Investigation of Mechano-Electrochemical Effects on Hydrogen Distribution at Corrosion Defects
Abstract
1. Introduction
2. Experimental Procedures
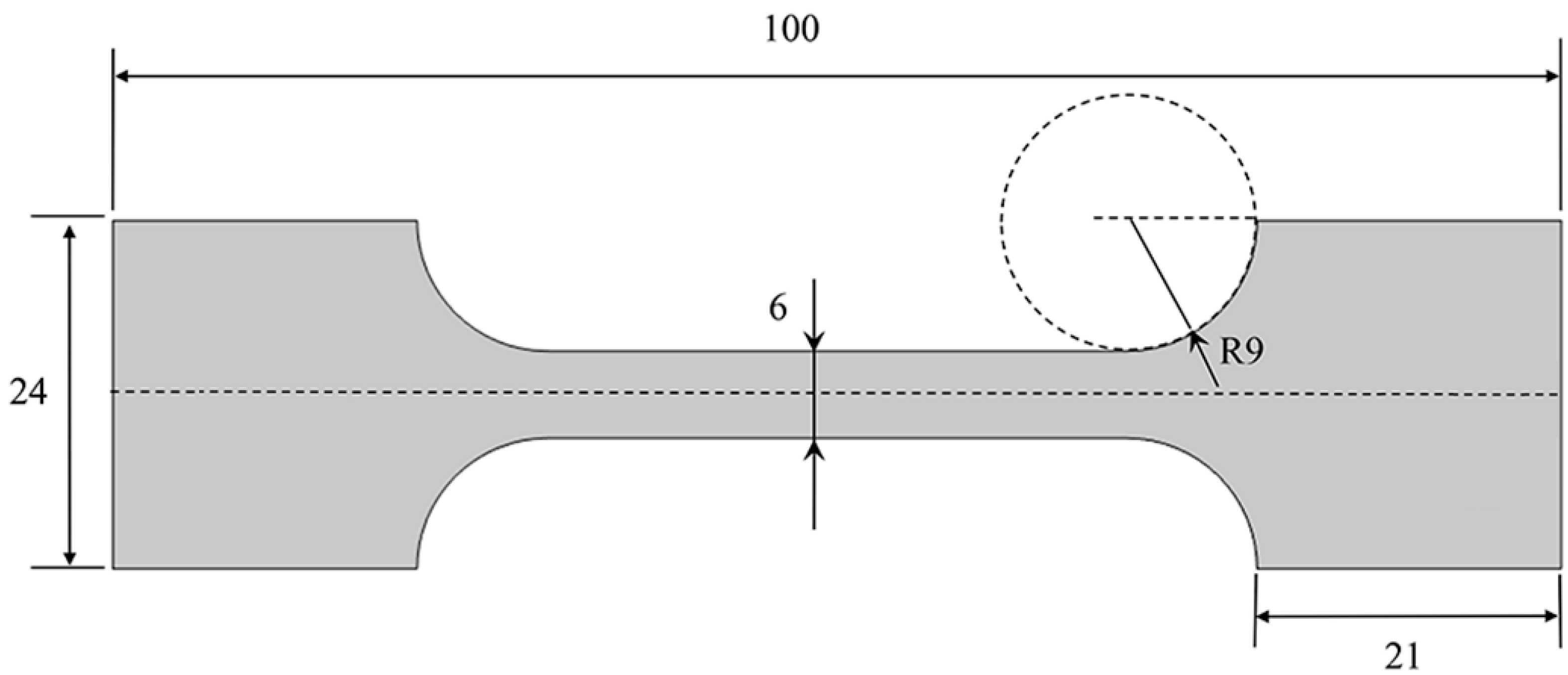
2.1. Materials and Tensile Tests
2.2. Electrochemical Measurements
2.2.1. Hydrogen Permeation Test
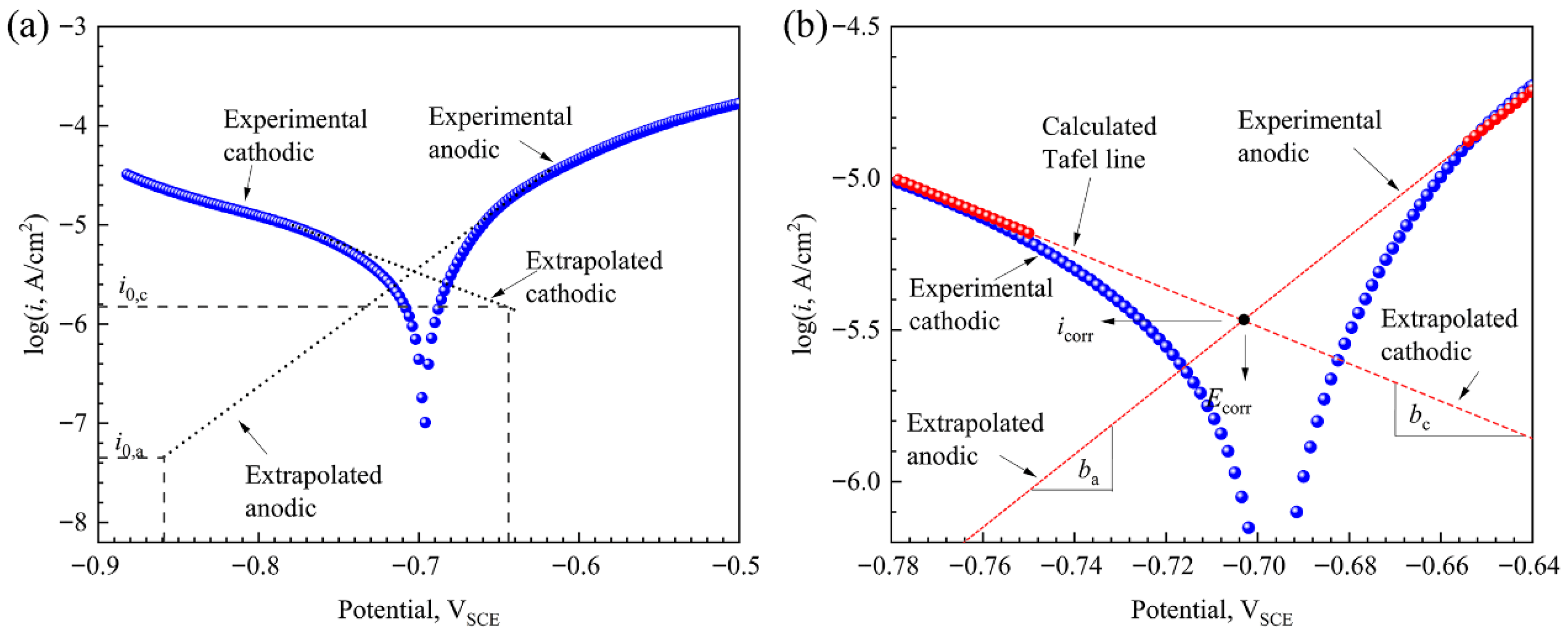
2.2.2. Potentiodynamic Polarization Tests
2.3. FE Simulation
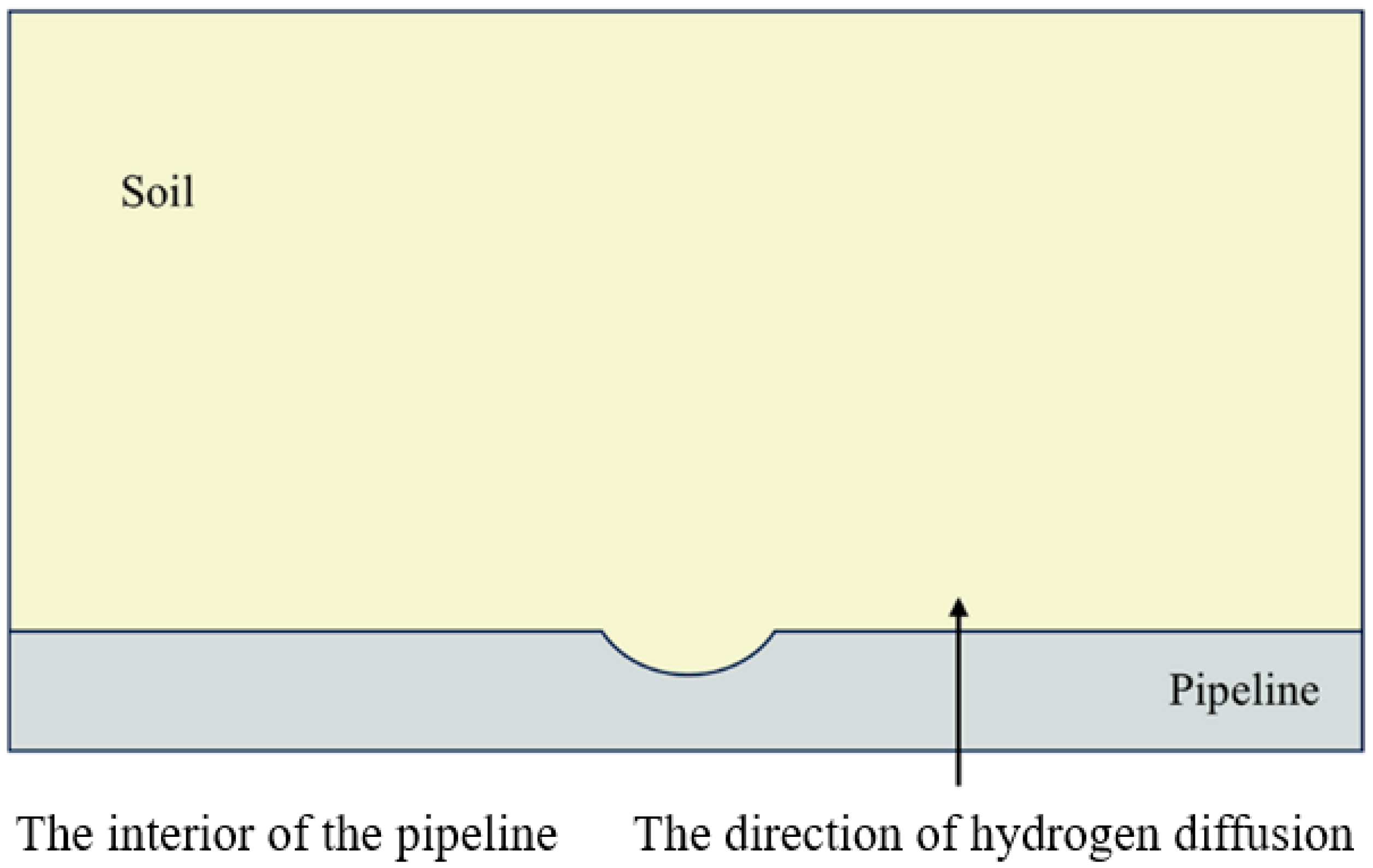
2.3.1. Initial and Boundary Conditions
2.3.2. Hydrogen Atom Distribution Simulation
2.3.3. M-E Effect and Hydrogen Distribution Simulation
Stress Field Simulation
Electrochemical Field Simulation
- (1)
- Anodic reaction
- (2)
- Cathodic reaction
Distribution of the Electrical Fields in a Solution
Multifield Coupling Simulation
3. Results
3.1. Tensile, Hydrogen Permeation, and Potentiodynamic Polarization Characteristics
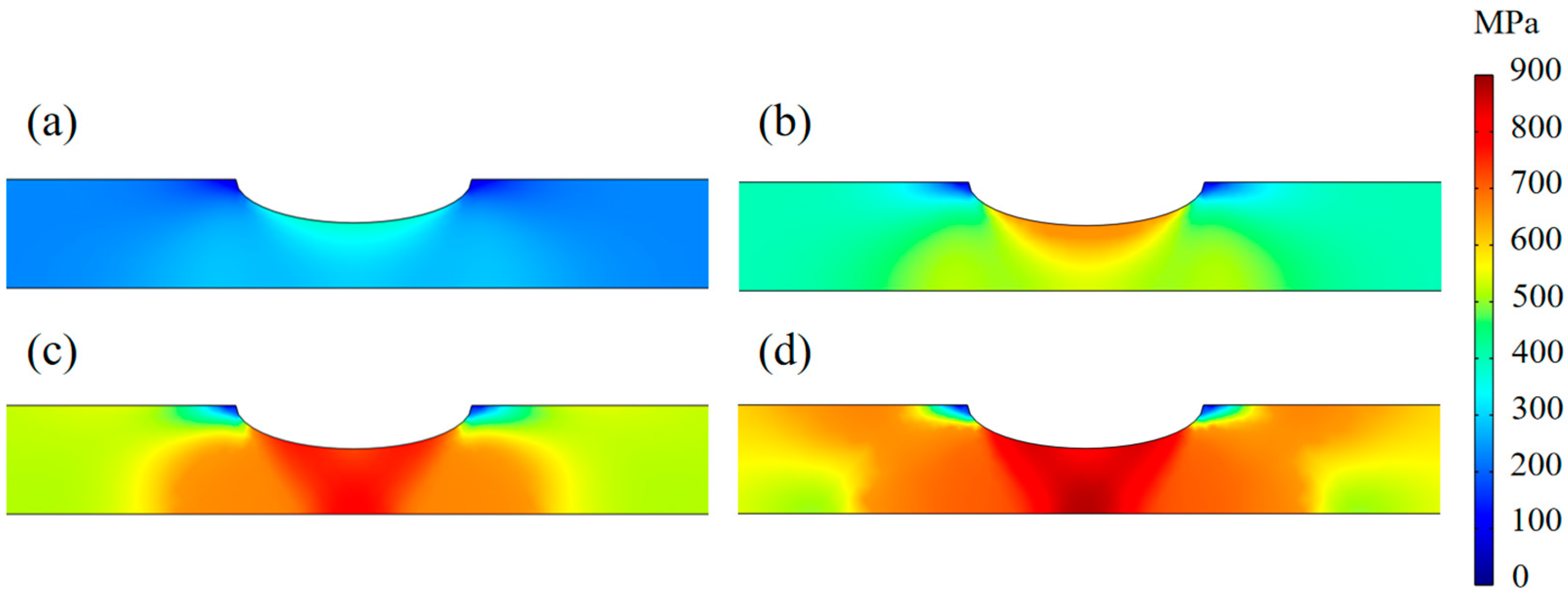
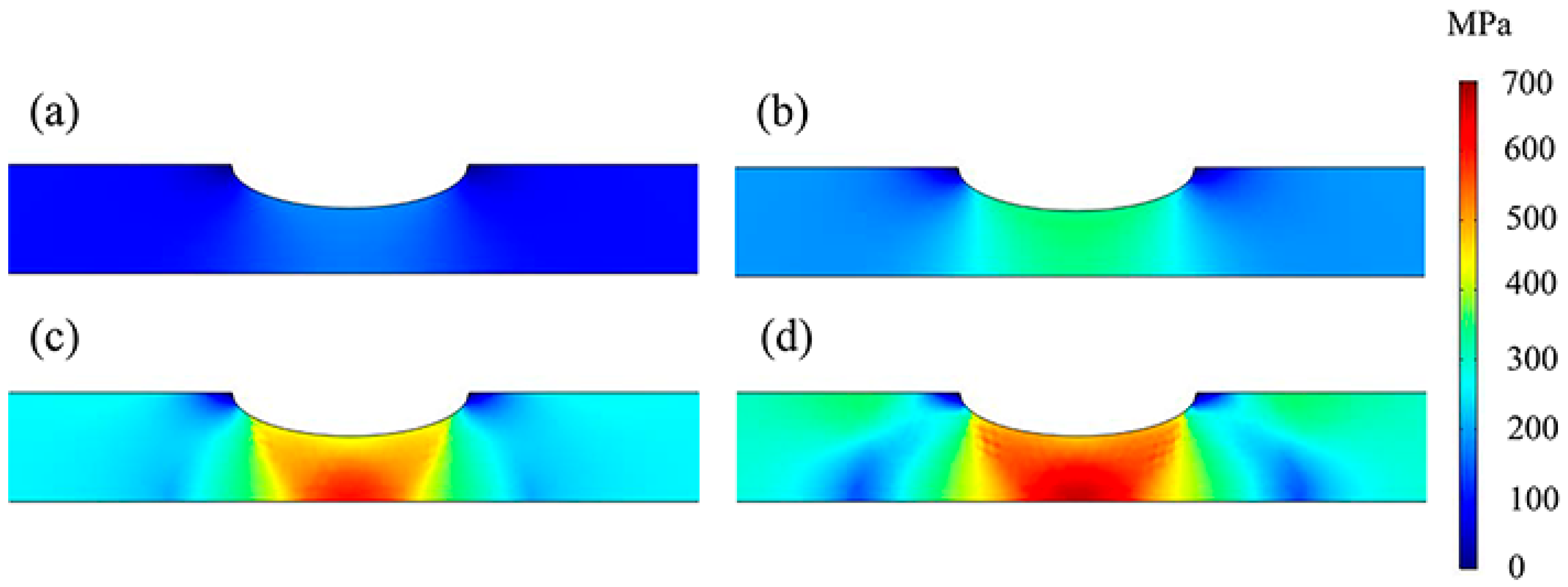
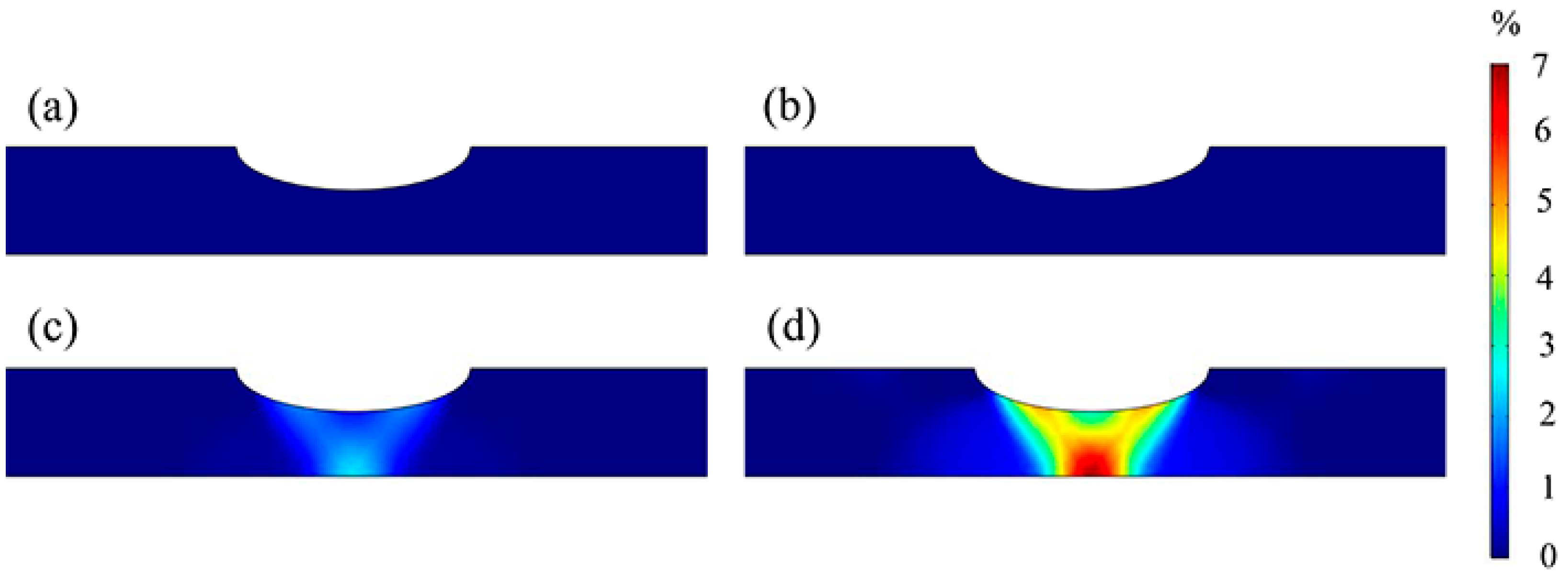
3.2. Stress and Strain Distributions
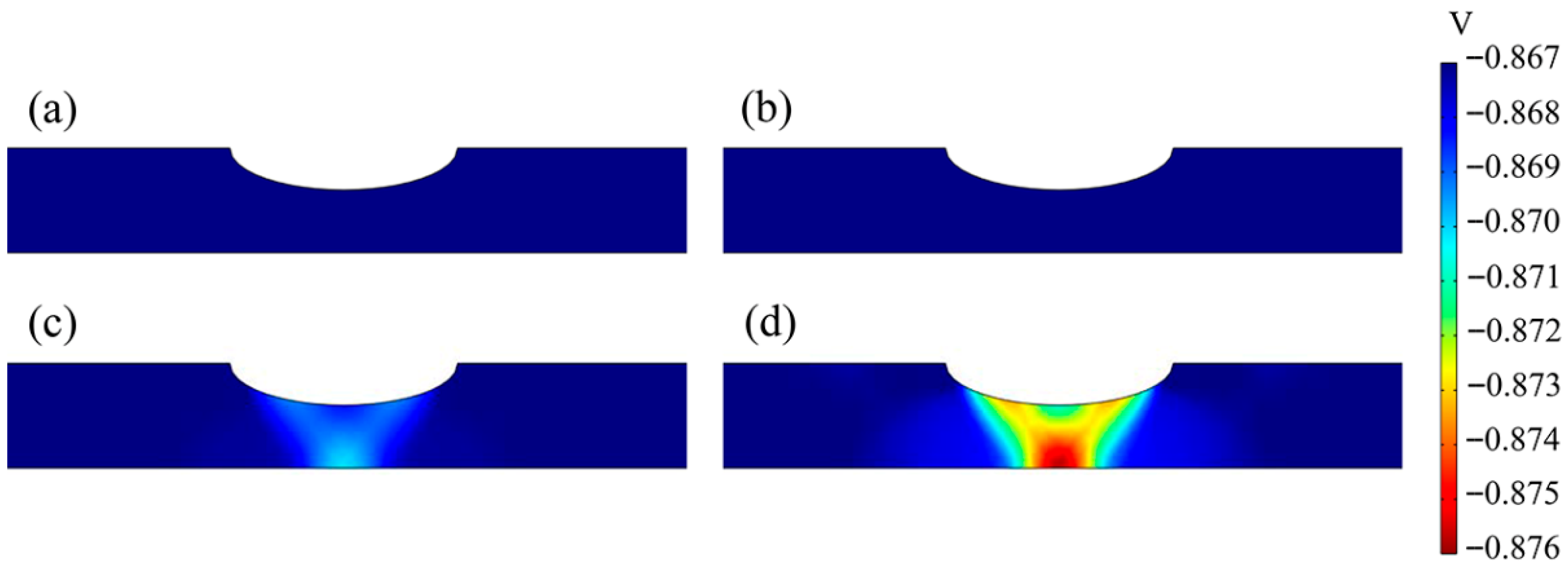
3.3. Anodic Equilibrium Potential and Cathodic Exchange Current Density Distributions
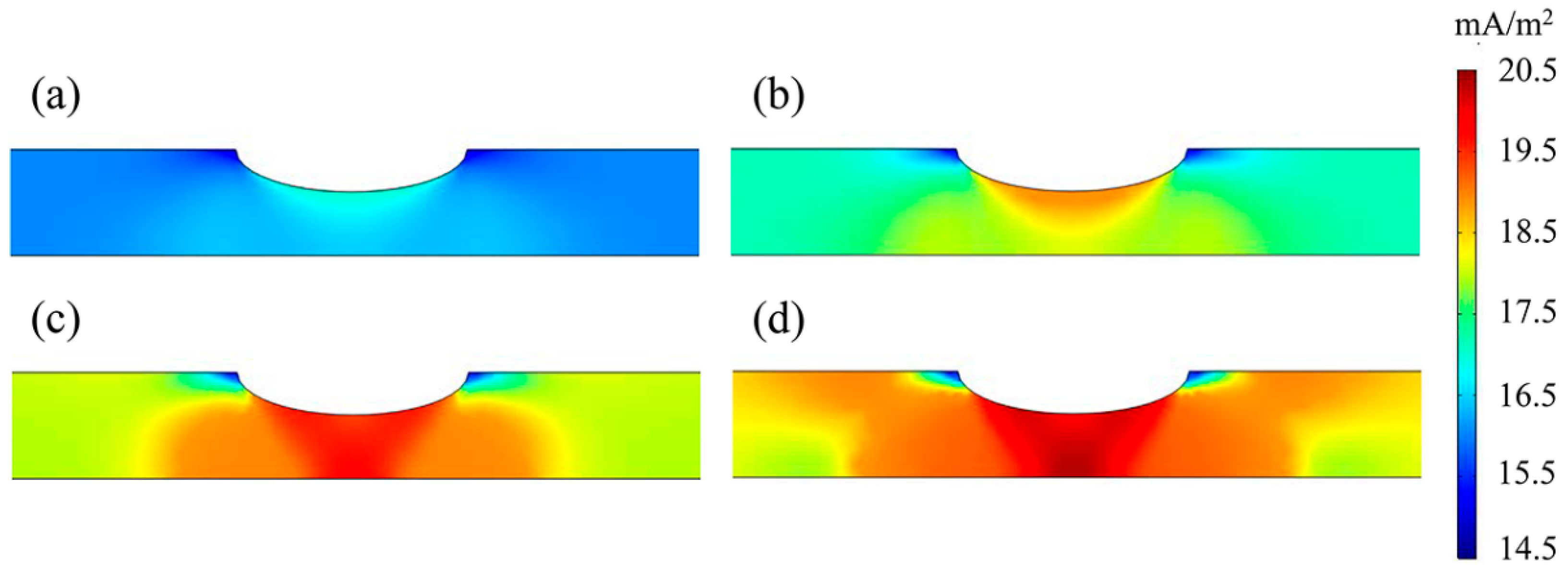
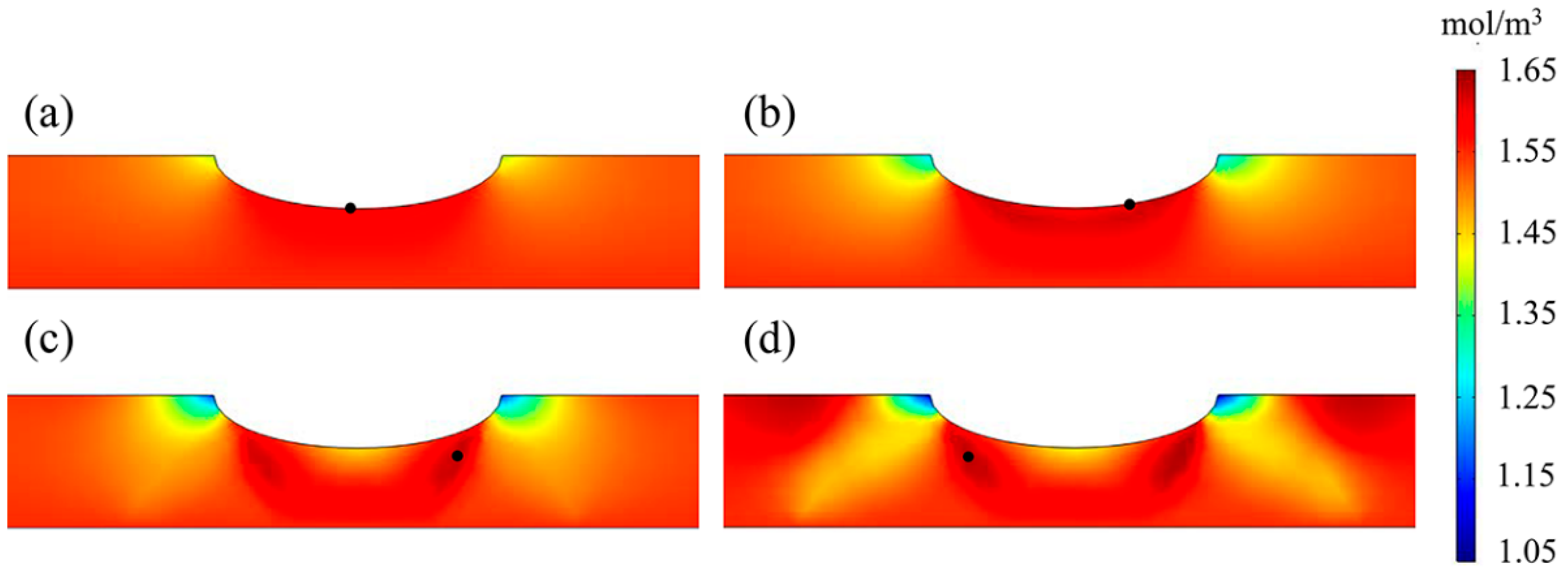
3.4. Hydrogen Distribution
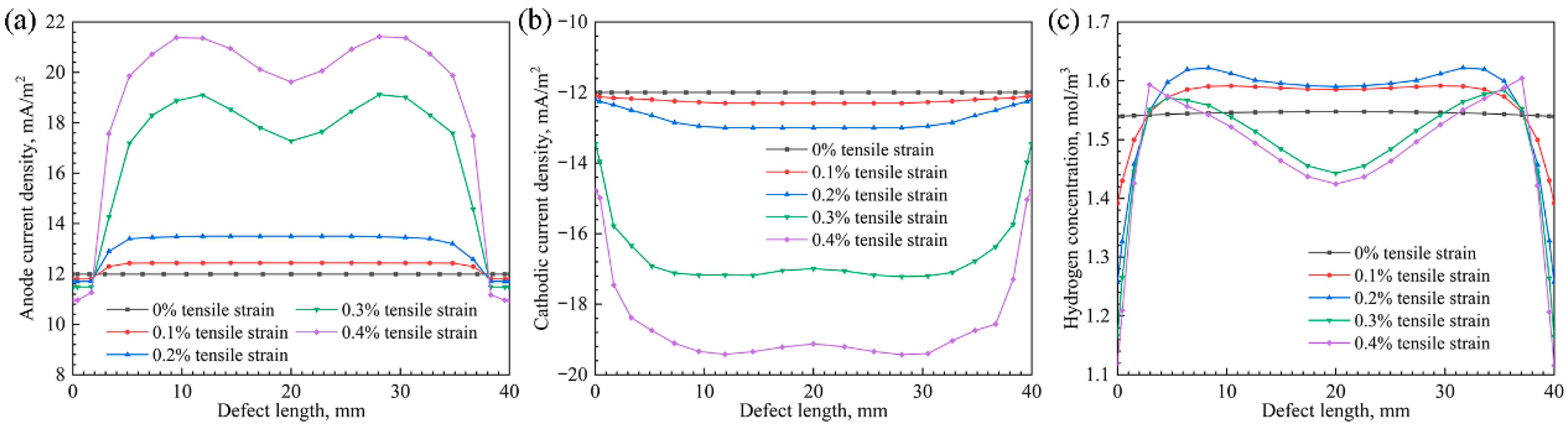
3.5. Linear Distribution of the Current Density and Hydrogen Concentration
4. Discussion
4.1. The Effect of Tensile Strain on the Anodic Equilibrium Potential and Cathodic Exchange Current Density
4.2. The Influence of the M-E Effect on the Anodic and Cathodic Current Densities
4.3. The Influence of the M-E Effect on the Hydrogen Distribution
5. Conclusions
- (1)
- The tensile strain influenced the cathodic and anodic reactions by altering the stress–strain distribution on the X80 pipeline. Under elastic deformation, the anodic equilibrium potential was uniform across the pipeline, while the maximum cathodic exchange current density was evident at the defect root. Under plastic deformation, the anodic equilibrium potential displayed a negative shift, while the maximum cathodic exchange current density deviated from the center.
- (2)
- The M-E effect induced anodic polarization in high-stress regions, which enhanced corrosion, while cathodic polarization occurs in low-stress regions, resulting in corrosion mitigation.
- (3)
- In a near-neutral solution environment, the hydrogen distribution state on the X80 pipeline was consistent with that of the stress–hydrogen diffusion coupling when considering the M-E effect. However, the presence of the M-E effect slightly increased the hydrogen concentration at the corrosion defect.
Author Contributions
Funding
Conflicts of Interest
References
- Faye, O.; Szpunar, J.; Eduok, U. A critical review on the current technologies for the generation, storage, and transportation of hydrogen. Int. J. Hydrogen Energy 2022, 47, 13771–13802. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Zhu, Y.-Q.; Song, W.; Wang, H.-B.; Qi, J.-T.; Zeng, R.-C.; Ren, H.; Jiang, W.-C.; Meng, H.-B.; Li, Y.-X. Advances in reducing hydrogen effect of pipeline steels on hydrogen-blended natural gas transportation: A systematic review of mitigation strategies. Renew. Sustain. Energy Rev. 2023, 189, 113950. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, H.; Liu, C.; Wang, C.; Fan, X.; Cheng, Y.F.; Li, Y. Effects of defect on the hydrogen embrittlement behavior of X80 pipeline steel in hydrogen-blended natural gas environments. Int. J. Hydrogen Energy 2024, 58, 158–173. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Du, C.; Cheng, Y. Effect of cathodic protection on corrosion of pipeline steel under disbonded coating. Corros. Sci. 2009, 51, 2242–2245. [Google Scholar] [CrossRef]
- Perdomo, J.; Song, I. Chemical and electrochemical conditions on steel under disbonded coatings: The effect of applied potential, solution resistivity, crevice thickness and holiday size. Corros. Sci. 2000, 42, 1389–1415. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z.; Li, X.; Du, C. Electrochemical characteristic and stress corrosion behavior of API X70 high-strength pipeline steel under a simulated disbonded coating in an artificial seawater environment. J. Electroanal. Chem. 2019, 845, 92–105. [Google Scholar] [CrossRef]
- Vanboven, G.; Chen, W.; Rogge, R. The role of residual stress in neutral pH stress corrosion cracking of pipeline steels. Part I: Pitting and cracking occurrence. Acta Mater. 2007, 55, 29–42. [Google Scholar] [CrossRef]
- Zhang, Z.; Guo, L.; Cheng, Y.F. Interaction between internal and external defects on pipelines and its effect on failure pressure. Thin–Walled Struct. 2021, 159, 107230. [Google Scholar] [CrossRef]
- Guo, S.; Xu, L.; Dong, S.; Cheng, Y.F. Finite element modeling of hydrogen atom diffusion and distribution at corrosion defect on aged pipelines transporting hydrogen. Int. J. Hydrogen Energy 2023, 48, 13566–13577. [Google Scholar] [CrossRef]
- Li, W.; Guo, T.; Xu, L.; Chen, L.; Jiang, B.; Wang, X.; Wu, H.; Qiao, L. Promotion of pitting corrosion at hydrogen-enriched α/γ phase boundaries in austenitic stainless steel weld joints. Acta Mater. 2022, 227, 117728. [Google Scholar] [CrossRef]
- Devanathan, M.A.V.; Stachurski, Z. The mechanism of hydrogen evolution on iron in acid solutions by determination of permeation rates. J. Electrochem. Soc. 1964, 111, 619–623. [Google Scholar] [CrossRef]
- Doyle, D.; Palumbo, G.; Aust, K.; El-Sherik, A.; Erb, U. The influence of intercrystalline defects on hydrogen activity and transport in nickel. Acta Met. Mater. 1995, 43, 3027–3033. [Google Scholar] [CrossRef]
- Cheng, Y.F. Fundamentals of hydrogen evolution reaction and its implications on near-neutral pH stress corrosion cracking of pipelines. Electrochim. Acta 2007, 52, 2661–2667. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, Y. Development of a finite element model for simulation and prediction of mechanoelectrochemical effect of pipeline corrosion. Corros. Sci. 2013, 73, 150–160. [Google Scholar] [CrossRef]
- Hafsi, Z.; Mishra, M.; Elaoud, S. Hydrogen embrittlement of steel pipelines during transients. Procedia Struct. Integr. 2018, 13, 210–217. [Google Scholar] [CrossRef]
- Krom, A.; Koers, R.; Bakker, A. Hydrogen transport near a blunting crack tip. J. Mech. Phys. Solids 1999, 47, 971–992. [Google Scholar] [CrossRef]
- Wijmans, J.; Baker, R. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Sofronis, P.; McMeeking, R. Numerical analysis of hydrogen transport near a blunting crack tip. J. Mech. Phys. Solids 1989, 37, 317–350. [Google Scholar] [CrossRef]
- McLellan, A.G.; Fyfe, W.S. Non-hydrostatic thermodynamics of chemical systems. Proc. R. Soc. Lond. A Math. Phys. Sci. 1970, 314, 443–455. [Google Scholar]
- Gutman, E.; Solovioff, G.; Eliezer, D. The mechanochemical behavior of type 316L stainless steel. Corros. Sci. 1996, 38, 1141–1145. [Google Scholar] [CrossRef]
- Wu, X.; Teng, M.; Jia, W.; Cai, J. Study on the mechanical properties of X80 pipeline steel under pre-charged high-pressure gaseous hydrogen. Int. J. Hydrogen Energy 2024, 84, 39–52. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, Y. Corrosion of X100 pipeline steel under plastic strain in a neutral pH bicarbonate solution. Corros. Sci. 2012, 64, 145–152. [Google Scholar] [CrossRef]
- Gutman, E.M. Mechanochemistry of Solid Surfaces; World Scientific Publishing Company: Singapore, 1994. [Google Scholar]
- Celikin, M. The role of dynamic transmission electron microscopy on the development of next generation magnesium alloys. Mater. Today 2015, 18, 472. [Google Scholar] [CrossRef]
- Saxena, A.; Findley, K. Modelling the Effects of Hydrogen Pressure on Fatigue Crack Growth Behavior in SA372 Pressure Vessel Steels. In Proceedings of the ASME 2022 Pressure Vessels & Piping Conference, Las Vegas, Nevada, 17–22 July 2022. [Google Scholar] [CrossRef]
- Toribio, J.; González, B.; Matos, J.-C. Numerical modeling of hydrogen embrittlement of pearlitic steel in the presence of blunt notches. Procedia Struct. Integr. 2021, 33, 1215–1218. [Google Scholar] [CrossRef]
- Sahal, M.; Creus, J.; Sabot, R.; Feaugas, X. The effects of dislocation patterns on the dissolution process of polycrystalline nickel. Acta Mater. 2006, 54, 2157–2167. [Google Scholar] [CrossRef]
- Dai, Y.Z.; Chiang, F.P. On the Mechanism of Plastic Deformation Induced Surface Roughness. J. Eng. Mater. Technol. 1992, 114, 432–438. [Google Scholar] [CrossRef]
- Huang, H.; Jia, C.; Guo, L. Effect of local corrosion on tensile behavior of steel plates. Structures 2022, 43, 977–989. [Google Scholar] [CrossRef]



| C | Si | Mn | P | S | Cr | Mo | Ni | Nb | V | Cu | B | Al |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.076 | 0.21 | 1.89 | 0.010 | 0.0022 | 0.27 | 0.28 | 0.055 | 0.082 | 0.029 | 0.059 | 0.0002 | 0.038 |
| Parameter | Value |
|---|---|
| The density of steel, ρ (g/cm3) | 7.85 [25] |
| Molar mass of steel, M (g/mol) | 56 |
| Partial molar volume of hydrogen, Vh (m3/mol) | 2 × 10−6 [26,27] |
V | A/cm2 | V | V | V/dec | V/dec | A/cm2 | A/cm2 |
|---|---|---|---|---|---|---|---|
| −0.704 | 3.373 × 10−6 | −0.859 | −0.644 | 0.162 | −0.083 | 4.508 × 10−8 | 1.496 × 10−6 |
| Physical Field | Hydrogen Concentration | ||||
|---|---|---|---|---|---|
| Tensile Strain | 0.1% | 0.2% | 0.3% | 0.4% | |
| mechano-chemical–H atom diffusion | minimum value | 1.392 | 1.258 | 1.165 | 1.116 |
| maximum value | 1.592 | 1.622 | 1.627 | 1.635 | |
| stress–H atom diffusion | minimum value | 1.382 | 1.248 | 1.157 | 1.107 |
| maximum value | 1.587 | 1.617 | 1.621 | 1.632 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, Z.; Tang, J.; Zheng, S.; Wang, F.; Bie, Q.; Kang, P.; Wang, X.; Guo, S.; Chen, L. Investigation of Mechano-Electrochemical Effects on Hydrogen Distribution at Corrosion Defects. Hydrogen 2025, 6, 69. https://doi.org/10.3390/hydrogen6030069
Dai Z, Tang J, Zheng S, Wang F, Bie Q, Kang P, Wang X, Guo S, Chen L. Investigation of Mechano-Electrochemical Effects on Hydrogen Distribution at Corrosion Defects. Hydrogen. 2025; 6(3):69. https://doi.org/10.3390/hydrogen6030069
Chicago/Turabian StyleDai, Zhixiang, Jiamin Tang, Sijia Zheng, Feng Wang, Qin Bie, Pengcheng Kang, Xinyi Wang, Shiwen Guo, and Lin Chen. 2025. "Investigation of Mechano-Electrochemical Effects on Hydrogen Distribution at Corrosion Defects" Hydrogen 6, no. 3: 69. https://doi.org/10.3390/hydrogen6030069
APA StyleDai, Z., Tang, J., Zheng, S., Wang, F., Bie, Q., Kang, P., Wang, X., Guo, S., & Chen, L. (2025). Investigation of Mechano-Electrochemical Effects on Hydrogen Distribution at Corrosion Defects. Hydrogen, 6(3), 69. https://doi.org/10.3390/hydrogen6030069






