Training Mode Comparisons on Cardiorespiratory, Body Composition and Metabolic Profile Adaptations in Reproductive Age Women: A Systemic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Research Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Selection of Studies
2.4. Data Assessment and Methodological Quality
2.5. Statistical Analysis
3. Results
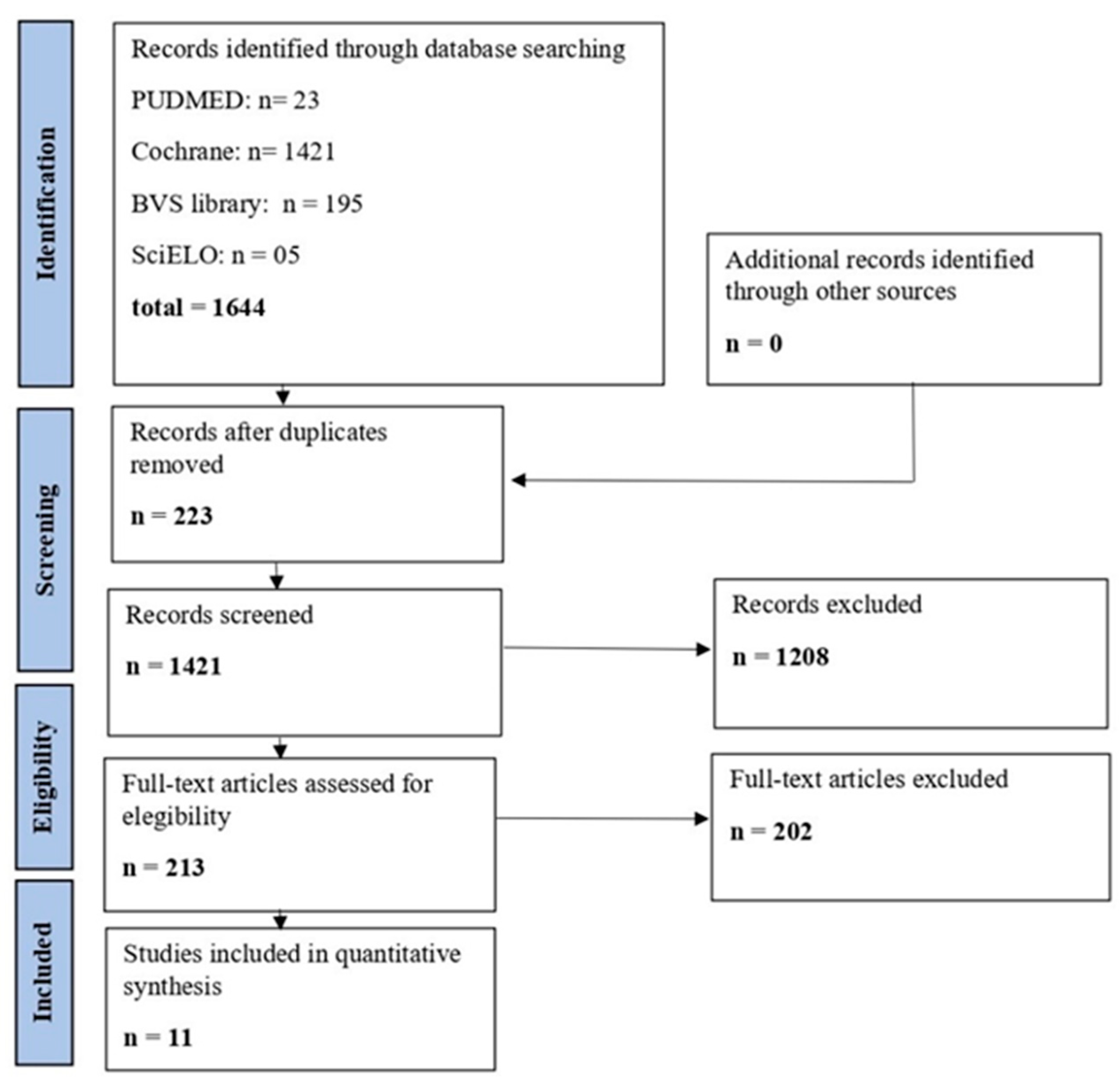
3.1. Study Selection
3.2. Study Characteristics
3.3. Meta-Analysis
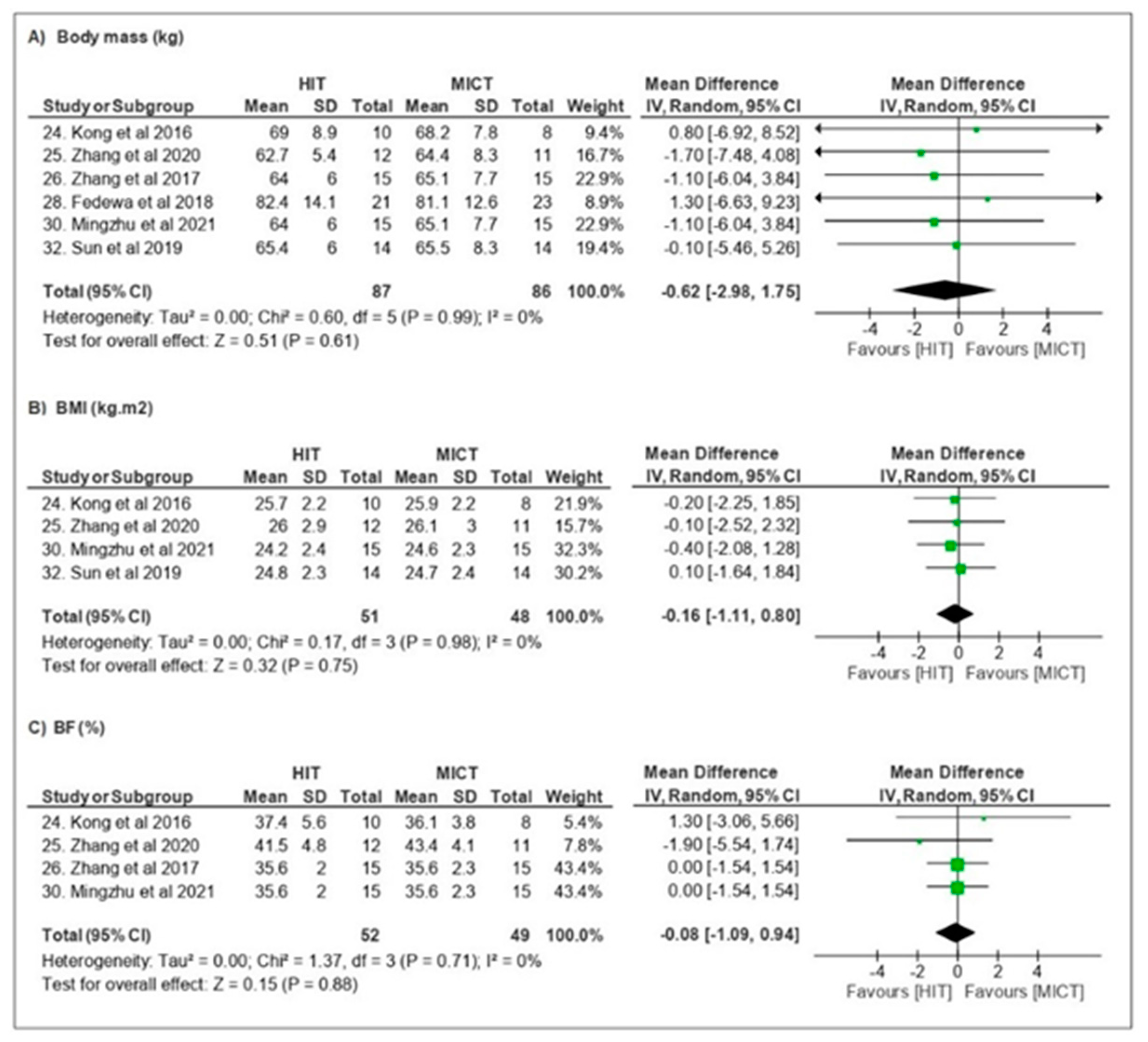
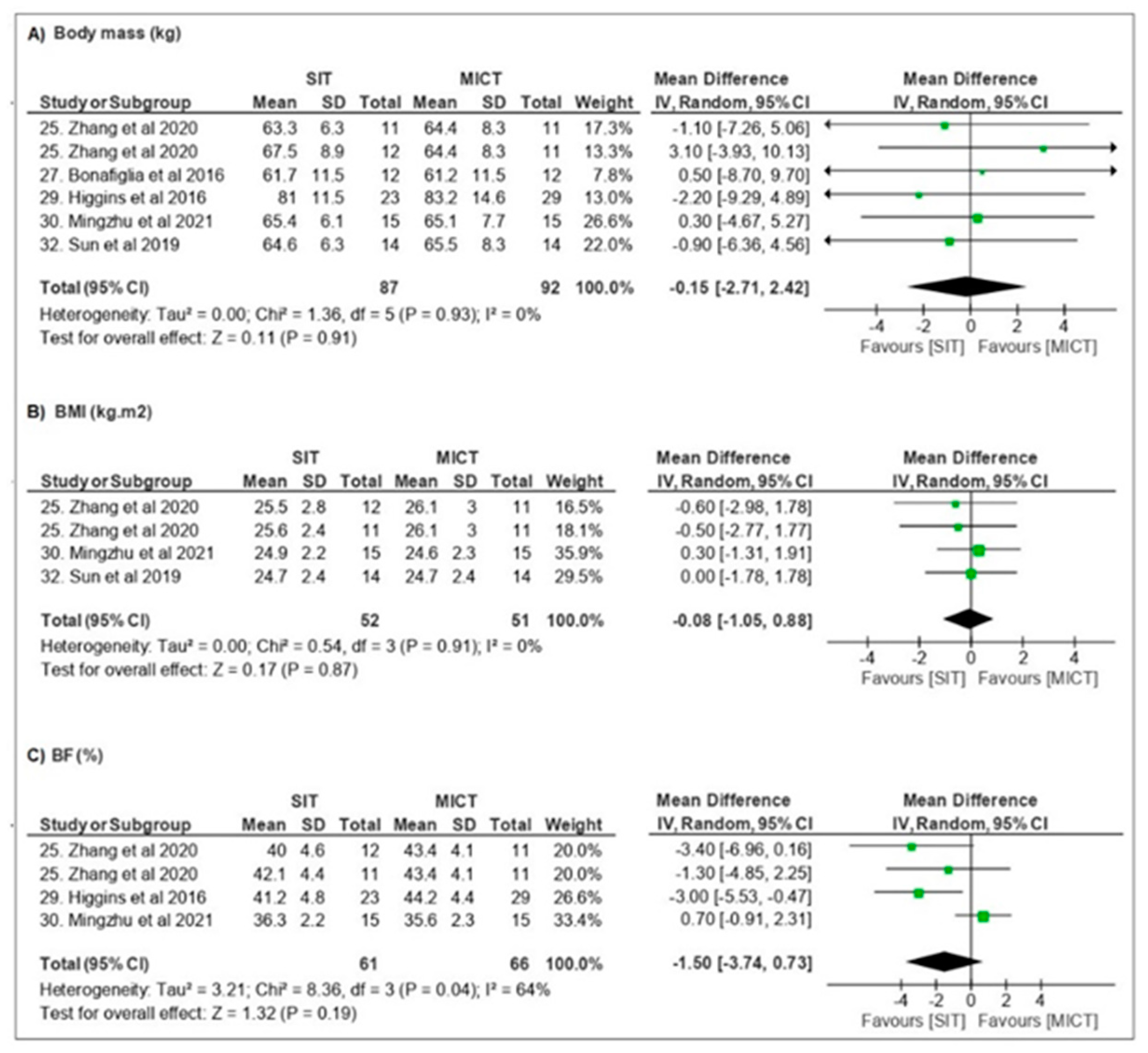
3.3.1. Body Composition
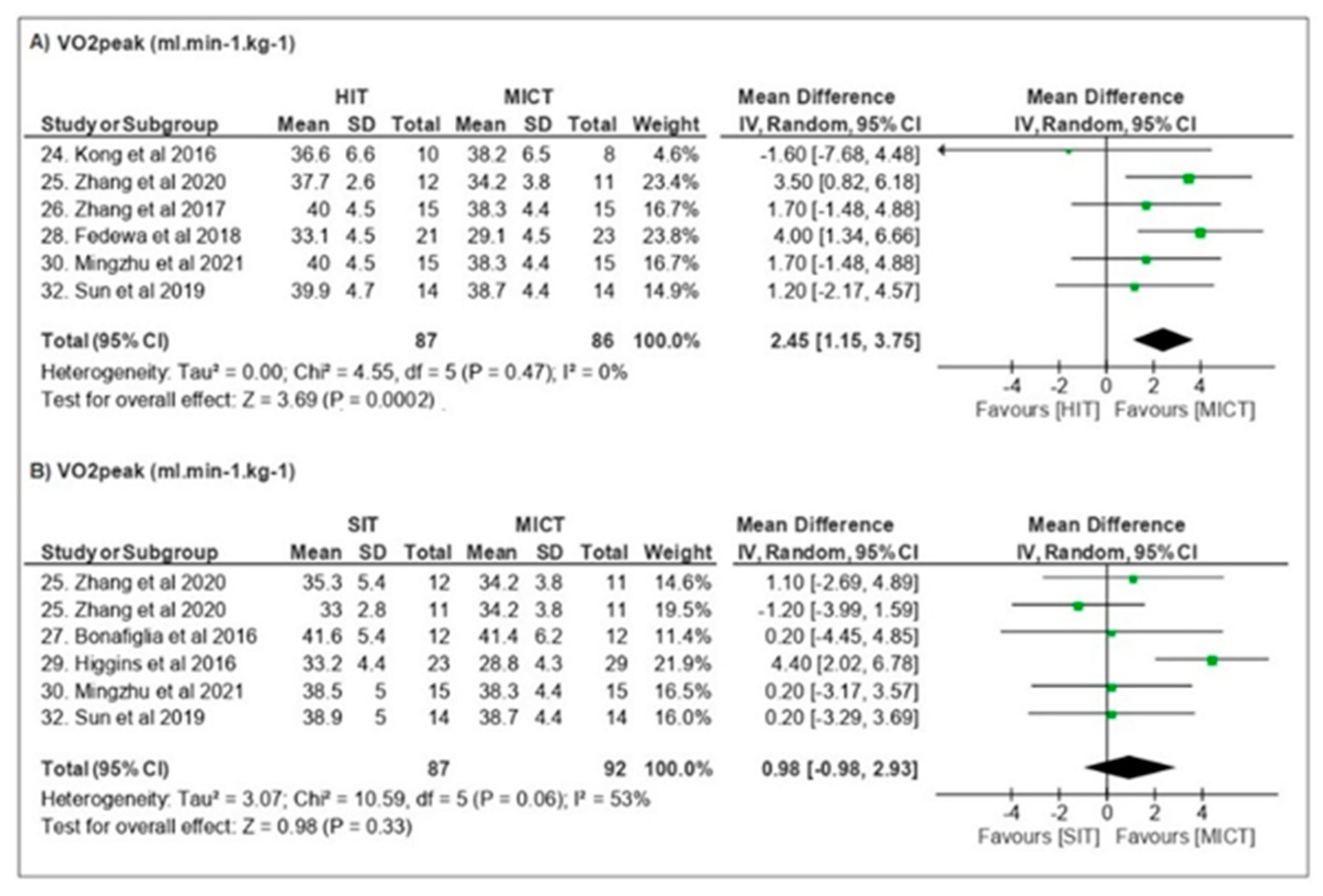
3.3.2. Cardiorespiratory Fitness
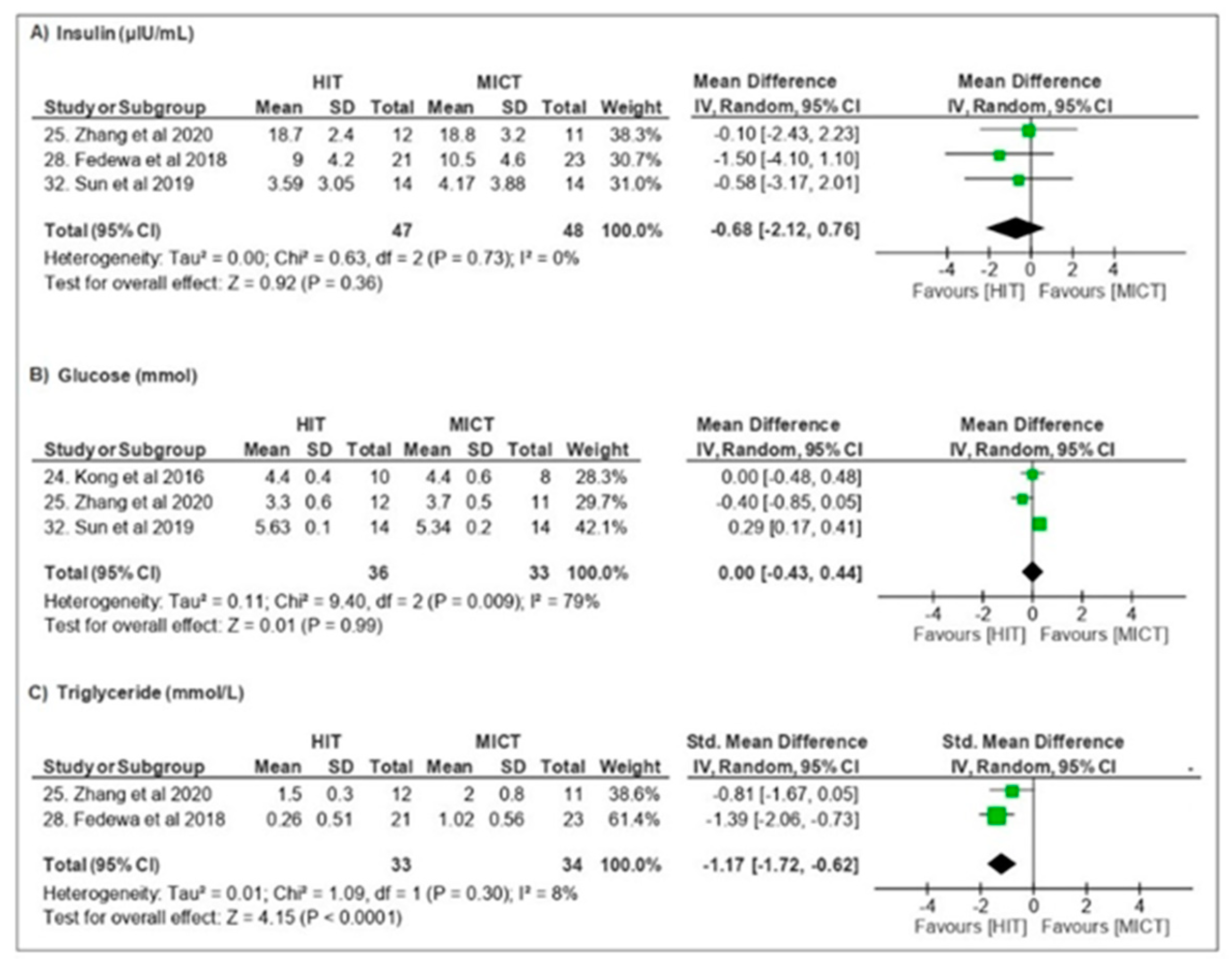
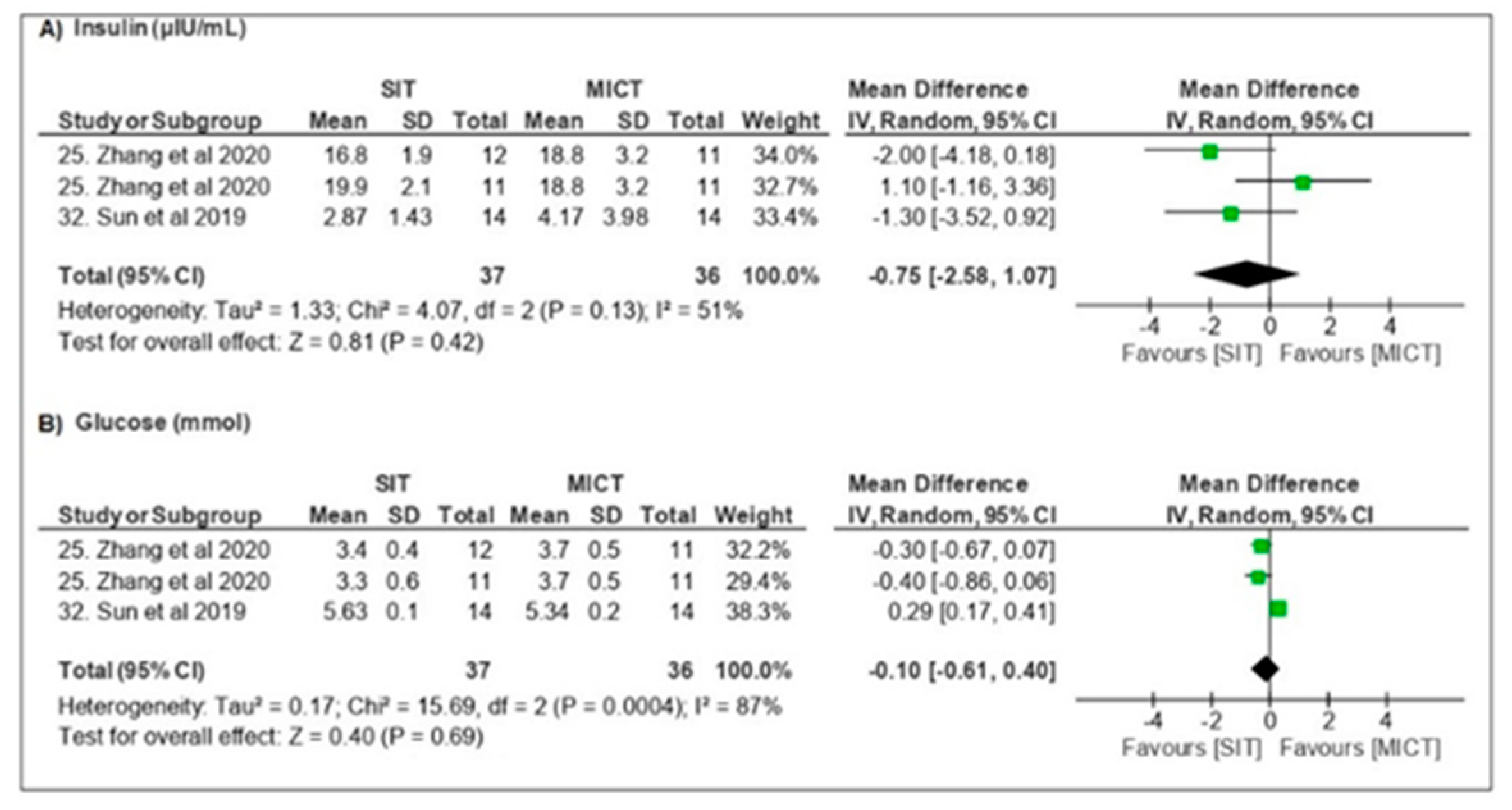
3.3.3. Metabolic Parameters
3.4. Risk of Bias Assessment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boileau, R.A.; Massey, B.H.; Misner, J.E. Body composition changes in adult men during selected weight training and jogging programs. Research Quarterly. Am. Assoc. Health Phys. Educ. Recreat. 1973, 44, 158–168. [Google Scholar] [CrossRef]
- Molmen-Hansen, H.E.; Stolen, T.; Tjonna, A.E.; Aamot, I.L.; Ekeberg, I.S.; Tyldum, G.A.; Wisloff, U.; Ingul, C.B.; Stoylen, A. Aerobic interval training reduces blood pressure and improves myocardial function in hypertensive patients. Eur. J. Prev. Cardiol. 2011, 19, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Pattyn, N.; Beulque, R.; Cornelissen, V. Aerobic interval vs. continuous training in patients with coronary artery disease or heart failure: An updated systematic review and meta-analysis with a focus on secondary outcomes. Sports Med. 2018, 48, 1189–1205. [Google Scholar] [CrossRef]
- Rosenblat, M.A.; Perrotta, A.S.; Thomas, S.G. Effect of high-intensity interval training versus sprint interval training on time-trial performance: A systematic review and meta-analysis. Sports Med. 2020, 50, 1145–1161. [Google Scholar] [CrossRef] [PubMed]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2013, 48, 1227–1234. [Google Scholar] [CrossRef]
- Astorino, T.A.; Schubert, M.M. Changes in fat oxidation in response to various regimes of high intensity interval training (HIIT). Eur. J. Appl. Physiol. 2018, 118, 51–63. [Google Scholar] [CrossRef]
- Martin-Smith, R.; Cox, A.; Buchan, D.S.; Baker, J.S.; Grace, F.; Sculthorpe, N. High Intensity Interval Training (HIIT) Improves Cardiorespiratory Fitness (CRF) in Healthy, Overweight and Obese Adolescents: A Systematic Review and Meta-Analysis of Controlled Studies. Int. J. Environ. Res. Public Health 2020, 17, 2955. [Google Scholar] [CrossRef]
- Dupuit, M.; Maillard, F.; Pereira, B.; Marquezi, M.L.; Lancha, A.H., Jr.; Boisseau, N. Effect of high intensity interval training on body composition in women before and after menopause: A meta-analysis. Exp. Physiol. 2020, 105, 1470–1490. [Google Scholar] [CrossRef]
- Gibala, M.J.; Little, J.P.; MacDonald, M.J.; Hawley, J.A. Physiological adaptations to low-volume, high-intensity interval training in health and disease. J. Physiol. 2012, 590, 1077–1084. [Google Scholar] [CrossRef]
- Scott, S.N.; Cocks, M.; Andrews, R.C. High-Intensity Interval Training Improves Aerobic Capacity Without a Detrimental Decline in Blood Glucose in People with Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2019, 104, 604–612. [Google Scholar] [CrossRef]
- Marquezi, M.L.; Agostinho, C.F.M.; Lima, F.R.; Aparecido, J.M.L.; Cascapera, M.S. Six hit treadmill sessions improve lipid oxidation and ventilatory threshold intensities. Rev. Bras. De Med. Do Esporte 2019, 25, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Rolid, K.; Andreassen, A.K.; Yardley, M. High-intensity interval training and health-related quality of life in de novo heart transplant recipients—results from a randomized controlled trial. Health Qual. Life Outcomes 2020, 18, 283. [Google Scholar] [CrossRef] [PubMed]
- Scribbans, T.D.; Edgett, B.A.; Vorobej, K.; Mitchell, A.S.; Joanisse, S.D.; Matusiak, J.B. Fibre-specific responses to endurance and low volume high intensity interval training: Striking similarities in acute and chronic adaptation. PLoS ONE 2014, 9, e98119. [Google Scholar] [CrossRef] [PubMed]
- Roxburgh, B.H.; Nolan, P.B.; Weatherwax, R.M.; Dalleck, L.C. Is moderate intensity exercise training combined with high intensity interval training more effective at improving cardiorespiratory fitness than moderate intensity exercise training alone? J. Sports Sci. Med. 2014, 13, 702–707. [Google Scholar]
- Wewege, M.; Van den Berg, R.; Ward, R.E.; Keech, A. The effects of high-intensity interval training vs. moderate-intensity continuous training on body composition in overweight and obese adults: A systematic review and meta-analysis. Obes. Rev. 2017, 18, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Yardley, J.E. Fasting May Alter Blood Glucose Responses to High-Intensity Interval Exercise in Adults With Type 1 Diabetes: A Randomized, Acute Crossover Study. Can. J. Diabetes 2020, 44, 727–733. [Google Scholar] [CrossRef]
- Alvarez, C.; Ramirez-Campillo, R.; Martinez-Salazar, C.; Mancilla, R.; Flores-Opazo, M.; Cano-Montoya, J. Low-volume high-intensity interval training as a therapy for type 2 diabetes. Int. J. Sports Med. 2016, 37, 723–729. [Google Scholar] [CrossRef]
- Wewege, M.A.; Ahn, D.; Yu, J.; Liou, K.; Keech, A. High-Intensity Interval Training for Patients with Cardiovascular Disease-Is It Safe? A Systematic Review. J. Am. Heart Assoc. 2018, 7, e009305. [Google Scholar] [CrossRef] [Green Version]
- Boisseau, N.; Isacco, L. Substrate metabolism during exercise: Sexual dimorphism and women’s specificities. Eur. J. Sport Sci. 2022, 22, 672–683. [Google Scholar] [CrossRef]
- Frientes, C.S.; Barros, R.; Cascapera, M.S.; Aparecido, J.M.L.; Marquezi, M.L. Six Hit Sessions Alters Substrate Oxidation and Lactate Concentration in Women. Rev. Soc. Cardiol. Estado São Paulo 2019, 2 (Suppl. 29), 236. Available online: http://files.bvs.br/upload/S/1413-9979/2013/v18n1/a3444.pdf (accessed on 20 April 2022).
- Marquezi, M.L.; Frientes, C.S.; Aparecido, J.M.L.; Cascapera, M.S.; Lancha, A.H., Jr. Hit effects on substrates oxidation rates of women in different phases of monthly ovarian cycle. Med. Sci. Sports Exerc. 2020, 52, 452–453. [Google Scholar] [CrossRef]
- Moher, D. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 4, 264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.P.V.; Silva, V.; Grande, A.J. Assessment of risk of bias in randomized controlled trials by the Cochrane Collaboration tool. Diagn. Trat. 2013, 18, 38–44. Available online: http://files.bvs.br/upload/S/1413-9979/2013/v18n1/a3444.pdf (accessed on 20 April 2022).
- Kong, Z.; Sun, S.; Liu, M.; Shi, Q. Short-Term high-intensity interval training on body composition and blood glucose in overweight and obese young women. J. Diabetes Res. 2016, 2016, 4073618. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tong, T.K.; Kong, Z.; Shi, Q.; Liu, Y.; Nie, J. Exercise training-induced visceral fat loss in obese women: The role of training intensity and modality. Scand. J. Med. Sci. Sports 2020, 31, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Tong, T.K.; Qiu, W.; Zhang, X.; Zhou, S.; Liu, Y.; He, Y. Comparable effects of high-intensity interval training and prolonged continuous exercise training on abdominal visceral fat reduction in obese young women. J. Diabetes Res. 2017, 2017, 5071740. [Google Scholar] [CrossRef]
- Bonafiglia, J.T.; Rotundo, M.P.; Whittall, J.P.; Scribbans, T.D.; Graham, R.B.; Gurd, B.J. Inter-Individual variability in the adaptive responses to endurance and sprint interval training: A randomized crossover study. PLoS ONE 2016, 11, e0167790. [Google Scholar] [CrossRef] [PubMed]
- Fedewa, M.V.; Hathaway, E.D.; Higgins, S.; Forehan, R.L.; Schmidt, M.D.; Evans, E.M. Moderate, but not vigorous, intensity exercise training reduces C-reactive protein. Acta Cardiol. 2017, 73, 283–290. [Google Scholar] [CrossRef]
- Higgins, S.; Fedewa, M.V.; Hathaway, E.D.; Schmidt, M.D.; Evans, E.M. Sprint interval and moderate-intensity cycling training differentially affect adiposity and aerobic capacity in overweight young-adult women. Appl. Physiol. Nutr. Metab. 2016, 41, 1177–1183. [Google Scholar] [CrossRef]
- Hu, M.; Kong, Z.; Sun, S.; Zou, L.; Shi, Q.; Chow, B.C.; Nie, J. Interval training causes the same exercise enjoyment as moderate-intensity training to improve cardiorespiratory fitness and body composition in young Chinese women with elevated BMI. J. Sports Sci. 2021, 39, 1677–1686. [Google Scholar] [CrossRef]
- Nazari, M.; Minasian, V.; Hovsepian, S. Effects of Two Types of Moderate- and High-Intensity Interval Training on Serum Salusin-α and Salusin-β Levels and Lipid Profile in Women with Overweight/Obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2020, 13, 1385–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, S.; Zhang, H.; Kong, Z.; Shi, Q.; Tong, T.K.; Nie, J. Twelve weeks of low volume sprint interval training improves cardio-metabolic health outcomes in overweight females. J. Sports Sci. 2018, 37, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- TaheriChadorneshin, H.; Cheragh-Birjandi, S.; Goodarzy, S.; Ahmadabadi, F. The impact of high intensity interval training on serum chemerin, tumor necrosis factor-alpha and insulin resistance in overweight women. Obes. Med. 2019, 14, 100101. [Google Scholar] [CrossRef]
- Tong, T.K.; Zhang, H.; Shi, H.; Liu, Y.; Ai, J.; Nie, J.; Kong, Z. Comparing time efficiency of sprint vs. high-intensity interval training in reducing abdominal visceral fat in obese young women: A randomized, controlled trial. Front. Physiol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Little, J.P.; Safdar, A.; Bishop, D.; Tarnopolsky, M.A.; Gibala, M.J. An acute bout of high-intensity interval training increases the nuclear abundance of PGC-1α and activates mitochondrial biogenesis in human skeletal muscle. Am. J. Physiol. 2011, 300, R1303–R1310. [Google Scholar] [CrossRef] [Green Version]
- Burgomaster, K.A.; Howarth, K.R.; Phillips, S.M.; Rakobowchuk, M.; Macdonald, M.J.; Mcgee, S.L.; Gibala, M.J. Similar metabolic adaptations during exercise after low volume sprint interval and traditional endurance training in humans. J. Physiol. 2008, 586, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Oosthuyse, T.; Bosch, A.N. The Effect of the Menstrual Cycle on Exercise Metabolism. Sports Med. 2010, 40, 207–227. [Google Scholar] [CrossRef]
- Olean-Oliveira, T.; Figueiredo, C.; de Poli, R.A.B.; Lopes, V.H.F.; Jimenez-Maldonado, A.; Lira, F.S.; Antunes, B.M. Menstrual cycle impacts adipokine and lipoprotein responses to acute high-intensity intermittent exercise bout. Eur. J. Appl. Physiol. 2022, 122, 103–112. [Google Scholar] [CrossRef]
- Elliott-Sale, K.J. The Effects of Oral Contraceptives on Exercise Performance in Women: A Systematic Review and Meta-analysis. Sports Med. 2020, 50, 1785–1812. [Google Scholar] [CrossRef]
- Wohlgemuth, K.J.; Arieta, L.R.; Brewer, G.J.; Hoselton, A.L.; Gould, L.M.; Smith-Ryan, A.E. Sex differences and considerations for female specific nutritional strategies: A narrative review. J. Int. Soc. Sports Nutr. 2021, 18, 27. [Google Scholar] [CrossRef]
- Broskey, N.T.; Tam, C.S.; Sutton, E.F.; Altazan, A.D.; Burton, J.H.; Ravussin, E.; Redman, L.M. Metabolic inflexibility in women with PCOS is similar to women with type 2 diabetes. Nutr. Metab. 2018, 15, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Storlien, L.; Oakes, N.D.; Kelley, D.E. Metabolic flexibility. Proc. Nutr. Soc. 2004, 63, 363–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.E.; Febbraio, M.A. Effect of ovarian hormones on mitochondrial enzyme activity in fat oxidation pathway of skeletal muscle. Am. J. Physiol. 2001, 281, E803–E808. [Google Scholar] [CrossRef]
- Redman, L.M.; Scroop, G.C.; Westlander, G.; Norman, R.J. Effect of a synthetic progestin on the exercise status of sedentary young women. J. Clin. Endocrinol. Metab. 2005, 90, 3830–3837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isacco, L.; Duche, P.; Boisseau, N. Influence of hormonal status on substrate utilization at rest and during exercise in the female population. Sports Med. 2012, 42, 327–342. [Google Scholar] [CrossRef]
- Braun, B.; Sharoff, C.; Chipkin, S.R.; Beaudoin, F. Effects of insulin resistance on substrate utilization during exercise in overweight women. J. Appl. Physiol. 2004, 97, 991–997. [Google Scholar] [CrossRef] [Green Version]

| Author | Purpose | Sample | Protocol | Results | |
|---|---|---|---|---|---|
| Kong et al. [24] | BM, BMI, TFM, TBF, %BF, TLM, regional body composition, VO2peak, PPO, GLU, Testosterone, Cortisol, GH, Leptin and FGF-21, RPE | n = 18 ♀ Overweight Obese Folicular stage Ø OC | 20–25 sessions 4–5 d/wk 5 weeks | MICT—40 min cycling at 65% VO2peak HIT—60 × 8 s cycling at ~90% VO2peak 12 s recovery | HIT = MICT ↔ body composition ↑ VO2peak/↑ PPO ↓ GLU ↔ systemic hormones RPE < HIT |
| Zhang et al. [25] | BM, TFM, %BF, regional body composition, AVFA, ASFA, VO2peak, GLU, TG, TC, INS, GH and EPI | n = 59 ♀ Obese Undefined Ø OC | 36–48 sessions 3–4 d/wk 12 weeks | CONT—no exercise MICT—cycling at 60% VO2peak (200 kJ) HIT—4 min cycling at ~90% VO2peak 3 min recovery (200 kJ) SITall-out-40 × 6 s cycling “all-out” sprint 9 s recovery SIT120—1 min cycling at ~120% VO2peak 1.5 min recovery (200 kJ) | All groups ↓ body composition HIT/SITs > MICT ↓ AVFA ↓ GH ↓ EPI SIT > time-efficient |
| Zhang et al. [26] | BM, TFM, %BF, regional body composition, AVFA, ASFA, VO2peak | n = 43 ♀ Obese Undefined Undefined | 36–48 sessions 3–4 d/wk 12 weeks | CONT—no exercise MICT—cycling at 60% VO2peak (300 kJ) HIT—4 min cycling at ~90% VO2peak 3 min recovery (300 kJ) | HIT = MICT ↓ BM/↓ TFM/↓ BF/ ↓ regional body composition ↓ AVFA/↓ ASFA ↑VO2peak |
| Bonafiglia et al. [27] | BM, VO2peak, LT, PPO, HRsubmax | n = 21 ♀ = 12/♂ = 9 Eutrophic Undefined Undefined | 12 sessions 4 d/wk 3 weeks | MICT—30 min cycling at ~65% VO2peak SIT—8 × 20 s cycling at ~170% VO2peak 10 s recovery | SIT = MICT ↑ VO2peak ↑ LT/↑PPO/↓ HRsubmax |
| Fedewa et al. [28] | BM, %BF, TC, HDL, LDL, TG, HOMA-IR, INS, CRP, VO2peak | n = 44 ♀ Overweight Obese Undefined Undefined | 18 sessions 3x/wk 6 weeks | MICT—20–30 min cycling at ~60–70% HRR HIT—5–7 × 30 s cycling at near-maximal 4 min active recovery | MICT ↓ CRP HIT ↓%BF ↑VO2peak |
| Higgins et al. [29] | BM, BMI, TFM, %BF, TLM, regional body composition, VO2peak | n = 52 ♀ Overweight Obese Undefined Undefined | 18 sessions 3x/wk 6 weeks | MICT—20–30 min cycling at ~60–70% HRR SIT—5–7 × 30 s cycling at “all out” 4 min active recovery | SIT > MICT ↔ BM/↓TFM/↓%BF ↑VO2peak |
| Mingzhu et al. [30] | BM, BMI, TFM, %BF, TLM, regional body composition, VO2peak, PACES | n = 66 ♀ Overweight Obese Undefined Ø OC | 36 sessions 3x/wk 12 weeks | CONT—no exercise MICT—~65 min cycling at ~60% VO2peak (200–300 kJ) HIT—4 min cycling at ~90% VO2peak 3 min recovery (300 kJ) SIT—80 × 6 s cycling “all-out” sprint 9 s recovery | HIT/SIT > MICT ↓ BM/↓ %BF ↑ VO2peak SIT > time-efficient |
| Nazari et al. [31] | BM, BMI, %BF, VO2peak, Salusin α e β, HDL, LDL, VLDL, TC, TG | n = 40 ♀ Overweight Obese Undefined Undefined | 24 sessions 3x/ws 8 weeks | CONT—no exercise MIIT—30 s cycling at 75–80% HRmax 30 s recovery HIT—30 s cycling at 90–95% HRmax 30 s recovery | HIT = MIIT ↓ TG/↓ VLDL/↑ HDL ↑ Salusin α/↓ Salusin β |
| Sun et al. [32] | BM, BMI, GLU, INS, HOME-IR, VO2peak, HRmax, RPE | n = 42 ♀ Overweight Stage control Ø OC | 36 sessions 3x/ws 12 weeks | MICT—~61 min cycling at 60% VO2peak HIT—~9 × 4 min cycling at 90% VO2peak 3 min recovery (~300 kJ) SIT—80 × 6 s cycling at 90–95% HRmax 9 s recovery (~300 kJ) | MICT ↓GLU HIT/SIT = MICT ↓ BM ↑ VO2peak HIT/SIT ↑ INS e ↑HOMA-IR SIT > time-efficient HOMA-IR |
| TaheriChadomeshin et al. [33] | BM, BMI, %BF, WHR, GLU, INS, HOME-IR, TC TG, HDL, LDL, VLDL, chemerin, TNF-α | n = 28 ♀ Overweight Undefined Undefined | 24 sessions 3x/ws 8 weeks | CONT—no exercise HIT—20 m × 30 s runnig at maximum speed 20 m × 30 s walking | HIT ↓ BMI/↓%BF ↓TC/↓TG/↓LDL/↑HDL ↓chemerin ↓TNF-α |
| Tong et al. [34] | BM, %BF, regional body composition, AVFA, ASFA, VO2peak, RPE | n = 46 ♀ Obese Undefined Ø OC | 36–48 sessions 3–4x/ws 12 weeks | CONT—no exercise HIT—4 min cycling at 90% VO2peak 3 min recovery (200–400 kJ) SIT—80 × 6 s cycling at “all out” 9 s recovery | HIT < SIT ↓ BM/↓%BF ↓ regional body composition ↓ AVFA/↓ ASFA, ↑ VO2peak |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aparecido, J.M.L.; Frientes, C.S.; Martins, G.L.; Santos, G.C.; Silva, J.D.A.; Rogeri, P.S.; Pires, R.S.; Amorim, T.S.; da Silva, T.D.O.; Santo, T.E.; et al. Training Mode Comparisons on Cardiorespiratory, Body Composition and Metabolic Profile Adaptations in Reproductive Age Women: A Systemic Review and Meta-Analysis. Obesities 2022, 2, 222-235. https://doi.org/10.3390/obesities2020018
Aparecido JML, Frientes CS, Martins GL, Santos GC, Silva JDA, Rogeri PS, Pires RS, Amorim TS, da Silva TDO, Santo TE, et al. Training Mode Comparisons on Cardiorespiratory, Body Composition and Metabolic Profile Adaptations in Reproductive Age Women: A Systemic Review and Meta-Analysis. Obesities. 2022; 2(2):222-235. https://doi.org/10.3390/obesities2020018
Chicago/Turabian StyleAparecido, Juliana Monique Lino, Caroline Santana Frientes, Gabriel Loureiro Martins, Gustavo C. Santos, Jennyfer D. Alves Silva, Patricia Soares Rogeri, Raquel S. Pires, Tatiane Santos Amorim, Thayná Donadei Oliveira da Silva, Thayná Espírito Santo, and et al. 2022. "Training Mode Comparisons on Cardiorespiratory, Body Composition and Metabolic Profile Adaptations in Reproductive Age Women: A Systemic Review and Meta-Analysis" Obesities 2, no. 2: 222-235. https://doi.org/10.3390/obesities2020018
APA StyleAparecido, J. M. L., Frientes, C. S., Martins, G. L., Santos, G. C., Silva, J. D. A., Rogeri, P. S., Pires, R. S., Amorim, T. S., da Silva, T. D. O., Santo, T. E., Boisseau, N., Lancha, A. H., Jr., & Marquezi, M. L. (2022). Training Mode Comparisons on Cardiorespiratory, Body Composition and Metabolic Profile Adaptations in Reproductive Age Women: A Systemic Review and Meta-Analysis. Obesities, 2(2), 222-235. https://doi.org/10.3390/obesities2020018








