Effects of Combining Shockwaves or Radiofrequency with Aerobic Exercise on Subcutaneous Adipose Tissue and Lipid Mobilization: A Randomized Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample and Eligibiliy Criteria
2.3. Instruments and Variables
2.3.1. Questionnaires
2.3.2. Anthropometric Measurements and Body Composition
2.3.3. Clinical Analyses
2.3.4. Shockwave Protocol
2.3.5. Radiofrequency Protocol
2.3.6. Aerobic Exercise Protocol
2.4. Data Collection
2.5. Sample Size Calculation and Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAT | Subcutaneous adipose tissue |
| TG | Triglycerides |
| ESWT | External shock wave therapy |
| RF | Radiofrequency |
| IL-6 | Interleukin-6 |
| BMI | Body mass index |
| IPAQ | International physical activity questionnaire |
| MET | Equivalent metabolic |
| FFQ | Food frequency questionnaire |
| HR | Heart rate |
References
- Duncan, D.I.; Kim, T.H.M.; Temaat, R. Quantification of Adipose Volume Reduction with a Prospective Study Analyzing the Application of External Radiofrequency Energy and High Voltage Ultrashort Pulse Duration Electrical Fields. J. Cosmet. Laser Ther. 2016, 18, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Crewe, C.; An, Y.A.; Scherer, P.E. The Ominous Triad of Adipose Tissue Dysfunction: Inflammation, Fibrosis, and Impaired Angiogenesis. J. Clin. Investig. 2017, 127, 74–82. [Google Scholar] [CrossRef]
- Maillard, F.; Pereira, B.; Boisseau, N. Effect of High-Intensity Interval Training on Total, Abdominal and Visceral Fat Mass: A Meta-Analysis. Sports Med. 2018, 48, 269–288. [Google Scholar] [CrossRef]
- Romero, M.; Zorzano, A. Role of Autophagy in the Regulation of Adipose Tissue Biology. Cell Cycle 2019, 18, 1435–1445. [Google Scholar] [CrossRef]
- Nassar, A.H.; Dorizas, A.S.; Shafai, A.; Sadick, N.S. A Randomized, Controlled Clinical Study to Investigate the Safety and Efficacy of Acoustic Wave Therapy in Body Contouring. Dermatol. Surg. 2015, 41, 366–370. [Google Scholar] [CrossRef]
- van Baak, M.A.; Mariman, E.C.M. Mechanisms of Weight Regain After Weight Loss—The Role of Adipose Tissue. Nat. Rev. Endocrinol. 2019, 15, 274–287. [Google Scholar] [CrossRef] [PubMed]
- Friedmann, D.P. A Review of the Aesthetic Treatment of Abdominal Subcutaneous Adipose Tissue: Background, Implications, and Therapeutic Options. Dermatol. Surg. 2015, 41, 18–34. [Google Scholar] [CrossRef] [PubMed]
- Thomaz, F.S.; John, O.D.; Sinha, P.; Shafie, S.R.; Worrall, S. The Metabolic Syndrome: An Overview and Proposed Mechanisms. Obesities 2024, 4, 226–255. [Google Scholar] [CrossRef]
- Leopold, J.A.; Antman, E.M. Obesity and Ideal Cardiovascular Health: Results from the My Research Legacy Study. Obesities 2021, 1, 36–48. [Google Scholar] [CrossRef]
- Kouidrat, Y.; Louhou, R.; Mondot, C.; Daami, I.; Amad, A.; Diouf, M. Quality of Life in Patients with Obesity: The Role of Multidisciplinary Rehabilitation Medicine. Obesities 2024, 4, 160–168. [Google Scholar] [CrossRef]
- Mazzoni, D.; Lin, M.J.; Dubin, D.P.; Khorasani, H. Review of Non-Invasive Body Contouring Devices for Fat Reduction, Skin Tightening and Muscle Definition. Australas. J. Dermatol. 2019, 60, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Pinto, H. Local Fat Treatments: Classification Proposal. Adipocyte 2016, 5, 22–26. [Google Scholar] [CrossRef]
- Alizadeh, Z.; Halabchi, F.; Mazaheri, R.; Abolhasani, M.; Tabesh, M. Review of the Mechanisms and Effects of Noninvasive Body Contouring Devices on Cellulite and Subcutaneous Fat. Int. J. Endocrinol. Metab. 2016, 14, e36727. [Google Scholar] [CrossRef]
- Modena, D.A.O.; da Silva, C.N.; Grecco, C.; Guidi, R.M.; Moreira, R.G.; Coelho, A.A.; Sant’Ana, E.; de Souza, J.R. Extracorporeal shockwave: Mechanisms of Action and Physiological Aspects for Cellulite, Body Shaping, and Localized Fat—Systematic Review. J. Cosmet. Laser Ther. 2017, 19, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Simplicio, C.L.; Purita, J.; Murrell, W.; Santos, G.S.; dos Santos, R.G.; Lana, J.F.S.D. Extracorporeal Shock Wave Therapy Mechanisms in Musculoskeletal Regenerative Medicine. J. Clin. Orthop. Trauma 2020, 11, S309–S318. [Google Scholar] [CrossRef]
- Franco, W.; Kothare, A.; Goldberg, D.J. Controlled Volumetric Heating of Subcutaneous Adipose Tissue Using a Novel Radiofrequency Technology. Lasers Surg. Med. 2009, 41, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Vale, A.L.; Pereira, A.S.; Morais, A.; Noites, A.; Mendonça, A.C.; Martins Pinto, J.; Vilarinho, R.; Carvalho, P. Effects of Radiofrequency on Adipose Tissue: A Systematic Review with Meta-Analysis. J. Cosmet. Dermatol. 2018, 17, 703–711. [Google Scholar] [CrossRef]
- Ahmadian, M.; Duncan, R.E.; Sul, H.S. The Skinny on Fat: Lipolysis and Fatty Acid Utilization in Adipocytes. Trends Endocrinol. Metab. 2009, 20, 424–428. [Google Scholar] [CrossRef]
- Mika, A.; Macaluso, F.; Barone, R.; Di Felice, V.; Sledzinski, T. Effect of Exercise on Fatty Acid Metabolism and Adipokine Secretion in Adipose Tissue. Front. Physiol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Hopewell, S.; Boutron, I.; Chan, A.-W.; Collins, G.S.; de Beyer, J.A.; Hróbjartsson, A.; Nejstgaard, C.H.; Østengaard, L.; Schulz, K.F.; Tunn, R.; et al. An Update to SPIRIT and CONSORT Reporting Guidelines to Enhance Transparency in Randomized Trials. Nat. Med. 2022, 28, 1740–1743. [Google Scholar] [CrossRef]
- Craig, C.L.; Marshall, A.L.; Sjöström, M.; Bauman, A.E.; Booth, M.L.; Ainsworth, B.E.; Pratt, M.; Ekelund, U.; Yngve, A.; Sallis, J.F.; et al. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003, 35, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Lopes, C.; Aro, A.; Azevedo, A.; Ramos, E.; Barros, H. Intake and Adipose Tissue Composition of Fatty Acids and Risk of Myocardial Infarction in a Male Portuguese Community Sample. J. Am. Diet. Assoc. 2007, 107, 276–286. [Google Scholar] [CrossRef]
- Bolanowski, M.; Nilsson, B.E. Assessment of Human Body Composition Using Dual-Energy X-Ray Absorptiometry and Bioelectrical Impedance Analysis. Med. Sci. Monit. 2001, 7, 1029–1033. [Google Scholar]
- Vale, A.L.; Pereira, A.S.; Morais, A.; de Carvalho, P.; Vilarinho, R.; Mendonça, A.; Noites, A. Effect of Four Sessions of Aerobic Exercise with Abdominal Radiofrequency in Adipose Tissue in Healthy Women: Randomized Control Trial. J. Cosmet. Dermatol. 2020, 19, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Borg, G.A.V. Psychophysical Bases of Perceived Exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Monahan, K.D.; Seals, D.R. Age-Predicted Maximal Heart Rate Revisited. J. Am. Coll. Cardiol. 2001, 37, 153–156. [Google Scholar] [CrossRef]
- ACSM. American College of Sports Medicine’s Exercise Testing and Prescription; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2023. [Google Scholar]
- Maher, J.M.; Markey, J.C.; Ebert-May, D. The Other Half of the Story: Effect Size Analysis in Quantitative Research. CBE—Life Sci. Educ. 2013, 12, 345–351. [Google Scholar] [CrossRef]
- Duncan, D.I.; Kim, T.H.M.; Temaat, R. A Prospective Study Analyzing the Application of Radiofrequency Energy and High-Voltage, Ultrashort Pulse Duration Electrical Fields on the Quantitative Reduction of Adipose Tissue. J. Cosmet. Laser Ther. 2016, 18, 257–267. [Google Scholar] [CrossRef]
- Adatto, M.A.; Adatto-Neilson, R.; Novak, P.; Krotz, A.; Haller, G. Body Shaping with Acoustic Wave Therapy AWT®/EPAT®: Randomized, Controlled Study on 14 Subjects. J. Cosmet. Laser Ther. 2011, 13, 291–296. [Google Scholar] [CrossRef]
- Ferraro, G.A.; De Francesco, F.; Cataldo, C.; Rossano, F.; Nicoletti, G.; D’Andrea, F. Synergistic Effects of Cryolipolysis and Shock Waves for Noninvasive Body Contouring. Aesthetic Plast. Surg. 2012, 36, 666–679. [Google Scholar] [CrossRef]
- Hexsel, D.; Camozzato, F.O.; Silva, A.F.; Siega, C. Acoustic Wave Therapy for Cellulite, Body Shaping and Fat Reduction. J. Cosmet. Laser Ther. 2017, 19, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, X.; Guo, A.; Liu, S.; Hu, G. Quantitative Assessments of Mechanical Responses Upon Radial Extracorporeal Shock Wave Therapy. Adv. Sci. 2018, 5, 1700797. [Google Scholar] [CrossRef]
- Sukubo, N.G.; Tibalt, E.; Respizzi, S.; Locati, M.; d’Agostino, M.C. Effect of Shock Waves on Macrophages: A Possible Role in Tissue Regeneration and Remodeling. Int. J. Surg. 2015, 24, 124–130. [Google Scholar] [CrossRef]
- Shoham, N.; Gefen, A. Mechanotransduction in Adipocytes. J. Biomech. 2012, 45, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, C.; Angehrn, F.; Sonnabend, O.; Voss, A. Impact of Extracorporeal Shock Waves on the Human Skin with Cellulite: A Case Study of an Unique Instance. Clin. Interv. Aging 2008, 3, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Schlaudraff, K.U.; Kiessling, M.C.; Császár, N.B.; Schmitz, C. Predictability of the Individual Clinical Outcome of Extracorporeal Shock Wave Therapy for Cellulite. Clin. Cosmet. Investig. Dermatol. 2014, 7, 171–183. [Google Scholar] [CrossRef]
- Christ, C.; Brenke, R.; Sattler, G.; Siems, W.; Novak, P.; Daser, A. Improvement in Skin Elasticity in the Treatment of Cellulite and Connective Tissue Weakness by Means of Extracorporeal Pulse Activation Therapy. Aesthetic Surg. J. 2008, 28, 538–544. [Google Scholar] [CrossRef]
- Angehrn, F.; Kuhn, C.; Voss, A. Can Cellulite Be Treated with Low-Energy Extracorporeal Shock Wave Therapy? Clin. Interv. Aging 2007, 2, 623–630. [Google Scholar] [CrossRef]
- Mariotto, S.; de Prati, A.C.; Cavalieri, E.; Amelio, E.; Marlinghaus, E.; Suzuki, H. Extracorporeal Shock Wave Therapy in Inflammatory Diseases: Molecular Mechanism That Triggers Anti-Inflammatory Action. Curr. Med. Chem. 2009, 16, 2366–2372. [Google Scholar] [CrossRef]
- van Hall, G. The Physiological Regulation of Skeletal Muscle Fatty Acid Supply and Oxidation During Moderate-Intensity Exercise. Sports Med. 2015, 45, 23–32. [Google Scholar] [CrossRef]
- Hayre, N.; Palm, M.; Jenkin, P. A Clinical Evaluation of a Next Generation, Non-Invasive, Selective Radiofrequency, Hands-Free, Body-Shaping Device. J. Drugs Dermatol. 2016, 15, 1557–1561. [Google Scholar] [PubMed]
- Mlosek, R.K.; Woźniak, W.; Malinowska, S.; Lewandowski, M.; Nowicki, A. The Effectiveness of Anticellulite Treatment Using Tripolar Radiofrequency Monitored by Classic and High-Frequency Ultrasound. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Suh, D.H.; Kim, C.M.; Lee, S.J.; Kim, H.; Yeom, S.K.; Ryu, H.J. Safety and Efficacy of a Non-Contact Radiofrequency Device for Body Contouring in Asians. J. Cosmet. Laser Ther. 2017, 19, 89–92. [Google Scholar] [CrossRef]
- Wanitphakdeedecha, R.; Sathaworawong, A.; Manuskiatti, W.; Sadick, N.S. Efficacy of Multipolar Radiofrequency with Pulsed Magnetic Field Therapy for the Treatment of Abdominal Cellulite. J. Cosmet. Laser Ther. 2017, 19, 205–209. [Google Scholar] [CrossRef]
- Levy, A.S.; Grant, R.T.; Rothaus, K.O. Radiofrequency Physics for Minimally Invasive Aesthetic Surgery. Clin. Plast. Surg. 2016, 43, 551–556. [Google Scholar] [CrossRef]
- Sadick, N.; Rothaus, K.O. Aesthetic Applications of Radiofrequency Devices. Clin. Plast. Surg. 2016, 43, 557–565. [Google Scholar] [CrossRef]
- Albornoz-Cabello, M.; Ibáñez-Vera, A.J.; De la Cruz-Torres, B. Efficacy of Monopolar Dielectric Transmission Radio Frequency in Panniculus Adiposus and Cellulite Reduction. J. Cosmet. Laser Ther. 2017, 19, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Boisnic, S.; Branchet, M.C.; Birnstiel, O.; Beilin, G. Clinical and Histopathological Study of the TriPollar Home-Use Device for Body Treatments. Eur. J. Dermatol. 2010, 20, 367–372. [Google Scholar] [CrossRef]
- Kaplan, H.; Gat, A. Clinical and Histopathological Results Following TriPollar™ Radiofrequency Skin Treatments. J. Cosmet. Laser Ther. 2009, 11, 78–84. [Google Scholar] [CrossRef]
- Levenberg, A. Clinical Experience with a TriPollar Radiofrequency System for Facial and Body Aesthetic Treatments. Eur. J. Dermatol. 2010, 20, 615–619. [Google Scholar]
- Rossmann, C.; Haemmerich, D. Review of Temperature Dependence of Thermal Properties, Dielectric Properties, and Perfusion of Biological Tissues at Hyperthermic and Ablation Temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Franco, W.; Kothare, A.; Ronan, S.J.; Grekin, R.C.; McCalmont, T.H. Hyperthermic Injury to Adipocyte Cells by Selective Heating of Subcutaneous Fat with a Novel Radiofrequency Device: Feasibility Studies. Lasers Surg. Med. 2010, 42, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Purdom, T.; Kravitz, L.; Dokladny, K.; Mermier, C. Understanding the Factors that Effect Maximal Fat Oxidation. J. Int. Soc. Sports Nutr. 2018, 15, 3. [Google Scholar] [CrossRef] [PubMed]
- Swift, D.L.; McGee, J.E.; Earnest, C.P.; Carlisle, E.; Nygard, M.; Johannsen, N.M. The Effects of Exercise and Physical Activity on Weight Loss and Maintenance. Prog. Cardiovasc. Dis. 2018, 61, 206–213. [Google Scholar] [CrossRef]
- Noites, A.; Vale, A.L.; Pereira, A.S.; Morais, A.; Vilarinho, R.; Carvalho, P.; Amorim, M.; Moreira, T.; Mendonça, A. Effect of an Aerobic Exercise Session Combined with Abdominal Radiofrequency on Lipolytic Activity in Women: Randomized Control Trial. J. Cosmet. Dermatol. 2020, 19, 638–645. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–22. [Google Scholar] [CrossRef]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on Cardiovascular Disease Prevention in Clinical Practice (Version 2012). The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by Representatives of Nine Societies and by Invited Experts). Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [CrossRef]
| EG 1 (n = 10) | EG2 (n = 11) | CG (n = 10) | Differences Between Groups, ANOVA (p Value) | |
|---|---|---|---|---|
| Age, years | 36.50 ± 9.3 | 38.27 ± 10.6 | 35.40 ± 11.1 | 0.816 |
| Height, m | 1.62 ± 0.65 | 1.61 ± 0.65 | 1.63 ± 5.04 | 0.724 |
| Body mass (kg) | 64.11 ± 6.18 | 61.66 ± 6.53 | 63.09 ± 10.88 | 0.786 |
| BMI, kg/m2 | 24.21 ± 1.81 | 23.68 ± 2.05 | 23.67 ± 3.99 | 0.882 |
| Waist/hip ratio | 0.84 ± 0.05 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.761 |
| Muscle mass, kg | 42.94 ± 3.66 | 42.01 ± 4.69 | 42.89 ± 4.60 | 0.858 |
| Fat mass, % | 29.28 ± 4.19 | 27.91 ± 5.11 | 27.43 ± 7.45 | 0.756 |
| IPAQ-METs, minutes/week | 1622.10 ± 1520.91 | 1381.27 ± 858.40 | 1100.60 ± 741.67 | 0.569 |
| FFQ | ||||
| Energy, (kcal/day) | 2071.04 ± 789.19 | 1999.24 ± 580.04 | 1901.06 ± 469.17 | 0.831 |
| Proteins, (g/day) | 92.80 ± 37.36 | 108.05 ± 28.79 | 92.66 ± 21.10 | 0.403 |
| Carbohydrates, (g/day) | 206.37 ± 95.91 | 215.31 ± 56.02 | 212.88 ± 62.82 | 0.960 |
| Total fat, (g/day) | 102.0 ± 47.32 | 80.32 ± 29.09 | 78.16 ± 23.79 | 0.249 |
| Saturated fat, (g/day) | 23.26 ± 7.34 | 21.91 ± 7.03 | 19.56 ± 5.69 | 0.471 |
| Monounsaturated fat, (g/day) | 48.09 ± 26.48 | 36.30 ± 15.73 | 37.47 ± 12.84 | 0.324 |
| Polyunsaturated fat, (g/day) | 23.27 ± 17.66 | 15.13 ± 6.04 | 14.66 ± 4.57 | 0.157 |
| Cholesterol, (mg/day) | 307.91 ± 67.45 | 361.84 ± 119.47 | 366.26 ± 153.97 | 0.480 |
| Sugars, (g/day) | 102.62 ± 59.55 | 89.01 ± 24.49 | 91.95 ± 34.45 | 0.740 |
| EG 1 (n = 10) | EG2 (n = 11) | CG (n = 10) | Differences Between Groups, ANOVA (p Value) | Effect Size (η2p) | Difference Between Moments, t Test (p Value) | |
|---|---|---|---|---|---|---|
| Body Mass, kg | ||||||
| M0 | 64.11 ± 6.18 | 61.66 ± 6.53 | 63.09 ± 10.88 | 0.786 | EG1: 1.000 | |
| M1 | 64.11 ± 6.42 | 61.59 ± 6.67 | 63.18 ± 10.46 | 0.768 | EG2: 0.718 | |
| M1-M0 (95% CI) | 0 ± 0.89 (−0.64; 0.63) | −0.07 ± 0.65 (−0.51; 0.36) | 0.09 ± 0.92 (−0.57; 0.75) | 0.903 | 0.007 | CG: 0.765 |
| Fat mass, % | ||||||
| M0 | 29.28 ± 4.19 | 27.91 ± 5.11 | 27.43 ± 7.45 | 0.756 | EG1: 0.680 | |
| M1 | 29.44 ± 3.78 | 28.47 ± 4.74 | 27.45 ± 6.61 | 0.693 | EG2: 0.089 | |
| M1-M0 (95% CI) | 0.16 ± 1.19 (−0.69; 1.00) | 0.56 ± 0.99 (−0.10; 1.23) | 0.02 ± 1.05 (−0.73; 0.77) | 0.492 | 0.049 | CG: 0.954 |
| Muscle mass, kg | ||||||
| M0 | 42.94 ± 3.66 | 42.01 ± 4.69 | 42.89 ± 4.60 | 0.858 | EG1: 0.702 | |
| M1 | 42.86 ± 3.69 | 41.9 ± 4.61 | 43.06 ± 4.76 | 0.810 | EG2: 0.649 | |
| M1-M0 (95% CI) | −0.08 ± 0.64 (−0.53; 0.38) | −0.11 ± 0.77 (−0.63; 0.41) | 0.17 ± 0.54 (−0.21; 0.55) | 0.582 | 0.001 | CG: 0.342 |
| Trunk fat mass, % | ||||||
| M0 | 24.09 ± 5.20 | 23.14 ± 5.27 | 21.7 ± 9.14 | 0.730 | EG1: 0.760 | |
| M1 | 23.87 ± 4.67 | 22.78 ± 5.84 | 21.76 ± 7.52 | 0.745 | EG2: 0.653 | |
| M1-M0 (95% CI) | −0.22 ± 2.21 (−1.80; 1.36) | −0.35 ± 2.54 (−2.06; 1.35) | 0.06 ± 1.83 (−1.25; 1.37) | 0.911 | 0.007 | CG: 0.920 |
| Visceral fat index | ||||||
| M0 | 4.6 ± 2.59 | 4.27 ± 1.90 | 3.8 ± 2.10 | 0.720 | EG1: 0.357 | |
| M1 | 4.0 ± 1.56 | 3.91 ± 1.97 | 3.7 ± 2.0 | 0.934 | EG2: 0.221 | |
| M1-M0 (95% CI) | −0.6 ± 1.96 (−2.0; 0.80) | −0.36 ± 0.92 (−0.98; 0.26) | −0.1 ± 0.32 (−0.33; 0.13) | 0.674 | 0.030 | CG: 0.343 |
| BMI, kg/m2 | ||||||
| M0 | 24.21 ± 1.81 | 23.68 ± 2.05 | 23.67 ± 3.99 | 0.882 | EG1: 0.769 | |
| M1 | 24.18 ± 1.87 | 23.65 ± 2.02 | 23.71 ± 3.81 | 0.890 | EG2: 0.740 | |
| M1-M0 (95% CI) | −0.03 ± 0.31 (−0.25; 0.19) | −0.03 ± 0.26 (−0.21; 1.15) | 0.04 ± 0.36 (−0.22; 0.29) | 0.852 | 0.011 | CG: 0.735 |
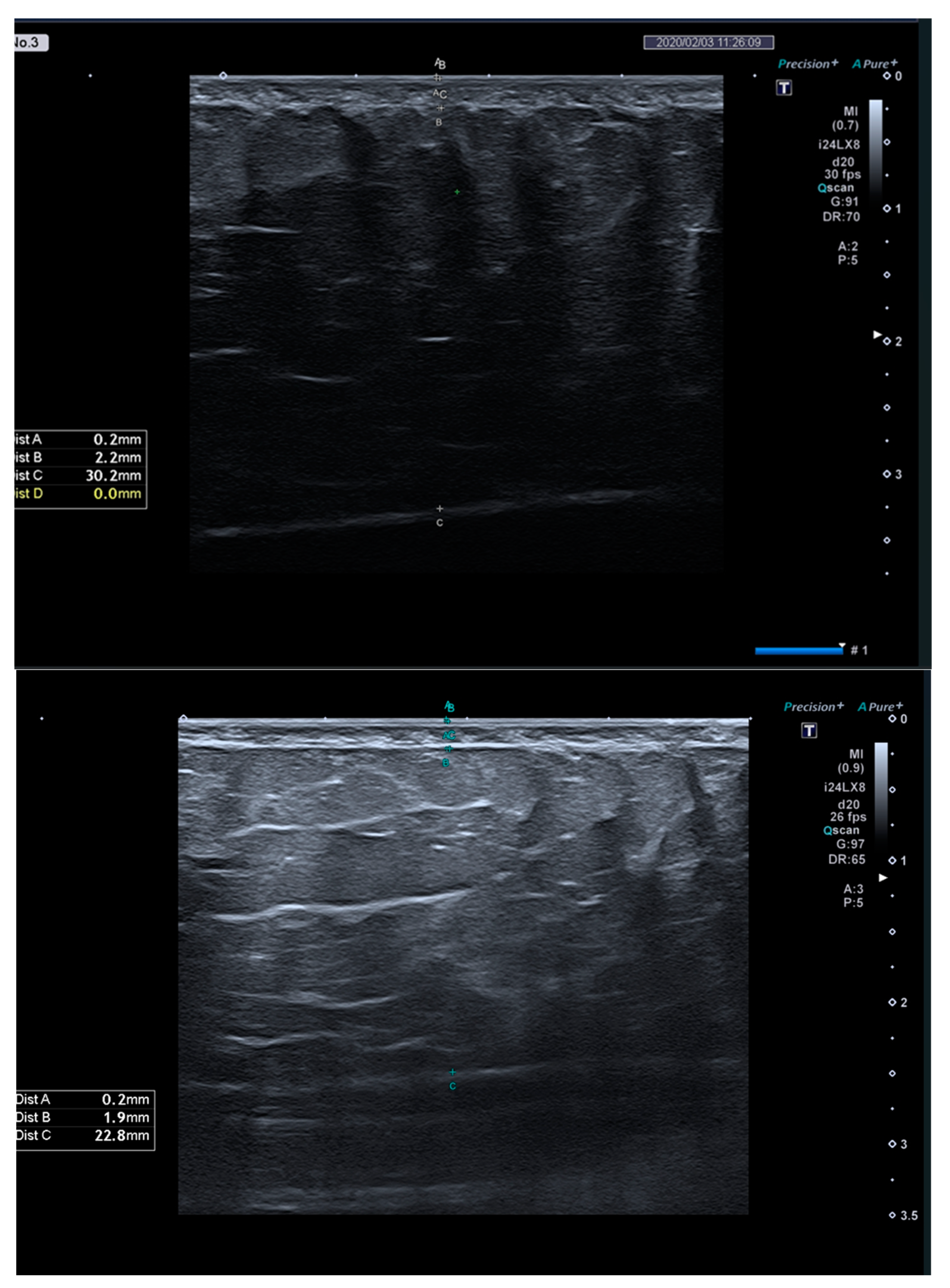
| SAT thickness, mm | ||||||
| M0 | 21.93 ± 6.66 | 17.42 ± 5.36 | 19.13 ± 10.30 | 0.410 | EG1: <0.001 | |
| M1 | 18.86 ± 7.01 | 14.29 ± 4.43 | 18.81 ± 10.07 | 0.282 | EG2: <0.001 | |
| M1-M0 (95% CI) | −3.07 ± 1.25 (−3.97; −2.17) | −3.13 ± 1.92 (−4.41; −1.84) | −0.32 ± 1.27 (−1.22; 0.59) | <0.001 a | 0.446 | CG: 0.449 |
| Waist-to-hip ratio | ||||||
| M0 | 0.84 ± 0.05 | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.761 | EG1: <0.001 | |
| M1 | 0.82 ± 0.05 | 0.82 ± 0.04 | 0.82 ± 0.04 | 0.987 | EG2: <0.001 | |
| M1-M0 (95% CI) | −0.02 ± 0.01 (−0.02; −0.01) | −0.02 ± 0.01 (−0.03; −0.01) | −0.01 ± 0.01 (−0.01; −0.003) | 0.001 b | 0.408 | CG: 0.004 |
| Mean Temperature, °C | ||||||
| M0 | 28.99 ± 3.19 | 30.4 ± 1.88 | 30.23 ± 1.79 | 0.350 | EG1: 0.744 | |
| M1 | 29.33 ± 0.82 | 30.02 ± 1.56 | 30.51 ± 1.85 | 0.218 | EG2: 0.536 | |
| M1-M0 (95% CI) | 0.34 ± 3.19 (−1.94; 2.62) | −0.38 ± 1.98 (−1.71; 0.95) | 0.28 ± 2.32 (−0.84; 0.97) | 0.768 | 0.019 | CG: 0.712 |
| EG 1 (n = 10) | EG2 (n = 11) | CG (n = 10) | Differences Between Groups, ANOVA (p Value) | Effect Size (η2p) | Difference Between Moments, t Test (p Value) | |
|---|---|---|---|---|---|---|
| Glycerol, mmol/L | ||||||
| M0 | 0.06 ± 0.03 | 0.05 ± 0.02 | 0.07 ± 0.04 | 0.255 | EG1: 0.169 | |
| M1 | 0.05 ± 0.02 | 0.04 ± 0.03 | 0.04 ± 0.01 | 0.760 | EG2: 0.855 | |
| M1-M0 (95% CI) | −0.01 ± 0.03 (−0.03; 0.01) | 0.00 ± 0.02 (−0.01; 0.01) | −0.03 ± 0.03 (−0.05; −0.002) | 0.127 | 0.142 | CG: 0.080 |
| Total cholesterol, mg/dL | ||||||
| M0 | 167.89 ± 41.87 | 149.25 ± 30.57 | 138.39 ± 28.1 | 0.176 | EG1: 0.657 | |
| M1 | 163.61 ± 26.66 | 162.55 ± 45.77 | 150.1 ± 28.67 | 0.646 | EG2: 0.086 | |
| M1-M0 (95% CI) | −4.28 ± 27.82 (−25.66; 17.11) | 13.31 ± 23.19 (−2.27; 28.98) | 11.71 ± 19.12 (−1.97; 25.34) | 0.214 | 0.108 | CG: 0.085 |
| Triglycerides, mg/dL | ||||||
| M0 | 69.72 ± 31.18 | 54.74 ± 20.42 | 47.39 ± 12.80 | 0.105 | EG1: 0.370 | |
| M1 | 75.58 ± 26.60 | 67.85 ± 34.44 | 50.72 ± 13.75 | 0.131 | EG2: 0.038 | |
| M1-M0 (95% CI) | 5.86 ± 18.50 (−8.37; 20.07) | 13.11 ± 18.25 (0.85; 25.37) | 3.33 ± 5.23 (−0.41; 7.07) | 0.328 | 0.079 | CG: 0.075 |
| HDL cholesterol, mg/dL | ||||||
| M0 | 52.81 ± 16.71 | 55.03 ± 9.06 | 50.11 ± 10.20 | 0.656 | EG1: 0.993 | |
| M1 | 52.79 ± 12.66 | 58.41 ± 13.27 | 52.8 ± 11.43 | 0.504 | EG2: 0.288 | |
| M1-M0 (95% CI) | −0.02 ± 7.77 (−5.99; 5.94) | 3.38 ± 9.99 (−3.33; 10.09) | 2.69 ± 9.03 (−3.77; 9.14) | 0.689 | 0.027 | CG: 0.371 |
| LDL cholesterol, mg/dL | ||||||
| M0 | 101.13 ± 29.27 | 83.27 ± 25.45 | 78.80 ± 20.91 | 0.148 | EG1: 0.445 | |
| M1 | 95.71 ± 20.28 | 90.58 ± 32.53 | 87.16 ± 22.78 | 0.777 | EG2: 0.078 | |
| M1-M0 (95% CI) | −5.43 ± 20.28 (−21.01; 10.16) | 7.31 ± 12.34 (−0.98; 15.60) | 8.35 ± 12.30 (−0.44; 17.16) | 0.108 | 0.152 | CG: 0.060 |
| EG 1 (n = 10) | EG2 (n = 11) | CG (n = 10) | Differences Between Groups, Kruskal–Wallis (p Value) | Difference Between Moments, Wilcoxon (p Value) | |
|---|---|---|---|---|---|
| IL-6, pg/mL | |||||
| M0 | 5.25 (3.28; 5.25) | 4.57 (3.23; 6.84) | 3.67 (1.15; 7.36) | 0.714 | EG1: 0.250 |
| M1 | 2.89 (1.92; 2.89) | 4.56 (3.53; 5.95) | 2.70 (1.45; 7.15) | 0.584 | EG2: 0.844 |
| M1–M0 | −2.36 (−2.62; −2.36) | −0.50 (−3.07; 2.72) | −0.57 (−3.58; 3.46) | 0.780 | CG: 1.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, L.; Neves, J.; Pereira, A.; Santiago, A.; Troia, S.; Vilarinho, R.; Amorim, M.M.; Noites, A. Effects of Combining Shockwaves or Radiofrequency with Aerobic Exercise on Subcutaneous Adipose Tissue and Lipid Mobilization: A Randomized Controlled Trial. Obesities 2025, 5, 31. https://doi.org/10.3390/obesities5020031
Marques L, Neves J, Pereira A, Santiago A, Troia S, Vilarinho R, Amorim MM, Noites A. Effects of Combining Shockwaves or Radiofrequency with Aerobic Exercise on Subcutaneous Adipose Tissue and Lipid Mobilization: A Randomized Controlled Trial. Obesities. 2025; 5(2):31. https://doi.org/10.3390/obesities5020031
Chicago/Turabian StyleMarques, Leila, Joana Neves, Ana Pereira, Ana Santiago, Sara Troia, Rui Vilarinho, Maria Manuela Amorim, and Andreia Noites. 2025. "Effects of Combining Shockwaves or Radiofrequency with Aerobic Exercise on Subcutaneous Adipose Tissue and Lipid Mobilization: A Randomized Controlled Trial" Obesities 5, no. 2: 31. https://doi.org/10.3390/obesities5020031
APA StyleMarques, L., Neves, J., Pereira, A., Santiago, A., Troia, S., Vilarinho, R., Amorim, M. M., & Noites, A. (2025). Effects of Combining Shockwaves or Radiofrequency with Aerobic Exercise on Subcutaneous Adipose Tissue and Lipid Mobilization: A Randomized Controlled Trial. Obesities, 5(2), 31. https://doi.org/10.3390/obesities5020031








