Association Between Coffee Consumption and Visceral Obesity: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Anthropometry
2.3. Statistical Analysis
2.4. Ethical Considerations
3. Results
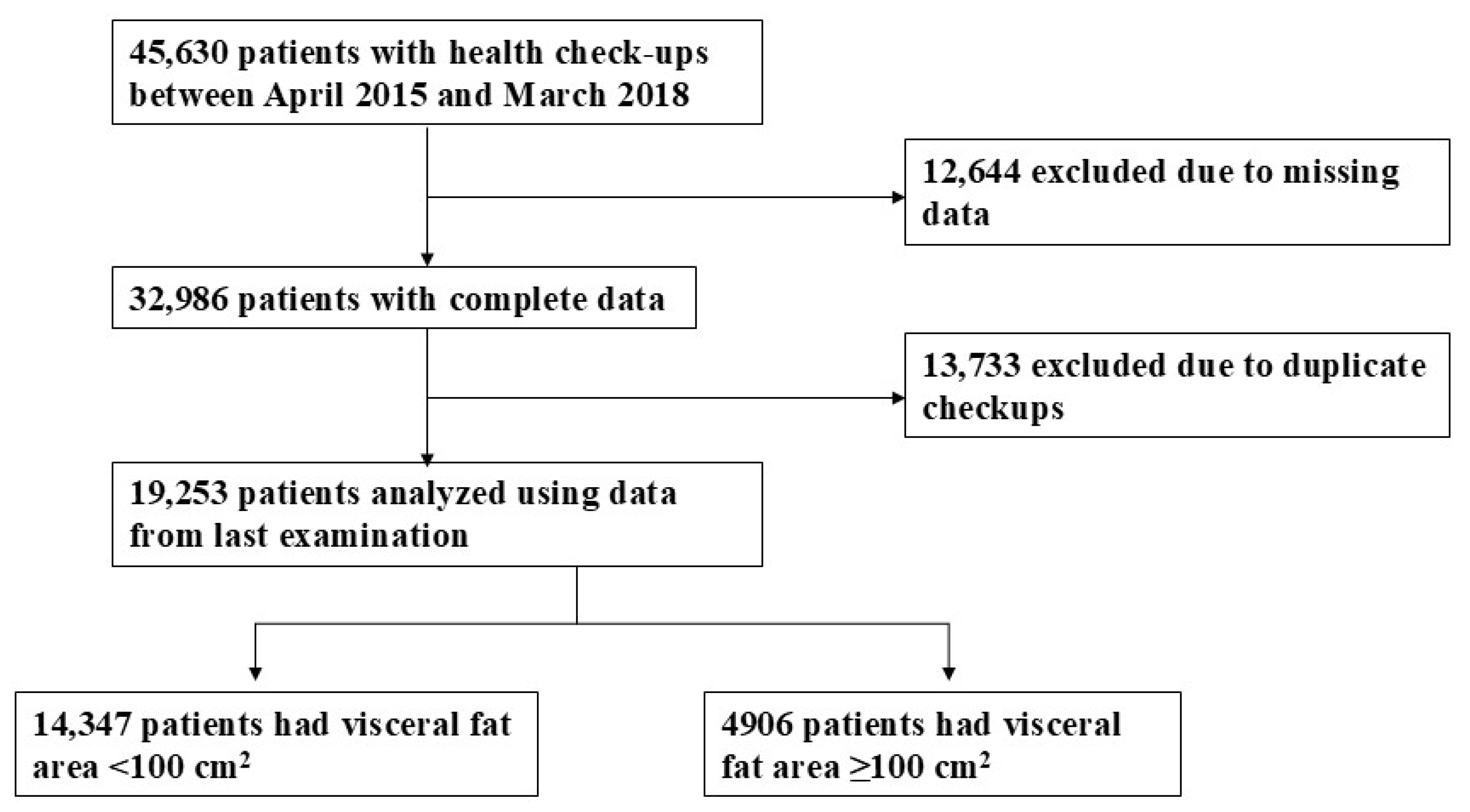
3.1. Study Participant Selection
3.2. Demographic Variables
3.3. Correlation of Coffee Consumption to Visceral Obesity
3.4. Multivariate Regression Analysis Models
3.5. Multiple Linear Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. Obesity and Overweight. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 6 January 2025).
- Milhem, F.; Komarnytsky, S. Progression to Obesity: Variations in Patterns of Metabolic Fluxes, Fat Accumulation, and Gastrointestinal Responses. Metabolites 2023, 13, 1016. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H.; Grundy, S.M.; Zimmet, P.Z. The metabolic syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Matsuzawa, Y.; Funahashi, T.; Kihara, S.; Shimomura, I. Adiponectin and Metabolic Syndrome. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Khan, L.K.; Sobush, K.; Keener, D.; Goodman, K.; Lowry, A.; Kakietek, J.; Zaro, S.; Centers for Disease Control and Prevention. Recommended community strategies and measurements to prevent obesity in the United States. MMWR Recomm. Rep. 2009, 58, 1–26. [Google Scholar]
- Nieber, K. The Impact of Coffee on Health. Planta Med. 2017, 83, 1256–1263. [Google Scholar] [CrossRef]
- Mills, C.E.; Flury, A.; Marmet, C.; Poquet, L.; Rimoldi, S.F.; Sartori, C.; Rexhaj, E.; Brenner, R.; Allemann, Y.; Zimmermann, D.; et al. Mediation of coffee-induced improvements in human vascular function by chlorogenic acids and its metabolites: Two randomized, controlled, crossover intervention trials. Clin. Nutr. 2017, 36, 1520–1529. [Google Scholar] [CrossRef]
- Skates, E.; Overall, J.; DeZego, K.; Wilson, M.; Esposito, D.; Lila, M.A.; Komarnytsky, S. Berries containing anthocyanins with enhanced methylation profiles are more effective at ameliorating high fat diet-induced metabolic damage. Food Chem. Toxicol. 2018, 111, 445–453. [Google Scholar] [CrossRef]
- Jackson, K.M.P.; Rathinasabapathy, T.; Esposito, D.; Komarnytsky, S. Structural constraints and importance of caffeic acid moiety for anti-hyperglycemic effects of caffeoylquinic acids from chicory. Mol. Nutr. Food Res. 2017, 61, 1601118. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Bes-Rastrollo, M.; Galvano, F.; Martinez-Gonzalez, M.A. Long-term coffee consumption is associated with decreased incidence of new-onset hypertension: A dose–response meta-analysis. Nutrients 2017, 9, 890. [Google Scholar] [CrossRef]
- Huxley, R.; Lee, C.M.Y.; Barzi, F.; Timmermeister, L.; Czernichow, S.; Perkovic, V.; Grobbee, D.E.; Batty, D.; Woodward, M. Coffee, decaffeinated coffee, and tea consumption in relation to incident type 2 diabetes mellitus: A systematic review with meta-analysis. Arch. Intern. Med. 2009, 169, 2053–2063. [Google Scholar] [CrossRef]
- Shang, F.; Li, X.; Jiang, X. Coffee consumption and risk of the metabolic syndrome: A meta-analysis. Diabetes Metab. 2016, 42, 80–87. [Google Scholar] [CrossRef]
- Hino, A.; Adachi, H.; Enomoto, M.; Furuki, K.; Shigetoh, Y.; Ohtsuka, M.; Kumagae, S.-I.; Hirai, Y.; Jalaldin, A.; Satoh, A.; et al. Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome. An epidemiological study in a general Japanese population. Diabetes Res. Clin. Pract. 2007, 76, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, E.T.; Cowan, L.D.; Fabsitz, R.R.; Howard, B.V. Coffee consumption and the incidence of type 2 diabetes in men and women with normal glucose tolerance: The Strong Heart Study. Nutr. Metab. Cardiovasc. Dis. 2011, 21, 418–423. [Google Scholar] [CrossRef]
- Koyama, T.; Maekawa, M.; Ozaki, E.; Kuriyama, N.; Uehara, R. Daily Consumption of Coffee and Eating Bread at Breakfast Time Is Associated with Lower Visceral Adipose Tissue and with Lower Prevalence of Both Visceral Obesity and Metabolic Syndrome in Japanese Populations: A Cross-Sectional Study. Nutrients 2020, 12, 3090. [Google Scholar] [CrossRef]
- Lee, K.Y.; Uchida, K.; Shirota, T.; Kono, S. Validity of a self-administered food frequency questionnaire against 7-day dietary records in four seasons. J. Nutr. Sci. Vitaminol. 2002, 48, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, T.; Mizoue, T.Y.T.; Tabata, S.; Ogawa, S.T.S.; Yamaguchi, K.; Shimizu, E.; Mineshita, M.; Kono, S. Coffee consumption and glucose tolerance status in middle-aged Japanese men. Diabetologia 2004, 47, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Belloc, N.B.; Breslow, L. Relationship of physical health status and health practices. Prev. Med. 1972, 1, 409–421. [Google Scholar] [CrossRef]
- Suzuki, A.; Angulo, P.; St Sauver, J.; Muto, A.; Okada, T.; Lindor, K. Light to moderate alcohol consumption is associated with lower frequency of hypertransaminasemia. Am. J. Gastroenterol. 2007, 102, 1912–1919. [Google Scholar] [CrossRef]
- Gunji, T.; Matsuhashi, N.; Sato, H.; Fujibayashi, K.; Okumura, M.; Sasabe, N.; Urabe, A. Light and moderate alcohol consumption significantly reduces the prevalence of fatty liver in the Japanese male population. Am. J. Gastroenterol. 2009, 104, 2189–2195. [Google Scholar] [CrossRef]
- Pierce, J.P.; Messer, K.; White, M.M.; Cowling, D.W.; Thomas, D.P. Prevalence of heavy smoking in California and the United States, 1965–2007. JAMA 2011, 305, 1106–1112. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef] [PubMed]
- Hiuge-Shimizu, A.; Kishida, K.; Funahashi, T.; Ishizaka, Y.; Oka, R.; Okada, M.; Suzuki, S.; Takaya, N.; Nakagawa, T.; Fukui, T.; et al. Absolute value of visceral fat area measured on computed tomography scans and obesity-related cardiovascular risk factors in large-scale Japanese general population (the VACATION-J study). Ann. Med. 2012, 44, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Jee, S.H.; He, J.; Appel, L.J.; Whelton, P.K.; Suh, I.I.; Klag, M.J. Coffee Consumption and Serum Lipids: A Meta-Analysis of Randomized Controlled Clinical Trials. Am. J. Epidemiol. 2001, 153, 353–362. [Google Scholar] [CrossRef]
- Bae, J.-H.; Park, J.-H.; Im, S.-S.; Song, D.-K. Coffee and health. Integr. Med. Res. 2014, 3, 189–191. [Google Scholar] [CrossRef]
- Matsuura, H.; Mure, K.; Nishio, N.; Kitano, N.; Nagai, N.; Takeshita, T. Relationship between coffee consumption and prevalence of metabolic syndrome among Japanese civil servants. J. Epidemiol. 2012, 22, 160–166. [Google Scholar] [CrossRef]
- Takami, H.; Nakamoto, M.; Uemura, H.; Katsuura, S.; Yamaguchi, M.; Hiyoshi, M.; Sawachika, F.; Juta, T.; Arisawa, K. Inverse Correlation Between Coffee Consumption and Prevalence of Metabolic Syndrome: Baseline Survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J. Epidemiol. 2013, 23, 12–20. [Google Scholar] [CrossRef]
- Lancaster, T.; Muir, J.; Silagy, C. The effects of coffee on serum lipids and blood pressure in a UK population. J. R. Soc. Med. 1994, 87, 506–507. [Google Scholar] [CrossRef]
- Kempf, K.; Herder, C.; Erlund, I.; Kolb, H.; Martin, S.; Carstensen, M.; Koenig, W.; Sundvall, J.; Bidel, S.; Kuha, S.; et al. Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: A clinical trial. Am. J. Clin. Nutr. 2010, 91, 950–957. [Google Scholar] [CrossRef]
- Greenberg, J.A.; Axen, K.V.; Schnoll, R.; Boozer, C.N. Coffee, tea and diabetes: The role of weight loss and caffeine. Int. J. Obes. 2005, 29, 1121–1129. [Google Scholar] [CrossRef]
- Higdon, J.V.; Frei, B. Coffee and Health: A Review of Recent Human Research. Crit. Rev. Food Sci. Nutr. 2006, 46, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez de Sotillo, D.V.; Hadley, M. Chlorogenic acid modifies plasma and liver concentrations of: Cholesterol, triacylglycerol, and minerals in (fa/fa) Zucker rats. J. Nutr. Biochem. 2002, 13, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Baspinar, B.; Eskici, G.; Ozcelik, A.O. How coffee affects metabolic syndrome and its components. Food Funct. 2017, 8, 2089–2101. [Google Scholar] [CrossRef]
- Arion, W.J.; Canfield, W.K.; Ramos, F.C.; Schindler, P.W.; Burger, H.J.; Hemmerle, H.; Schubert, G.; Below, P.; Herling, A.W. Chlorogenic acid and hydroxynitrobenzaldehyde: New inhibitors of hepatic glucose 6-phosphatase. Arch. Biochem. Biophys. 1997, 339, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Wang, J.; Ballevre, O.; Luo, H.; Zhang, W. Antihypertensive effects and mechanisms of chlorogenic acids. Hypertens. Res. 2012, 35, 370–374. [Google Scholar] [CrossRef]
- Matsuzawa, Y. Therapy Insight: Adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 2006, 3, 35–42. [Google Scholar] [CrossRef]
- Yamashita, K.; Yatsuya, H.; Muramatsu, T.; Toyoshima, H.; Murohara, T.; Tamakoshi, K. Association of coffee consumption with serum adiponectin, leptin, inflammation and metabolic markers in Japanese workers: A cross-sectional study. Nutr. Diabetes 2012, 2, e33–e36. [Google Scholar] [CrossRef]
- Williams, C.J.; Fargnoli, J.L.; Hwang, J.J.; van Dam, R.M.; Blackburn, G.L.; Hu, F.B.; Mantzoros, C.S. Coffee consumption is associated with higher plasma adiponectin concentrations in women with or without type 2 diabetes: A prospective cohort study. Diabetes Care 2008, 31, 504–507. [Google Scholar] [CrossRef]
- Imatoh, T.; Tanihara, S.; Miyazaki, M.; Momose, Y.; Uryu, Y.; Une, H. Coffee consumption but not green tea consumption is associated with adiponectin levels in Japanese males. Eur. J. Nutr. 2011, 50, 279–284. [Google Scholar] [CrossRef]
- Mure, K.; Maeda, S.; Mukoubayashi, C.; Mugitani, K.; Iwane, M.; Kinoshita, F.; Mohara, O.; Takeshita, T. Habitual coffee consumption inversely associated with metabolic syndrome-related biomarkers involving adiponectin. Nutrition 2013, 29, 982–987. [Google Scholar] [CrossRef]
- Grosso, G.; Stepaniak, U.; Micek, A.; Topor-Mądry, R.; Pikhart, H.; Szafraniec, K.; Pająk, A. Association of daily coffee and tea consumption and metabolic syndrome: Results from the Polish arm of the HAPIEE study. Eur. J. Nutr. 2015, 54, 1129–1137. [Google Scholar] [CrossRef] [PubMed]
- Van Soeren, M.H.; Graham, T.E. Effect of caffeine on metabolism, exercise endurance, and catecholamine responses after withdrawal. J. Appl. Physiol. 1998, 85, 1493–1501. [Google Scholar] [CrossRef] [PubMed]
- Fisone, G.; Borgkvist, A.; Usiello, A. Caffeine as a psychomotor stimulant: Mechanism of action. Cell Mol. Life Sci. 2004, 61, 857–872. [Google Scholar] [CrossRef]
- Astrup, A.; Toubro, S.; Cannon, S.; Hein, P.; Breum, L.; Madsen, J. Caffeine: A double-blind, placebo-controlled study of its thermogenic, metabolic, and cardiovascular effects in healthy volunteers. Am. J. Clin. Nutr. 1990, 51, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Murase, T.; Misawa, K.; Minegishi, Y.; Aoki, M.; Ominami, H.; Suzuki, Y.; Shibuya, Y.; Hase, T. Coffee polyphenols suppress diet-induced body fat accumulation by downregulating SREBP-1c and related molecules in C57BL/6J mice. Am. J. Physiol. Metab. 2011, 300, E122–E133. [Google Scholar] [CrossRef]
- Oboh, G.; Agunloye, O.M.; Adefegha, S.A.; Akinyemi, A.J.; Ademiluyi, A.O. Caffeic and chlorogenic acids inhibit key enzymes linked to type 2 diabetes (in vitro): A comparative study. J. Basic. Clin. Physiol. Pharmacol. 2015, 26, 165–170. [Google Scholar] [CrossRef]
| Variable | |
|---|---|
| n | 19,253 |
| Age (years), mean (SD) | 53.3 (9.8) |
| Sex (male), n (%) | 15,120 (78.5) |
| Body mass index (kg/m2), mean (SD) | 23.5 (3.5) |
| Waist circumference (cm), mean (SD) | 84.6 (9.4) |
| Visceral fat area (cm2), mean (SD) | 74.0 (49.7) |
| Atherosclerotic complications | |
| Cardiovascular disease (yes), n (%) | 312 (1.6) |
| Cerebrovascular disease (yes), n (%) | 292 (1.5) |
| Hypertension-related factors | |
| Systolic blood pressure (mmHg), mean (SD) | 126.8 (19.0) |
| Diastolic blood pressure (mmHg), mean (SD) | 78.2 (12.9) |
| Antihypertensive drug use (yes), n (%) | 3510 (18.2) |
| High blood pressure (yes), n (%) | 9530 (49.5) |
| Lipid-related items | |
| High-density lipoprotein cholesterol (mg/dL), mean (SD) | 59.0 (15.4) |
| Low-density lipoprotein cholesterol (mg/dL), mean (SD) | 116.6 (29.0) |
| Triglycerides (mg/dL), mean (SD) | 114.1 (81.3) |
| Antidyslipidemic drug use (yes), n (%) | 1992 (10.3) |
| Metabolic lipid disorder (yes), n (%) | 6042 (31.4) |
| Diabetes-related items | |
| Fasting plasma glucose (mg/dL), mean (SD) | 102.7 (20.2) |
| HbA1c, mean (SD) | 5.7 (0.7) |
| Antidiabetic drug use (yes), n (%) | 1026 (5.3) |
| Metabolic glucose disorder (yes), n (%) | 3709 (19.3) |
| Lifestyle characteristics | |
| Daily coffee consumption (cups/day), mean (SD) | 1.7 (1.3) |
| None, n (%) | 3018 (15.7) |
| 1 or 2 cups/day, n (%) | 11,483 (59.6) |
| 3 or 4 cups/day, n (%) | 3917 (20.3) |
| ≥5 cups/day, n (%) | 835 (4.3) |
| Daily green tea consumption (cups/day), mean (SD) | 1.4 (1.5) |
| Alcohol consumption | |
| Nondrinker, n (%) | 8747 (45.4) |
| Light drinker, n (%) | 4159 (21.6) |
| Moderate drinker, n (%) | 3447 (17.9) |
| Heavy drinker, n (%) | 2900 (15.1) |
| Exercise frequency per week | |
| None, n (%) | 8533 (44.3) |
| Seldom, n (%) | 2996 (15.6) |
| Sometimes, n (%) | 1746 (9.1) |
| Frequently, n (%) | 5978 (31.0) |
| Smoking status | |
| Nonsmoker, n (%) | 14,932 (77.6) |
| Light smoker, n (%) | 427 (2.2) |
| Moderate smoker, n (%) | 1904 (9.9) |
| Heavy smoker, n (%) | 1990 (10.3) |
| Sleeping hours (≥7 h), n (%) | 6493 (33.7) |
| Breakfast every morning (yes), n (%) | 15,984 (83.0) |
| Snack between meals (no), n (%) | 75 (0.4) |
| Variable | Visceral Fat Area | |||
|---|---|---|---|---|
| <100 cm2 | ≥100 cm2 | p Value | ||
| n | 14,347 | 4906 | ||
| Age (years), mean (SD) | 52.4 (9.8) | 55.9 (9.2) | <0.05 | |
| Sex (male), n (%) | 10,558 (73.6) | 4562 (93.0) | <0.05 | |
| Body mass index (kg/m2), mean (SD) | 22.4 (2.7) | 26.75 (3.4) | <0.05 | |
| Waist circumference (cm), mean (SD) | 81.4 (7.4) | 94.1 (8.1) | <0.05 | |
| Atherosclerotic complications | ||||
| Cardiovascular disease (yes), n (%) | 173 (1.2) | 139 (2.8) | <0.05 | |
| Cerebrovascular disease (yes), n (%) | 189 (1.3) | 103 (2.1) | <0.05 | |
| Hypertension-related factors | ||||
| Systolic blood pressure (mmHg), mean (SD) | 124.1 (18.6) | 134.5 (18.0) | <0.05 | |
| Diastolic blood pressure (mmHg), mean (SD) | 76.4 (12.7) | 83.5 (12.2) | <0.05 | |
| Antihypertensive drug use (yes), n (%) | 1737 (12.1) | 1773 (36.1) | <0.05 | |
| High blood pressure (yes), n (%) | 5910 (41.2) | 3620 (73.8) | <0.05 | |
| Lipid-related items | ||||
| High-density lipoprotein cholesterol (mg/dL), mean (SD) | 61.8 (15.5) | 50.9 (11.7) | <0.05 | |
| Low-density lipoprotein cholesterol (mg/dL), mean (SD) | 114.8 (28.6) | 121.8 (29.6) | <0.05 | |
| Triglycerides (mg/dL), mean (SD) | 99.1 (66.2) | 157.8 (102.9) | <0.05 | |
| Antidyslipidemic drug use (yes), n (%) | 1093 (7.6) | 899 (18.3) | <0.05 | |
| Metabolic lipid disorder (yes), n (%) | 3276 (22.8) | 2766 (56.4) | <0.05 | |
| Diabetes-related items | ||||
| Fasting plasma glucose (mg/dL), mean (SD) | 99.5 (16.4) | 112.1 (26.3) | <0.05 | |
| HbA1c, mean (SD) | 5.6 (0.5) | 6.0 (0.9) | <0.05 | |
| Antidiabetic drug use (yes), n (%) | 454 (3.2) | 572 (11.7) | <0.05 | |
| Metabolic glucose disorder (yes), n (%) | 1843 (12.8) | 1866 (38.0) | <0.05 | |
| Lifestyle characteristics | ||||
| Daily coffee consumption (cups/day), mean (SD) | 1.8 (1.3) | 1.6 (1.3) | <0.05 | |
| None, n (%) | 2071 (14.4) | 947 (19.3) | <0.05 | * |
| 1 or 2 cups/day, n (%) | 8630 (60.2) | 2853 (58.2) | ||
| 3 or 4 cups/day, n (%) | 3005 (20.9) | 912 (18.6) | ||
| ≥5 cups/day, n (%) | 641 (4.5) | 194 (4.0) | ||
| Daily green tea consumption (cups/day), mean (SD) | 1.4 (1.5) | 1.5 (1.5) | <0.05 | |
| Alcohol consumption | ||||
| Nondrinker, n (%) | 6816 (47.5) | 1931 (39.4) | <0.05 | * |
| Light drinker, n (%) | 3161 (22.0) | 998 (20.3) | ||
| Moderate drinker, n (%) | 2471 (17.2) | 976 (19.9) | ||
| Heavy drinker, n (%) | 1899 (13.2) | 1001 (20.4) | ||
| Exercise frequency per week | ||||
| None, n (%) | 6102 (42.5) | 2431 (49.6) | <0.05 | * |
| Seldom, n (%) | 2230 (15.5) | 766 (15.6) | ||
| Sometimes, n (%) | 1352 (9.4) | 394 (8.0) | ||
| Frequently, n (%) | 4663 (32.5) | 1315 (26.8) | ||
| Smoking status | ||||
| Nonsmoker, n (%) | 11,345 (79.1) | 3587 (73.1) | <0.05 | * |
| Light smoker, n (%) | 329 (2.3) | 98 (2.0) | ||
| Moderate smoker, n (%) | 1364 (9.5) | 540 (11.0) | ||
| Heavy smoker, n (%) | 1309 (9.1) | 681 (13.9) | ||
| Sleeping hours ( ≥ 7 h), n (%) | 4782 (33.3) | 1711 (34.9) | 0.05 | |
| Breakfast every morning (yes), n (%) | 11,982 (83.5) | 4002 (81.6) | <0.05 | |
| Snack between meals (no), n (%) | 55 (0.4) | 20 (0.4) | 0.79 | |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p Value | OR (95% CI) | p Value | OR (95% CI) | p Value | |
| Daily coffee consumption | ||||||
| 1 or 2 cups/day | 0.71 (0.64–0.78) | <0.05 | 0.72 (0.65–0.79) | <0.05 | 0.75 (0.68–0.83) | <0.05 |
| 3 or 4 cups/day | 0.61 (0.55–0.68) | <0.05 | 0.62 (0.55–0.69) | <0.05 | 0.67 (0.59–0.75) | <0.05 |
| ≥5 cups/day | 0.58 (0.48–0.70) | <0.05 | 0.57 (0.47–0.68) | <0.05 | 0.65 (0.53–0.80) | <0.05 |
| Continuous variable | 0.89 (0.87–0.92) | <0.05 | 0.89 (0.87–0.92) | <0.05 | 0.91 (0.89–0.94) | <0.05 |
| β Coefficient (SE) | p Value | |
|---|---|---|
| Model 1 | −2.70 (0.25) | <0.05 |
| Model 2 | −2.76 (0.26) | <0.05 |
| Model 3 | −1.86 (0.23) | <0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayashi, Y.; Sasabe, N.; Taniguchi, H.; Gunji, T. Association Between Coffee Consumption and Visceral Obesity: A Cross-Sectional Study. Obesities 2025, 5, 16. https://doi.org/10.3390/obesities5010016
Hayashi Y, Sasabe N, Taniguchi H, Gunji T. Association Between Coffee Consumption and Visceral Obesity: A Cross-Sectional Study. Obesities. 2025; 5(1):16. https://doi.org/10.3390/obesities5010016
Chicago/Turabian StyleHayashi, Yoshinori, Noriko Sasabe, Hiroshi Taniguchi, and Toshiaki Gunji. 2025. "Association Between Coffee Consumption and Visceral Obesity: A Cross-Sectional Study" Obesities 5, no. 1: 16. https://doi.org/10.3390/obesities5010016
APA StyleHayashi, Y., Sasabe, N., Taniguchi, H., & Gunji, T. (2025). Association Between Coffee Consumption and Visceral Obesity: A Cross-Sectional Study. Obesities, 5(1), 16. https://doi.org/10.3390/obesities5010016






