Influence of Catalytic Support on Hydrogen Production from Glycerol Steam Reforming
Abstract
1. Introduction
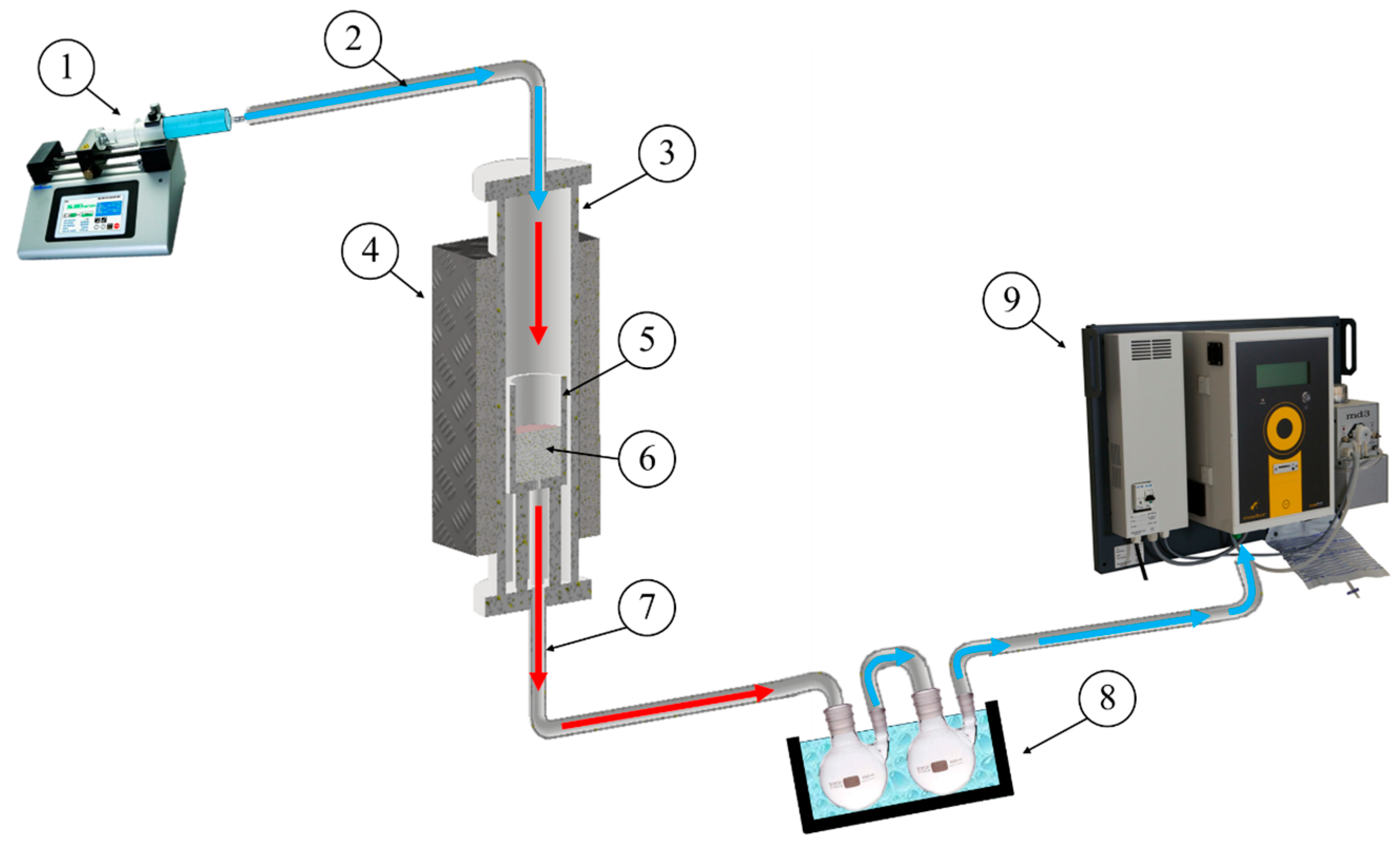
2. Glycerol Reforming Process
3. Materials and Methods
4. Results and Discussion
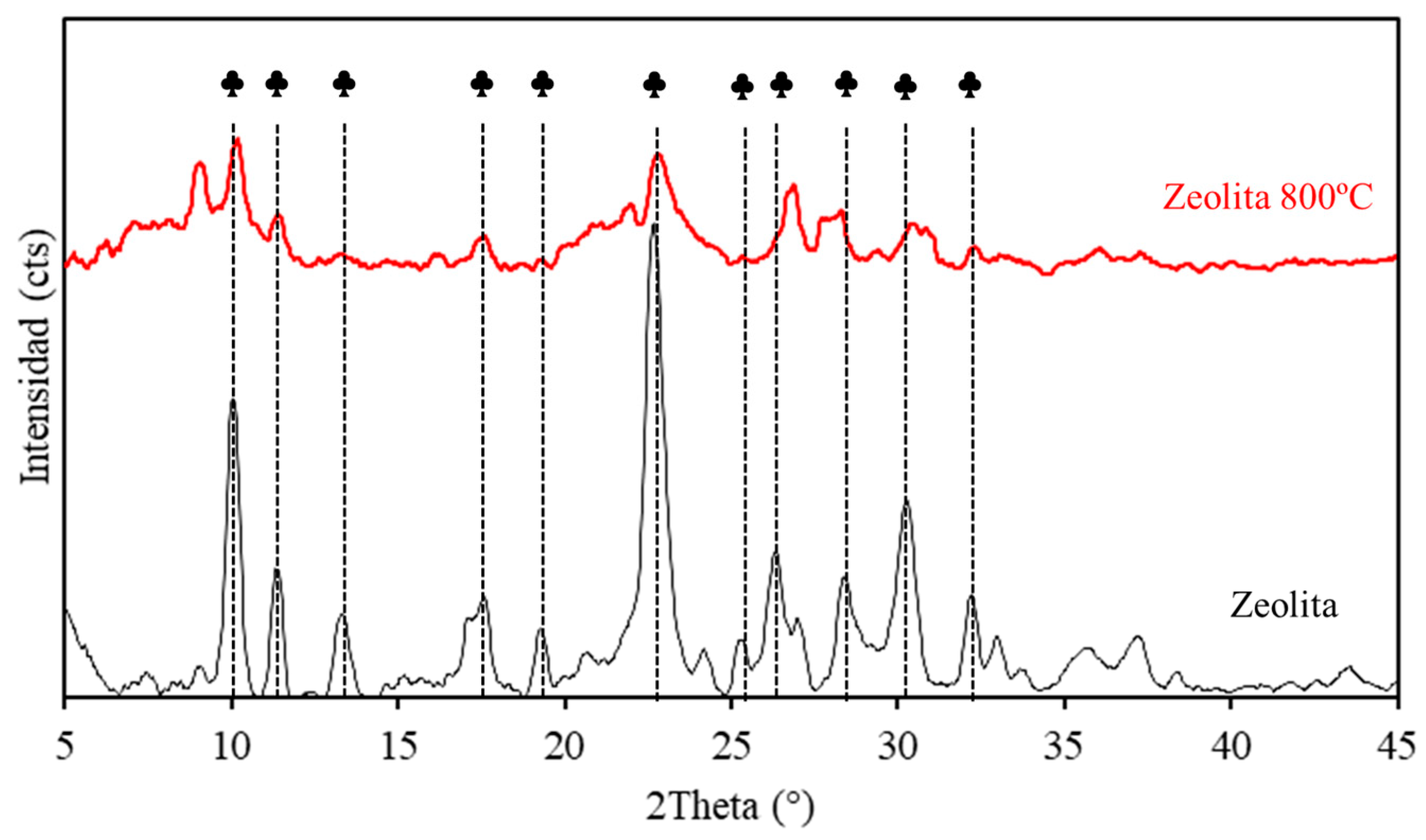
4.1. Supports Without Ni
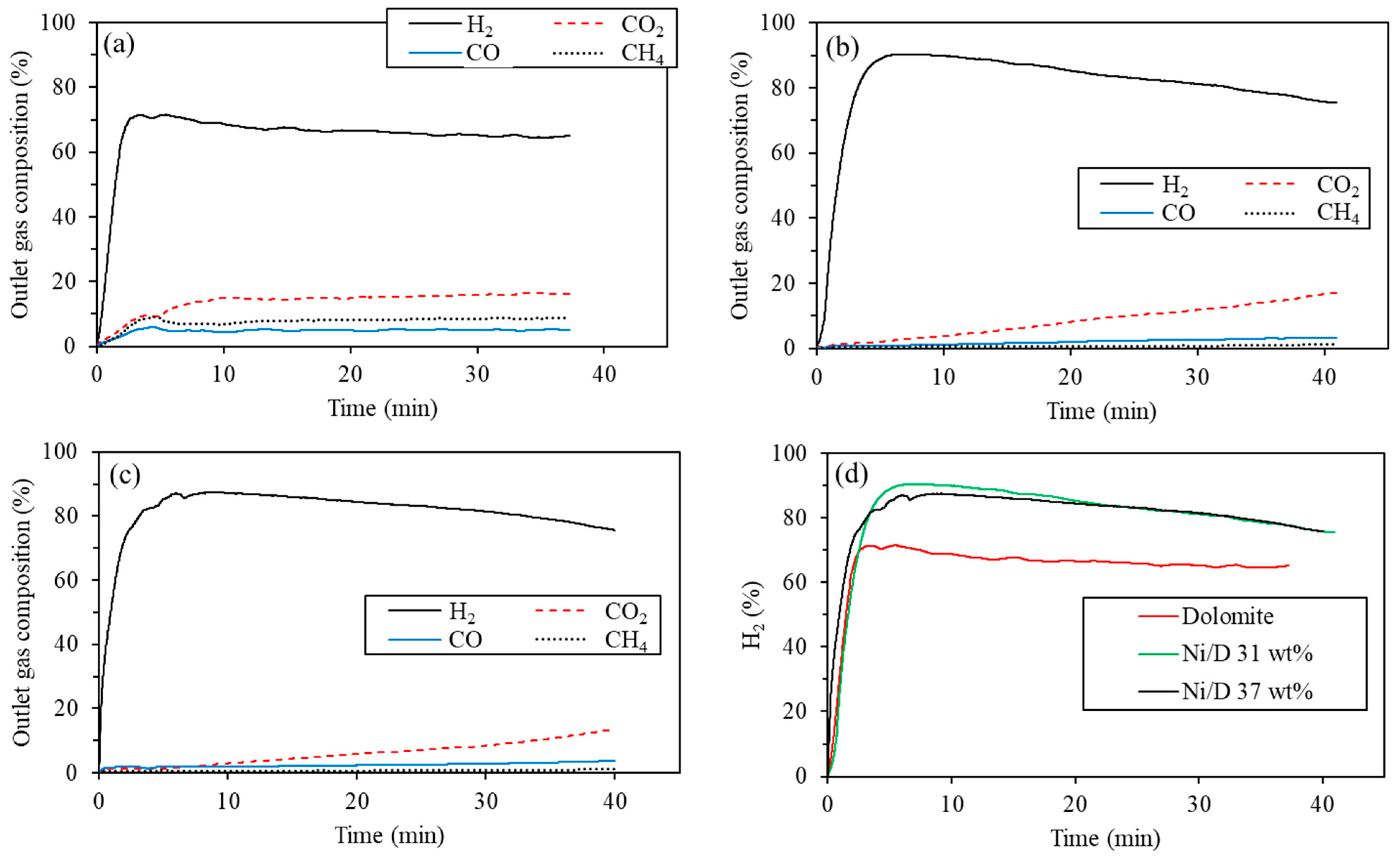
4.2. Supports with Different Concentrations of Ni
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Energy Institute. Statistical Review of World Energy 2025, 74th ed.; Energy Institute: London, UK, 2025. [Google Scholar]
- Martino, M.; Ruocco, C.; Meloni, E.; Pullumbi, P.; Palma, V. Main Hydrogen Production Processes: An Overview. Catalysts 2021, 11, 547. [Google Scholar] [CrossRef]
- Schwengber, C.A.; Alves, H.J.; Schaffner, R.A.; Da Silva, F.A.; Sequinel, R.; Bach, V.R.; Ferracin, R.J. Overview of Glycerol Reforming for Hydrogen Production. Renew. Sustain. Energy Rev. 2016, 58, 259–266. [Google Scholar] [CrossRef]
- Tao, X.; Zhao, Y.; Wang, S.; Li, C.; Li, R. Recent Advances and Perspectives for Solar-Driven Water Splitting Using Particulate Photocatalysts. Chem. Soc. Rev. 2022, 51, 3561–3608. [Google Scholar] [CrossRef]
- Song, H.; Luo, S.; Huang, H.; Deng, B.; Ye, J. Solar-Driven Hydrogen Production: Recent Advances, Challenges, and Future Perspectives. ACS Energy Lett. 2022, 7, 1043–1065. [Google Scholar] [CrossRef]
- Yu, L.; Ning, M.; Wang, Y.; Yuan, C.; Ren, Z. Direct Seawater Electrolysis for Hydrogen Production. Nat. Rev. Mater. 2025. [Google Scholar] [CrossRef]
- Mishra, A.; Park, H.; El-Mellouhi, F.; Suk Han, D. Seawater Electrolysis for Hydrogen Production: Technological Advancements and Future Perspectives. Fuel 2024, 361, 130636. [Google Scholar] [CrossRef]
- Maceiras, R.; Feijoo, J.; Alfonsín, V.; Pérez, L.; Álvarez-Feijoo, M.A.; Falcón, P.; Vallejo, J.P. Influence of Alumina Fixed-Bed in Steam Reforming of Glycerol for Hydrogen Production. Energy Rep. 2023, 9, 309–315. [Google Scholar] [CrossRef]
- Gujar, J.P.; verma, A.; Modhera, B. Optimizing Glycerol Conversion to Hydrogen: A Critical Review of Catalytic Reforming Processes and Catalyst Design Strategies. Int. J. Hydrogen Energy 2025, 109, 823–850. [Google Scholar] [CrossRef]
- Florin, N.H.; Harris, A.T. Enhanced Hydrogen Production from Biomass with in Situ Carbon Dioxide Capture Using Calcium Oxide Sorbents. Chem. Eng. Sci. 2008, 63, 287–316. [Google Scholar] [CrossRef]
- Diglio, M.; Contento, I.; Impemba, S.; Berretti, E.; Della Sala, P.; Oliva, G.; Naddeo, V.; Caporali, S.; Primo, A.; Talotta, C.; et al. Hydrogen Production from Formic Acid Decomposition Promoted by Gold Nanoparticles Supported on a Porous Polymer Matrix. Energy Fuels 2025, 39, 14320–14329. [Google Scholar] [CrossRef]
- Erdemir, D.; Dincer, I. A Quicker Route to Hydrogen Economy with Ammonia. Int. J. Hydrogen Energy 2024, 82, 1230–1237. [Google Scholar] [CrossRef]
- Coşkuner Filiz, B.; Civelek Yörüklü, H.; Açıkalın, K.; Demirci, U.B.; Kantürk Figen, A. Boron-Based Hydrogen Storage Materials towards Power-to-X Technology on the Path to Carbon Neutrality. Int. J. Hydrogen Energy 2023, 48, 39389–39407. [Google Scholar] [CrossRef]
- Goren, A.Y.; Temiz, M.; Erdemir, D.; Dincer, I. The Role of Effective Catalysts for Hydrogen Production: A Performance Evaluation. Energy 2025, 315, 134257. [Google Scholar] [CrossRef]
- Ghaffari Saeidabad, N.; Noh, Y.S.; Alizadeh Eslami, A.; Song, H.T.; Kim, H.D.; Fazeli, A.; Moon, D.J. A Review on Catalysts Development for Steam Reforming of Biodiesel Derived Glycerol; Promoters and Supports. Catalysts 2020, 10, 910. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.; Nichio, N.N. Hydrogen and/or Syngas from Steam Reforming of Glycerol. Study of Platinum Catalysts. Int. J. Hydrogen Energy 2010, 35, 8912–8920. [Google Scholar] [CrossRef]
- Lin, K.H.; Chang, A.C.C.; Lin, W.H.; Chen, S.H.; Chang, C.Y.; Chang, H.F. Autothermal Steam Reforming of Glycerol for Hydrogen Production over Packed-Bed and Pd/Ag Alloy Membrane Reactors. Int. J. Hydrogen Energy 2013, 38, 12946–12952. [Google Scholar] [CrossRef]
- Lai, H.J.; Liu, Y.C.; Nachimuthu, S.; Lin, S.D.; Jiang, J.C. Small Iridium Clusters Supported on TiO2 as Catalysts for Intensifying Low-Temperature Methane Activation and Reforming. Chem. Eng. J. 2024, 492, 152352. [Google Scholar] [CrossRef]
- Kuznetsov, V.V.; Vitovsky, O.V.; Gasenko, O.A. Methane Steam Reforming in an Annular Microchannel with Rh/Al2O3 Catalyst. J. Eng. Thermophys. 2009, 18, 187–196. [Google Scholar] [CrossRef]
- Namioka, T.; Saito, A.; Inoue, Y.; Park, Y.; Min, T.J.; Roh, S.A.; Yoshikawa, K. Hydrogen-Rich Gas Production from Waste Plastics by Pyrolysis and Low-Temperature Steam Reforming over a Ruthenium Catalyst. Appl. Energy 2011, 88, 2019–2026. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Douvartzides, S.L.; Siakavelas, G.I.; Tzounis, L.; Polychronopoulou, K.; Goula, M.A. The Relationship Between Reaction Temperature and Carbon Deposition on Nickel Catalysts Based on. Catalysts 2019, 9, 676. [Google Scholar] [CrossRef]
- Cheng, C.K.; Foo, S.Y.; Adesina, A.A. H2-Rich Synthesis Gas Production over Co/Al2O3 Catalyst via Glycerol Steam Reforming. Catal. Commun. 2010, 12, 292–298. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.I.; Papageridis, K.N.; Motta, D.; Dimitratos, N.; Sebastian, V.; Polychronopoulou, K.; Goula, M.A. The Effect of Noble Metal (M: Ir, Pt, Pd) on M/Ce2O3-γ-Al2O3 Catalysts for Hydrogen Production via the Steam Reforming of Glycerol. Catalysts 2020, 10, 790. [Google Scholar] [CrossRef]
- Liguras, D.K.; Kondarides, D.I.; Verykios, X.E. Production of Hydrogen for Fuel Cells by Steam Reforming of Ethanol over Supported Noble Metal Catalysts. Appl. Catal. B Environ. 2003, 43, 345–354. [Google Scholar] [CrossRef]
- López-Tenllado, F.J.; Estévez, R.; Hidalgo-Carrillo, J.; López-Fernández, S.; Urbano, F.J.; Marinas, A. Hydrogen Photo-Production from Glycerol on Platinum, Gold and Silver-Modified TiO2-USY62 Catalysts. Catal. Today 2022, 390–391, 92–98. [Google Scholar] [CrossRef]
- Marques, T.M.F.; Morais, R.N.; Nobre, F.X.; Rocha, J.M.; Ghosh, A.; Soares, T.A.S.; Viana, B.C.; Machado, G.; Costa, J.C.S.; De Matos, J.M.E. Hydrogen Production from Aqueous Glycerol Using Titanate Nanotubes Decorated with Au Nanoparticles as Photocatalysts. An. Acad. Bras. Cienc. 2019, 91, 1–15. [Google Scholar] [CrossRef]
- Pompeo, F.; Santori, G.F.; Nichio, N.N. Hydrogen Production by Glycerol Steam Reforming with Pt/SiO2 and Ni/SiO2 Catalysts. Catal. Today 2011, 172, 183–188. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Arafat, Y.; Ibrahim, A.A.; Shaikh, H.; Atia, H.; Abasaeed, A.E.; Armbruster, U.; Al-Fatesh, A.S. Highly Selective Syngas/H2 Production via Partial Oxidation of CH4 Using (Ni, Co and Ni-Co)/ZrO2 -Al2O3 Catalysts: Influence of Calcination Temperature. Processes 2019, 7, 141. [Google Scholar] [CrossRef]
- Thyssen, V.V.; Maia, T.A.; Assaf, E.M. Cu and Ni Catalysts Supported on γ-Al2O3 and SiO2 Assessed in Glycerol Steam Reforming Reaction. J. Braz. Chem. Soc. 2015, 26, 22–31. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Papageridis, K.N.; Siakavelas, G.; Tzounis, L.; Goula, M.A. Effect of Active Metal Supported on SiO2 for Selective Hydrogen Production from the Glycerol Steam Reforming Reaction. BioResources 2016, 11, 10173–10189. [Google Scholar] [CrossRef]
- Abiso, A.M.; Fasanya, O.O.; Suleiman, M.Y.; Atta, A.Y.; Dutta, J.; Jibril, B.E.-Y. Advances in Copper-Based Catalysts for Sustainable Hydrogen Production via Methanol Steam Reforming. Chem. Eng. J. Adv. 2024, 19, 100625. [Google Scholar] [CrossRef]
- Impemba, S.; Provinciali, G.; Filippi, J.; Salvatici, C.; Berretti, E.; Caporali, S.; Banchelli, M.; Caporali, M. Engineering the Heterojunction between TiO2 and In2O3 for Improving the Solar-Driven Hydrogen Production. Int. J. Hydrogen Energy 2024, 63, 896–904. [Google Scholar] [CrossRef]
- Zheng, X.; Song, Y.; Liu, Y.; Yang, Y.; Wu, D.; Yang, Y.; Feng, S.; Li, J.; Liu, W.; Shen, Y.; et al. ZnIn2S4-Based Photocatalysts for Photocatalytic Hydrogen Evolution via Water Splitting. Coord. Chem. Rev. 2023, 475, 214898. [Google Scholar] [CrossRef]
- Iriondo, A.; Cambra, J.F.; Güemez, M.B.; Barrio, V.L.; Requies, J.; Sánchez-Sánchez, M.C.; Navarro, R.M. Effect of ZrO2 Addition on Ni/Al2O3 Catalyst to Produce H2 from Glycerol. Int. J. Hydrogen Energy 2012, 37, 7084–7093. [Google Scholar] [CrossRef]
- Li, G.; Hu, L.; Hill, J.M. Comparison of Reducibility and Stability of Alumina-Supported Ni Catalysts Prepared by Impregnation and Co-Precipitation. Appl. Catal. A Gen. 2006, 301, 16–24. [Google Scholar] [CrossRef]
- El Doukkali, M.; Iriondo, A.; Cambra, J.F.; Gandarias, I.; Jalowiecki-Duhamel, L.; Dumeignil, F.; Arias, P.L. Deactivation Study of the Pt and/or Ni-Based γ-Al2O3 Catalysts Used in the Aqueous Phase Reforming of Glycerol for H2 Production. Appl. Catal. A Gen. 2014, 472, 80–91. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Papageridis, K.N.; Baklavaridis, A.; Tzounis, L.; Goula, G.; Yentekakis, I.V.; Polychronopoulou, K.; Goula, M.A. The Effect of WO3 Modification of ZrO2 Support on the Ni-Catalyzed Dry Reforming of Biogas Reaction for Syngas Production. Front. Environ. Sci. 2017, 5, 66. [Google Scholar] [CrossRef]
- Shao, S.; Shi, A.-W.; Liu, C.-L.; Yang, R.-Z.; Dong, W.-S. Hydrogen Production from Steam Reforming of Glycerol over Ni/CeZrO Catalysts. Fuel Process. Technol. 2014, 125, 1–7. [Google Scholar] [CrossRef]
- Alessio, H.J.; Pestana, G.L.; Comelli, R.A.; Grau, J.M. Hydrogen Production via Aqueous Phase Reforming of Glycerol over Ni-Co/γ-Al2O3 Catalysts: Effect of Support Modification with Lanthanides and Alkaline Earth Metals. Fuel 2025, 404, 136217. [Google Scholar] [CrossRef]
- Luo, N.; Ouyang, K.; Cao, F.; Xiao, T. Hydrogen Generation from Liquid Reforming of Glycerin over Ni–Co Bimetallic Catalyst. Biomass Bioenergy 2010, 34, 489–495. [Google Scholar] [CrossRef]
- Profeti, L.P.R.; Dias, J.A.C.; Assaf, J.M.; Assaf, E.M. Hydrogen Production by Steam Reforming of Ethanol over Ni-Based Catalysts Promoted with Noble Metals. J. Power Sources 2009, 190, 525–533. [Google Scholar] [CrossRef]
- Karimi, S.; Bibak, F.; Meshkani, F.; Rastegarpanah, A.; Deng, J.; Liu, Y.; Dai, H. Promotional Roles of Second Metals in Catalyzing Methane Decomposition over the Ni-Based Catalysts for Hydrogen Production: A Critical Review. Int. J. Hydrogen Energy 2021, 46, 20435–20480. [Google Scholar] [CrossRef]
- Iriondo, A.; Barrio, V.L.; Cambra, J.F.; Arias, P.L.; Güemez, M.B.; Navarro, R.M.; Sánchez-Sánchez, M.C.; Fierro, J.L.G. Hydrogen Production from Glycerol Over Nickel Catalysts Supported on Al2O3 Modified by Mg, Zr, Ce or La. Top. Catal. 2008, 49, 46–58. [Google Scholar] [CrossRef]
- Soares, R.R.; Simonetti, D.A.; Dumesic, J.A. Glycerol as a Source for Fuels and Chemicals by Low-Temperature Catalytic Processing. Angew. Chemie Int. Ed. 2006, 45, 3982–3985. [Google Scholar] [CrossRef]
- Bepari, S.; Kuila, D. Steam Reforming of Methanol, Ethanol and Glycerol over Nickel-Based Catalysts-A Review. Int. J. Hydrogen Energy 2020, 45, 18090–18113. [Google Scholar] [CrossRef]
- Luo, N.; Zhao, X.; Cao, F.; Xiao, T.; Fang, D. Thermodynamic Study on Hydrogen Generation from Different Glycerol Reforming Processes. Energy Fuels 2007, 21, 3505–3512. [Google Scholar] [CrossRef]
- Alberton, A.L.; Souza, M.M.V.M.; Schmal, M. Carbon Formation and Its Influence on Ethanol Steam Reforming over Ni/Al2O3 Catalysts. Catal. Today 2007, 123, 257–264. [Google Scholar] [CrossRef]
- Maceiras, R.; Feijoo, J.; Perez-Rial, L.; Alfonsin, V.; Falcon, P. Study of Natural Zeolites for Hydrogen Purification: CO2 Adsorption Capacity and Kinetic Mechanism. Fuel 2024, 376, 132732. [Google Scholar] [CrossRef]
- van Heijst, J.; Martin-Calvo, A.; Calero, S. Hydrogen Recovery from Steam Methane Reforming Using the ITQ-12 Zeolite. Sep. Purif. Technol. 2024, 350, 127895. [Google Scholar] [CrossRef]
- Maceiras, R.; Feijoo, J.; Alfonsin, V.; Alvarez-Feijoo, M.A.; Azofra, L. Enhanced CO2 Adsorption for Hydrogen Purification Using Calcined Zeolite: Process Analysis and Kinetic Study. Microporous Mesoporous Mater. 2025, 399, 113869. [Google Scholar] [CrossRef]
- Yao, D.; Yang, H.; Chen, H.; Williams, P.T. Investigation of Nickel-Impregnated Zeolite Catalysts for Hydrogen/Syngas Production from the Catalytic Reforming of Waste Polyethylene. Appl. Catal. B Environ. 2018, 227, 477–487. [Google Scholar] [CrossRef]
- Grasa, G.S.; Abanades, J.C. CO2 Capture Capacity of CaO in Long Series of Carbonation/Calcination Cycles. Ind. Eng. Chem. Res. 2006, 45, 8846–8851. [Google Scholar] [CrossRef]
- Sun, P.; Grace, J.R.; Lim, C.J.; Anthony, E.J. The Effect of CaO Sintering on Cyclic CO2 Capture in Energy Systems. AIChE J. 2007, 53, 2432–2442. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, L.; Yang, Z.; He, Z. An Experiment Study of Biomass Steam Gasification over NiO/Dolomite for Hydrogen-Rich Gas Production. Int. J. Hydrogen Energy 2017, 42, 76–85. [Google Scholar] [CrossRef]
- Gallucci, K.; Stendardo, S.; Foscolo, P.U. CO2 Capture by Means of Dolomite in Hydrogen Production from Syn Gas. Int. J. Hydrogen Energy 2008, 33, 3049–3055. [Google Scholar] [CrossRef]
- Weitkamp, J.; Hunger, M. Chapter 22—Acid and Base Catalysis on Zeolites. In Introduction to Zeolite Science and Practice; Čejka, J., van Bekkum, H., Corma, A., Schüth, F., Eds.; Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2007; Volume 168, pp. 787–835. [Google Scholar] [CrossRef]
- Feijoo, J.; Gomez-Villalba, L.S.; de los Ríos, A.; Fort, R. Electroprecipitation of Inorganic Borates, with Different Solubility, within Monumental Stones to Avoid Fungal Colonization. Constr. Build. Mater. 2023, 368, 130435. [Google Scholar] [CrossRef]
- de Rosario, I.; Rivas, T.; Buceta, G.; Feijoo, J.; Mosquera, M.J. Surfactant-Synthesized Consolidants Applied To A Granitic Medieval Necropolis In NW Spain. Laboratory And In Situ Effectiveness Evaluation. Int. J. Archit. Herit. 2017, 11, 1166–1176. [Google Scholar]
- Feijoo, J.; de Rosario, I.; Rivas, T.; Mosquera, M.J.; Benavides, R. Influence of a Pre-Consolidation Treatment on the Desalination Effectiveness of a Highly Deteriorated Granite Façade of Medieval Age. Int. J. Archit. Herit. 2023, 17, 1965–1983. [Google Scholar] [CrossRef]
- Mambetova, M.; Dossumov, K.; Baikhamurova, M.; Yergaziyeva, G. Sorbents Based on Natural Zeolites for Carbon Dioxide Capture and Removal of Heavy Metals from Wastewater: Current Progress and Future Opportunities. Processes 2024, 12, 2071. [Google Scholar] [CrossRef]
- Yaşyerli, S.; Ar, İ.; Doğu, G.; Doğu, T. Removal of Hydrogen Sulfide by Clinoptilolite in a Fixed Bed Adsorber. Chem. Eng. Process. Process Intensif. 2002, 41, 785–792. [Google Scholar] [CrossRef]
- Kuldeyev, E.; Seitzhanova, M.; Tanirbergenova, S.; Tazhu, K.; Doszhanov, E.; Mansurov, Z.; Azat, S.; Nurlybaev, R.; Berndtsson, R. Modifying Natural Zeolites to Improve Heavy Metal Adsorption. Water 2023, 15, 2215. [Google Scholar] [CrossRef]
- Shamsuddin, M.R.; Asikin-Mijan, N.; Marliza, T.S.; Miyamoto, M.; Uemiya, S.; Yarmo, M.A.; Taufiq-Yap, Y.H. Promoting Dry Reforming of Methane via Bifunctional NiO/Dolomite Catalysts for Production of Hydrogen-Rich Syngas. RSC Adv. 2021, 11, 6667–6681. [Google Scholar] [CrossRef] [PubMed]
- Zulqarnain; Kim, S.; Hyuk Chun, D.; Yoo, J.; Kim, S.; Im, H.; Choi, H.; Lim, J. Improving the Activity and Long-Term Durability of the Ni/Al2O3 Catalyst in the Low-Temperature Steam Reforming of Highly Concentrated Volatile Organic Compounds. Energy Convers. Manag. X 2024, 24, 100819. [Google Scholar] [CrossRef]
- Bastan, F.; Kazemeini, M.; Larimi, A.; Maleki, H. Production of Renewable Hydrogen through Aqueous-Phase Reforming of Glycerol over Ni/Al2O3MgO Nano-Catalyst. Int. J. Hydrogen Energy 2018, 43, 614–621. [Google Scholar] [CrossRef]
- Md Radzi, M.R.; Azizan, M.T.; Daud, N.F.D.M.; Topek, M.A.; Abidin, S.Z. Aqueous Phase Reforming of Sorbitol over Ca Doped Ni/Al2O3 for Value-Added Chemicals Production. Mater. Today Proc. 2018, 5, 21728–21736. [Google Scholar] [CrossRef]
- Kiren, B.; Ayas, N. Nickel Modified Dolomite in the Hydrogen Generation from Sodium Borohydride Hydrolysis. Int. J. Hydrogen Energy 2022, 47, 19702–19717. [Google Scholar] [CrossRef]
- Guo, Y.; Azmat, M.U.; Liu, X.; Wang, Y.; Lu, G. Effect of Support’s Basic Properties on Hydrogen Production in Aqueous-Phase Reforming of Glycerol and Correlation between WGS and APR. Appl. Energy 2012, 92, 218–223. [Google Scholar] [CrossRef]

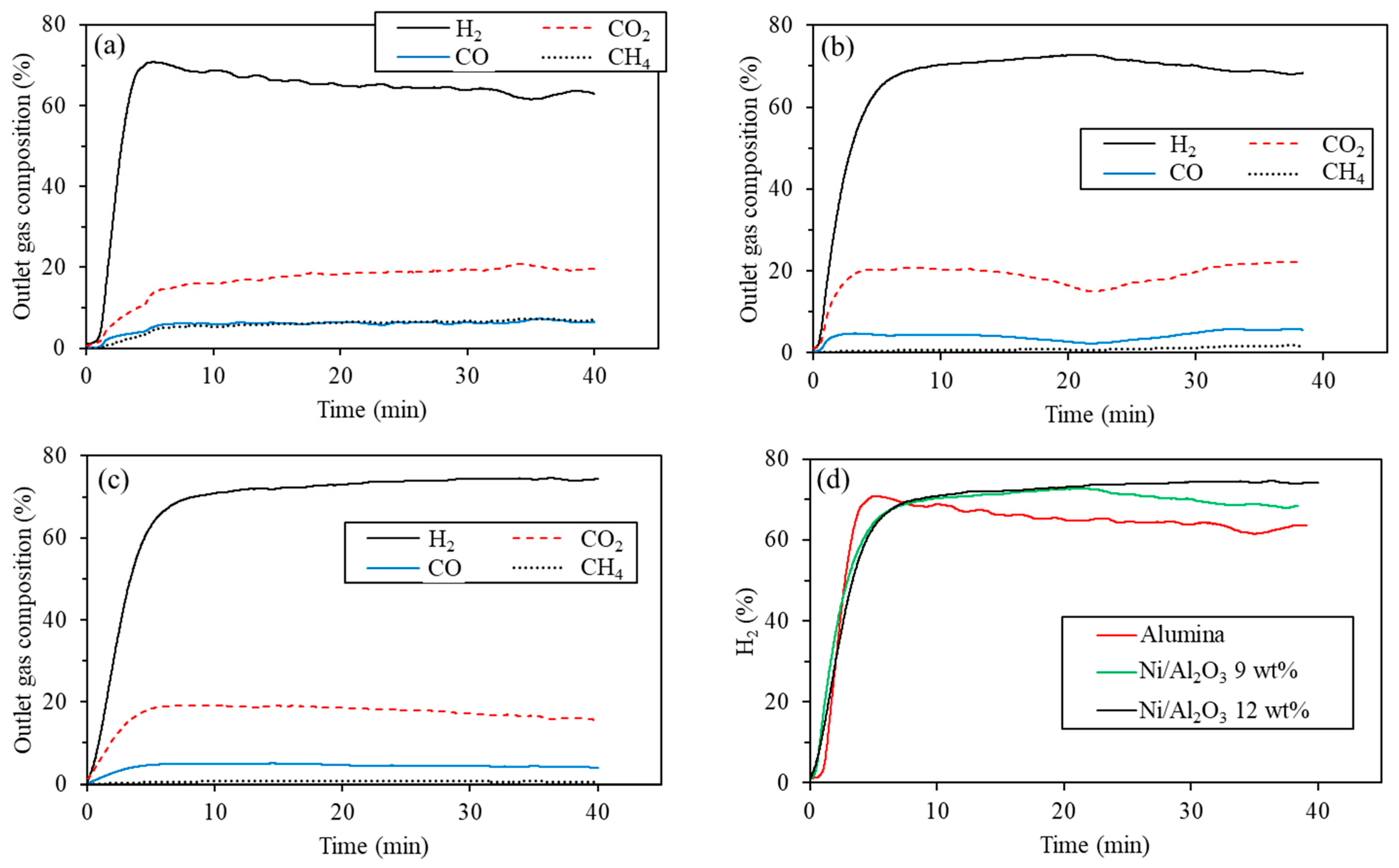
| Alumina | H2 | CO2 | CO | CH4 |
| Maximum (%) | 70.8 | 20.8 | 7.3 | 7.4 |
| Average (%) | 62.0 | 16.8 | 5.8 | 5.7 |
| Dolomite | H2 | CO2 | CO | CH4 |
| Maximum (%) | 71.6 | 16.6 | 5.9 | 9.2 |
| Average (%) | 65.1 | 13.8 | 4.8 | 7.7 |
| Zeolite | H2 | CO2 | CO | CH4 |
| Maximum (%) | 54.2 | 20.9 | 9.6 | 8.7 |
| Average (%) | 51.0 | 18.2 | 7.5 | 7.4 |
| Ni(NO3)2 Concentration | 10 wt% | 12 wt% |
|---|---|---|
| Alumina | 9% | 12% |
| Dolomite | 31% | 37% |
| Alumina | H2 | CO2 | CO | CH4 |
| Maximum (%) | 70.8 | 20.8 | 7.3 | 7.4 |
| Average (%) | 62.0 | 16.8 | 5.8 | 5.7 |
| Ni/Al2O3 9 wt% | H2 | CO2 | CO | CH4 |
| Maximum (%) | 72.8 | 22.3 | 5.9 | 1.8 |
| Average (%) | 66.2 | 18.7 | 4.1 | 0.9 |
| Ni/Al2O3 12 wt% | H2 | CO2 | CO | CH4 |
| Maximum (%) | 76.0 | 19.2 | 5.0 | 0.8 |
| Average (%) | 70.1 | 16.4 | 4.2 | 0.6 |
| Dolomite | H2 | CO2 | CO | CH4 |
| Maximum (%) | 71.6 | 16.6 | 5.9 | 9.2 |
| Average (%) | 65.1 | 13.8 | 4.8 | 7.7 |
| Ni/D 31 wt% | H2 | CO2 | CO | CH4 |
| Maximum (%) | 90.3 | 16.7 | 3.3 | 1.2 |
| Average (%) | 81.0 | 8.0 | 2.0 | 0.7 |
| Ni/D 37 wt% | H2 | CO2 | CO | CH4 |
| Maximum (%) | 87.5 | 13.6 | 3.6 | 1.1 |
| Average (%) | 81.3 | 5.9 | 2.4 | 0.7 |
| Support | Maximum (%) | Average (%) | Efficiency (%) |
|---|---|---|---|
| Alumina | 70.8 | 62.0 | - |
| Ni/Al2O3 9 wt% | 72.8 | 66.2 | 6.8 |
| Ni/Al2O3 12 wt% | 76.0 | 70.1 | 13.1 |
| Dolomite | 71.6 | 65.1 | - |
| Ni/D 31 wt% | 90.3 | 81.0 | 24.4 |
| Ni/D 37 wt% | 87.5 | 81.3 | 24.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feijoo, J.; Maceiras, R.; Alfonsín, V.; Aly, N.; de la Fuente, A. Influence of Catalytic Support on Hydrogen Production from Glycerol Steam Reforming. Hydrogen 2025, 6, 88. https://doi.org/10.3390/hydrogen6040088
Feijoo J, Maceiras R, Alfonsín V, Aly N, de la Fuente A. Influence of Catalytic Support on Hydrogen Production from Glycerol Steam Reforming. Hydrogen. 2025; 6(4):88. https://doi.org/10.3390/hydrogen6040088
Chicago/Turabian StyleFeijoo, Jorge, Rocío Maceiras, Victor Alfonsín, Nevin Aly, and Alejandro de la Fuente. 2025. "Influence of Catalytic Support on Hydrogen Production from Glycerol Steam Reforming" Hydrogen 6, no. 4: 88. https://doi.org/10.3390/hydrogen6040088
APA StyleFeijoo, J., Maceiras, R., Alfonsín, V., Aly, N., & de la Fuente, A. (2025). Influence of Catalytic Support on Hydrogen Production from Glycerol Steam Reforming. Hydrogen, 6(4), 88. https://doi.org/10.3390/hydrogen6040088








