The Role of Whey Protein in Maintaining Fat-Free Mass and Promoting Fat Loss After 18 Months of Bariatric Surgery
Abstract
1. Introduction
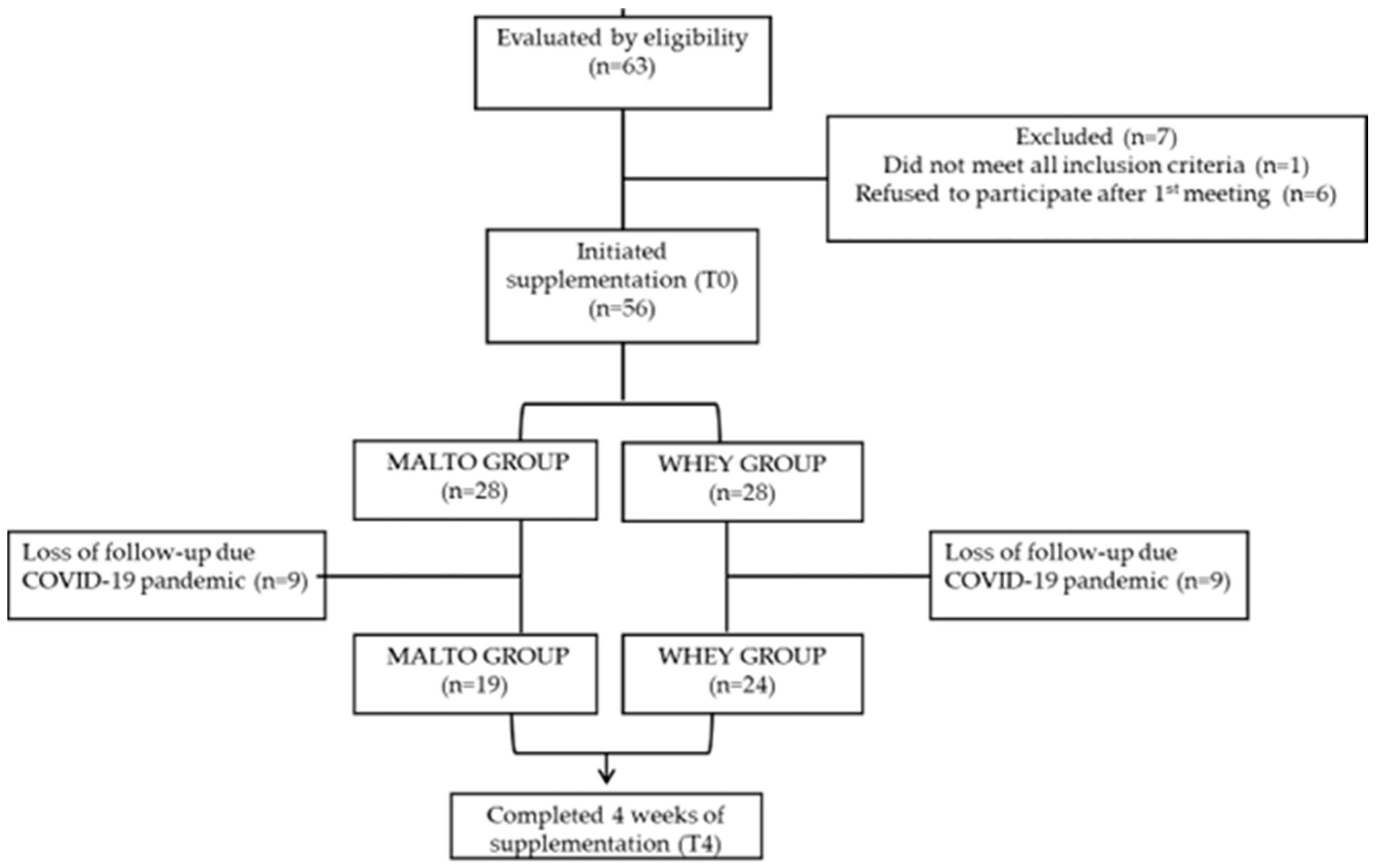
2. Methods
2.1. Study Protocol
2.2. Body Composition
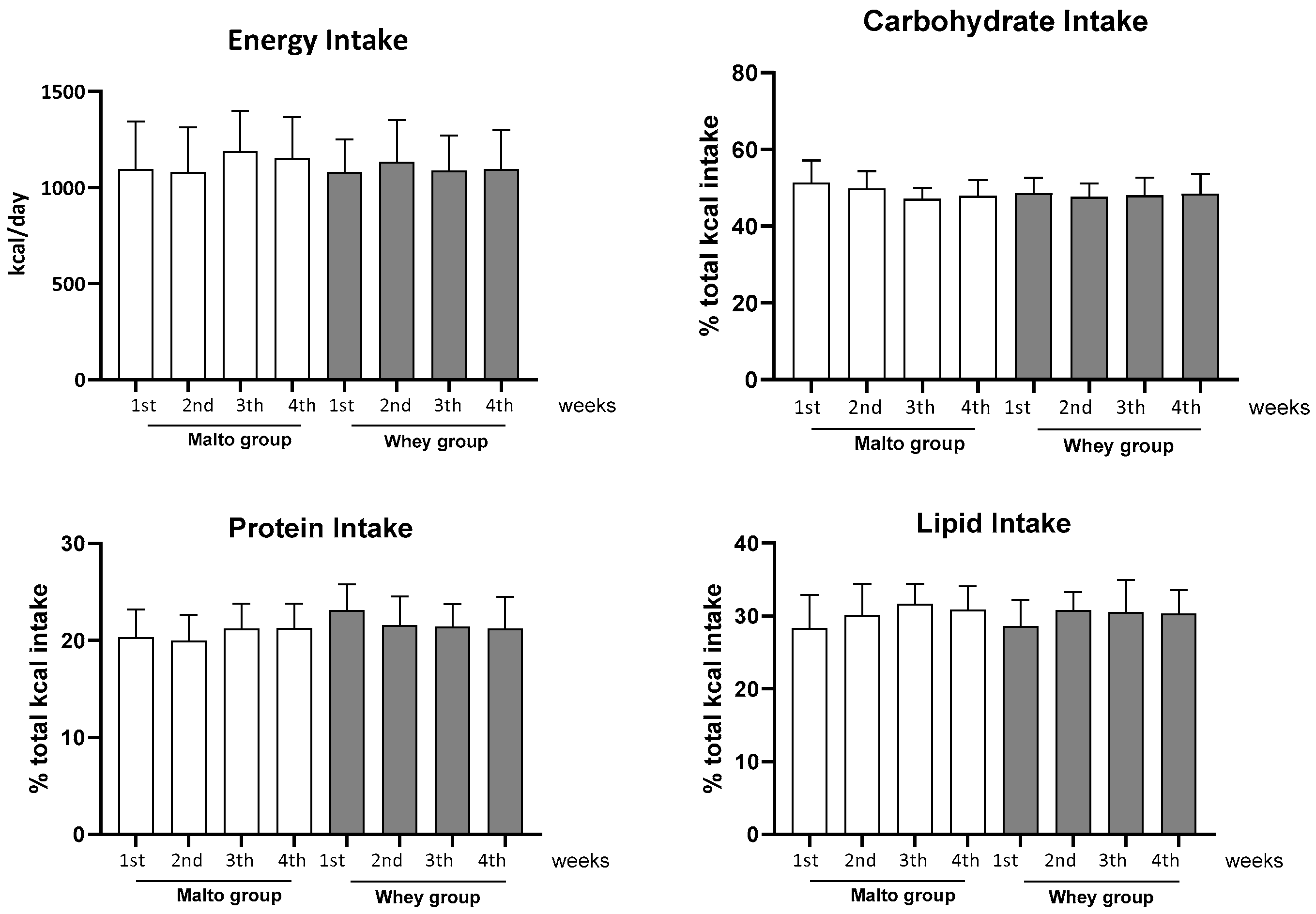
2.3. Food Intake
2.4. Indirect Calorimetry and Physical Activity
2.5. Physical Activity
2.6. Muscle Thickness Assessment
2.7. Muscle Strength
2.8. Statistical Analysis
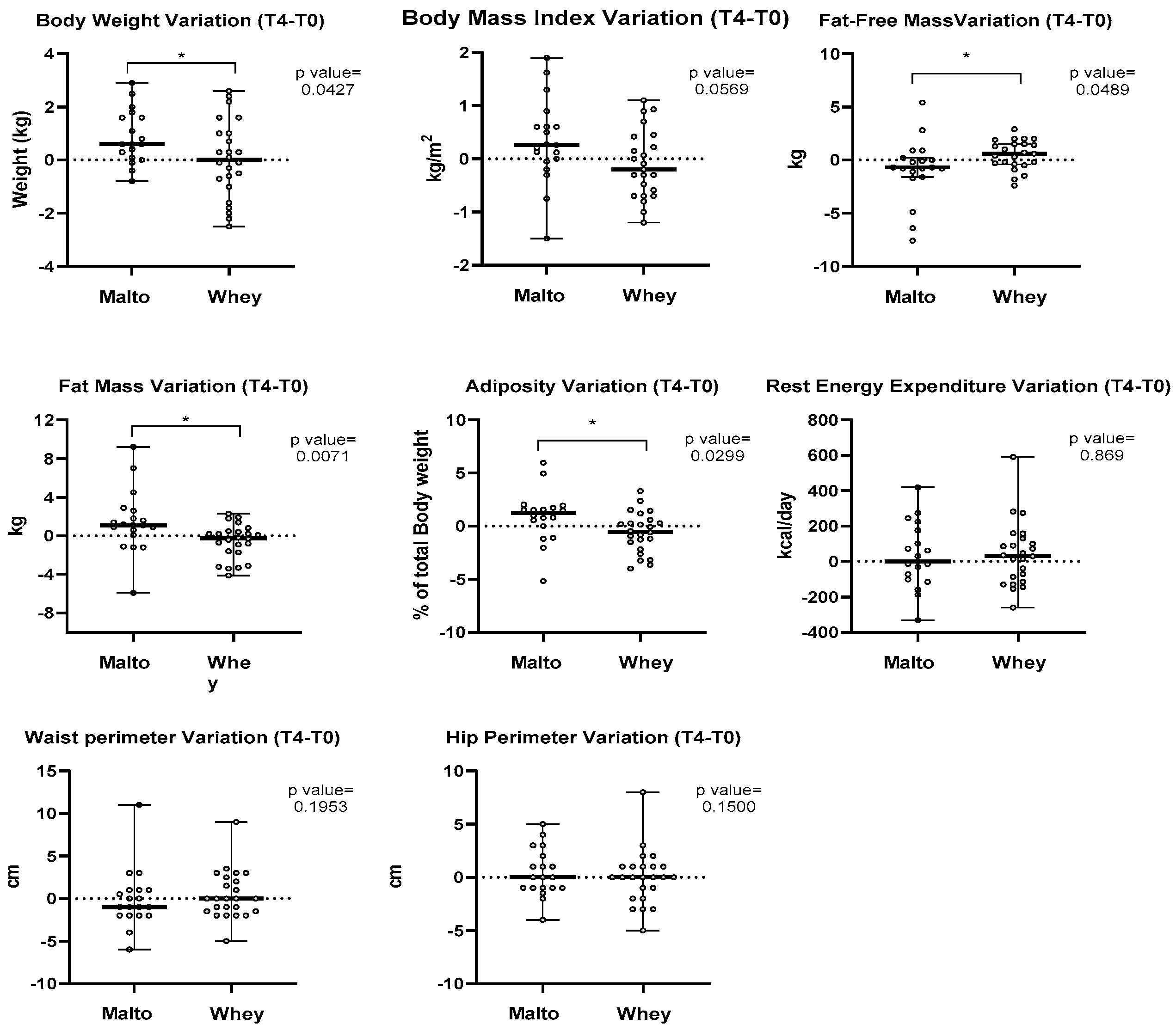
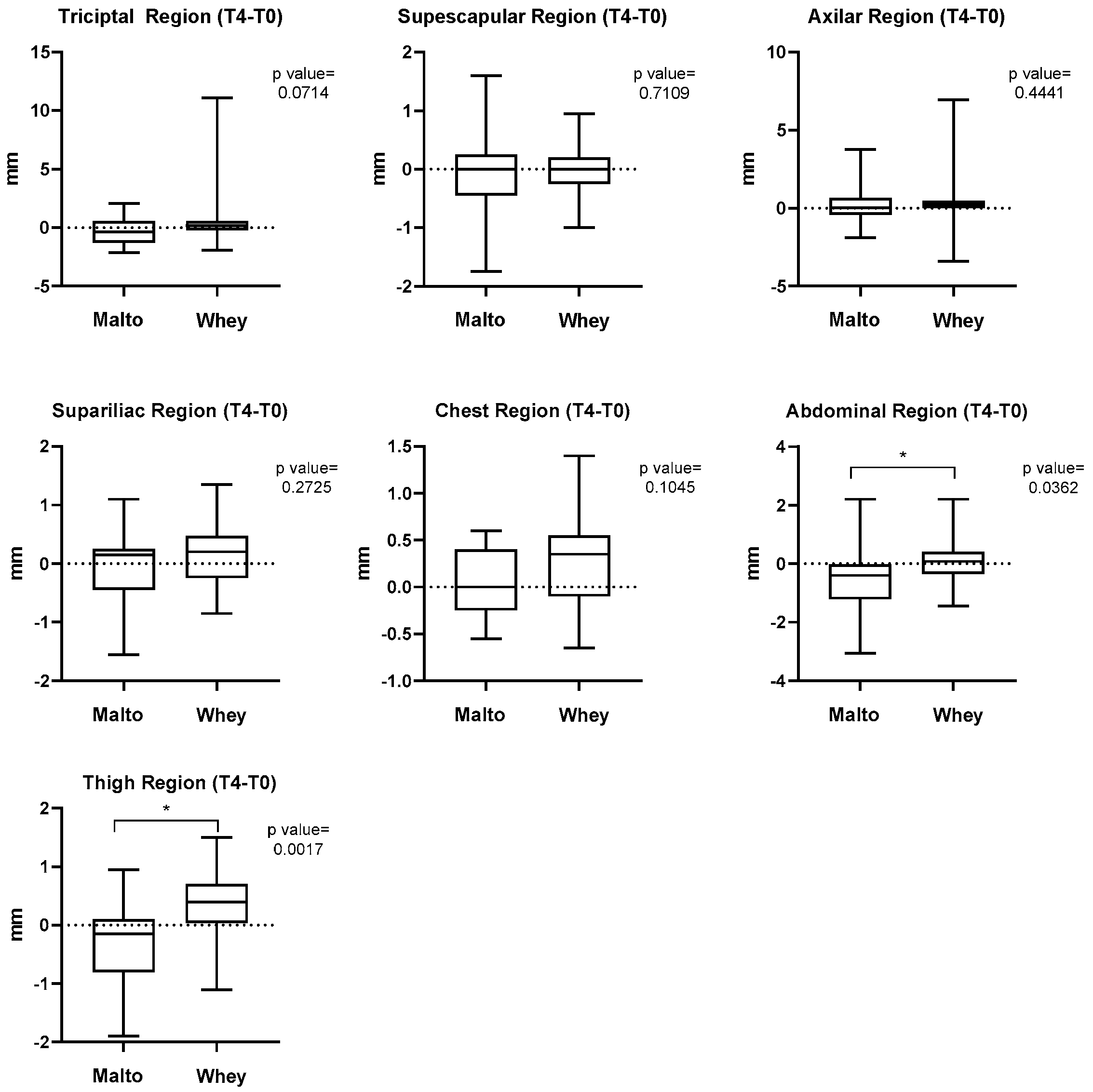
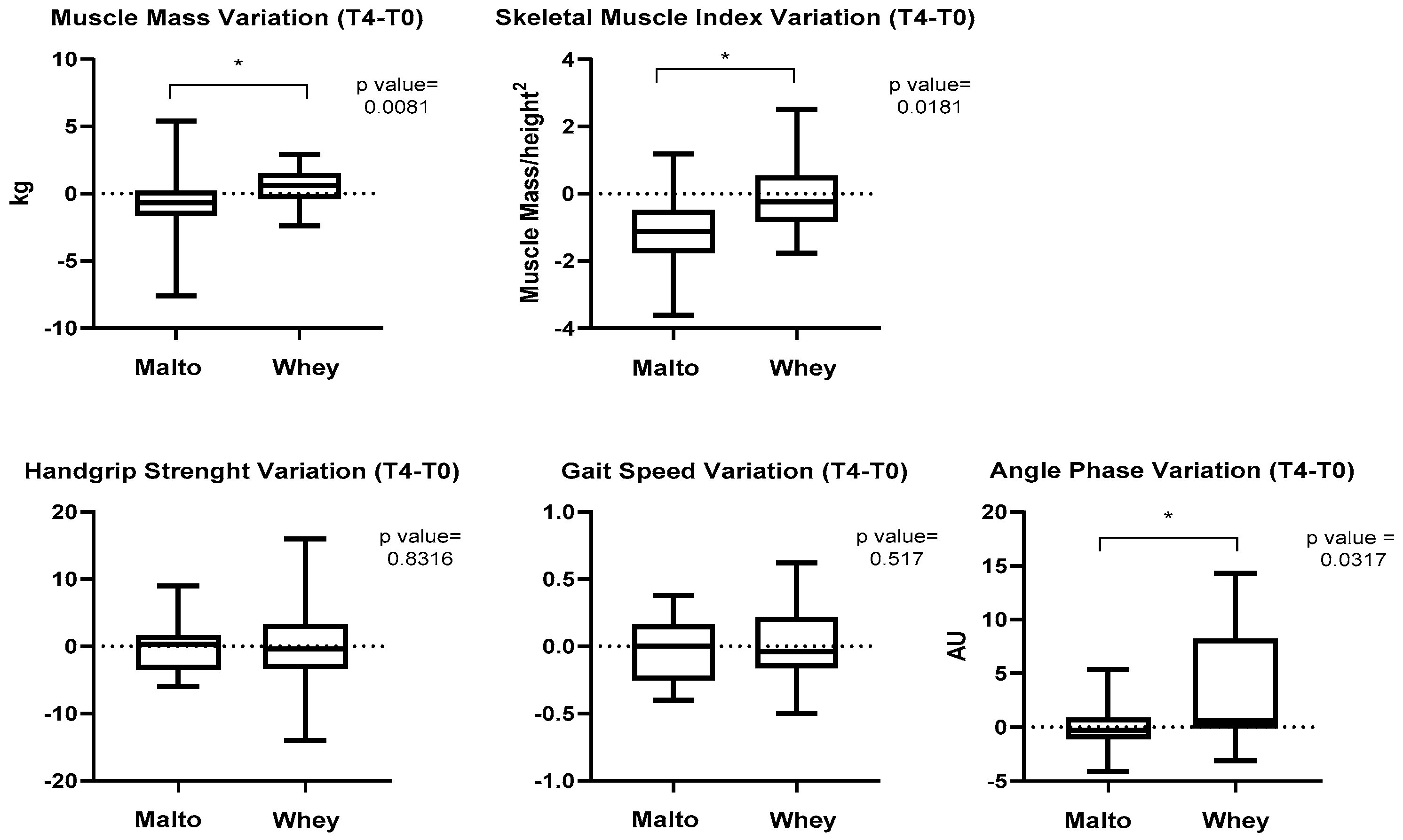
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rubino, F.; Cummings, D.E.; Eckel, R.H.; Cohen, R.V.; Wilding, J.P.H.; Brown, W.A.; Stanford, F.C.; Batterham, R.L.; Farooqi, I.S.; Farpour-Lambert, N.J.; et al. Definition and diagnostic criteria of clinical obesity. Lancet Diabetes Endocrinol. 2025, 13, 221–262, Erratum in Lancet Diabetes Endocrinol. 2025, 13, e6. [Google Scholar] [CrossRef] [PubMed]
- Lupoli, R.; Lembo, E.; Saldalamacchia, G.; Avola, C.K.; Angrisani, L.; Capaldo, B. Bariatric surgery and long-term nutritional issues. World J. Diabetes 2017, 8, 464–474. [Google Scholar] [CrossRef]
- Martínez, M.C.; Meli, E.F.; Candia, F.P.; Filippi, F.; Vilallonga, R.; Cordero, E.; Hernández, I.; Eguinoa, A.Z.; Burgos, R.; Vila, A.; et al. The Impact of Bariatric Surgery on the Muscle Mass in Patients with Obesity: 2-Year Follow-up. Obes. Surg. 2022, 32, 625–633. [Google Scholar] [CrossRef]
- Aguas-Ayesa, M.; Yárnoz-Esquíroz, P.; Olazarán, L.; Gómez-Ambrosi, J.; Frühbeck, G. Precision nutrition in the context of bariatric surgery. Rev. Endocr. Metab. Disord. 2023, 24, 979–991. [Google Scholar] [CrossRef]
- Capella, J.F.; Capella, R.F. An assessment of vertical banded gastroplasty-Roux-en-Y gastric bypass for the treatment of morbid obesity. Am. J. Surg. 2002, 183, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.C.; Lee, C.G.; Torabi, M.R.; Lohrmann, D.K. The longitudinal trajectory of post-surgical % total weight loss among middle-aged women who had undergone bariatric surgery. Prev. Med. Rep. 2016, 5, 200–204. [Google Scholar] [CrossRef]
- Henao Carrillo, D.C.; Gómez, A.M.; Muñoz, O.M.; Rubio, C.; Rodríguez, N.; Ursida, V.; Forero, A.M.; Pinzón, F.; Mikler, R. Factors associated with different patterns of weight change after bariatric surgery: A longitudinal study. Obes. Sci. Pract. 2023, 9, 477–483. [Google Scholar] [CrossRef]
- Resende, C.M.M.; Durso, D.F.; Borges, K.B.G.; Pereira, R.M.; Rodrigues, G.K.D.; Rodrigues, K.F.; Silva, J.L.P.; Rodrigues, E.C.; Franco, G.R.; Alvarez-Leite, J.I. The polymorphism rs17782313 near MC4R gene is related with anthropometric changes in women submitted to bariatric surgery over 60 months. Clin. Nutr. 2018, 37, 1286–1292. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, G.K.; Resende, C.M.; Durso, D.F.; Rodrigues, L.A.; Silva, J.L.; Reis, R.C.; Pereira, S.S.; Ferreira, D.C.; Franco, G.R.; Alvarez-Leite, J. A single FTO gene variant rs9939609 is associated with body weight evolution in a multiethnic extremely obese population that underwent bariatric surgery. Nutrition 2015, 31, 1344–1350. [Google Scholar] [CrossRef] [PubMed]
- Barati-Boldaji, R.; Esmaeilinezhad, Z.; Babajafari, S.; Kazemi, A.; Clark, C.C.T.; Mazidi, M.; Ofori-Asenso, R.; Haghighat, N.; Shafiee, M.; Mazloomi, S.M. Bariatric surgery reduces branched-chain amino acids’ levels: A systematic review and meta-analysis. Nutr. Res. 2021, 87, 80–90. [Google Scholar] [CrossRef]
- Mastino, D.; Robert, M.; Betry, C.; Laville, M.; Gouillat, C.; Disse, E. Bariatric Surgery Outcomes in Sarcopenic Obesity. Obes. Surg. 2016, 26, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Brasil. Ministério da Saúde (MS). Secretaria de Vigilância em Saúde. Departamento de Análise em Saúde e Vigilância de Doenças não Transmissíveis. Vigitel Brasil 2021: Vigilância de Fatores de Risco e Proteção Para Doenças Crônicas por Inquérito Telefônico: Estimativas Sobre Frequência e Distribuição Sociodemográfica de Fatores de Risco e Proteção Para Doenças Crônicas nas Capitais dos 26 Estados Brasileiros e no Distrito Federal em 2021; MS: Brasília, Brazil, 2022. [Google Scholar]
- Wagner, D.R. Ultrasound as a tool to assess body fat. J. Obes. 2013, 2013, 280713. [Google Scholar] [CrossRef] [PubMed]
- Lauretani, F.; Russo, C.R.; Bandinelli, S.; Bartali, B.; Cavazzini, C.; Di Iorio, A.; Corsi, A.M.; Rantanen, T.; Guralnik, J.M.; Ferrucci, L. Age-associated changes in skeletal muscles and their effect on mobility: An operational diagnosis of sarcopenia. J. Appl. Physiol. 2003, 95, 1851–1860. [Google Scholar] [CrossRef]
- Drummen, M.; Tischmann, L.; Gatta-Cherifi, B.; Adam, T.; Westerterp-Plantenga, M. Dietary Protein and Energy Balance in Relation to Obesity and Co-morbidities. Front. Endocrinol. 2018, 9, 443. [Google Scholar] [CrossRef]
- Schollenberger, A.E.; Karschin, J.; Meile, T.; Küper, M.A.; Königsrainer, A.; Bischoff, S.C. Impact of protein supplementation after bariatric surgery: A randomized controlled double-blind pilot study. Nutrition 2016, 32, 186–192. [Google Scholar] [CrossRef]
- Brody, F.; Flood, M.; Richards, N.G.; Vaziri, K.; Garey, C.; LeBrun, C. A novel single agent for nutritional supplementation following Roux-en-Y gastric bypass. J. Laparoendosc. Adv. Surg. Tech. 2013, 23, 596–600. [Google Scholar] [CrossRef]
- Moon, J.; Koh, G. Clinical Evidence and Mechanisms of High-Protein Diet-Induced Weight Loss. J. Obes. Metab. Syndr. 2020, 29, 166–173. [Google Scholar] [CrossRef]
- Lamarca, F.; Vieira, F.T.; Lima, R.M.; Nakano, E.Y.; da Costa, T.H.M.; Pizato, N.; Dutra, E.S.; de Carvalho, K.M.B. Effects of Resistance Training With or Without Protein Supplementation on Body Composition and Resting Energy Expenditure in Patients 2-7 Years PostRoux-en-Y Gastric Bypass: A Controlled Clinical Trial. Obes. Surg. 2021, 31, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Cereda, E.; Turri, A.; Klersy, C.; Cappello, S.; Ferrari, A.; Filippi, A.R.; Brugnatelli, S.; Caraccia, M.; Chiellino, S.; Borioli, V.; et al. Whey protein isolate supplementation improves body composition, muscle strength, and treatment tolerance in malnourished advanced cancer patients undergoing chemotherapy. Cancer Med. 2019, 8, 6923–6932. [Google Scholar] [CrossRef]
- Ranjbar, M.; Fallah, M.; Djafarian, K.; Mohammadi, H.; Mohammadi Farsani, G.; Shab-Bidar, S. The effects of protein supplementation on body composition after bariatric surgery: A systematic review and meta-analysis of randomized controlled trials. Obesity 2025, 33, 1027–1036. [Google Scholar] [CrossRef]
- Bergia, R.E., 3rd; Hudson, J.L.; Campbell, W.W. Effect of whey protein supplementation on body composition changes in women: A systematic review and meta-analysis. Nutr. Rev. 2018, 76, 539–551. [Google Scholar] [CrossRef] [PubMed]
- Taselaar, A.E.; Boes, A.J.; de Bruin, R.W.F.; Kuijper, T.M.; Van Lancker, K.; van der Harst, E.; Klaassen, R.A. PROMISE: Effect of protein supplementation on fat-free mass preservation after bariatric surgery, a randomized double-blind placebo-controlled trial. Trials 2023, 24, 717. [Google Scholar] [CrossRef] [PubMed]
- Luijpers, C.L.H.; Nuijten, M.A.H.; Groenhuijzen, E.J.; van Hogezand, L.L.; Monpellier, V.M.; Eijsvogels, T.M.H.; Hopman, M.T.E. Protein Supplement Tolerability and Patient Satisfaction after Bariatric Surgery. Obes. Surg. 2024, 34, 3866–3875. [Google Scholar] [CrossRef]
- King, W.C.; Belle, S.H.; Hinerman, A.S.; Mitchell, J.E.; Steffen, K.J.; Courcoulas, A.P. Patient Behaviors and Characteristics Related to Weight Regain After Roux-en-Y Gastric Bypass: A Multicenter Prospective Cohort Study. Ann. Surg. 2020, 272, 1044–1052. [Google Scholar] [CrossRef]
- Oppert, J.M.; Bellicha, A.; Roda, C.; Bouillot, J.L.; Torcivia, A.; Clement, K.; Poitou, C.; Ciangura, C. Resistance Training and Protein Supplementation Increase Strength After Bariatric Surgery: A Randomized Controlled Trial. Obesity 2018, 26, 1709–1720. [Google Scholar] [CrossRef] [PubMed]
- Streb, A.R.; Hansen, F.; Gabiatti, M.P.; Tozetto, W.R.; Del Duca, G.F. Phase angle associated with different indicators of health-related physical fitness in adults with obesity. Physiol. Behav. 2020, 225, 113104. [Google Scholar] [CrossRef]
- Manoel, R.; Venâncio, F.A.; Miguel, G.P.S.; Haraguchi, F.K.; Pedrosa, R.G. A Higher Phase Angle Is Associated with Greater Metabolic Equivalents in Women 1 Year After Bariatric Surgery. Obes. Surg. 2022, 32, 2003–2009. [Google Scholar] [CrossRef]
- Alvarez-Leite, J.I. Nutrient deficiencies secondary to bariatric surgery. Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 569–575. [Google Scholar] [CrossRef]
| Composition: | Maltodextrin | Whey Protein | ||
|---|---|---|---|---|
| In 100 g | In 30 g | In 100 g | In 30 g | |
| Energy Value | 372 kcal | 111.6 kcal | 375 kcal | 112.5 kcal |
| Carbohydrate | 93 g | 27.9 g | 0 g | 0 g |
| Protein | 0 g | 0 g | 87 g | 26.1 g |
| Fat | 0 g | 0 g | 0 g | 0 g |
| Sodium | 0 g | 0 g | 155 mg | 46.5 mg |
| Parameters | Malto T0 | Whey T0 | ||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age (years): | 47.60 | 7.45 | 46.00 | 8.26 |
| Height (m) | 1.63 | 0.05 | 1.60 | 0.06 |
| Pre-surgical weight (kg): | 125.5 | 21.35 | 126.7 | 15.63 |
| 1 BMI at surgery (kg/m2): | 48.72 | 7.30 | 48.17 | 5.79 |
| 1-year post-surgical weight (kg): | 89.51 | 23.91 | 88.15 | 11.24 |
| Parameters | Malto | Whey | ||||
|---|---|---|---|---|---|---|
| No Activity | <150 min/wk | >150 min/wk | No Activity | <150 min/wk | >150 min/wk | |
| Frequency | 42.2% | 31.5% | 26.3% | 37.5% | 37.5% | 25% |
| Aerobic Exercises | - | 21% | 15.8% | - | 29.2% | 16.7% |
| Anaerobic Exercises | - | 10.5% | 10.5% | - | 8.3% | 8.3% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henriques, H.K.F.; Kattah, F.M.; Piccolo, M.S.; dos Santos, E.A.; de Araújo Ventura, L.H.; Cerqueira, F.R.; Vieira, C.M.A.F.; Leite, J.I.A. The Role of Whey Protein in Maintaining Fat-Free Mass and Promoting Fat Loss After 18 Months of Bariatric Surgery. Obesities 2025, 5, 42. https://doi.org/10.3390/obesities5020042
Henriques HKF, Kattah FM, Piccolo MS, dos Santos EA, de Araújo Ventura LH, Cerqueira FR, Vieira CMAF, Leite JIA. The Role of Whey Protein in Maintaining Fat-Free Mass and Promoting Fat Loss After 18 Months of Bariatric Surgery. Obesities. 2025; 5(2):42. https://doi.org/10.3390/obesities5020042
Chicago/Turabian StyleHenriques, Hirla Karen Fialho, Fabiana Martins Kattah, Matheus Soares Piccolo, Elandia Aparecida dos Santos, Lucas Haniel de Araújo Ventura, Flávia Rodrigues Cerqueira, Claudia Maria Andrade Fernandes Vieira, and Jacqueline Isaura Alvarez Leite. 2025. "The Role of Whey Protein in Maintaining Fat-Free Mass and Promoting Fat Loss After 18 Months of Bariatric Surgery" Obesities 5, no. 2: 42. https://doi.org/10.3390/obesities5020042
APA StyleHenriques, H. K. F., Kattah, F. M., Piccolo, M. S., dos Santos, E. A., de Araújo Ventura, L. H., Cerqueira, F. R., Vieira, C. M. A. F., & Leite, J. I. A. (2025). The Role of Whey Protein in Maintaining Fat-Free Mass and Promoting Fat Loss After 18 Months of Bariatric Surgery. Obesities, 5(2), 42. https://doi.org/10.3390/obesities5020042






