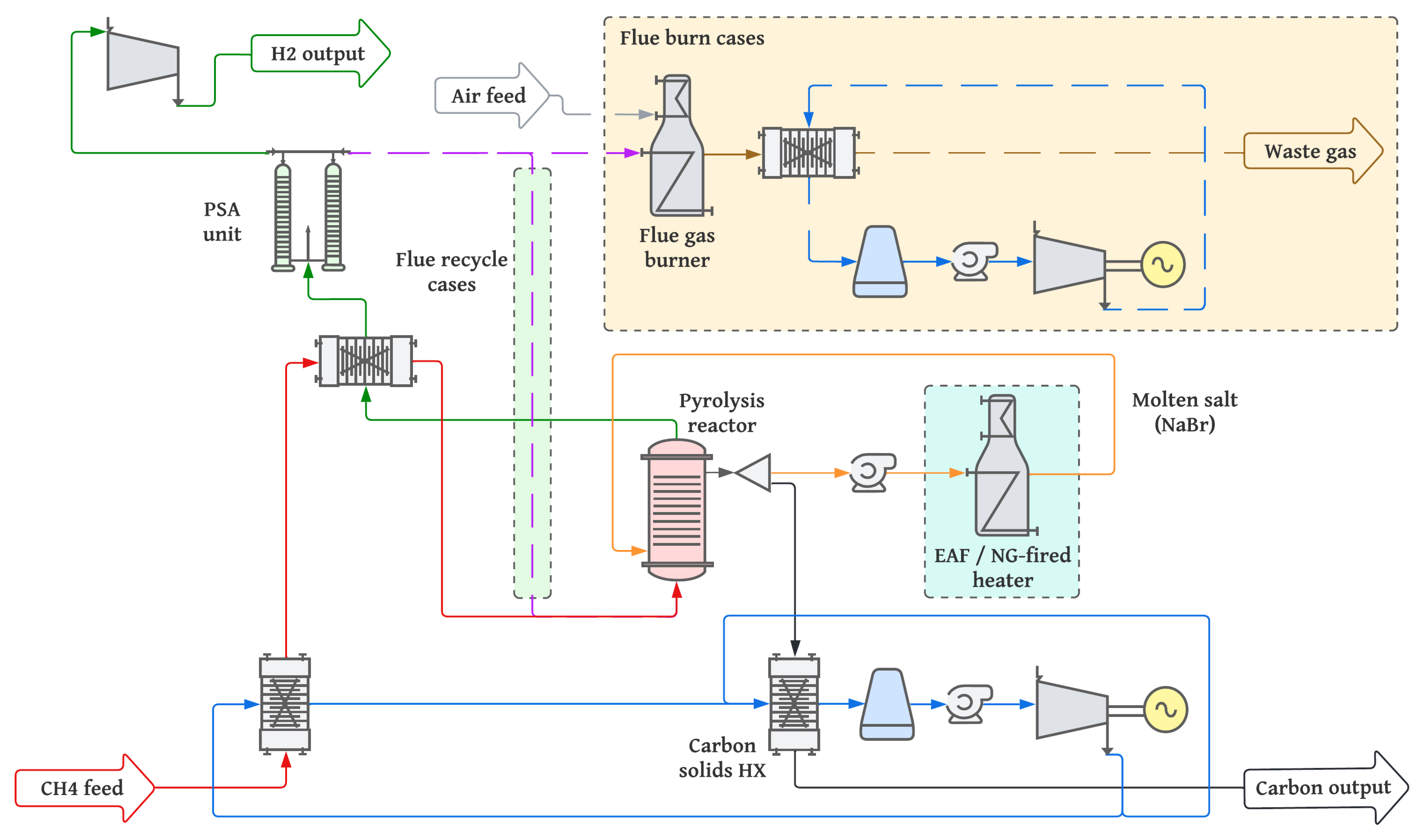
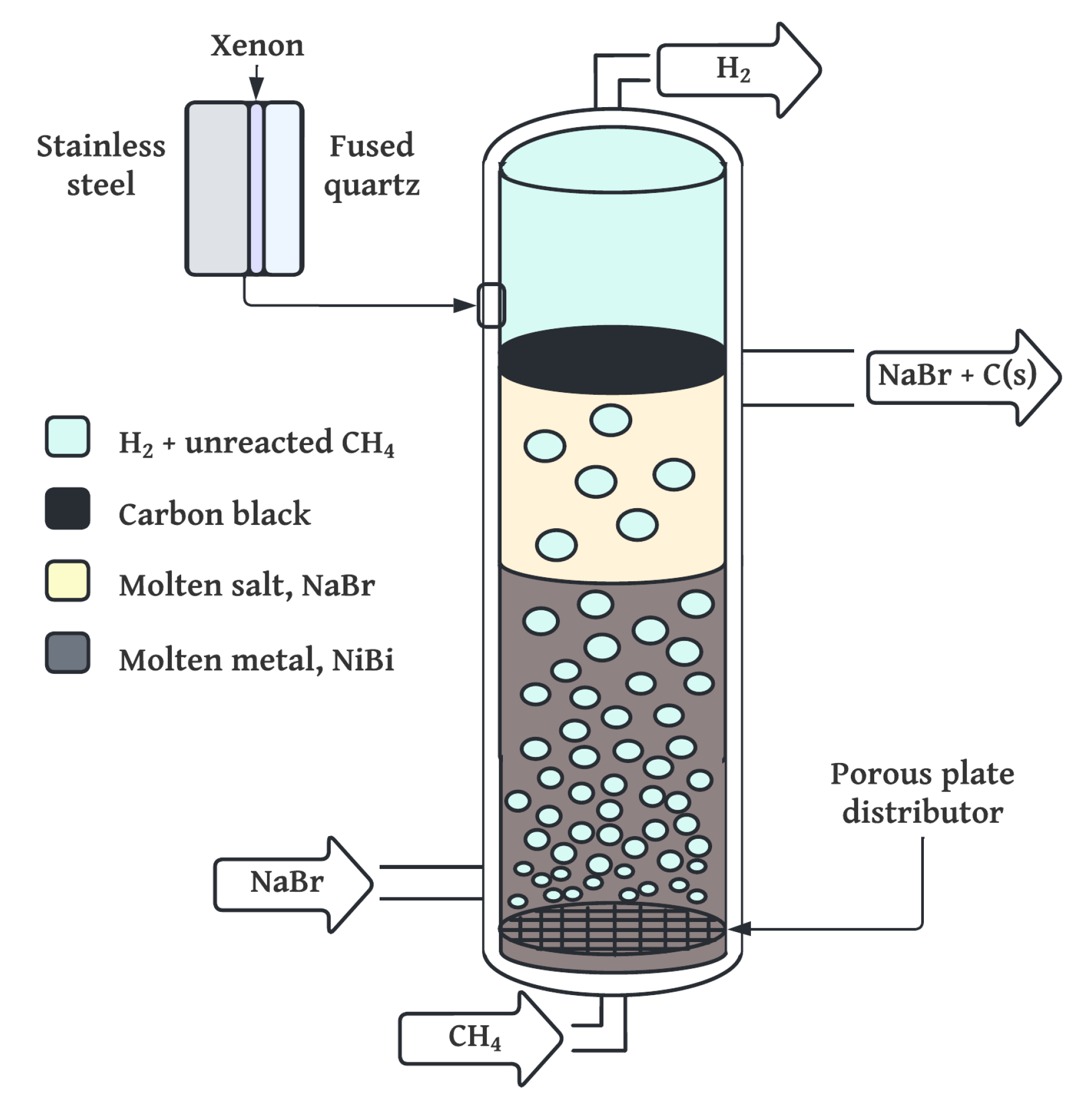
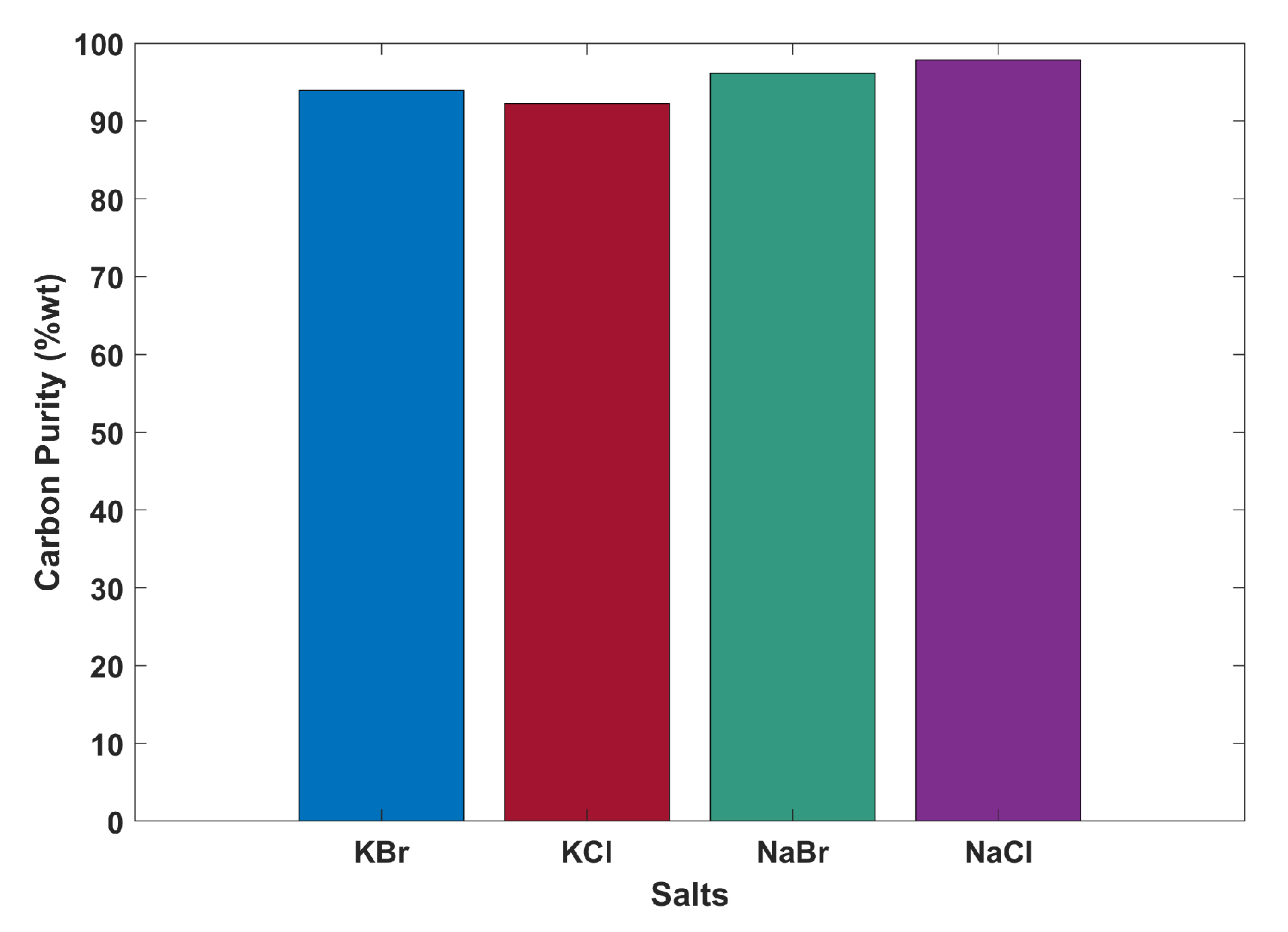
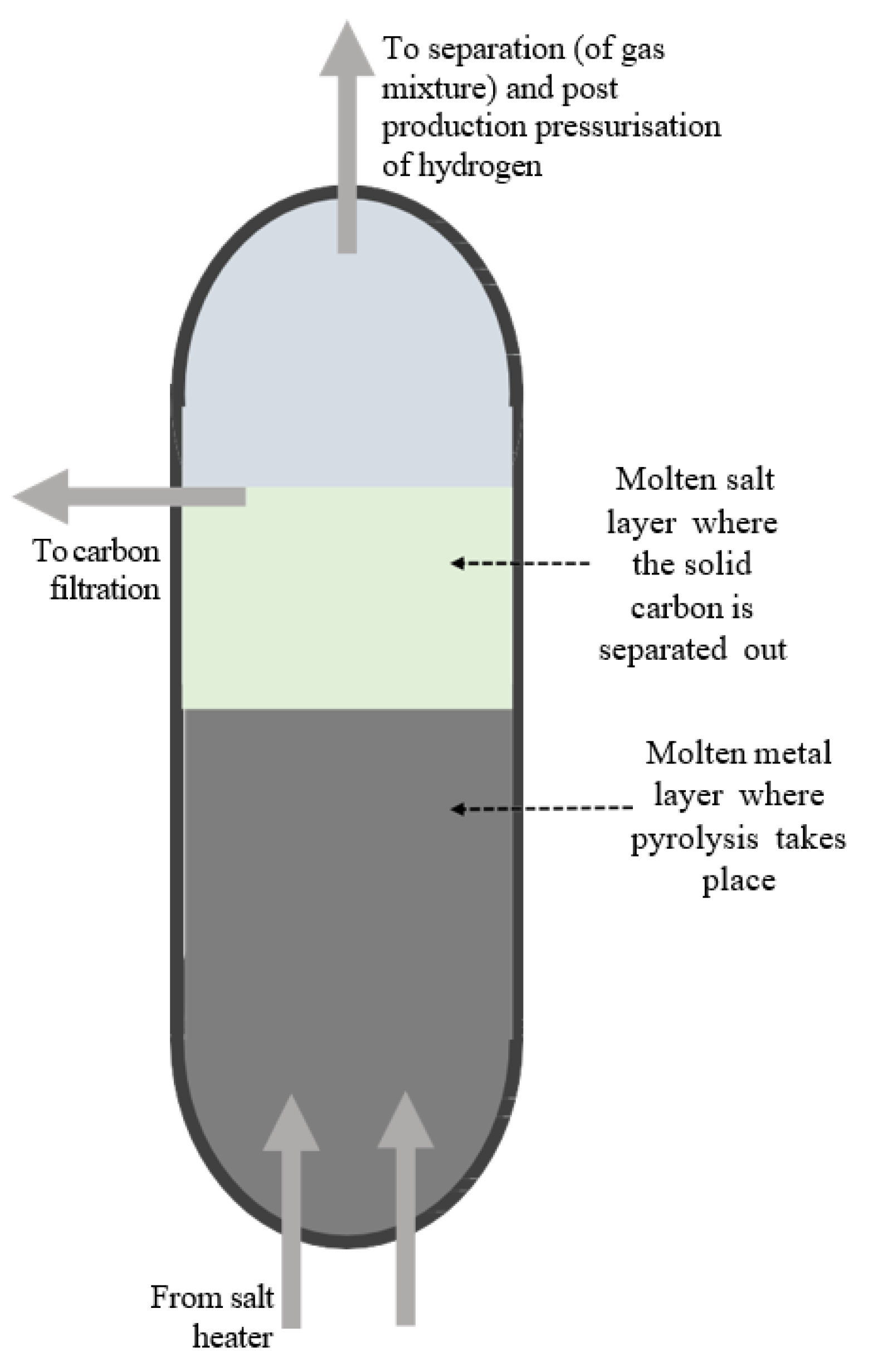
4.1. Thermodynamic Assessment
Table 9 summarises the thermodynamic performance of the 200 kta
−1 H
2 plant according to the plant indexes defined in
Table 4. Mass and thermal flows of the CH
4 feedstock and H
2 and carbon products are also shown, along with a breakdown of the power consumed by electrical components.
It is immediately clear that cases 1 and 2, in which purge gases of the PSA unit are combusted to raise steam, are significantly higher consumers of methane feedstock than cases 3 and 4, which recycle purge gases to the reactor. This is primarily a result of unreacted methane leaving the pyrolysis vessel after only one pass during which 12% of methane remains unconverted. The continuous recycling of these purge gases in cases 3 and 4 allows the methane conversion to converge on 100%. This is shown by the H
2 yield index parameter for case 4, which shows a hydrogen output of two moles for every one mole of methane, since this scenario does not consume methane elsewhere in the process. An equivalent 200 kta
−1 H
2 SMR facility consumes 658 kta
−1 CH
4 [
36].
The advantage of combusting purge gases is apparent by the electricity generation provided by these setups. Whereas cases 3 and 4 are net consumers of electricity, cases 1 and 2 are net producers, 1040 MW and 720 MW, respectively. That said, it is apparent that the electricity produced in cases 1 and 2 is not sufficient to cover the increased energy consumption represented by higher methane input, as shown by the heat rate index. Thus the process efficiency of these cases, expressed as the lower heating value of hydrogen as a fraction of total energy consumption, is only 23.9% for case 1 and 26.2% for case 2.
Although case 3 uses a flue gas recycle setup, the methane used by the fired heater to supply heat to the pyrolysis vessel reduces its thermal efficiency compared to case 4. However, this also means it consumes less electric power than the EAF setup proposed in case 4, consuming only 11 MW.
It can be seen that all cases in which methane is combusted in air produce significant CO
2 emissions, especially cases 1 and 2 at 25 kg
CO2 and 20 kg
CO2 per kilogram H
2. A study into the impact of hydrogen decarbonisation on industry [
5] found that SMR emits between 8 and 12 kg
CO2/kg
H2, while coal gasification emits around 20 kg
CO2/kg
H2. Meanwhile, as electricity producers, cases 1 and 2 are more CO
2 intensive than the current world average for power grids at 0.48 kg
CO2/kWh
e [
5]. It is clear by these comparisons that a CCS system should be employed in scenarios 1 and 2 to reduce their environmental impact compared to conventional methods of hydrogen and subsequent electricity production.
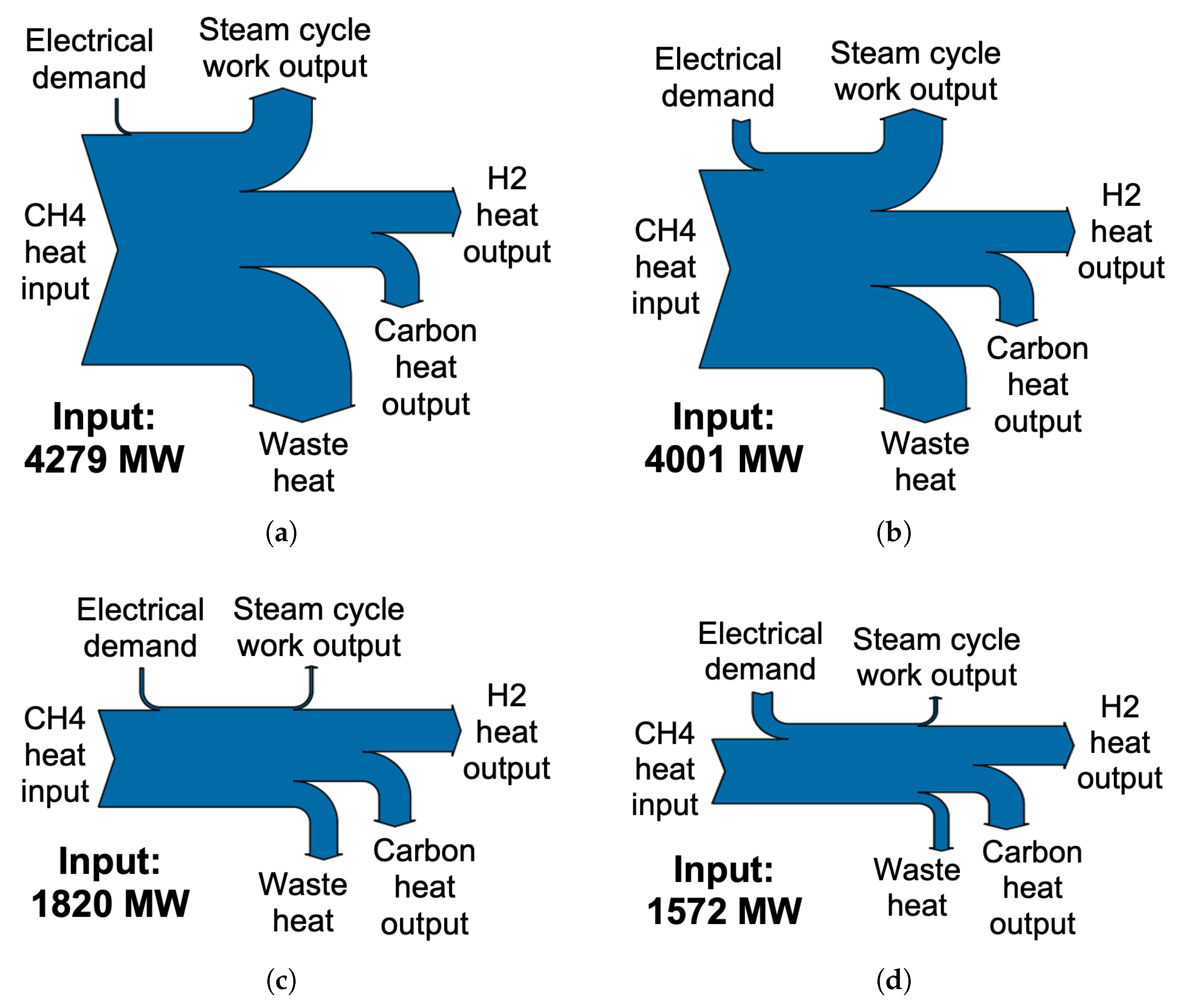
Figure 7 shows the process heat flows of each case scenario considered, each for the same H
2 output and calculated by the lower heating values of each component. Significant waste heat in cases 1 and 2 is a result of the typically low thermal efficiency, around 45%, of electricity production from conventional steam cycles [
37].
4.2. Techno-Economic Assessment
The full results of the techno-economic analysis can be found in
Table 10. Listed for each case scenario are the bare erected costs, operating expenditure and total capital expenditure.
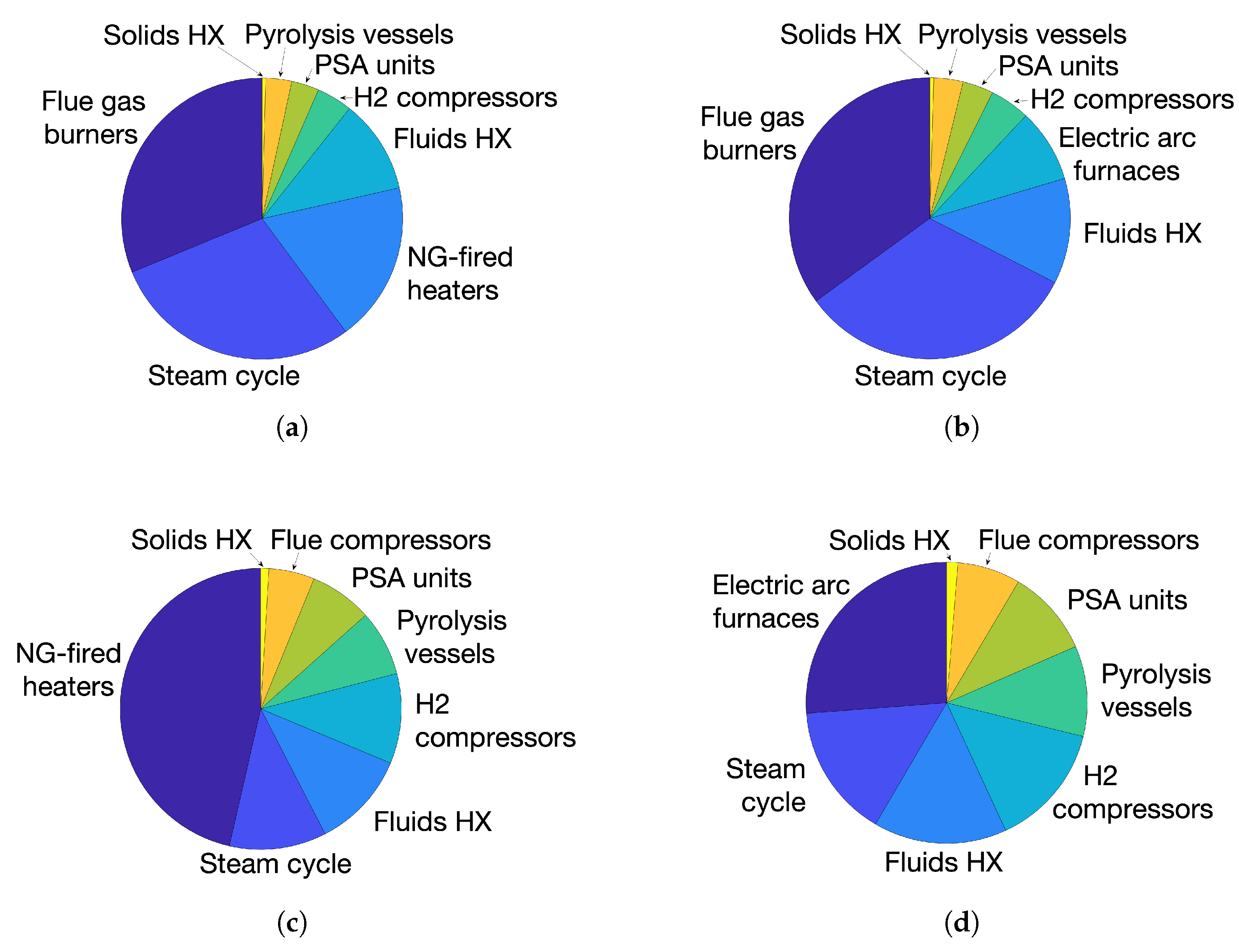
Figure 8 represents graphically the split of BEC.
The impact of electricity production on BEC from cases 1 and 2 is evident by the cost of the steam cycle components, primarily multiple intermediate and low-pressure turbines. In each of these cases the steam cycle can be seen to make up around 30% of the total BEC, whereas the figure is roughly half that for cases 3 and 4.
The other significant cost of cases 1 and 2 above the others is that of the burners required to combust the purge gases. Although such burners are well-established in industry, it is their sheer scale, producing over a gigawatt of electricity in case 1, which makes them a significant cost factor.
The total operating expenditure of a methane pyrolysis plant is found to be between 198 and 390 M$/year. It can be seen that the OPEX in each case is dominated by four factors: CH4 price, electricity price, CO2 tax and the sale value of solid carbon black. Although the electricity export of cases 1 and 2 more than negates the high feedstock cost, they both suffer a heavy CO2 tax at the base case cost of 75 $/tonneCO2. The sale of the carbon black product recovers a portion of the operating expenses in all cases, given that the carbon is of sufficient quality to be sold at 300 $/tonne.
The high operating temperature (1100 °C) and pressure (20 bar) of the LMBCR system impose technical challenges that impact both capital and operational expenditures. From a CAPEX perspective, reactor materials must withstand prolonged thermal and chemical stress, necessitating the use of specialty alloys, high-purity ceramics, and insulation, all of which increase fabrication cost. Additionally, high-pressure piping, sealing components, and safety systems require enhanced compliance with pressure vessel standards, further elevating installation costs.
On the OPEX side, sustaining these conditions demands significant thermal energy input, particularly in the natural gas-fired or electrically heated variants. Electricity-based heating (e.g., via resistive or plasma arc methods) introduces energy inefficiencies and cost variability, while combustion-based heating adds emissions unless offset by flue gas recycling or CCS. Nonetheless, these elevated conditions are essential for high methane conversion and carbon purity, particularly in molten-phase reactors where kinetic limitations are mitigated at high temperatures.
In comparison, solid-state methane pyrolysis reactors, such as fluidised beds and fixed beds, typically operate at lower temperatures (800–1000 °C) and atmospheric pressure but face severe catalyst deactivation due to carbon deposition and thermal sintering. Plasma-based MP reactors achieve CH4 cracking at similar or higher temperatures (2000–3000 °C) but suffer from extremely high electricity demand (>10–15 kWh/kg H2), leading to very high OPEX and a need for renewable electricity to maintain decarbonisation benefits.
To mitigate OPEX and improve energy efficiency in the LMBCR system, heat integration strategies can be employed. These include using the sensible heat of hot solid carbon (≈900 °C) to preheat incoming methane feed via a solid–gas recuperator and recovering waste heat from flue gases through steam generation for auxiliary processes or electricity generation. Such integration could reduce the net thermal input and enhance overall process efficiency, offsetting the economic penalty of high operating temperatures.
The levelised cost of hydrogen given base case assumptions is found to be between 1.29
$/kg for the most cost-effective design, case 4, and 2.99
$/kg for the least cost-effective, case 1. Case scenario 2 gives an LCOH of 2.73
$/kg, and case 3 1.53
$/kg. These results show cases 3 and 4 to be competitive with SMR processes incorporating CCS technology, which typically costs 1.20–2.10
$/kg according to a 2019 study by the International Energy Agency [
38]. The results are also comparable to Parkinson et al.’s 2018 findings of 1.40
$/kg for a design of methane pyrolysis in molten metal and molten salt [
36]. All case scenarios considered are cheaper compared to electrolysis, currently considered the cleanest method of hydrogen production but costing between 3.00 and 6.55
$/kg [
10].
4.3. Sensitivity Analysis
A sensitivity analysis has been carried out on the components of operating expenditure that were either judged to be subject to significant uncertainty or found to be particularly influential on the LCOH. Although some elements of CAPEX are subject to price fluctuations, such as steel price for the manufacture of pyrolysis vessels, none were found to have a greater impact on the LCOH than the factors expressed in
Table 11.
In this study, a single-pass reactor model was used as a baseline for comparison; however, industrial-scale methane pyrolysis systems typically employ multi-pass configurations to enhance conversion efficiency. In a multi-pass system, the unconverted CH4–H2 mixture exiting the reactor is cooled and separated, and the residual methane is re-circulated into the reactor inlet. This iterative process gradually increases the overall CH4 conversion, approaching equilibrium limits over multiple cycles. As the hydrogen fraction increases with each pass, the forward reaction rate decreases due to product inhibition. Nonetheless, thermodynamic equilibrium progressively shifts in favour of hydrogen production under high temperatures and low partial pressures of methane. Future work could integrate a detailed recycle loop model to assess the trade-offs between conversion, compression energy, and heat integration requirements.
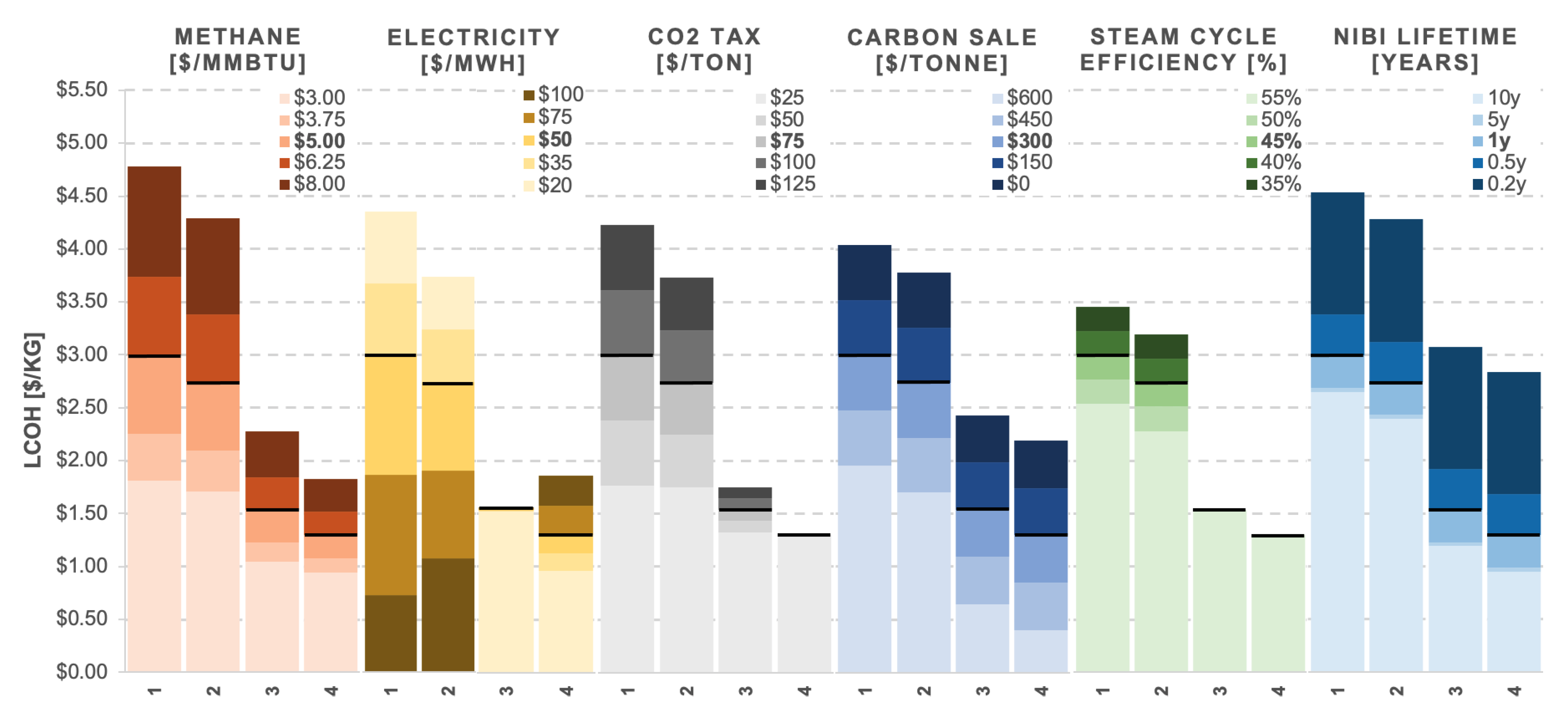
Figure 9 shows the result of the sensitivity testing, with each parameter tested as the single independent variable in the analysis. All other values remained at the base case assumptions. What is immediately evident is that case scenario 4, in which heat is supplied to the molten salt by an EAF and flue gases are recycled back into the reactor, is both the most cost-effective and least sensitive design. It is the least affected by fluctuations in methane price and steam cycle efficiency and is not impacted by CO
2 tax since it has zero emissions. Cases 3 and 4, which are less sensitive to methane price, are likely to become more advantageous in the future as natural gas supplies continue to deplete and as it comes under greater price pressure from environmental regulation.
Case 3 is least sensitive to electricity price since its net use is close to zero, staying between 1.51 and 1.55 $/kg. As such, it could be favourable in an environment in which electricity prices are particularly unstable. Should the electricity price rise above 75 $/MWh, cases 1 and 2 would gain sufficient revenue by exporting back to the grid to become competitive with cases 3 and 4, with all scenarios between 1.50 and 2.00 $/kg. Case 1’s sensitivity to electricity price is the most significant of all sensitivities tested across the design proposals.
The level at which a CO2 tax in the US will be set in the coming years is unknown. However, if the aims of the Paris Agreement are to be respected, it is infeasible to imagine that such a tax would not impact the plant over its 30-year operational period. As such, the minimum tested value is 25 $/tonne, ranging up to 125 $/tonne. It can be seen that those cases which emit greater CO2 emissions are more greatly affected, with a 100 $/tonne tax pushing case 1 above 3.50 $/kg. This is an element to consider should legislation shift towards further increasing emissions tax as a form of environmental regulation.
The most consistently sensitive factor in the analysis is the value of the solid carbon product. A conservative base case estimate of 300
$/tonne may not represent the true value of the carbon if it were to be formed of the nanotube-like structures found by Rahimi [
15]. That said, the quality of the product at such a scale and post-processing is unknown. Furthermore and equally impactful is the size of the carbon black market globally. Although on a small scale it may be feasible to sell the product for a good price, the scale of production would soon reduce its value by saturating the market, which is currently estimated at 16,400 kilotonnes annually, most of which is not domestic to the US [
39]. Uses of carbon black outside of the tyre industry are also to be considered, such as a reducing agent for the production of silicon-carbide and as an addition or substitute for coke in metallurgical processes [
39]. Given the potential market saturation of multiple plants of this size, the sale price has been varied between 600
$/kg for early adopter plants and 0
$/kg for n
th -of-a-kind plants. Even for the best scenario in case 4, ‘selling’ carbon black at 0
$/kg would raise the LCOH to 2.18
$/kg, rendering it uncompetitive against SMR with CCS.
Although nickel-bismuth was chosen as a catalyst because it doesn’t deactivate, there is the possibility that the inventory will need to be replenished at regular intervals, especially given that this technology has not been tested at such a scale. The intervals in the sensitivity analysis have been varied between approximately 10 weeks and 10 years. Should the inventory lifetime be between ten weeks and half a year, case 1 would cost 3.37–4.53 $/kg and case 4 from 1.67–2.83 $/kg.
It can be seen from this analysis that recycling flue gases back into the reactor is not only more cost-effective than burning them but results in lower sensitivity to all parameters tested. The choice of heating method, by NG-fired heater or electric arc furnace, is less consequential economically but still found to be sensitive to methane and electricity prices.
Although not considered directly in this work, it can be concluded from this analysis that a likely design scenario is one in which the majority of flue gases are recycled back into the reactor to increase overall conversion rates, while the remainder are combusted for electricity generation. This could be designed in such a way that the plant’s electricity use is net zero, removing its sensitivity to electricity prices. Furthermore, should electricity prices rise such that it becomes profitable to be an exporter, the setup could be dynamically adjusted to reflect this. A carbon capture and storage system would likely need to be costed into the plant in this case to reduce CO2 emissions and make it environmentally justifiable. Of the proposed scenarios, an electric arc furnace would be the most cost-effective heating method.