1. Introduction
The origin of life on Earth remains one of the most profound questions in the field of science. Among the various proposed scenarios, hydrothermal systems—including oceanic hydrothermal vents [
1], as well as open ponds and terrestrial hot mineral springs [
2], are rich in chemical ions and compounds. Their primary energy sources are chemical and geothermal [
3]. In these settings, the combination of heat, and mineral catalysis hot water sources on land [
4] and in hydrothermal vents [
5], along with hydrogen ions (H
+) [
6] and molecular hydrogen (H
2) [
7] may have driven the essential biomolecular structures.
Hydration on the Earth’s mantle introduces protons (H
+), which enhance the mobility of Fe
2+ and Mg
2+ ions within the olivine crystal structure by generating cationic defects. These structural modifications promote redox reactions in the presence of water and sulfate ions, offering a plausible pathway for the generation of molecular hydrogen (H
2) within the deep lithosphere [
8]. As a potent reducing agent, H
2 could have played a key role in creating and sustaining chemically favorable conditions for the emergence and early life.
In addition, reactions between hydrogen- and oxygen-bearing minerals in the Earth’s mantle may have led to the in situ formation of water, further contributing to the geochemical conditions favorable for prebiotic chemistry [
9]. Furthermore, the accumulation and retention of hydrogen within mantle minerals may have helped maintain a reduced atmosphere on the early Earth. Thermochemical and planetary modeling studies indicate that, under scenarios involving intense volcanic outgassing and limited hydrogen escape to space, molecular hydrogen could constitute 10–30% of the early Earth’s atmosphere [
10].
The oldest known water on Earth dates to around 2.7 billion years. It was discovered over 2 km underground in the Canadian Shield at the Kidd Creek Mine, its age was confirmed through isotopic analysis, and it contains H
2, CH
4, and other reducing gases [
11]. The molecular hydrogen is over 50 vol.%.
The earliest discoveries of biogenic graphite date back to 3.8 billion years ago [
12]. The oldest confirmed evidence of living organisms is found in stromatolites, dated to 3.5 billion years [
13]. Szostak and co-authors emphasized that geothermal energy could help overcome thermodynamic barriers in chemical evolution by creating localized environments conducive to the synthesis of organic molecules [
14]. Subsequent research published in 2010 suggested that the likelihood of life emerging was higher in hot, mineral-rich freshwater springs and ponds [
15,
16].
Two of the most prebiotically significant amino acids, glycine and alanine, have been repeatedly identified in meteorites, such as Murchison [
17,
18] and Murrey [
19], and glycine in the Wild-2 comet [
20]. The oldest grains in the Murchison meteorite are 7 billion years old, making them the oldest known solid material ever found on Earth [
18]. The proofs for glycine and alanine were found in two Martian meteorites—Nakhea [
21] and Allan Hills (ALH 84001) [
22]. The presence of amino acids in various cosmic objects highlights their ubiquity. It supports the idea that they existed both in interstellar space and during the early evolution of the Solar System. Together with inorganic ions and compounds, and under favorable conditions such as liquid water and energy, they could have contributed to the formation of life’s chemical precursors. The origin of life is widely believed to have been facilitated by the spontaneous assembly of inorganic and simple organic molecules in early aquatic environments, such as open hot mineral sources, ponds, and hydrothermal vents. Among these molecules, amino acids played a pivotal role as the building blocks of peptides and protocellular structures. Numerous experimental models have been developed to simulate protocell formation under conditions thought to resemble those on the early Earth. Fox demonstrated that amino acids subjected to thermal condensation form proteinoid microspheres with rudimentary membrane-like behavior [
23]. Nakashima and Fox demonstrate that amino acids such as glycine, phenylalanine, proline, and lysine can form peptides through single or multiple ATP-driven reactions when added to suspensions of nucleus proteinoids microspheres, suggesting a prebiotic pathway for peptide formation in compartment-like environments [
24]. Gözen’s study demonstrates the spontaneous formation of lipid-based protocell structures on solid mineral surfaces, where Ca
2+ ions enhanced membrane adhesion and compartment formation [
25]. In this model, amphiphilic films deposited on mineral surfaces spontaneously form vesicles with bilayer membranes, which exhibit fusion, budding, and pseudo-division—features closely resembling living cells. However, their reliance on pre-synthesized lipids introduces uncertainty, as the abiotic availability and stability of amphiphiles remain debated. Quantum chemical simulations of Rimola et al. show that glycine can undergo surface-mediated self-organization on mineral substrates via hydrogen bonding, proton transfer, and thermal fluctuations, leading to linear and clustering assemblies [
26]. Thermal polycondensation, described by Mougkogiannis and Adamatzky, demonstrated that glycine and alanine heated with montmorillonite clay yield oligopeptides with up to ten residues—supporting a plausible non-enzymatic route for proteinoid-like polymer formation under early Earth’s conditions [
27]. Additional studies further expand the experimental foundation for prebiotic chemistry. Stanley Miller’s pioneering experiment in 1953 demonstrated that amino acids, such as glycine and alanine, can spontaneously form under reducing atmospheric conditions using electric discharge [
28]. In 2015, Ignatov and Mosin demonstrated a linear relationship between the mass of the sediments and the number of carbon atoms [
29] in the Miller–Wilson experiments [
30], with glycine and alanine identified as key components of the resulting organic compounds. Juan Oro (1961) provided early evidence that alanine and other amino acids can be synthesized through Strecker-type reactions from formaldehyde, ammonia, and hydrogen cyanide, as molecules abundant in prebiotic environments [
31]. In 1997, Bujdák and Rode showed that alanine can form peptide bonds under wet–dry cycles facilitated by clay minerals, offering a geochemically plausible pathway for early peptide synthesis in hydrothermal conditions [
32]. Under hydrothermal conditions at a temperature of hot water around 150 °C, amino acids, such as glycine, alanine, aspartic acid, and serine, can be synthesized from simple molecules such as CO
2, H
2, and NH
3. The results show the big potential of abiotic synthesis in hot water [
33]. A terrestrial hot spring scenario proposed by Damer and Deamer (2015) incorporates wet–dry cycling and mineral-assisted polymerization [
34]. This model combines realistic cycling with spontaneous compartmentalization, offering a geologically consistent route toward protocell emergence. The scenario proposed by Ignatov for the origin of life since 2010 involves open ponds with hot mineral water [
15]. The investigation presented in this paper reveals that glycine and alanine can spontaneously form stable membrane-free microspheres under conditions with hot mineral water, in the presence of calcium and clay [
2], supporting a plausible prebiotic pathway toward compartmentalization. The model relies solely on amino acids, such as glycine and alanine, calcium carbonate, and clay minerals in hydrogen-rich water. The resulting microspheres display membrane-like boundaries, internal zoning (confirmed by optical microscopy, TEM, and SSIM), and morphological consistency at 75 °C. Unlike lipid-based systems, this model is completely non-lipid and non-genetic, built from minimal components and geochemically realistic settings like serpentinized springs.
As demonstrated with laboratory experiments by Miller and Urey (1959) [
28], Damer and Deamer (2020) [
4], and Ignatov et al. (2025) [
2,
7], hot water enriched with hydrogen in the primordial hydrosphere provides a reducing environment. The experiments show that amino acids can be structured from inorganic compounds under similar conditions.
As demonstrated by the laboratory experiments of Miller and Urey (1959) [
28], as well by more recent studies of Damer and Deamer (2020) [
4], and Ignatov et al. (2025) [
2,
7], hot water enriched with hydrogen, such as in the primordial hydrosphere, provides a reducing environment. Under such conditions, experiments have shown that amino acids can be formed from inorganic compounds.
The primary aim of this study is to assess whether simple amino acids, such as glycine and alanine, can self-organize into morphologically stable, compartmentalized microspheres under conditions of hot mineral water enriched in hydrogen and divalent ions. Using phase-contrast microscopy, TEM, and SSIM, this study aims to demonstrate that such prebiotic compartmentalization can emerge without the need for lipids or nucleic acids, providing a minimalistic, geochemically plausible route to protocell formation on the early Earth.
2. Materials and Methods
2.1. Amino Acid Analysis
The amino acids glycine and alanine of the hydrogen-enriched biomass were analyzed on Biotronic LC-5001 (230 × 3.2) column (Eppendorf-Nethleler-Hinz, Hamburg, Germany) with a UR-30 sulfunated sterine resin (Beckmann-Spinco, Brea, CA, USA) as a stationary phase, at the temperature 25 °C; the mobile phase—0.2 N; sodium-citrate buffer (pH = 2.5); the granule diameter—25 µm; working pressure—50–60 atm; the eluent input rate—18.5 mL·h
−1; the ninhydrin input rate—9.25 mL·h
−1; detection—579 and λ
= 440 (for profine) [
35].
2.2. Glycine and Alanine Prepared from Brevibacterium Methylicum
The synthesis of deuterium-labeled alanine was performed using the Gram-positive facultative methylotrophic bacterium
Brevibacterium Methylicum [
35,
36]. The strain was cultured aerobically in a semi-synthetic medium containing 0.02% deuterium oxide (D
2O), with [
2H]-methanol as the sole carbon and energy source. The ammonium sulfate was used as the nitrogen source. Cultivation was carried out in Erlenmeyer flasks at 30 °C with shaking at 150 rpm for 72 h. Following incubation, the bacterial biomass was harvested by centrifugation and subjected to acid hydrolysis (6 N HCl, 24 h, 110 °C). The resulting amino acid mixture was purified using ion-exchange chromatography (Dowex 50WX8, H
+ form; Dow Chemical Company, Midland, MI, USA). Alanine was identified and analyzed by LC-MS and
1H/
2H NMR to confirm the degree of deuterium incorporation [
37]. Glycine was extracted from the hydrolyzed bacterial biomass using ion-exchange chromatography, following microbial cultivation in a deuterium-rich medium with [
2H]-methanol and D
2O.
2.3. Formation of Microspheres from Glycine and Alanine
The study by Albertsson et al. (2014) estimates the D/H ratio in early Solar nebula water to be approximately 160 ppm or 0.016%, consistent with the present-day value found in the Earth’s ocean water [
38].
Microspheres were obtained by solution by mixing alanine and glycine in a 1:1 molar ratio in 99.98% H2O and 0.02% D2O with added hydrogen. The microspheres of the control solution were obtained without added hydrogen. The control solution was with the exact composition of glycine and alanine (1:1 molar ratio in 99.98% H2O and 0.02% D2O) without added hydrogen. The solution was gently heated to 75 °C (348.15 K) and maintained at this temperature with continuous stirring at 150 rpm for 10 min to simulate a hot mineral source. The solution was cooled to room temperature and observed under a phase-contrast microscope (Olympus CX33, 40xPH objective) at 1000× magnification.
Glycine and alanine used in this study were obtained via microbial synthesis using Brevibacterium Methylicum in D2O-containing medium or as chemical standards. Their incorporation into structures was supported by isotopic data (1H/2H NMR and LC-MS). Glycine and alanine were prepared by Oleg V. Mosin (1966–2016) in the Biotechnology Department at Moscow State University of Applied Biotechnology, Moscow 109316, Russian Federation.
2.4. Solution for Glycine and Alanine with Hydrogen Enrichment
A solution containing 0.05 g of magnesium powder, 0.1 g of glycine, 0.1 g of alanine, and 50 mg of sodium bicarbonate (NaHCO3) was prepared in 100 mL of distilled water. The mixture was incubated at 75 °C for 10 min to allow the gradual release of molecular hydrogen (H2) and stabilization of pH = 8.2. The resulting solution was used to observe the self-assembly of amino acids under simulated hot water conditions.
2.5. Structural Similarity Index Measure (SSIM)
To evaluate the internal organization of the glycine/alanine-based microsphere, a modified structural similarity approach was implemented based on the methodology introduced by Mougkogiannis and Adamatzky [
27]. A high-resolution image of a microsphere was segmented into a 10 × 10 grid, generating 100 subdomains interpreted as putative sub-neural (SN) zones. Pairwise structural similarity values were computed using SSIM. It is a perceptually derived metric that quantified local texture and structural coherence between image regions [
39].
2.6. Transmission Electron Microscopy (TEM) Imaging
High-resolution imaging of the glycine/alanine microspheres was performed using a PEM-125K transmission electron microscope (ZAO ELMI, Saint Petersburg, Russia). This instrument operates at an accelerating voltage of 125 kV and provides a spatial resolution of approximately 0.3–0.5 nm.
Specimens were deposited onto standard carbon-coated copper grids and air-dried. The samples were introduced into a vacuum chamber and analyzed under high vacuum conditions. The electron beam was thermionically generated and focused via an electronic lens system. Imaging was conducted at magnification up ×10,000, depending on the sample and contrast requirements.
Image was recorded using a digital CCD camera system integrated into the PEM-125K, allowing for enhanced structural visualization and morphological assessment of the spherical microspheres.
Samples were prepared by drop-casting 10 µL of the aqueous glycine/alanine solution onto carbon-coated 300-mesh copper TEM grids (Agar Scientific, Rotherham, UK), followed by air-drying at ambient conditions. No staining or coating was applied to preserve the native morphology of the microspheres. TEM micrographs were recorded in low-magnification mode for whole-structure visualization.
2.7. MD-Inspired Molecular Packing Model
This is a static concentration-based approximation conceptually inspired by molecular dynamics but not involving real simulations or interaction potentials [
40], combined with volumetric concentration-based methods commonly applied in systems biology and prebiotic modeling [
34]. To estimate the theoretical maximum number of amino acid molecules within the single microsphere (0.5 µm diameter), a molecular dynamics (MD)-inspired approximation is used. This calculation draws conceptually from molecular dynamics simulations but does not involve time-dependent integration of particle trajectories or interaction potentials. The approximation is based on scaling known or assumed concentrations (c) over a defined system volume (V), using Avogadro’s number to calculate total molecular counts: where
Nmol is the estimated number of molecules.
c is the concentration in mol·L−1;
V is the modeled volume in liters.
NA = 6.022 × 1023 mol−1.
For glycine and alanine, concentrations were based on typical intracellular and model system levels. Water molecules were calculated assuming full volumetric occupancy at mol·L−1. Experimental counts were derived from known sample concentrations and actual measured volumes. The “membrane” values reflect hypothetical partial transport scenarios, while “MD model” values correspond to particle counts with scaled up simulation box settings.
2.8. Estimation of Refractive Index and Osmotic Pressure
Phase-contrast microscopy was used to evaluate the optical properties of the 12.2 µm protostructure. A visible contrast between the interior and surrounding medium indicated a difference in refractive index. Using published calibration data and assuming a reference value of water at 75 °C (348.15 K), the internal refractive index was estimated to be slightly higher, with Δn ≈ 0.012. The optical difference corresponds to a solute concentration gradient between the interior and the medium, estimated using standard index values for amino acid solutions. Based on this, the resulting pressure difference was calculated to be approximately Δπ ≈ 2490 Pa, indicating a chemically asymmetric microenvironment capable of driving passive transport processes.
3. Results
3.1. Conditions for Creation of Microsphere from Water, Glycine, and Alanine
3.1.1. Temperature and Gibbs Free Energy
The temperature of 75 °C for the experiments was chosen because it corresponds to the conditions in real hot mineral springs, such as in Rupite, Bulgaria (73.4 °C), and to the optimum of
Hydrogenobacter thermophilus, which assimilates inorganic compounds for the synthesis of organic compounds [
41].
The temperature range 70–75 °C is relevant to the thermophilic organism
H. thermophilus [
41,
42], which thrives in hot mineral springs. This bacterium oxidizes inorganic substrates such, as hydrogen and sulfur compounds, and synthesizes organic molecules via autotrophic pathways. The temperature was selected to simulate plausible geothermal conditions on early Earth, under which prebiotic molecular interactions could occur.
Elevated temperatures increase molecular mobility and entropy, which, according to the Gibbs free energy equation (ΔG = ΔH − TΔS), may facilitate molecular interactions. Still, a favorable Gibbs energy (ΔG < 0) depends on the specific enthalpic and entropic parameters of the reaction system. However, such temperature-induced aggregation should be interpreted with caution, as it does not necessarily imply biological relevance without further molecular characterization. These temperature conditions mimic hydrothermal systems where non-enzymatically molecular organization could emerge. Nonetheless, it distributes thermodynamically favorable aggregation.
3.1.2. Hydrophobic Effect, and Ionic and Electrostatic Interactions
It also facilitates temporary disruption of the water lattice (H2O structure), allowing the molecules to rearrange into an energy-minimizing shape—the sphere.
Alanine has a methyl group (–CH3), which is slightly hydrophobic. Glycine is small and polar.
Under alkaline conditions (pH = 8.2), amino acids, such as glycine and alanine, predominantly exist in their zwitterionic forms (–NH
3+/–COO
−), which allows for electrostatic interactions and potential clustering in aqueous environments [
43]. Alanine, with its small hydrophobic side chain, and glycine, due to its compact structure, can engage in weak non-covalent interactions, particularly in heated solutions.
While spherical assemblies were observed under phase-contrast microscopy, such formations may arise from physicochemical effects such as concentration, temperature, and ionic strength, rather than from electron microscopy, spectroscopy, or dynamic light scattering, and it is necessary to confirm whether these are genuine protocell analogs or simple aggregates.
3.2. Microstrutures from Alanine and Glycine
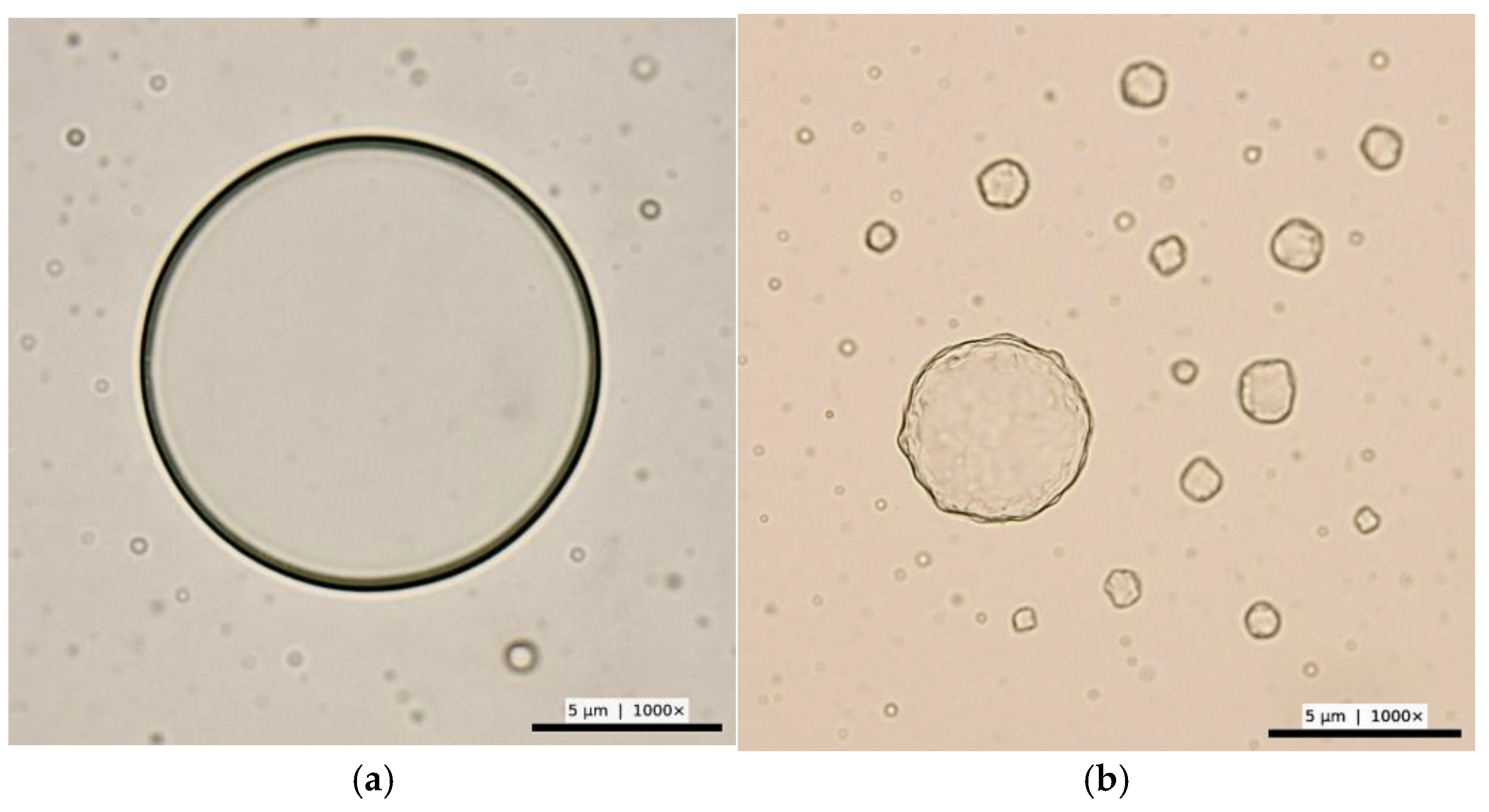
Upon microscopic examination, spherical microstructures were observed in heated glycine/alanine solution with hydrogen, with diameters ranging from approximately 0.25 to 12.2 µm (
Figure 1a).
Figure 1b demonstrates the microspheres in alanine/glycine solution without hydrogen ranging from approximately 0.25 to 6.2 µm. These structures appeared as optically dense, rounded aggregates under contrast phase microscopy.
During observation of the spherical microstructures using a phase-contrast microscope, a clearly defined membrane-like boundary was detected around the microspheres. This membrane exhibited optical contrast consistent with differences in density and refractive index between the interior and the surrounding medium. The visible boundary confirms the presence of a self-organized lipid-like or amino-base envelope serving as a primitive compartment. These spherical assemblies likely result from physicochemical aggregation processes and may serve as simple models for prebiotic compartment formation, although there is no evidence of functionally equivalent protocells.
Figure 2 shows that at pH 8.2, glycine and alanine exist in their zwitterionic forms, where the positively charged amino groups (–NH
3+) and the negatively charged carboxyl group (–COO
−) orient toward the aqueous environment, forming a hydrophilic outer surface.
The methyl group (–CH3) in alanine is hydrophobic and tends to point inward within the microsphere, contributing to a water-repellent internal barrier.
The combination of polar functional groups in glycine and alanine, with their differential hydrophobicity, enables self-assembly into a structure where hydrophobic regions interact with the external solution, while hydrophobic domains help define a primitive compartment as a protomembrane-like boundary.
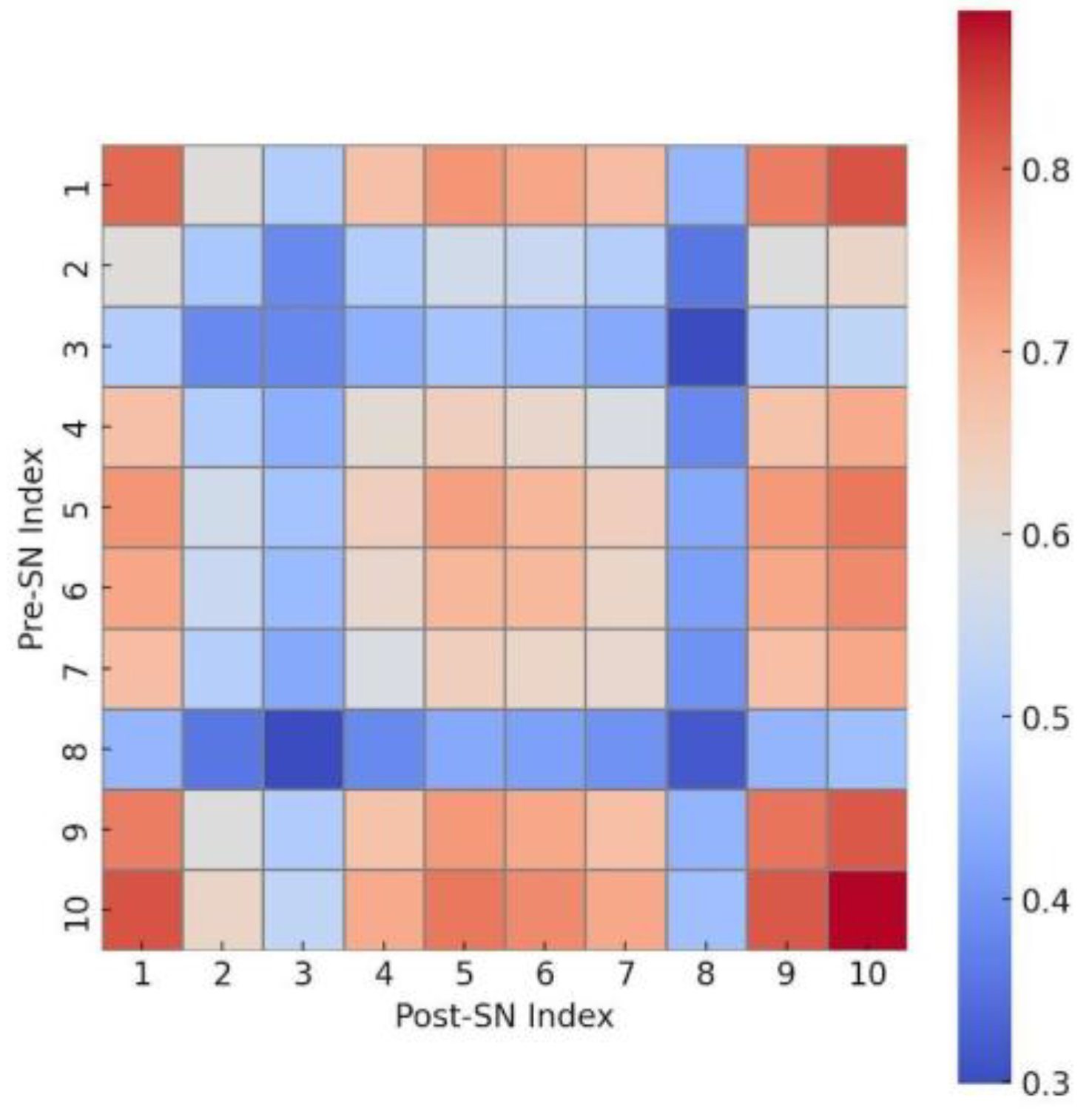
3.3. SSIM Zoning and Interaction Matrix Analysis
Figure 3 shows the functional interaction matrix derived from the Structural Similarity Index Measure (SSIM)-based zoning of the glycine/alanine. The 10 × 10 heatmap reveals high structural similarity (SSIM > 0.8) between peripheral zones, suggesting a well-defined boundary layer. In contrast, the central zones exhibit lower similarity values (SSIM ≈ 0.3–0.4), indicating morphological differentiation within the photosphere.
The SSIM-based pattern revealed peripheral structural similarity and central heterogeneity, though this pattern likely reflects differences in density or imaging contrast rather than functional compartmentalization. These results support the hypothesis that the microsphere exhibits early features of compartmentalization and functional zoning, analogous to protocellular organization.
3.4. Transmission Electron Microscopy (TEM) Image of Glycine/Alanine-Based Microsphere
TEM image of glycine/alanine-based microsphere, acquired using PEM-125K transmission electron image (
Figure 4).
The image reveals a spherical microsphere with a sharply defined boundary, suggesting a denser boundary region, but not sufficient to confirm the presence of a selective or biologically functional membrane. The observed morphology shows high circular symmetry and internal homogeneity, characteristic of self-assembled microspheres. The estimation of microsphere is approximately 12.2 μm. Magnification 10,000×.
The spherical morphology and sharply defined outer boundary suggest the presence of a membrane-like envelope. The structure exhibits high circular symmetry and internal homogeneity, supporting its interpretation as an abiotic photosphere with protocellular features. The estimated real diameter of the structure is 12.2 µm.
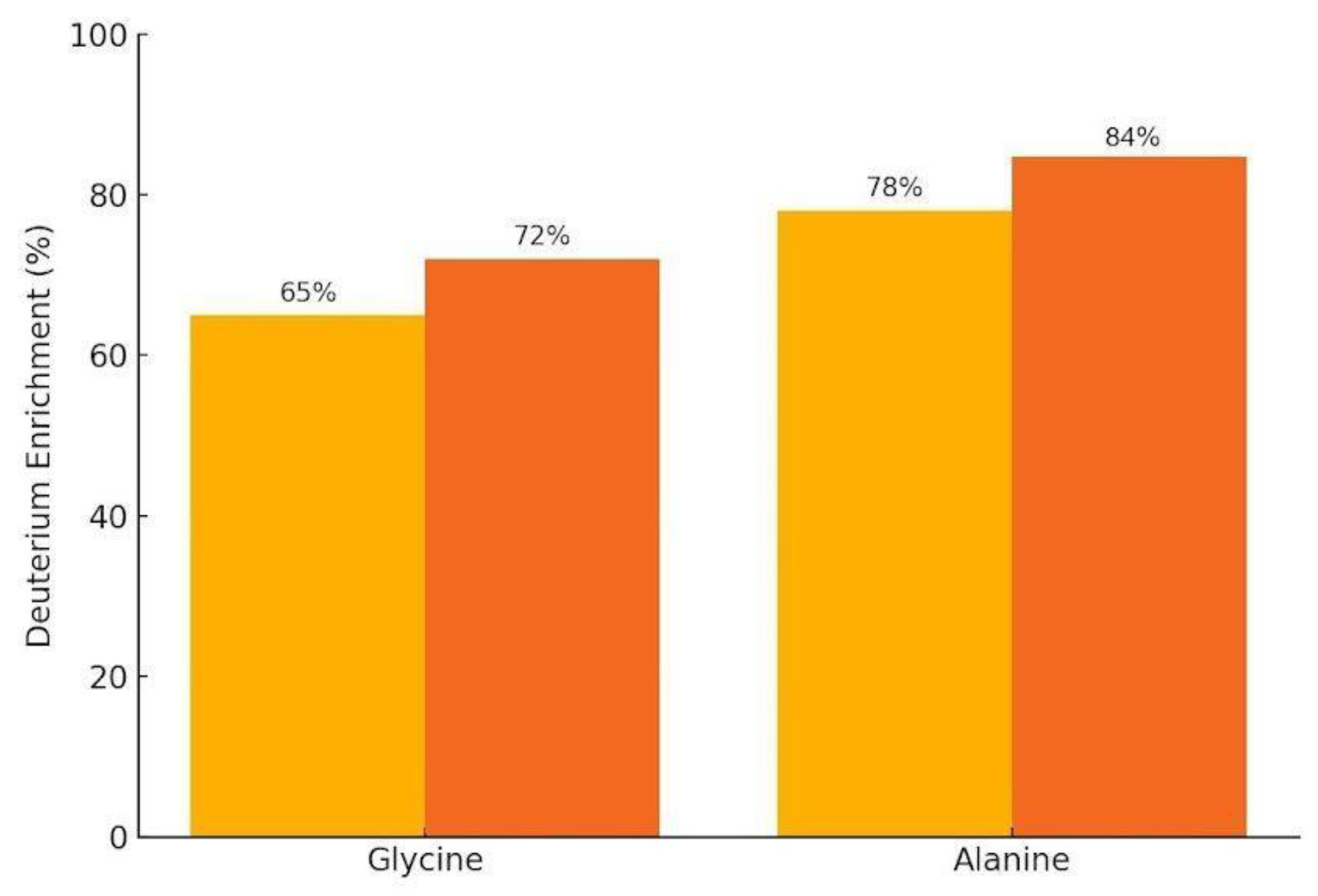
3.5. Estimation of Molecular Counts Using MD-Inspired Approximation
To estimate the number of molecules per species (glycine, alanine, Mg
2+, HCO
3−, and H
2O), they were applied to a molecular (MD)—(
Figure 5).
Deuterium incorporation into glycine and alanine was quantified using 1H/2H NMR with internal calibration and confirmed by LC-MS isotopic peak analysis. The yellow bars correspond to the control solution (without added hydrogen), and the orange bars to the hydrogen treated solution.
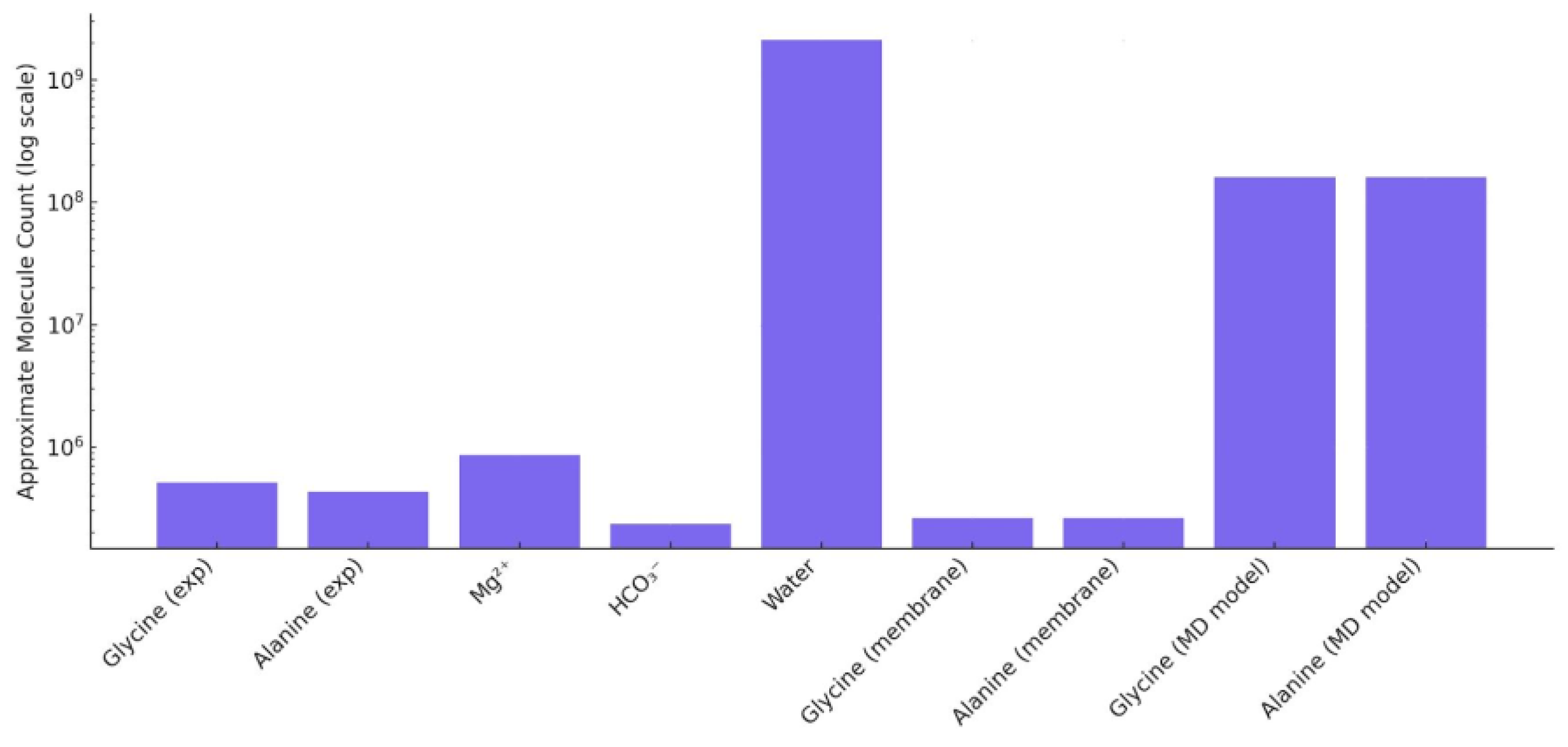
3.6. Approximate Molecular Counts Across Systems
Figure 6 demonstrates the estimated molecular counts for key species across three conceptual frameworks: experimental measurements (“exp”), membrane-based transport models (“membrane”), and simplified simulations (“MD model”) (
Figure 6). All values are presented on a logarithmic scale to accommodate the broad range of magnitudes. Water dominates all systems (~10
9), consistent with its solvent role and high molar concentration.
Glycine and alanine reach values of (~106) in experimental systems, while “membrane” transport approximations yield slightly lower counts, reflecting barrier-limited diffusions. Notably, the “MD model” estimates present significantly higher values (~108) for glycine and alanine, resulting from scaled simulation volumes and representative concentrations used in MD-inspired approximation.
These results illustrate the expected variation across physical, modeled, and computational systems. The MD-values do not represent exact molecular simulations but serve as analytically computed estimates of molecular content based on simplified concentration-driven assumptions.
3.7. Osmotic Pressure Calculated from Refractive Index Contrast
In a control experiment with sodium chloride [
44] and sea water from the Black Sea [
45], osmosis and diffusion were observed in pure water. The processes were possible because the sizes of the pores are bigger than the size of ions. Osmotic pressure and electrical conductivity are indicators for processes behind both osmosis and diffusion. There are two fundamental mechanisms by which living cells exchange matter and energy with their environment while maintaining internal organization and chemical non-equilibrium.
Phase contrast microscopy of the 12.2 µm microsphere revealed a distinct refractive index contrast between the interior and the surrounding medium. Based on the observed phase intensity profile and supported by published calibration data, and assuming an external refractive index of n ≈ 1.333, the interior refractive index is estimated at n ≈ 1.345. The refractive index difference was Δn ≈ 0.012. Applying a refractive index increment based on the weighted contribution of glycine and alanine (dn/dc ≈ 0.0139 L/mol), this corresponds to a solute concentration difference in Δc ≈ 0.861 mol·L−1 between the inner compartment and the external aqueous environment.
According to the van’t Hoff equation, this yields an osmotic pressure difference [
46]:
where R = 8.314 J·mol
−1·K
−1 and T = 348.15 K (75 °C), and the resulting osmotic pressure difference was calculated to be Δπ ≈ 2490 Pa.
This pressure gradient is significant at the microscale and supports the presence of a chemically asymmetric system capable of sustaining directional water flux and passive molecular accumulation.
4. Discussion
The glycine- and alanine-based structures reported in this study represent alternative microspheres that are closer to the actual conditions of prebiotic chemistry, linking mineral-driven redox processes with amino acid self-organization.
Our results suggest that glycine- and alanine-based microspheres could have acted as primitive compartments under such redox conditions. These structures can be viewed as an alternative to other studied prebiotic models. For comparison, lipid vesicles are generally divided into the following: (i) fatty acid vesicles, considered prebiotically plausible; (ii) phospholipid vesicles, which are more stable and resemble modern cell membranes; and (iii) polymer vesicles.
Szostak and colleagues demonstrated that fatty acid vesicles can form abiotically and self-assemble into permeable compartments that allow molecular exchange, making them plausible protocell models [
14]. Phospholipid giant unilamellar vesicles provide stable cell-like compartments capable of encapsulating enzymes and genetic material, and are widely used in synthetic biology [
47].
Amphiphilic polymer vesicles are more robust than lipid vesicles and offer additional functional possibilities for constructing synthetic cells [
48].
Coacervate droplets, formed by the phase separation of charged biomolecules, can concentrate nucleic acids and peptides, and serve as dynamic protocell models [
49].
Taken together, these comparisons highlight that glycine/alanine microspheres should be considered alongside fatty acid vesicles, phospholipid vesicles, polymer vesicles, and coacervated as potential pathways for prebiotic compartmentalization. This strengthens the relevance of our findings to the broader field of origin-of-life research.
5. Conclusions
The experiments simulated shallow hydrothermal environments, such as mineral-rich hot springs or geothermal ponds, where surface water reaches 70–75 °C and maintains an alkaline pH, which are conditions that plausibly existed on early Earth.
Glycine and alanine, incubated under hot liquid conditions, spontaneously formed stable, membraneless microspheres. Phase-contrast and electron microscopy confirmed the spherical organization.
A measurable refractive index contrast was detected between the microsphere interior and the surrounding medium. This contrast corresponds to a concentration difference derived from known optical properties of amino acids. Quantitative image analysis showed consistent size distribution and compartmentalization.
Molecular modeling suggested directional orientation of zwitterionic groups, where polar termini remained exposed to water. Structural integrity was maintained without lipid membranes.
Experimental conditions mimicked those of plausible early Earth environments, characterized by neutral-to-alkaline pH and moderate heat. Overall, the results support a prebiotic pathway for the formation of organized amino acid-based microstructures.
This article was written in memory of Oleg V. Mosin (1966–2016).