energy status. Whereas, in obese states, where NAD+ availability is diminished and SIRT1 activity impaired, this axis may become uncoupled, resulting in chrono-disruption and metabolic inflexibility [33,34].
Importantly, skeletal muscle circadian clocks are not isolated. They operate within an integrated temporal network involving endocrine signals (e.g., cortisol, insulin), nutrient flux, and neural cues [35]. While the central clock in the SCN primarily governs behavioural rhythms, the muscle clock responds predominantly to feeding–fasting cycles and activity-induced contraction [36,37]. This decentralised regulation allows muscle to anticipate metabolic demands and synchronise tissue-level metabolism with ongoing environmental conditions.
There is now emerging evidence that skeletal muscle clocks may influence other tissues through myokine secretion and metabolic cross-talk. Although the mechanisms are incompletely understood, studies suggest that muscle-specific clock disruption can lead to altered gene expression in liver and adipose tissue, indicating a systemic effect of muscle circadian misalignment [38]. This reinforces the concept that skeletal muscle acts not just as a passive responder to circadian signals but as a central node in the body’s temporal metabolic network.
Together, this work positions skeletal muscle as a core regulator of circadian metabolic health. Its intrinsic clock coordinates local energy metabolism and systemic homeostasis. As such, maintaining robust circadian rhythms in skeletal muscle represents a potential strategy to help mitigate metabolic disease risk. However, the dysfunction of the intrinsic clock mechanism in skeletal muscle contributes significantly to the pathophysiology of obesity, insulin resistance, and circadian misalignment. A deeper understanding of the molecular interactions between clock components and metabolic signalling pathways in muscle is essential for understanding the impact of such a relationship and for the development of effective chronotherapeutic interventions in obesity and metabolic disorders like type 2 diabetes. Circadian disruption has also been implicated in the development of sarcopenia, with evidence showing that core clock components regulate muscle protein turnover and mitochondrial maintenance. Age-related dampening of circadian rhythms in skeletal muscle may therefore contribute to anabolic resistance and metabolic decline associated with sarcopenia [39].
3. Circadian Misalignment Contributes to Obesity and Metabolic Disorders
Circadian misalignment, characterised by the desynchronisation of behavioural rhythms from endogenous clocks, has emerged as a critical contributor to the pathogenesis of metabolic disorders, particularly obesity and type 2 diabetes mellitus (T2DM). Skeletal muscle, which accounts for approximately 80% of postprandial glucose uptake [40], and a large portion of whole-body energy expenditure, plays a central role in this process due to its intrinsic circadian clock system. Disruption of this system impairs temporal regulation of substrate metabolism, reduces insulin sensitivity, and reprograms metabolic gene networks. Chrono-disruption also substantially reshapes mitochondrial dynamics [41]. Thus, obese individuals exhibit blunted mitochondrial fusion–fission cycles and dampened respiratory oscillations across 24 h, whereas lean counterparts maintain rhythmic mitochondrial network integrity that aligns with respiratory capacity peaks [42].
Experimental models of circadian disruption have further demonstrated that peripheral clocks such as those in skeletal muscle are sensitive to misaligned behavioural cues, including altered feeding and sleep–wake cycles. A human study involving 14 healthy men subjected to a 3.5-day simulated night-shift protocol demonstrated that insulin sensitivity was reduced by 23% (non-oxidative glucose disposal: 23.7 vs. 18.4 mg/kg/min, p = 0.024), while fasting glucose and free fatty acids were elevated. Skeletal muscle biopsies revealed significant transcriptomic reprogramming, notably upregulation of genes related to PPAR signalling and fatty acid metabolism, as well as altered expression of clock genes such as PER2, CRY1, and BMAL1 [13]. This implies a temporal uncoupling between tissue molecular clocks and metabolic pathways, leading to substrate competition and impaired glucose handling at the cellular level.
There is evidence to show that at the systemic level, misaligned feeding and sleeping schedules perturb hormone regulation [43]. In rodent studies, a high-fat diet administered during resting/light periods has been shown to lead to exaggerated weight gain compared to identical feeding patterns in the active/dark period [44]. Similar circadian disruptions attenuate leptin-mediated satiety signalling and elevate ghrelin levels, which have been shown to promote overeating [43]. Interestingly, genetic chrono-disruption, for example, Clock and Per2 knockout in mice, has been shown to predispose the animals to obesity, exacerbated by an inability to regulate feeding rhythms [45], further linking behavioural disruption and metabolic disorder to the molecular clock.
In skeletal muscle-specific Bmal1 knockout (KO) mice, deletion of this non-redundant core clock gene results in profound metabolic derangements. These include downregulation of GLUT4, HK2, and PDH, essential for glucose uptake and utilisation, leading to reduced insulin-stimulated glucose disposal [9]. Moreover, muscle-specific Bmal1 loss reduces mitochondrial oxidative capacity and disrupts the normal rhythmic separation of carbohydrate and lipid metabolism, suggesting the muscle clock coordinates temporal fuel partitioning [46]. In complementary in vitro studies, siRNA-mediated CLOCK knockdown in human myotubes reduces insulin-stimulated glucose uptake, increases expression of fatty acid transporters like CD36, and impairs rhythmic secretion of metabolic myokines [5,15]. Highlighting the fundamental role of the intrinsic muscle clock in regulating glucose pathways and substrate metabolism to maintain insulin sensitivity.
Among these myokines, irisin has emerged as a key exercise-induced factor with potential circadian relevance. Secreted in response to PGC-1α activation during physical activity, enhances thermogenesis, and improves systemic glucose homeostasis. Beyond its metabolic effects, irisin has also been linked to neuroprotective and anti-inflammatory functions, positioning it as a promising mediator of exercise-induced health benefits [47]. Although its circadian regulation remains underexplored, emerging evidence suggests that its expression and secretion may follow diurnal patterns, potentially coupling muscle clock function with systematic metabolic adaptation.
Lipid overload, a hallmark of obesity and insulin resistance, exacerbates circadian dysfunction in muscle [16]. Exposure of synchronised myotubes to palmitate alters the expression of BMAL1, PER3, CRY2, and DBP, while simultaneously reducing the amplitude and period of lipid metabolite oscillations [16]. These effects are also mirrored in human skeletal muscle cells derived from patients with T2DM, where expression of key clock components such as REV-ERBα and REV-ERBβ is blunted and metabolic genes under clock control, including NAMPT and SIRT1, display dampened rhythmicity. These data suggest that diabetogenic conditions lead to a state of circadian inflexibility, impairing the muscle’s ability to adapt its metabolism to environmental cues.
Notably, metabolic impairments due to circadian misalignment are not limited to glucose pathways. Disruptions in lipid metabolism, including altered phospholipid and sphingolipid rhythmicity, have been identified in skeletal muscle under circadian disruption [48]. Approximately 20% of muscle lipids exhibit [16] diurnal oscillations, and many of these are lost following CLOCK gene knockdown. These lipid rhythms are believed to contribute to changes in membrane fluidity and receptor signalling, influencing insulin sensitivity and nutrient uptake, which are significantly disrupted with circadian clock knockout.
Circadian misalignment also alters systemic hormonal and metabolic regulation. Rodent studies show that feeding during the resting phase, rather than the active phase results in significant weight gain, hyperinsulinemia, and lipid accumulation, despite equivalent caloric intake [49]. In humans, chronic sleep deprivation and misaligned feeding schedules impair insulin sensitivity, elevate circulating lipids, and modify energy expenditure patterns [14,50]. Moreover, sleep loss selectively impairs skeletal muscle clocks without significantly affecting adipose clock components, highlighting tissue-specific vulnerability.
Collectively, these data support the notion that skeletal muscle clock dysfunction, whether from behavioural misalignment, intrinsic genetic defects, or nutrient overload, serves as a nexus for the development of metabolic disease. In this sense, circadian disruption also leads to loss of rhythmicity in mitochondrial respiration which has direct effects on fuel preference switching and glucose uptake, promoting insulin resistance and systemic metabolic inflexibility [51]. Importantly, the disruption of muscle clocks under conditions of obesity and even in T2DM appears to be reversible. A longitudinal study, took skeletal muscle biopsies from patients before and after Roux-en-Y gastric bypass and revealed partial restoration of clock gene expression post-intervention, including normalisation of BMAL1 and PER2 rhythms. Although it must be acknowledged results are varied depending on biopsy timing [52]. Nevertheless, these findings suggest that interventions targeting circadian realignment may improve skeletal muscle metabolism even in advanced metabolic disease.
Finally, there has been increasing adoption of emerging therapeutic strategies aiming at restoring circadian synchrony in tissues like skeletal muscle using non-photic time cues such as exercise and timed feeding [53]. Given the plasticity of muscle clocks and their central role in whole-body metabolic regulation, aligning skeletal muscle rhythms with behaviour may prove essential in treating and ultimately reversing the metabolic derangements associated with obesity and T2DM.
4. Exercise Can Reset Circadian Rhythms to Improve Metabolic Health
Exercise exerts powerful effects on the circadian system and skeletal muscle metabolism, functioning not only as a behavioural output of the clock but also as a non-photic zeitgeber capable of entraining peripheral oscillators [54]. Unlike the suprachiasmatic nucleus (SCN), which is primarily entrained by light, skeletal muscle clocks are particularly sensitive to contraction-induced cues such as exercise, nutrient flux, and metabolic stress. Emerging evidence now firmly supports the role of exercise in restoring and amplifying circadian rhythmicity, especially under conditions of metabolic dysfunction such as obesity and type 2 diabetes (T2DM), where intrinsic clock gene oscillations and associated metabolic outputs are disrupted. Through its capacity to reprogram gene expression, metabolism, and cellular signalling in a time-of-day-dependent manner, exercise represents a potent chronotherapeutic intervention for circadian misalignment and related metabolic disorders (Figure 2).
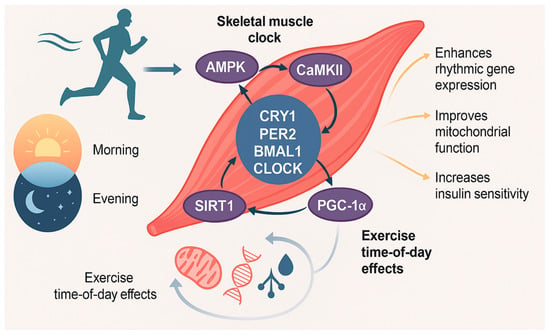
Figure 2.
Mechanisms of exercise-mediated circadian reprogramming. Some of molecular pathways through which exercise entrains the skeletal muscle circadian clock, emphasising time-of-day-dependent effects. Key signalling pathways include AMPK activation, which promotes PER2 degradation and CRY1 nuclear translocation; SIRT1 activation via NAD+, which deacetylates core clock proteins and co-activators like BMAL1 and PGC-1α; and CaMKII activation via contraction-induced calcium flux, enhancing BMAL1 transcription and mitochondrial biogenesis. These converging pathways facilitate clock reprogramming and improve metabolic gene expression. The illustration also contrasts morning versus evening exercise outcomes, highlighting enhanced mitochondrial function, insulin sensitivity, and metabolic flexibility when exercise is aligned with the active phase. Together, these mechanisms support exercise as a potent circadian zeitgeber, capable of restoring rhythmicity and improving metabolic health in disrupted or disease states.
Rodent and human studies consistently demonstrate that the metabolic and circadian effects of exercise are highly time-dependent. In mice, both acute and chronic bouts of exercise during the late active phase elicit greater improvements in exercise capacity, substrate utilisation, and gene expression than equivalent training performed earlier in the day. Ezagouri et al. [55] found that exercise capacity, glycolytic flux, and fatty acid oxidation exhibit strong diurnal variation, and this variance was abolished in Per1/2 knockout mice, indicating that the intrinsic muscle clock gates performance through temporal regulation of metabolism. Consistent with these findings, Adamovich et al. [17] demonstrated that exercise-induced improvements in running capacity, glucose tolerance, and muscle mitochondrial biogenesis are significantly greater when training is aligned with the active phase. This benefit was markedly attenuated in skeletal muscle-specific Bmal1 knockout and Clock mutant mice, underscoring the requirement of an intact circadian clock for optimal exercise adaptation. These impairments extended to systemic metabolism as well, with reduced exercise-induced transcriptional remodelling observed in the liver and adipose tissue of clock-deficient animals. Given that obesity and T2DM are associated with diminished clock amplitude and altered phase relationships in skeletal muscle, these findings suggest that time-of-day-matched exercise may be particularly effective in restoring clock-controlled metabolic pathways and enhancing therapeutic responses in metabolically compromised individuals.
Recent work has shown that the benefits of exercise on circadian and metabolic function extend beyond transcriptional outputs, encompassing chromatin-level modifications that further reinforce clock-dependent adaptations. For example, Viggars et al. [38] showed that training increases chromatin accessibility at promoters of key circadian and metabolic genes in a BMAL1-dependent manner. These epigenetic changes were associated with improved mitochondrial function, reduced ROS, and enhanced expression of metabolic genes across the circadian cycle, suggesting that exercise acts at both transcriptional and chromatin levels to remodel the circadian–metabolic interface. These muscle-specific Bmal1-deficient mice also failed to exhibit training-induced improvements in the liver and adipose tissue transcriptome, suggesting that muscle clocks may exert endocrine-like control over other peripheral tissues.
In support of in vivo findings, in vitro studies further demonstrate the plasticity of the skeletal muscle clock and its responsiveness to physiologically relevant stimuli. CLOCK knockdown in human primary myotubes impairs insulin-stimulated glucose uptake, elevates CD36 expression, and disrupts the rhythmicity of lipid metabolites, clear hallmarks of circadian-metabolic dysfunction [16]. However, these impairments can be partially reversed by AMPK activation or electrical pulse stimulation, which mimics contractile activity, reinstating elements of rhythmic gene expression and improving substrate handling [56]. These results highlight that the circadian machinery within muscle remains functionally adaptable and can be re-entrained through targeted metabolic or mechanical cues. Notably, exercise may not only reset phase and improve synchrony but also amplify circadian amplitude, an effect associated with enhanced metabolic robustness [57]. This may be particularly beneficial in conditions such as obesity and T2DM, where circadian rhythmicity is often dampened, and restoring clock amplitude could be critical to improving future metabolic outcomes.
At the molecular level, exercise has been shown to entrain the muscle clock through multiple converging pathways that link mechanical and metabolic cues to circadian regulation. AMPK activation in response to energetic stress promotes CRY1 phosphorylation and degradation, destabilises the CRY1-PER2 complex, and thereby modulates the pace of the molecular clock [58]. Concurrently, contraction-induced calcium flux activates CaMKII and PGC-1α, both of which upregulate BMAL1 transcription and stimulate mitochondrial biogenesis [59]. Another key mediator is SIRT1, a NAD+-dependent deacetylase that regulates clock protein acetylation and serves as a molecular mediator connecting redox state to circadian control [60]. This SIRT1–BMAL1–PGC1α axis forms a crucial signalling hub through which exercise can reinforce rhythmic gene expression and metabolic adaptation.
In obesity and T2DM, this pathway is frequently impaired due to NAD+ depletion and oxidative stress, resulting in reduced clock gene amplitude and metabolic inflexibility [61,62]. However, targeted exercise interventions can restore SIRT1 activity and circadian transcriptional output. For instance, Hansen et al. [11] reported that patients with T2DM exhibited blunted REV-ERBα/β and NAMPT rhythmicity in skeletal muscle, which was partially recovered following structured lifestyle interventions including exercise. Further support comes from Saner et al. [19], who demonstrated in healthy individuals that a single session of endurance exercise induced significant changes in skeletal muscle gene expression across both metabolic and clock pathways, including increased levels of PGC1α, NR4A3, and PER2. These effects occurred in parallel with enhanced mitochondrial respiration and substrate utilisation, indicating that exercise acts not only as a metabolic stimulus but also as a zeitgeber capable of influencing the circadian programme in human muscle. Although the study focused on healthy participants, the responsiveness of circadian genes to exercise even in basal states suggests preserved plasticity that may extend to metabolically impaired populations [19]. Together, these data position exercise as a molecular re-entrainment strategy that can overcome metabolic inertia by reactivating core circadian pathways in skeletal muscle.
Multi-tissue transcriptomic analyses have shown that exercise synchronises not only clocks in skeletal muscle, but also within adipose tissue. In an 8-week intervention study by Dreher et al. [63], both tissues displayed convergent yet tissue-specific adaptations. Skeletal muscle showed enrichment in genes related to oxidative phosphorylation and circadian transcription, while adipose tissue displayed reduced expression of lipogenic and inflammatory genes alongside restored rhythmicity of NR1D1 and PER2. These adaptations were accompanied by improved metabolic flexibility, indicating that exercise can remodel circadian transcriptional architecture in a systemic, tissue-coordinated fashion. These coordinated adaptations across metabolically active tissues highlight the potential of exercise not only to restore local clock function but to recalibrate whole-body circadian homeostasis, offering a powerful strategy to counteract the multi-organ dysregulation seen in obesity and T2DM.
Translational human studies have produced similar findings, highlighting that the metabolic and circadian benefits of exercise are preserved even in individuals with insulin resistance. Shen et al. [18] demonstrated that a single session of moderate-intensity aerobic exercise in both insulin-sensitive and insulin-resistant participants upregulated skeletal muscle expression of core clock genes PER1, PER2, and NR1D1, alongside key metabolic regulators such as PGC1α and GLUT4. These molecular changes were accompanied by acute enhancements in fatty acid oxidation and glucose handling, suggesting that skeletal muscle clocks retain a degree of plasticity and responsiveness to exercise despite metabolic impairment. This is particularly important in the context of obesity and T2DM, where circadian gene amplitude and phase coherence are often diminished.
Further supporting the relevance of circadian timing, time-of-day-specific training protocols have revealed that the effectiveness of exercise interventions are modulated by temporal alignment with the endogenous clock. In a crossover trial by Savikj et al. [20], men with T2DM experienced significantly greater improvements in glycaemic control and mitochondrial respiration following afternoon high-intensity interval training (HIIT) compared to morning sessions. These metabolic benefits were linked to enhanced expression of genes involved in mitochondrial biogenesis and lipid oxidation during the later part of the day, suggesting that training during periods of peak circadian metabolic activity may optimise therapeutic outcomes. Similarly, Sato et al. [64] reported that afternoon exercise improved glucose uptake and insulin sensitivity more effectively than morning sessions, reinforcing the idea that synchronising physical activity with the circadian phase of skeletal muscle enhances both metabolic efficiency and clinical efficacy. Together, these findings underscore the potential of personalised, time-of-day-targeted exercise interventions as a strategy to restore circadian alignment and improve metabolic health in insulin-resistant populations.
To further elucidate how exercise timing influences circadian alignment, Youngstedt et al. [65] developed human phase response curves to exercise, demonstrating that physical activity induces predictable shifts in circadian phase depending on the timing of the bout. Morning exercise performed between 07:00 and 10:00 advances the circadian phase, whereas late-evening activity between 19:00 and 22:00 induces phase delays. These effects are highly relevant for clinical populations with circadian disruption: early-morning training may be particularly beneficial for individuals with delayed sleep–wake rhythms, such as adolescents, shift workers, or those experiencing social jetlag, while evening sessions might support those with advanced sleep phase syndromes or metabolic conditions. Importantly, these phase-shifting effects are modulated by factors including chronotype, prior light exposure, and sleep pressure, highlighting the need for personalised exercise chronotherapy. Moreover, combining exercise with other non-photic zeitgebers may augment its entrainment potential. For instance, Chaix et al. [66] demonstrated that time-restricted feeding synergises with scheduled exercise to restore circadian alignment and improve metabolic flexibility in mice. Such findings support the rationale for integrated circadian interventions incorporating optimally timed physical activity, feeding–fasting cycles, and sleep hygiene as a holistic strategy to correct misaligned rhythms and improve metabolic outcomes in individuals with obesity and type 2 diabetes.
Together, these findings establish exercise as a central and versatile regulator of circadian biology with far-reaching implications for metabolic health. By directly targeting skeletal muscle clocks through mechanical and/or metabolic pathways, exercise can not only restore rhythmic gene expression, but enhances clock amplitude, synchrony, and tissue cross-talk. These benefits are retained in metabolically impaired states such as obesity and T2DM, underscoring the therapeutic value of physical activity even in the context of circadian dysfunction. Critically, the timing of exercise emerges as a key determinant of its efficacy, with evidence supporting time-of-day-specific enhancements in mitochondrial function, insulin sensitivity, and circadian phase resetting. This temporal sensitivity highlights the need to move beyond generic exercise prescriptions and toward personalised, chronotype-informed interventions. When optimally timed and potentially combined with other zeitgebers such as feeding schedules, exercise can realign peripheral clocks, re-establish systemic metabolic flexibility, and ultimately serve as a potent chronotherapeutic tool in the prevention and treatment of circadian-related metabolic disease.
While these findings support a holistic strategy to correct misaligned rhythms, incorporating optimally timed physical activity, feeding-fasting cycles, and sleep hygiene. It is important to acknowledge that such interventions must be applied with caution. Chronotherapeutic tools, much like pharmacological agents, carry the potential for both benefit and harm. For instance, excessively restrictive feeding windows, mistimed exercise, or inappropriate use of light or sleep promoting agents may inadvertently exacerbate circadian misalignment or be contraindicated in certain clinical populations, such as individuals with psychiatric or sleep disorders. Therefore, further research is required to define evidence-based protocols that maximise therapeutic efficacy while minimising adverse effects or unintended interactions among zeitgebers.
5. What-Omics Techniques Can Teach Us in Circadian Obesity Research
Obesity and type 2 diabetes (T2DM) are complex metabolic disorders influenced not only by lifestyle factors but also by the temporal misalignment of endogenous circadian rhythms with environmental cues [67]. Multi-omic technologies, e.g., epigenomics, transcriptomics, metabolomics, proteomics, etc., have emerged as powerful tools to dissect the molecular underpinnings of these diseases, particularly in the context of circadian disruption. These approaches enable a systems-level understanding of how clock dysfunction perturbs metabolic homeostasis, identifying new biomarkers, regulatory nodes, and therapeutic targets.
Circadian biology is characterised by highly dynamic, tissue-specific oscillations in gene expression, protein activity, and metabolite levels. In skeletal muscle, for instance, over 20% of total transcripts exhibit diurnal rhythmicity, many of which govern pathways central to energy metabolism, mitochondrial dynamics, and insulin sensitivity [5,23]. Disruption of these rhythms due to high-fat feeding, sleep deprivation, or genetic deletion of clock genes such as BMAL1, leads to profound metabolic dysfunction [9,49]. However, multi-omic profiling has helped to map these perturbations and reveal the temporal architecture of metabolic regulation [24].
Recent studies have begun to integrate multi-omic layers to explore the circadian control of exercise responses, which are relevant given the role of timed physical activity as a chronotherapeutic intervention. In a comprehensive mouse study by Maier et al. [68], combined transcriptomic, proteomic, and phosphoproteomic analyses revealing that early daytime exercise induced anabolic and regenerative pathways in skeletal muscle, whereas late-day training preferentially activated catabolic and stress-related signalling cascades. This temporal partitioning of signalling events suggests that the benefits of exercise are partly circadian-phase dependent. Despite robust transcriptional effects, these authors observed only modest changes in core clock gene expression, suggesting that exercise-induced metabolic reprogramming can occur without necessarily resetting the molecular clock, which is consistent with findings in similar models (e.g., [55]).
In human studies, proteomic and metabolomic shifts have also been reported in response to exercise at different times of day. For example, Dyar et al. [23] showed that circadian misalignment blunts rhythmic gene expression in metabolically active tissues and alters time-of-day-dependent responses to physical activity. These effects were pronounced in individuals with obesity, where the amplitude of rhythmic transcription and mitochondrial function was reduced. Such findings suggest that metabolic flexibility, and its restoration through chronotherapeutic exercise, may require a personalised approach based on circadian phenotype or chronotype [64].
Metabolomics has further highlighted specific metabolites and pathways altered by circadian disruption. For example, the AMPK-activating metabolite ZMP is induced by exercise in a time-dependent manner, with peak levels correlating with enhanced glycolysis and fatty acid oxidation during the active phase [55]. This provides a mechanistic link between energy sensing, the molecular clock, and exercise capacity. Moreover, rhythmic metabolite signatures in plasma and muscle can serve as accessible biomarkers of circadian alignment, which could inform timing of exercise or pharmacological interventions.
The integration of omics data across tissues has also shed light on inter-organ communication in circadian metabolism. Using multi-tissue multi-omic approaches, Wang et al. [69] demonstrated that obesity-prone and obesity-resistant mice exhibit distinct circadian regulation of hepatic and muscular gene networks, implicating disrupted inter-tissue signalling in the pathogenesis of metabolic disease. Notably, these alterations were not confined to traditional metabolic pathways but extended to inflammatory, fibrotic, and transcriptional control mechanisms as well, indicating broader circadian disruption that compromises systemic homeostasis and highlights the power to discover novel pathways through which temporal misalignment may drive metabolic dysfunction.
In conclusion, these multi-omic approaches have illuminated how circadian disruption contributes to metabolic disease, revealing new time-dependent regulatory circuits in skeletal muscle and beyond. These insights pave the way for temporally targeted interventions, whether via exercise, feeding, or pharmacology, to combat obesity and metabolic disorders. The future of circadian metabolic research lies in combining these data streams with machine learning to identify predictive signatures of disease and response. Longitudinal, wearable-integrated studies will be key in linking molecular rhythms to behavioural outputs and clinical outcomes. With the growing accessibility of multi-omics platforms and bioinformatics pipelines, personalised chrono-medicine is becoming increasingly feasible.
Looking ahead, several important research questions must be addressed to advance the clinical application of exercise-based chronotherapy. These include determining the optimal timing of exercise across different chronotypes and metabolic phenotypes, identifying reliable biomarkers of circadian alignment and therapeutic responsiveness, and characterising the interactive effects of physical activity, feeding schedules, and sleep timing on peripheral clock function. Furthermore, large-scale, longitudinal trials are needed to evaluate the long-term safety, efficacy, and personalisation of multi-zeitgeber interventions in diverse populations. Addressing these questions will be essential to translate circadian biology into practical and effective strategies for preventing and managing metabolic disease.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Partch, C.L.; Green, C.B.; Takahashi, J.S. Molecular Architecture of the Mammalian Circadian Clock. Trends Cell Biol. 2014, 24, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Schibler, U.; Gotic, I.; Saini, C.; Gos, P.; Curie, T.; Emmenegger, Y.; Sinturel, F.; Gosselin, P.; Gerber, A.; Fleury-Olela, F.; et al. Clock-Talk: Interactions between central and peripheral circadian oscillators in mammals. Cold Spring Harb. Symp. Quant. Biol. 2016, 80, 223–232. [Google Scholar] [CrossRef]
- Zurlo, F.; Larson, K.; Bogardus, C.; Ravussin, E. Skeletal Muscle Metabolism Is a Major Determinant of Resting Energy Expenditure. J. Clin. Investig. 1990, 86, 1423–1427. [Google Scholar] [CrossRef]
- Perrin, L.; Loizides-Mangold, U.; Chanon, S.; Gobet, C.; Hulo, N.; Isenegger, L.; Weger, B.; Migliavacca, E.; Charpagne, A.; Betts, J.; et al. Transcriptomic analyses reveal rhythmic and CLOCK-driven pathways in human skeletal muscle. Elife 2018, 7, e34114. [Google Scholar] [CrossRef] [PubMed]
- Dyar, K.A.; Hubert, M.J.; Mir, A.A.; Ciciliot, S.; Lutter, D.; Greulich, F.; Quagliarin, F.; Kleinert, M.; Fischer, K.; Eichmann, T.; et al. Transcriptional programming of lipid and amino acid metabolism by the skeletal muscle circadian clock. PLoS Biol. 2018, 16, e2005886. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Zierath, J.R. Circadian rhythms and exercise—Re-setting the clock in metabolic disease. Nat. Rev. Endocrinol. 2019, 15, 197–206. [Google Scholar] [CrossRef]
- Hodge, B.A.; Zhang, X.; Gutierrez-Monreal, M.A.; Cao, Y.; Hammers, D.; Yao, Z.; Wolff, C.; Du, P.; Kemler, D.; Judge, A.; et al. MYOD1 functions as a clock amplifier as well as a critical co-factor for downstream circadian gene expression in muscle. Elife 2019, 8, e43017. [Google Scholar] [CrossRef]
- Dyar, K.A.; Ciciliot, S.; Wright, L.E.; Biensø, R.; Tagliazucchi, G.; Patel, V.; Forcato, M.; Paz, M.; Gudiksen, A.; Solagna, F.; et al. Muscle insulin sensitivity and glucose metabolism are controlled by the intrinsic muscle clock. Mol. Metab. 2014, 3, 29–41. [Google Scholar] [CrossRef]
- Harfmann, B.D.; Schroder, E.A.; Kachman, M.T.; Hodge, B.; Zhang, X.; Esser, K. Muscle-specific loss of Bmal1 leads to disrupted tissue glucose metabolism and systemic glucose homeostasis. Skelet. Muscle 2016, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Timmers, S.; Moonen-Kornips, E.; Duez, H.; Staels, B.; Hesselink, M.; Schrauwen, P. Synchronized human skeletal myotubes of lean, obese and type 2 diabetic patients maintain circadian oscillation of clock genes. Sci. Rep. 2016, 6, 35047. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, B.M.; Altıntaş, A.; Bsmith, J.A.; Sardon-Puig, L.; Zhang, X.; Basse, A.; Laker, R.; Gao, H.; Liu, Z.; Dollet, L.; et al. Disrupted circadian oscillations in type 2 diabetes are linked to altered rhythmic mitochondrial metabolism in skeletal muscle. Sci. Adv. 2021, 7, eabi9654. [Google Scholar] [CrossRef]
- Wefers, J.; Van Moorsel, D.; Hansen, J.; Connell, N.; Havekes, B.; Hoeks, J.; Van Marken Lichtenbelt, W.D.; Duez, H.; Phielix, E.; Kalsbeek, A.; et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA 2018, 115, 7789–7794. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Schönke, M.; Orzechowski Westholm, J.; Mi, J.; Chibalin, A.; Voisin, S.; Osler, M.; Vogel, H.; Hornaeus, K.; Dickson, S.; et al. Acute sleep loss results in tissue-specific alterations in genome-wide DNA methylation state and metabolic fuel utilization in humans. Sci. Adv. 2018, 4, eaar8590. [Google Scholar] [CrossRef]
- Gutierrez-Monreal, M.A.; Harmsen, J.F.; Schrauwen, P.; Esser, K. Ticking for Metabolic Health: The Skeletal-Muscle Clocks. Obesity 2020, 28, S46–S54. [Google Scholar] [CrossRef]
- Gachon, F.; Loizides-Mangold, U.; Petrenko, V.; Dibner, C. Glucose homeostasis: Regulation by peripheral circadian clocks in rodents and humans. Endocrinology 2017, 158, 1074–1084. [Google Scholar] [CrossRef]
- Adamovich, Y.; Dandavate, V.; Ezagouri, S.; Manella, G.; Zwighaft, Z.; Sobel, J.; Kuperman, Y.; Golik, M.; Auerbach, A.; Itkin, M.; et al. Clock proteins and training modify exercise capacity in a daytime-dependent manner. Proc. Natl. Acad. Sci. USA 2021, 118, e2101115118. [Google Scholar] [CrossRef]
- Shen, B.; Ma, C.; Wu, G.; Liu, H.; Chen, L.; Yang, G. Effects of exercise on circadian rhythms in humans. Front. Pharmacol 2023, 14, 1282357. [Google Scholar] [CrossRef]
- Saner, N.J.; Lee, M.J.; Pitchford, N.W.; Kuang, J.; Roach, G.; Gamham, A.; Stokes, T.; Phillips, S.; Bishop, D.; Bartlett, J. The effect of sleep restriction, with or without high-intensity interval exercise, on myofibrillar protein synthesis in healthy young men. J. Physiol. 2020, 598, 1523–1536. [Google Scholar] [CrossRef]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Bjornholm, M.; Fritz, T.; Krook, A.; Zierath, J.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Pendergrast, L.A.; Lundell, L.S.; Ehrlich, A.M.; Ashcroft, S.; Schonke, M.; Basse, A.; Krook, A.; Treebak, J.; Dollet, L.; Zierath, J. Time of day determines postexercise metabolism in mouse adipose tissue. Proc. Natl. Acad. Sci. USA 2023, 120, e2218510120. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care 2009, 32 (Suppl. S2), S157. [Google Scholar] [CrossRef] [PubMed]
- Dyar, K.A.; Lutter, D.; Artati, A.; Cegila, N.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of Circadian Metabolism Reveals System-wide Coordination and Communication between Clocks. Cell 2018, 174, 1571–1585.e11. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Lahens, N.F.; Ballance, H.I.; Hughes, M.E.; Hogenesch, J.B. A circadian gene expression atlas in mammals: Implications for biology and medicine. Proc. Natl. Acad. Sci. USA 2014, 111, 16219–16224. [Google Scholar] [CrossRef] [PubMed]
- Bass, J.; Takahashi, J.S. Circadian Integration of Metabolism and Energetics. Science (1979) 2010, 330, 1349–1354. [Google Scholar] [CrossRef]
- Green, C.B.; Takahashi, J.S.; Bass, J. The Meter of Metabolism. Cell 2008, 134, 728–742. [Google Scholar] [CrossRef]
- Martin, R.A.; Viggars, M.R.; Esser, K.A. Metabolism and exercise: The skeletal muscle clock takes centre stage. Nat. Rev. Endocrinol. 2023, 19, 272–284. [Google Scholar] [CrossRef]
- Schroder, E.A.; Esser, K.A. Circadian rhythms, skeletal muscle molecular clocks, and exercise. Exerc. Sport. Sci. Rev. 2013, 41, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Riley, L.A.; Zhang, X.; Douglas, C.M.; Mijares, J.; Hammers, D.; Wolff, C.; Wood, N.; Olafson, H.; Du, P.; Labeit, S.; et al. The skeletal muscle circadian clock regulates titin splicing through RBM20. Elife 2022, 11, e76478. [Google Scholar] [CrossRef]
- Harmsen, J.-F.; Kotte, M.; Habets, I.; Bosschee, F.; Frenken, K.; Jorgensen, J.; De Kam, S.; Moonen-Kornips, E.; Cissen, J.; Doligkeit, D.; et al. Exercise training modifies skeletal muscle clock gene expression but not 24-hour rhythmicity in substrate metabolism of men with insulin resistance. J. Physiol. 2024, 602, 6417–6433. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Mistretta, J.; Scheer, F. Effects of the internal circadian system and circadian misalignment on glucose tolerance in chronic shift workers. J. Clin. Endocrinol. Metab. 2016, 101, 1066–1074. [Google Scholar] [CrossRef]
- Perrin, L.; Loizides-Mangold, U.; Skarupelova, S.; Pulimeno, P.; Chanon, S.; Robert, M.; Bouzakri, K.; Modoux, C.; Roux-Lombard, P.; Vidal, H.; et al. Human skeletal myotubes display a cell-autonomous circadian clock implicated in basal myokine secretion. Mol. Metab. 2015, 4, 834–845. [Google Scholar] [CrossRef] [PubMed]
- Um, J.H.; Pendergast, J.S.; Springer, D.A.; Foretz, M.; Viollet, B.; Brown, A.; Kim, M.; Yamazaki, S.; Chung, J. AMPK regulates circadian rhythms in a tissue- and isoform-specific manner. PLoS ONE 2011, 6, e18450. [Google Scholar] [CrossRef]
- Ramanathan, C.; Kathale, N.D.; Liu, D.; Lee, C.; Freeman, D.; Hogenesch, J.; Cao, R.; Liu, A. mTOR signaling regulates central and peripheral circadian clock function. PLoS Genet. 2018, 14, e1007369. [Google Scholar] [CrossRef]
- Peek, C.B.; Affinati, A.H.; Ramsey, K.M.; Kuo, H.; Yu, W.; Sena, L.; Ilkayeva, O.; Marcheva, B.; Kobayashi, Y.; Omura, C.; et al. Circadian clock NAD+ cycle drives mitochondrial oxidative metabolism in mice. Science (1979) 2013, 342, 1243417. [Google Scholar] [CrossRef] [PubMed]
- Wolff, G.; Esser, K.A. Scheduled exercise phase shifts the circadian clock in skeletal Muscle. Med. Sci. Sports Exerc. 2012, 44, 1663–1670. [Google Scholar] [CrossRef]
- Kemler, D.; Wolff, C.A.; Esser, K.A. Time-of-day dependent effects of contractile activity on the phase of the skeletal muscle clock. J. Physiol. 2020, 598, 3631–3644. [Google Scholar] [CrossRef]
- Viggars, M.R.; Berko, H.E.; Hesketh, S.J.; Wolff, C.; Gutierrez-Monreal, M.; Martin, R.; Jennings, I.; Huo, Z.; Esser, K. Skeletal muscle BMAL1 is necessary for transcriptional adaptation of local and peripheral tissues in response to endurance exercise training. Mol. Metab. 2024, 86, 101980. [Google Scholar] [CrossRef] [PubMed]
- de Alencar Silva, B.S.; Uzeloto, J.S.; Lira, F.S.; Pereira, T.; Coelho-E-Silva, M.J.; Caseiro, A. Exercise as a peripheral circadian clock resynchronizer in vascular and skeletal muscle aging. Int. J. Environ. Res. Public Health 2021, 18, 12949. [Google Scholar] [CrossRef]
- Defronzo, R.A.; Jacot, E.; Jequier, E.; Maeder, E.; Wahren, J.; Felber, J. The Effect of Insulin on the Disposal of Intravenous Glucose Results from Indirect Calorimetry and Hepatic and Femoral Venous Catheterization. Diabetes 1981, 30, 1000–1007. [Google Scholar] [CrossRef]
- Jacobi, D.; Liu, S.; Burkewitz, K.; Kory, N.; Knudsen, N.; Alexander, R.; Unluturk, U.; Li, X.; Kong, X.; Hyde, A.; et al. Hepatic Bmal1 regulates rhythmic mitochondrial dynamics and promotes metabolic fitness. Cell Metab. 2015, 22, 709–720. [Google Scholar] [CrossRef]
- Gemmink, A.; Daemen, S.; Wefers, J.; Hansen, J.; van Moorsel, D.; Astuti, P.; Jorgensen, J.; Kornips, E.; Schaart, G.; Hoeks, J.; et al. Twenty-four hour rhythmicity in mitochondrial network connectivity and mitochondrial respiration; a study in human skeletal muscle biopsies of young lean and older individuals with obesity. Mol. Metab. 2023, 72, 101727. [Google Scholar] [CrossRef]
- Scheer, F.A.J.L.; Hilton, M.F.; Mantzoros, C.S.; Shea, S. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA 2009, 106, 4453–4458. [Google Scholar] [CrossRef] [PubMed]
- Arble, D.M.; Bass, J.; Laposky, A.D.; Vitaterna, M.; Turek, F. Circadian timing of food intake contributes to weight gain. Obesity 2009, 17, 2100–2102. [Google Scholar] [CrossRef] [PubMed]
- Turek, F.W.; Joshu, C.; Kohsaka, A.; Lin, E.; Ivanova, G.; McDearmon, E.; Laposky, A.; Losee-Olson, S.; Easton, A.; Jensen, D.; et al. Obesity and metabolic syndrome in circadian Clock mutant nice. Science (1979) 2005, 308, 1043–1045. [Google Scholar] [CrossRef]
- Hodge, B.A.; Wen, Y.; Riley, L.A.; Zhang, X.; England, J.; Harfmann, B.; Schroder, E.; Esser, K. The endogenous molecular clock orchestrates the temporal separation of substrate metabolism in skeletal muscle. Skelet. Muscle 2015, 5, 17. [Google Scholar] [CrossRef]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.; Rasbach, K.; Bostrom, E.; Choi, J.; Long, J.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 481, 463–468. [Google Scholar] [CrossRef]
- Eckel-Mahan, K.L.; Patel, V.R.; De Mateo, S.; Orozco-Solis, R.; Cegila, N.; Sahar, S.; Dilag-Penilla, S.; Dyar, K.; Baldi, P.; Sassone-Corsi, P. Reprogramming of the circadian clock by nutritional challenge. Cell 2013, 155, 1464–1478. [Google Scholar] [CrossRef]
- Kohsaka, A.; Laposky, A.D.; Ramsey, K.M.; Estrada, C.; Joshu, C.; Kobayashi, Y.; Turek, F.; Bass, J. High-Fat Diet Disrupts Behavioral and Molecular Circadian Rhythms in Mice. Cell Metab. 2007, 6, 414–421. [Google Scholar] [CrossRef]
- Buxton, O.M.; Pavlova, M.; Reid, E.W.; Wang, W.; Simonson, D.; Alder, G. Sleep restriction for 1 week reduces insulin sensitivity in healthy men. Diabetes 2010, 59, 2126–2133. [Google Scholar] [CrossRef]
- De Goede, P.; Wefers, J.; Brombacher, E.C.; Schrauwen, P.; Kalsbeek, A. Circadian rhythms in mitochondrial respiration. J. Mol. Endocrinol. 2018, 60, R115–R130. [Google Scholar] [CrossRef]
- Puig, L.S.; Valera-Alberni, M.; Cantó, C.; Pillon, N. Circadian rhythms and mitochondria: Connecting the dots. Front. Genet. 2018, 9, 452. [Google Scholar] [CrossRef]
- Acosta-Rodríguez, V.A.; Rijo-Ferreira, F.; Green, C.B.; Takahashi, J. Importance of circadian timing for aging and longevity. Nat. Commun. 2021, 12, 2862. [Google Scholar] [CrossRef]
- Hesketh, S.J.; Esser, K.A. The clockwork of champions: Influence of circadian biology on exercise performance. Free Radic. Biol. Med. 2024, 224, 78–87. [Google Scholar] [CrossRef]
- Ezagouri, S.; Zwighaft, Z.; Sobel, J.; Baillieul, S.; Doutreleau, S.; Ladeuix, B.; Golik, M.; Verges, S.; Asher, G. Physiological and Molecular Dissection of Daily Variance in Exercise Capacity. Cell Metab. 2019, 30, 78–91.e4. [Google Scholar] [CrossRef]
- Aoyama, S.; Shibata, S. Time-of-Day-Dependent Physiological Responses to Meal and Exercise. Front. Nutr. 2020, 7, 18. [Google Scholar] [CrossRef]
- Hitrec, T.; Petit, C.; Cryer, E.; Muir, C.; Tal, N.; Fustin, J.; Hughes, A.; Piggins, H. Timed exercise stabilizes behavioral rhythms but not molecular programs in the brain’s suprachiasmatic clock. iScience 2023, 26, 106002. [Google Scholar] [CrossRef] [PubMed]
- Lamia, K.A.; Sachdeva, U.M.; DiTacchio, L.; Williams, E.C.; Alvarez, J.G.; Egan, D.F.; Vasquez, D.S.; Juguilon, H.; Panda, S.; Shaw, R.J.; et al. AMPK Regulates the Circadian Clock by Cryptochrome Phosphorylation and Degradation. Science (1979) 2009, 326, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.D.; Lamia, K.A. AMPK at the crossroads of circadian clocks and metabolism. Mol. Cell Endocrinol. 2013, 366, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.; Sassone-Corsi, P. The NAD+-Dependent Deacetylase SIRT1 Modulates CLOCK-Mediated Chromatin Remodeling and Circadian Control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef]
- Cantó, C.; Houtkooper, R.H.; Pirinen, E.; Youn, D.; Oosterveer, M.; Cen, Y.; Fernandez-Marcos, P.; Yamamoto, H.; Andreux, P.; Cettour-Rose, P.; et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012, 15, 838–847. [Google Scholar] [CrossRef]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide mononucleotide, a key NAD + intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef]
- Dreher, S.I.; Irmler, M.; Pivovarova-Ramich, O.; Kessler, K.; Jurchott, K.; Sticht, C.; Fritsche, L.; Schneeweiss, P.; Machann, J.; Pfeiffer, A.; et al. Acute and long-term exercise adaptation of adipose tissue and skeletal muscle in humans: A matched transcriptomics approach after 8-week training-intervention. Int. J. Obes. 2023, 47, 313–324. [Google Scholar] [CrossRef]
- Sato, S.; Basse, A.L.; Schönke, M.; Chen, S.; Samad, M.; Altintas, A.; Laker, R.; Dalbram, E.; Barres, R.; Baldi, P.; et al. Time of Exercise Specifies the Impact on Muscle Metabolic Pathways and Systemic Energy Homeostasis. Cell Metab. 2019, 30, 92–110.e4. [Google Scholar] [CrossRef] [PubMed]
- Youngstedt, S.D.; Elliott, J.A.; Kripke, D.F. Human circadian phase–response curves for exercise. J. Physiol. 2019, 597, 2253–2268. [Google Scholar] [CrossRef] [PubMed]
- Chaix, A.; Lin, T.; Le, H.D.; Chang, M.; Panda, S. Time-Restricted Feeding Prevents Obesity and Metabolic Syndrome in Mice Lacking a Circadian Clock. Cell Metab. 2019, 29, 303–319.e4. [Google Scholar] [CrossRef] [PubMed]
- Depner, C.M.; Stothard, E.R.; Wright, K.P. Metabolic consequences of sleep and circadian disorders. Curr. Diab. Rep. 2014, 14, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Maier, G.; Delezie, J.; Westermark, P.O.; Santos, G.; Ritz, D.; Handschin, C. Transcriptomic, proteomic and phosphoproteomic underpinnings of daily exercise performance and Zeitgeber activity of training in mouse muscle. J. Physiol. 2022, 600, 769–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lin, J.; Duan, M.; He, J.; Halizere, S.; Chen, N.; Chen, X.; Jiao, Y.; He, W.; Dyar, K.; et al. Multi-omics reveals different signatures of obesity-prone and obesity-resistant mice. iMetaOmics 2025, 2, e59. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).