Alternative Dietary Strategies to Modulate Obesity and Improve Metabolic Health in Aging: A Comparative Narrative Review
Abstract
:1. Introduction
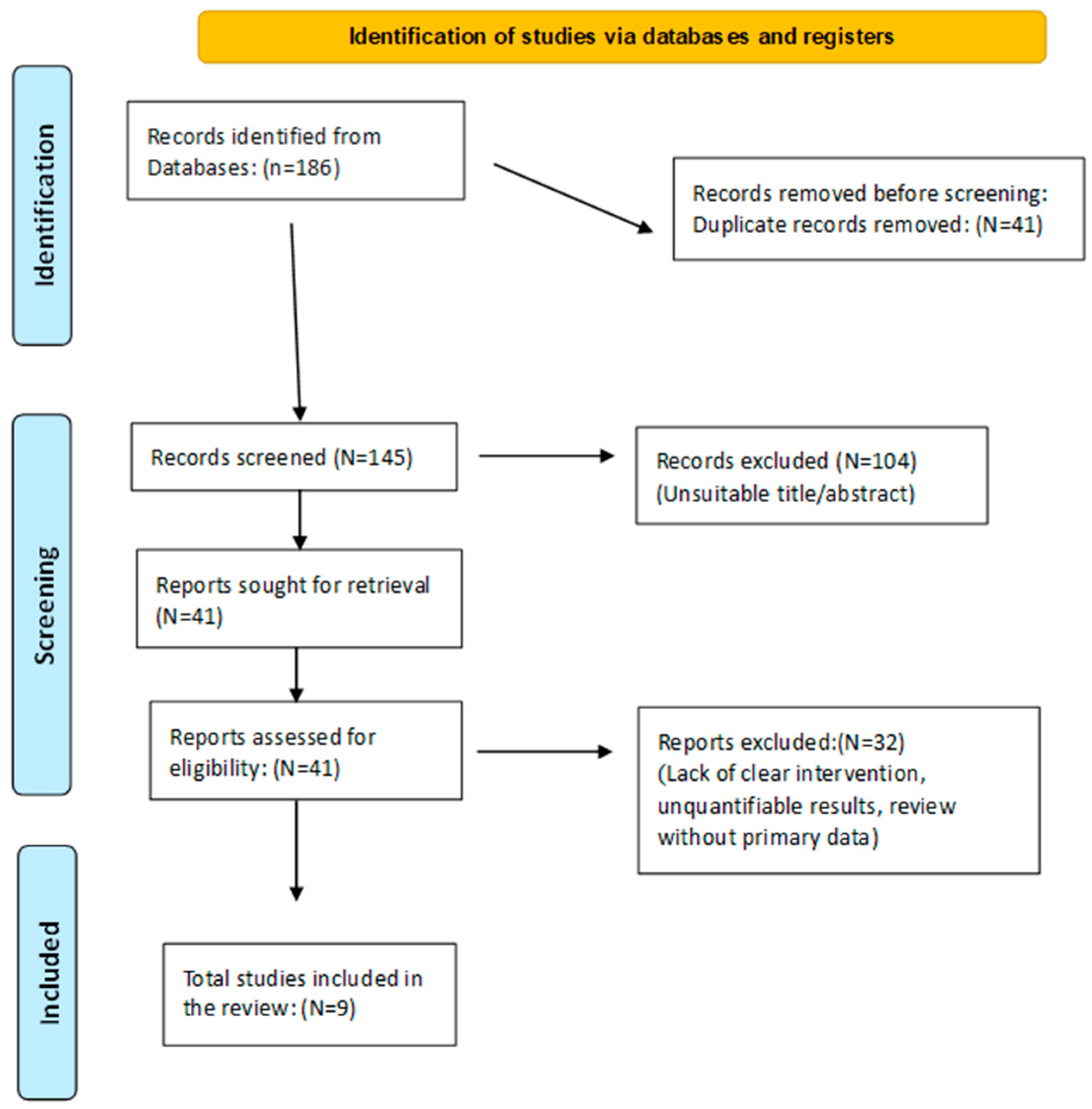
2. Materials and Methods
- Experimental studies in animals or clinical/observational trials in humans.
- Well-defined dietary interventions: FMDs (fasting-mimicking diets), TRF (time-restricted feeding), or PAAR (protein and amino acid restriction).
- Quantifiable outcomes related to markers of healthy aging or metabolic health (body weight, IGF-1, insulin, lipids, inflammation, longevity).
3. Results and Discussion
4. Discussion
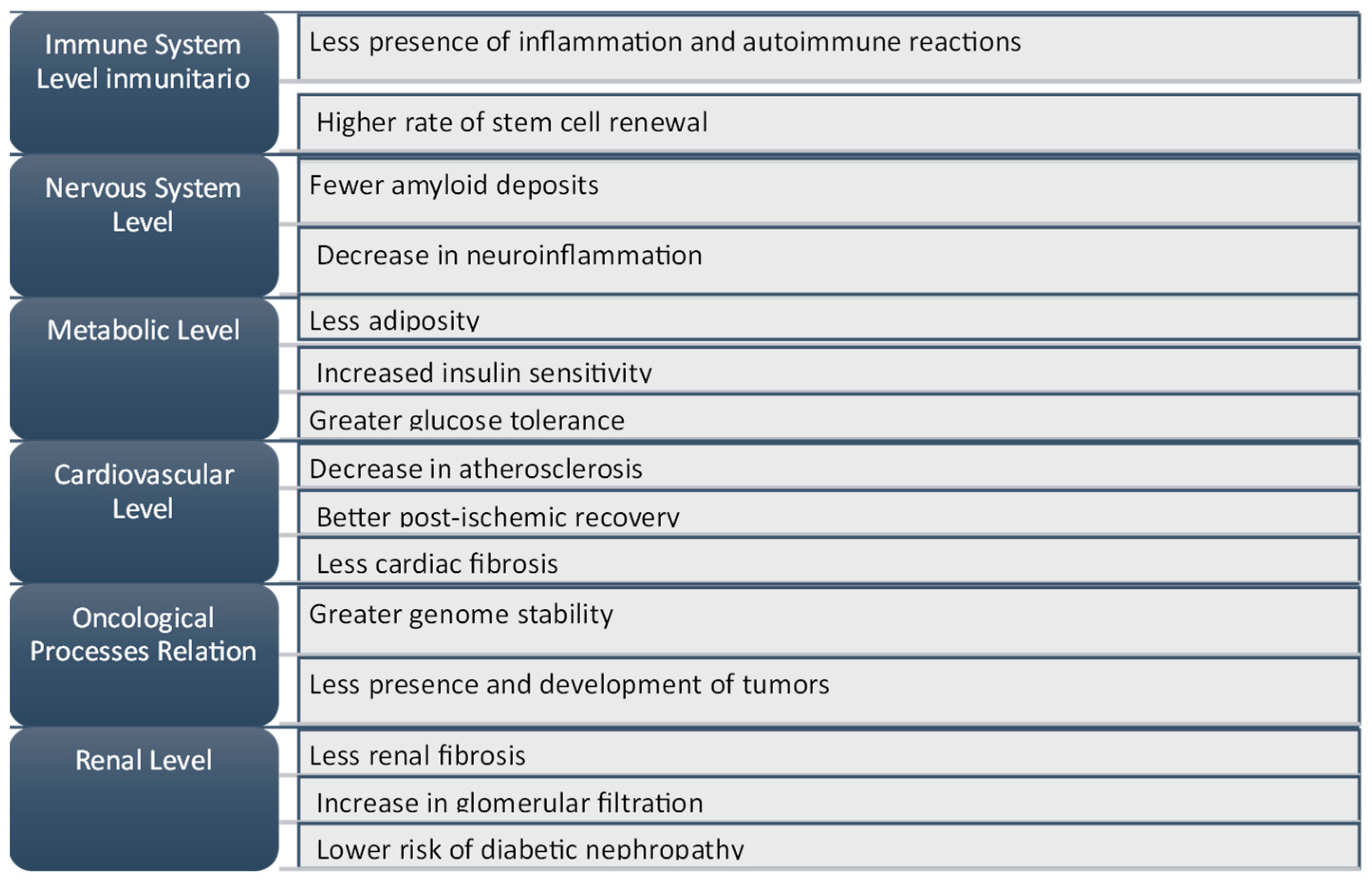
- Increased proteostasis;
- Enhanced autophagy and mitophagy;
- Greater efficiency in repairing DNA damage;
- Reduced oxidative stress;
- Decreased senescent cell population;
- Increased stem cell renewal capacity.
- Evaluated Dietary Patterns
4.1. Fasting-Mimicking Diets (FMDs)
4.2. Time-Restricted Feeding (TRF)
4.3. Dietary Protein and Amino Acid Restriction
- The mTORC1 complex and autophagy;
- The metabolism of S-adenosylmethionine (SAM) and glycine N-methyltransferase (Gnmt);
- Fibroblast growth factor 21 (FGF21);
- Growth hormone/insulin-like growth factor-1 (GH/IGF-1) signaling;
- Hydrogen sulfide;
- Oxidative stress and inflammation.
- Comparison of the Three Dietary Patterns
4.4. Study Limitations
5. Conclusions
Future Research
- Long-term clinical trials that simultaneously analyze the effects on life expectancy and quality of life, as well as the incidence rates of chronic diseases in individuals with and without obesity. A potential study could involve older adults with obesity and metabolic syndrome, assessing the evolution of inflammation biomarkers, autophagy, and insulin sensitivity under FMD, TRF, and PAAR protocols. These trials should be designed to yield both statistically and clinically significant outcomes.
- Differentiation by age groups and sex to determine whether the impact of each dietary strategy varies depending on life stage and hormonal profile. For example, a study in postmenopausal women could evaluate whether methionine and BCAA restriction enhances metabolic flexibility and reduces the risk of sarcopenia.
- An evaluation of the gut microbiota and its evolution in response to dietary interventions, given its key role in metabolism regulation and chronic inflammation. A human study could compare the microbiota composition before and after 12 weeks of TRF, FMD, or PAAR, assessing changes in key metabolites, their relationship with longevity, and the incidence of age-related diseases. Given its role as a key modulator of immune and metabolic responses, understanding microbiota dynamics may clarify individual variability in response to dietary strategies.
- Design of combined dietary protocols (e.g., TRE + PAAR) to determine synergies that improve adherence and metabolic control, particularly in obese populations. One possible study could analyze pre-diabetic patients undergoing TRF or FMD with a low-methionine diet to assess improvements in insulin sensitivity and low-grade inflammation. Such combined approaches may enhance clinical adherence and optimize therapeutic outcomes in metabolic disorders.
- Personalization based on biomarkers (lipid profile, leptin, adiponectin levels, or inflammation markers) to identify individuals with a higher probability of a favorable response. An example could be a study analyzing the relationship between baseline IGF-1 levels and the response to intermittent caloric restriction in individuals with obesity and insulin resistance. In addition to classical biomarkers, future studies could explore the relevance of epigenetic markers and inflammatory load to personalize dietary interventions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Martin, C.K.; Bhapkar, M.; Pittas, A.G.; Pieper, C.F.; Das, S.K.; Williamson, D.A.; Scott, T.; Redman, L.M.; Stein, R.; Gilhooly, C.H.; et al. Comprehensive assessment of long-term effects of reducing intake of energy (CALERIE) phase 2 study group, effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: The CALERIE 2 randomized clinical trial. JAMA Intern. Med. 2016, 176, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Stekovic, S.; Hofer, S.J.; Tripolt, N.; Aon, M.A.; Royer, P.; Pein, L.; Stadler, J.T.; Pendl, T.; Prietl, B.; Url, J.; et al. Alternate day fasting improves physiological and molecular markers of aging in healthy, non-obese humans. Cell Metab. 2020, 31, 878–881. [Google Scholar] [CrossRef]
- Lee, M.B.; Hill, C.M.; Bitto, A.; Kaeberlein, M. Antiaging diets: Separating fact from fiction. Science 2021, 374, eabe7365. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Murillo-Cancho, A.F.; Lozano-Paniagua, D.; Manzano-Agugliaro, F.; Nievas-Soriano, B.J. Worldwide research on calorie restriction in aging: A bibliometric study. Nutr. Clin. Diet. Hosp. 2024, 44, 131–141. [Google Scholar] [CrossRef]
- Brandhorst, S.; Choi, I.Y.; Wei, M.; Cheng, C.W.; Sedrakyan, S.; Navarrete, G.; Dubeau, L.; Yap, L.P.; Park, R.; Vinciguerra, M.; et al. A Periodic Diet that Mimics Fasting Promotes Multi-System Regeneration, Enhanced Cognitive Performance, and Healthspan. Cell Metab. 2015, 22, 86–99. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fontana, L.; Partridge, L. Promoting health and longevity through diet: From model organisms to humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef]
- Longo, V.D.; Mattson, M.P. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014, 19, 181–192. [Google Scholar] [CrossRef]
- Madeo, F.; Carmona-Gutierrez, D.; Hofer, S.J.; Kroemer, G. Caloric restriction mimetics against age-associated disease: Targets, mechanisms, and therapeutic potential. Cell Metab. 2019, 29, 592–610. [Google Scholar] [CrossRef]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity, and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1015. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Mitchell, S.J.; de Cabo, R.; Le Couteur, D.G. Macronutrients and caloric intake in health and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Murillo, A.F. ¿Es Posible Retrasar el Envejecimiento? Análisis Pormenorizado de las Potenciales Intervenciones Terapéuticas. Ph.D. Thesis, Universidad de Almería, Almería, Spain, 2024. [Google Scholar]
- Green, C.L.; Lamming, D.W.; Fontana, L. Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol. 2022, 23, 56–73. [Google Scholar] [CrossRef]
- Fanti, M.; Mishra, A.; Longo, V.D.; Brandhorst, S. Alimentación restringida en el tiempo, ayuno intermitente y dietas que imitan el ayuno en la pérdida de peso. Curr. Obes. Rep. 2021, 10, 70–80. [Google Scholar] [CrossRef]
- Caffa, I.; Spagnolo, V.; Vernieri, C.; Valdemarin, F.; Becherini, P.; Wei, M.; Brandhorst, S.; Zucal, C.; Driehuis, E.; Ferrando, L.; et al. Fasting-mimicking diet and hormone therapy induce breast cancer regression. Nature 2020, 583, 620–624. [Google Scholar] [CrossRef]
- Wei, S.; Han, R.; Zhao, J.; Wang, S.; Huang, M.; Wang, Y.; Chen, Y. Intermittent administration of a fasting-mimicking diet intervenes in diabetes progression, restores β cells and reconstructs gut microbiota in mice. Nutr. Metab. 2018, 15, 80. [Google Scholar] [CrossRef]
- Rangan, P.; Choi, I.; Wei, M.; Navarrete, G.; Guen, E.; Brandhorst, S.; Enyati, N.; Pasia, G.; Maesincee, D.; Ocon, V.; et al. Fasting-mimicking diet modulates microbiota and promotes intestinal regeneration to reduce inflammatory bowel disease pathology. Cell Rep. 2019, 26, 2704–2719.e6. [Google Scholar] [CrossRef]
- Zhou, Z.-L.; Jia, X.B.; Sun, M.F.; Zhu, Y.L.; Qiao, C.M.; Zhang, B.P.; Zhao, L.-P.; Yang, Q.; Cui, C.; Chen, X.; et al. Neuroprotection of fasting mimicking diet on MPTP-induced Parkinson’s disease mice via gut microbiota and metabolites. Neurotherapeutics 2019, 16, 741–760. [Google Scholar] [CrossRef]
- Boccardi, V.; Pigliautile, M.; Guazzarini, A.G.; Mecocci, P. The potential of fasting-mimicking diet as a preventive and curative strategy for Alzheimer’s disease. Biomolecules 2023, 13, 1133. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- de Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, M.J.; Manoogian, E.N.C.; Zadourian, A.; Lo, H.; Fakhouri, S.; Shoghi, A.; Wang, X.; Fleischer, J.G.; Navlakha, S.; Panda, S.; et al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020, 31, 92–104.e5. [Google Scholar] [CrossRef]
- Anton, S.D.; Lee, S.A.; Donahoo, W.T.; McLaren, C.; Manini, T.; Leeuwenburgh, C.; Pahor, M. The effects of time restricted feeding on overweight, older adults: A pilot study. Nutrients 2019, 11, 1500. [Google Scholar] [CrossRef] [PubMed]
- Sutton, E.F.; Beyl, R.; Early, K.S.; Cefalu, W.T.; Ravussin, E.; Peterson, C.M. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018, 27, 1212–1221.e13. [Google Scholar] [CrossRef]
- Duregon, E.; Pomatto-Watson, L.C.; Bernier, M.; Price, N.L.; de Cabo, R. Intermittent fasting: From calories to time restriction. Geroscience 2021, 43, 1083–1092. [Google Scholar] [CrossRef]
- Yin, Z.; Klionsky, D.J. Intermittent time-restricted feeding promotes longevity through circadian autophagy. Autophagy 2022, 18, 471–472. [Google Scholar] [CrossRef]
- Ulgherait, M.; Midoun, A.M.; Park, S.J.; Gatto, J.A.; Tener, S.J.; Siewert, J.; Klickstein, N.; Canman, J.C.; Ja, W.W.; Shirasu-Hiza, M. Circadian autophagy drives iTRF-mediated longevity. Nature 2021, 598, 353–358. [Google Scholar] [CrossRef]
- Speakman, J.R.; Mitchell, S.E.; Mazidi, M. Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp. Gerontol. 2016, 86, 28–38. [Google Scholar] [CrossRef]
- Simpson, S.J.; Le Couteur, D.G.; James, D.E.; George, J.; Gunton, J.E.; Solon-Biet, S.M.; Raubenheimer, D. The geometric framework for nutrition as a tool in precision medicine. Nutr. Healthy Aging 2017, 4, 217–226. [Google Scholar] [CrossRef]
- Solon-Biet, S.M.; Mitchell, S.J.; Coogan, S.C.; Cogger, V.C.; Gokarn, R.; McMahon, A.C.; Raubenheimer, D.; de Cabo, R.; Simpson, S.J.; Le Couteur, D.G. Dietary protein to carbohydrate ratio and caloric restriction: Comparing metabolic outcomes in mice. Cell Rep. 2015, 11, 1529–1534. [Google Scholar] [CrossRef]
- Navarrete Santos, A.; Jacobs, K.; Simm, A.; Glaubitz, N.; Horstkorte, R.; Hofmann, B. Dicarbonyls induce senescence of human vascular endothelial cells. Mech. Ageing Dev. 2017, 166, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Ferraz-Bannitz, R.; Beraldo, R.A.; Peluso, A.A.; Dall, M.; Babaei, P.; Foglietti, R.C.; Martins, L.M.; Gomes, P.M.; Marchini, J.S.; Suen, V.M.M.; et al. Dietary protein restriction improves metabolic dysfunction in patients with metabolic syndrome in a randomized, controlled trial. Nutrients 2022, 14, 2670. [Google Scholar] [CrossRef]
- Song, M.; Fung, T.T.; Hu, F.B.; Willett, W.C.; Longo, V.D.; Chan, A.T.; Giovannucci, E.L. Association of animal and plant protein intake with all-cause and cause-specific mortality. JAMA Intern. Med. 2016, 176, 1453–1463. [Google Scholar] [CrossRef]
- Wolk, A. Potential health hazards of eating red meat. J. Intern. Med. 2017, 281, 106–122. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Cummings, N.E.; Arriola Apelo, S.I.; Neuman, J.C.; Kasza, I.; Schmidt, B.A.; Cava, E.; Spelta, F.; Tosti, V.; Syed, F.A.; et al. Decreased consumption of branched-chain amino acids improves metabolic health. Cell Rep. 2016, 16, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Flores-Guerrero, J.L.; Oste, M.C.J.; Kieneker, L.M.; Gruppen, E.G.; Wolak-Dinsmore, J.; Otvos, J.D.; Connelly, M.A.; Bakker, S.J.L.; Dullaart, R.P.F. Plasma branched-chain amino acids and risk of incident type 2 diabetes: Results from the PREVEND prospective cohort study. J. Clin. Med. 2018, 7, 513. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvadó, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma branched-chain amino acids and incident cardiovascular disease in the PREDIMED trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef]
- Kitada, M.; Ogura, Y.; Monno, I.; Koya, D. The impact of dietary protein intake on longevity and metabolic health. EBioMedicine 2019, 43, 632–640. [Google Scholar] [CrossRef]
- Plaisance, E.P.; Greenway, F.L.; Boudreau, A.; Hill, K.L.; Johnson, W.D.; Krajcik, R.A.; Perrone, C.E.; Orentreich, N.; Cefalu, W.T.; Gettys, T.W. Dietary methionine restriction increases fat oxidation in obese adults with metabolic syndrome. J. Clin. Endocrinol. Metab. 2011, 96, E836–E840. [Google Scholar] [CrossRef] [PubMed]
| Author | Dietary Strategy | Model | Population/Species | Study Duration | Variables Measured | Key Findings |
|---|---|---|---|---|---|---|
| Matson et al. (2017) [3] | TRF | Human | Adults with obesity | 12 weeks | Weight, HOMA-IR, blood pressure | Weight: −4–6%; HOMA-IR: −25%; systolic BP: −7 mmHg |
| Brandhorst et al. (2015) [7] | FMD | Human | Overweight adults | 3 cycles/3 months | Glucose, IGF-1, leptin | IGF-1: −15%; leptin: −20%; fasting glucose: −11 mg/dL; no lean mass loss |
| Fontana et al. (2015) [8] | PAAR | Animal | C57BL/6 mice | 6 months | Longevity, visceral fat | Lifespan: +30%; visceral fat: −20% |
| Longo et al. (2014) [9] | FMD | Animal | Mice | 4-day cycles every 2 weeks | Autophagy, pancreatic regeneration | Enhanced beta-cell regeneration; increased autophagy |
| Madeo et al. (2019) [10] | PAAR | Animal | Mice (low methionine) | 6 months | Inflammation markers (IL-6, TNF-α), IGF-1 | IL-6 and TNF-α: −30%; IGF-1: −25% |
| Mattson el al. (2018) [11] | FMD | Animal | Mice | Intermittent | Cognitive function | Dopaminergic neuron loss: −30%; neuroprotection in Parkinson model |
| Panda S. (2016) [12] | TRF | Animal | Obese mice | 12 weeks | Weight, LDL cholesterol | Weight: −10%; LDL: −20% |
| Levine et al. (2014) [13] | TRF | Human | Older adults with frailty | 4 weeks | BMI, gait speed | BMI: -5%; gait speed: +5% |
| Solon-Biet et al. (2015) [14] | PAAR | Human | Adults aged 50–65 (observational) | Cross-sectional | Cancer mortality, IGF-1 | Cancer mortality: −75%; IGF-1: −20%; lower LDL and triglycerides |
| Aspect | Fasting-Mimicking Diets (FMDs) | Time-Restricted Feeding (TRF) | Dietary Protein and Amino Acid Restriction (PAAR) |
|---|---|---|---|
| Description | Diets that mimic the effects of prolonged fasting but allow for controlled intake during specific periods [8,9]. | Restriction of food intake to a reduced feeding window (typically 6–10 h per day) without caloric reduction [3]. | Reduction in total protein intake or specific amino acids, such as methionine and branched-chain amino acids (BCAAs) [10]. |
| Benefits | |||
| In humans | Reduced glucose, increased ketones, potential cancer prevention [3,7]. | Reduced lipids and BP; weight loss; improved insulin sensitivity [12,13]. | Lower cancer incidence and metabolic improvement in low-protein diets [7,14]. |
| In animals | Increased ketosis and autophagy; improved mitochondrial function; reduction in IGF-1 [9,10,11]. | Improved insulin sensitivity and autophagy [3]. | Reduced adiposity, improved insulin sensitivity, reduced inflammation [7,8]. |
| Limitations | |||
| In humans | Adherence issues, preliminary longevity data [3,10,11,14]. | More trials are needed; adherence varies with time-window length. Requires circadian alignment for benefits [12,13]. | Protein reduction must be personalized, especially in older adults. Sarcopenia risks are present with excessive restriction [7,14]. |
| In animals | Demonstrated benefits, but not always translatable [9]. | ||
| Examples of Studies | |||
| In humans | Showing benefits in breast cancer patients [7]. | Improved mobility and weight reduction in older adults [13]. | Longevity and reduced disease with low-protein diets [10,14]. |
| In animals | Reduced metabolic and neurodegenerative diseases [8,9]. | Prevented obesity and improved glucose tolerance [23]. | Reduced cancer risk with protein restriction in middle age [7]. |
| Aspect | Fasting-Mimicking Diets (FMDs) | Time-Restricted Feeding (TRF) | Dietary Protein and Amino Acid Restriction (PAAR) |
|---|---|---|---|
| Effect on Longevity | |||
| In humans | Evidence is still preliminary [8,9]. | Some benefits shown in middle-aged individuals [11,13]. | More modest evidence, especially in middle-aged adults [10]. |
| In animals | Increases longevity [8,9]. | Improves metabolic health markers associated with longevity [8]. | Extends lifespan by up to 30–50% depending on protocol [10,14]. |
| Chronic Disease Prevention | |||
| In humans | Decreases IGF-1 and glucose, improves risk markers [7]. | Reduces BP and lipids, improves HOMA-IR [24,25]. | Lowers LDL, triglycerides, and cancer risk in middle-aged adults [7,14]. |
| In animals | Reduces incidence of neurodegenerative and metabolic diseases [7]. | Prevents obesity, improves insulin sensitivity [12]. | Reduces visceral adiposity and systemic inflammation [10]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murillo-Cancho, A.F.; Lozano-Paniagua, D.; Nievas-Soriano, B.J. Alternative Dietary Strategies to Modulate Obesity and Improve Metabolic Health in Aging: A Comparative Narrative Review. Obesities 2025, 5, 30. https://doi.org/10.3390/obesities5020030
Murillo-Cancho AF, Lozano-Paniagua D, Nievas-Soriano BJ. Alternative Dietary Strategies to Modulate Obesity and Improve Metabolic Health in Aging: A Comparative Narrative Review. Obesities. 2025; 5(2):30. https://doi.org/10.3390/obesities5020030
Chicago/Turabian StyleMurillo-Cancho, Antonio Fernando, David Lozano-Paniagua, and Bruno José Nievas-Soriano. 2025. "Alternative Dietary Strategies to Modulate Obesity and Improve Metabolic Health in Aging: A Comparative Narrative Review" Obesities 5, no. 2: 30. https://doi.org/10.3390/obesities5020030
APA StyleMurillo-Cancho, A. F., Lozano-Paniagua, D., & Nievas-Soriano, B. J. (2025). Alternative Dietary Strategies to Modulate Obesity and Improve Metabolic Health in Aging: A Comparative Narrative Review. Obesities, 5(2), 30. https://doi.org/10.3390/obesities5020030









