Dry-Oxidative Reforming of Biogas for Hydrogen Generation over Ca and Mg-Promoted Titania-Supported Nickel Catalyst
Abstract
1. Introduction
2. Materials and Methods
2.1. Catalyst Synthesis
2.2. Catalyst Characterization
2.3. Catalytic Reforming
3. Results and Discussions
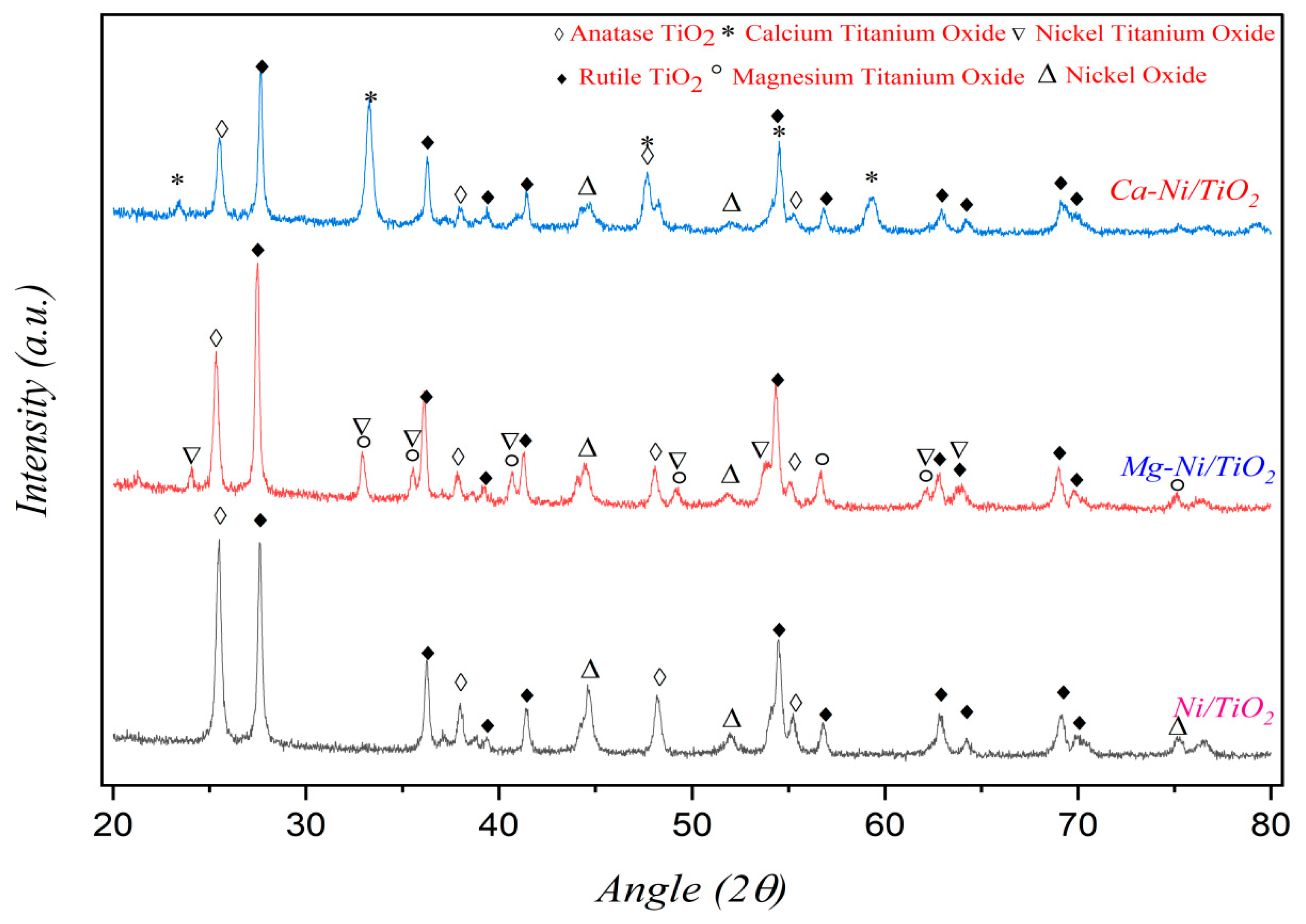
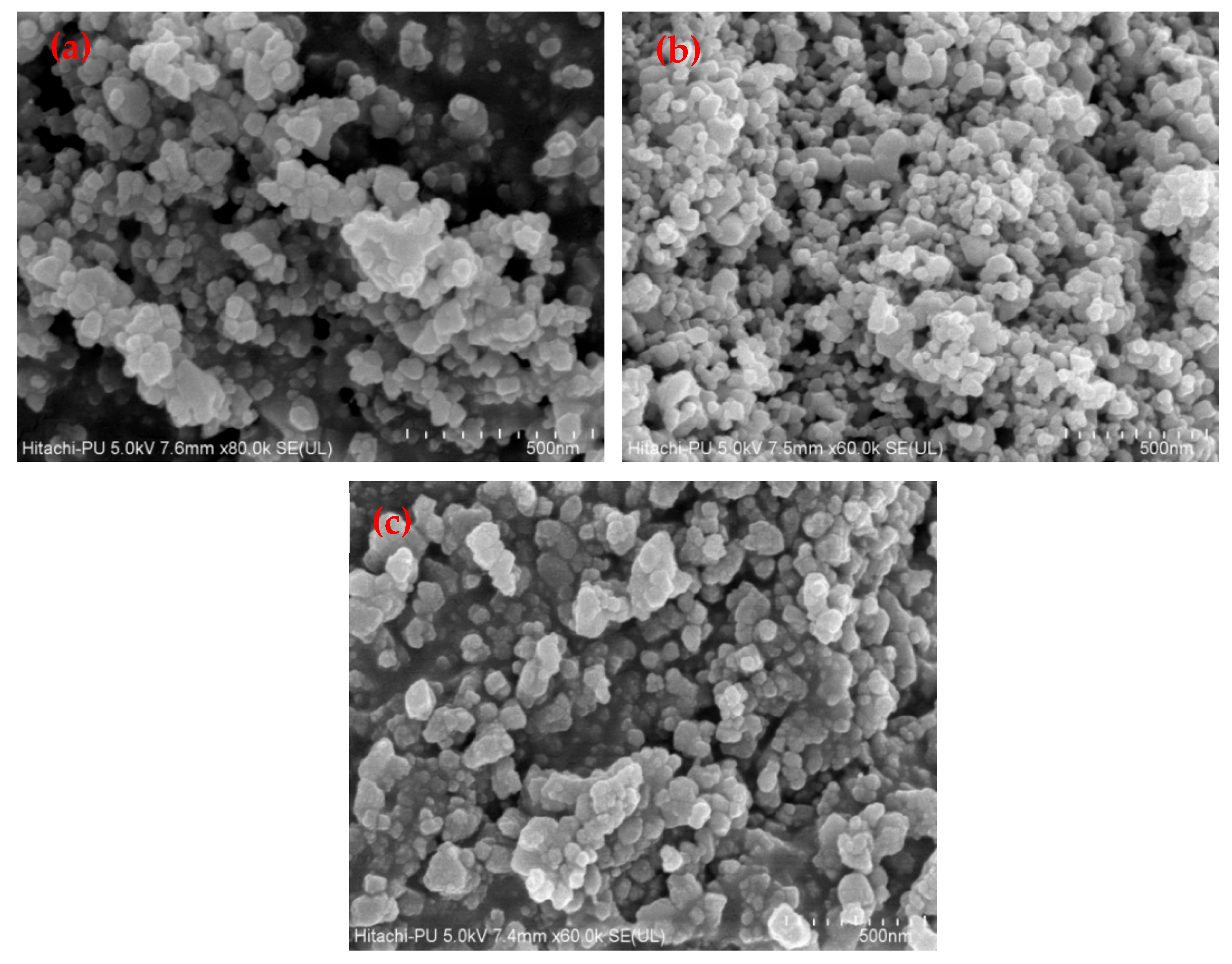
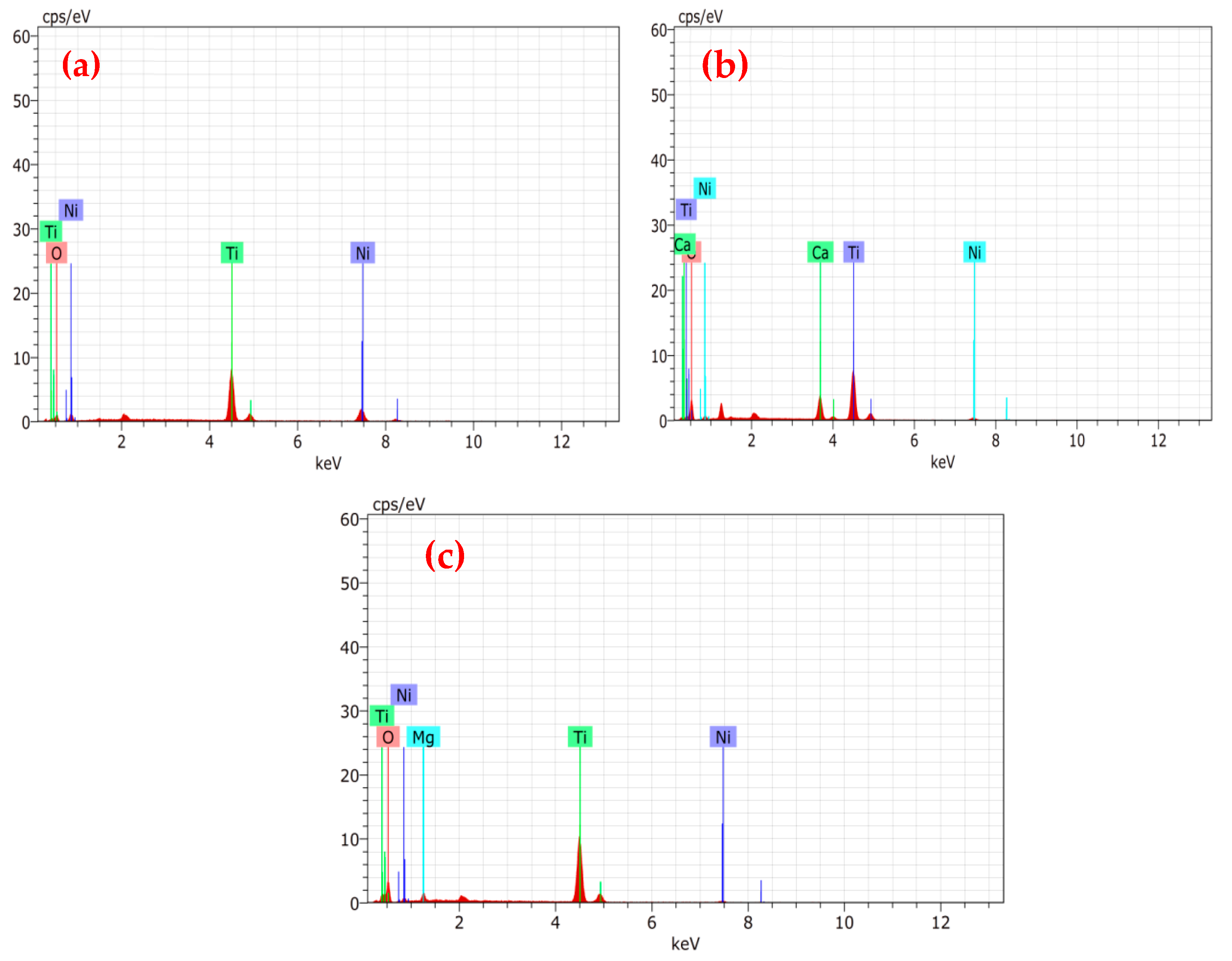
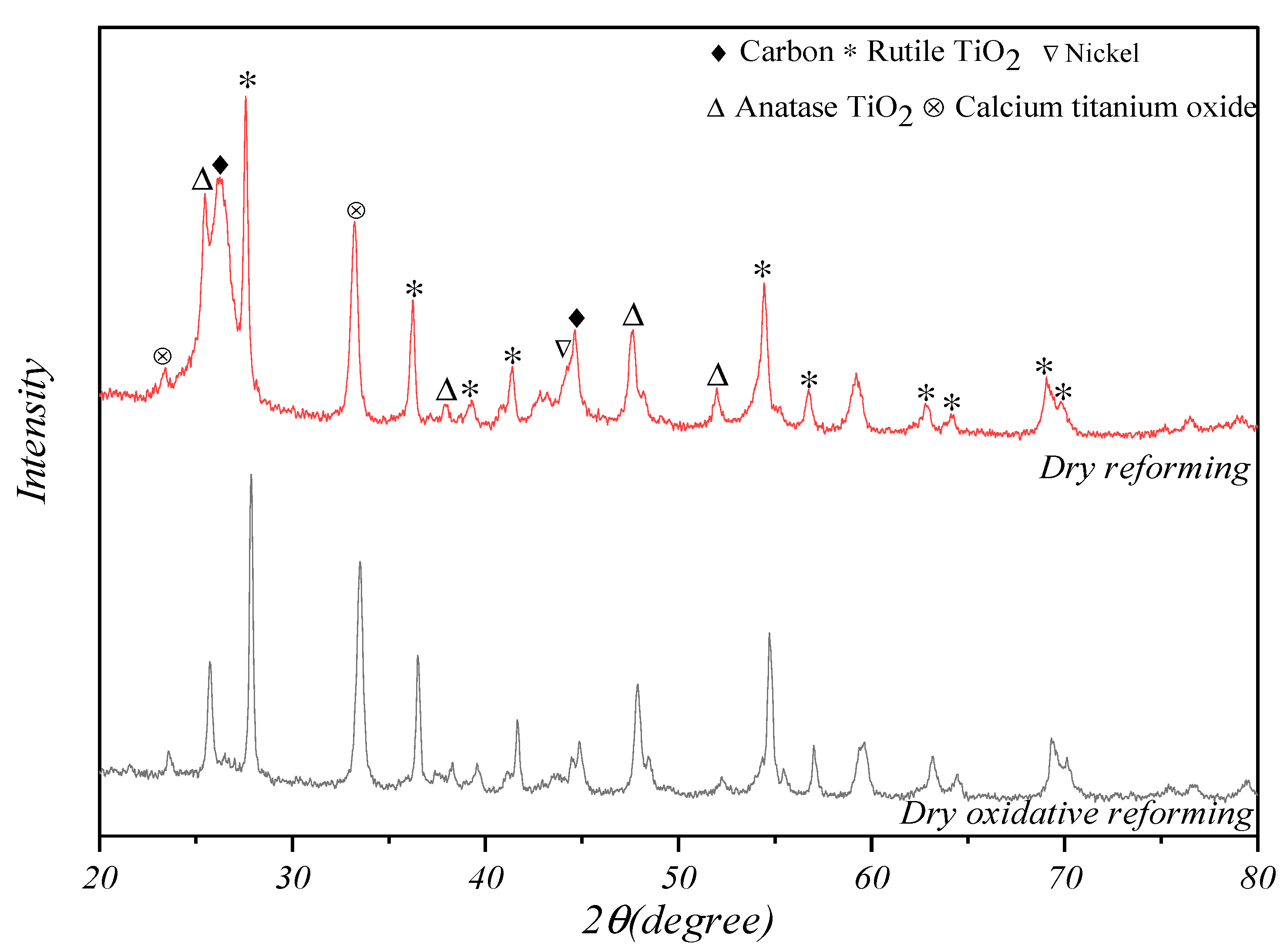
3.1. Characterization of Catalysts
3.2. Catalytic Activity
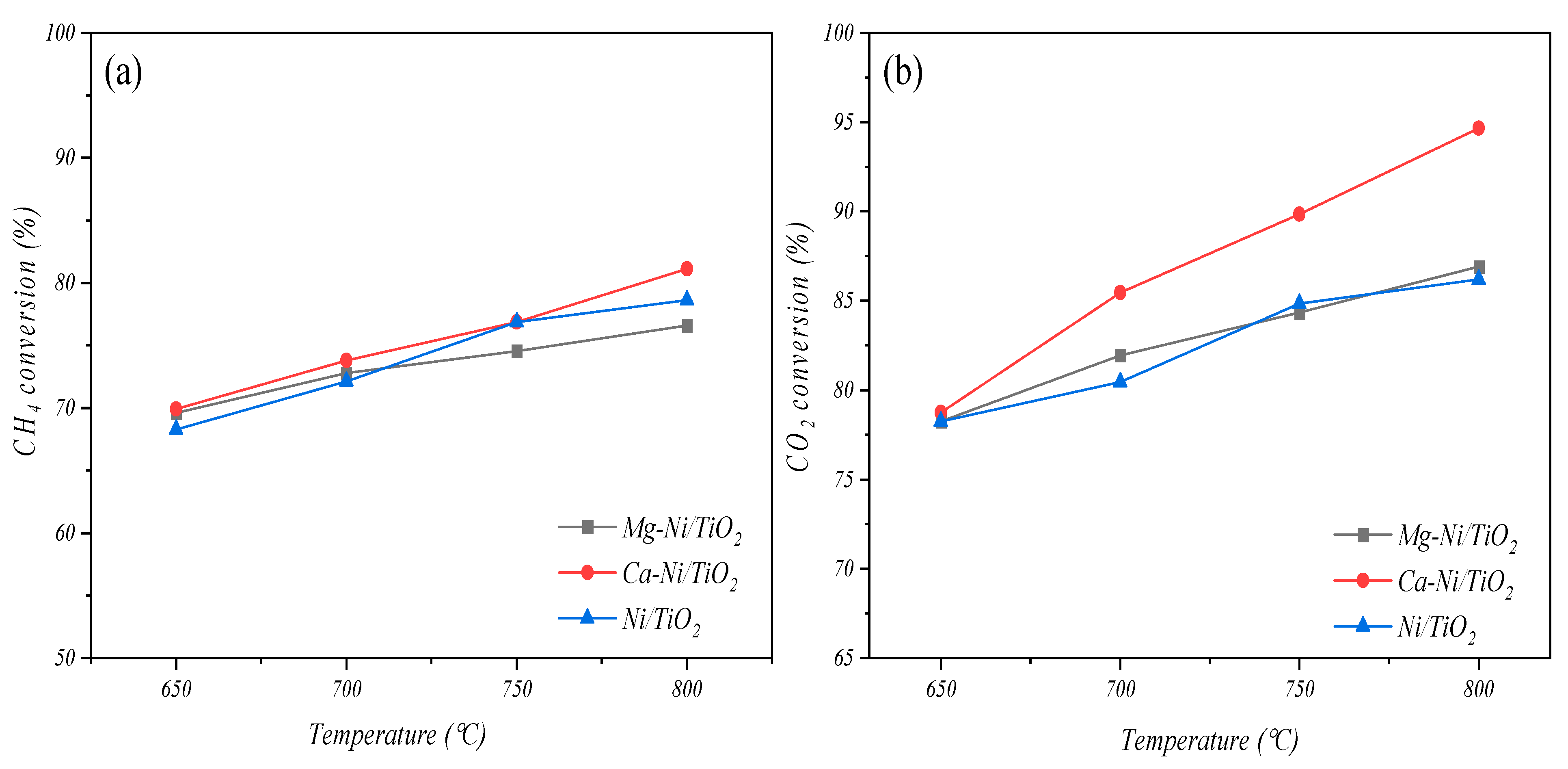
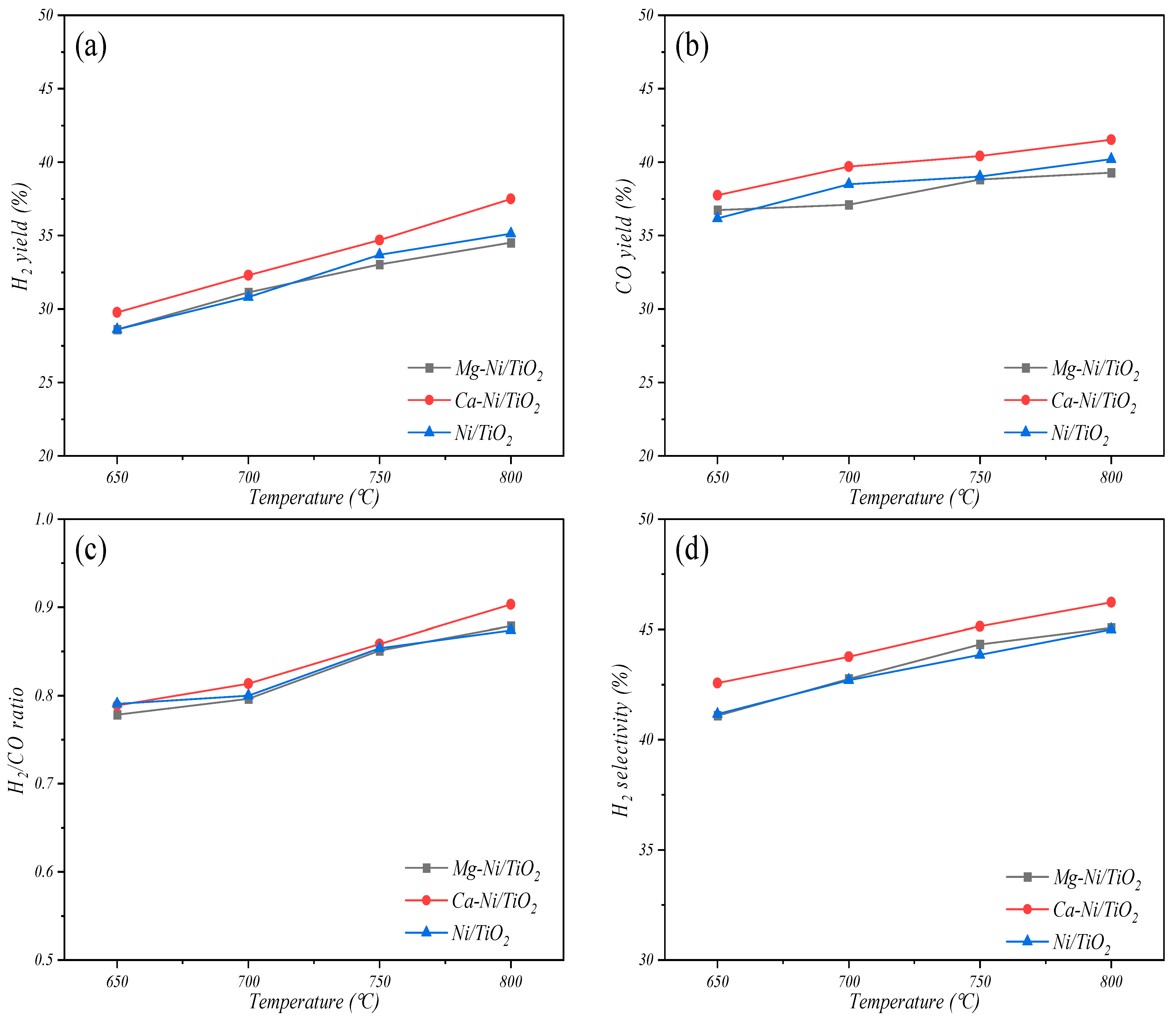
3.2.1. Effects of Temperature on Catalytic Activity
3.2.2. Effects of O2/CH4 Ratio on Catalytic Activity
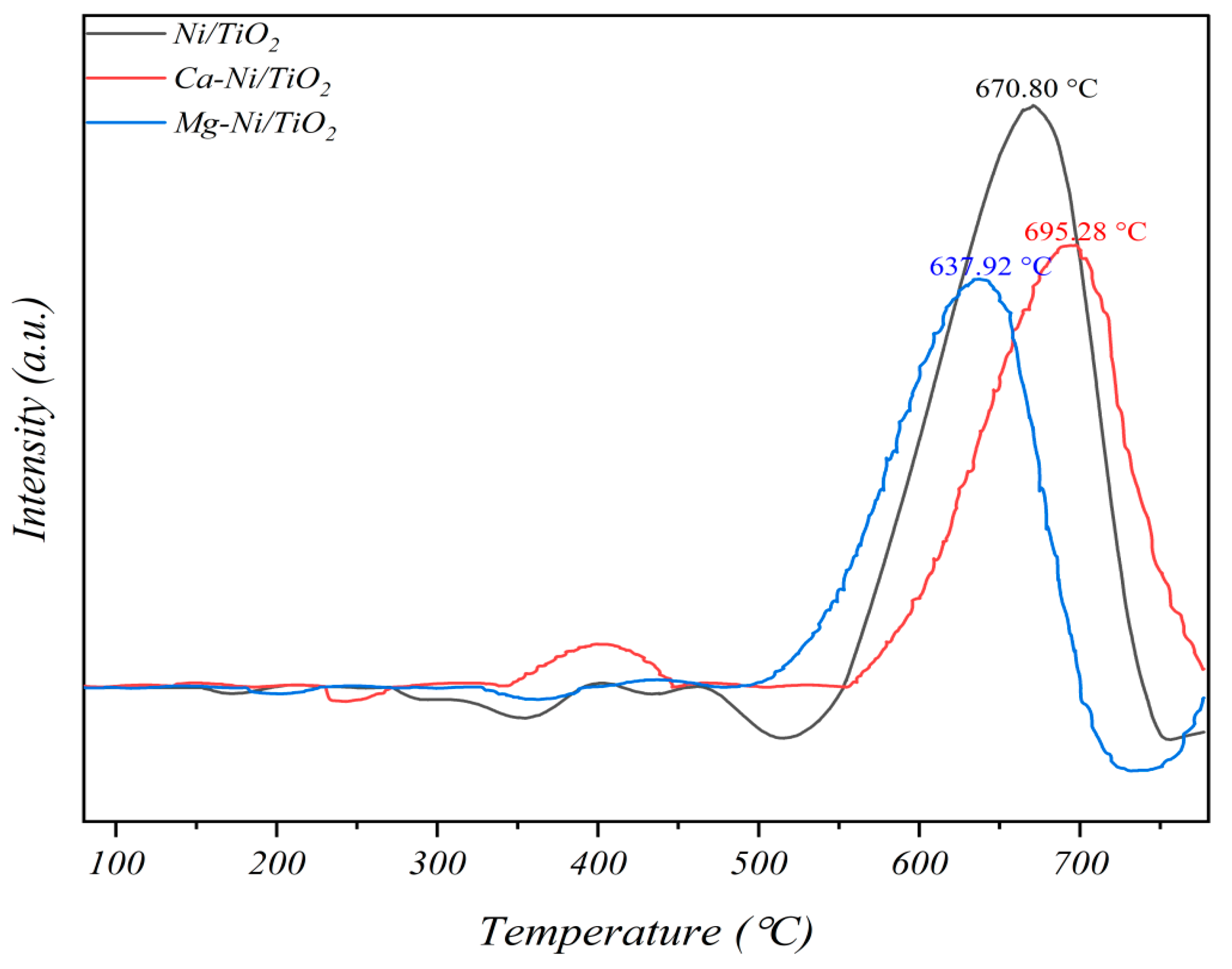
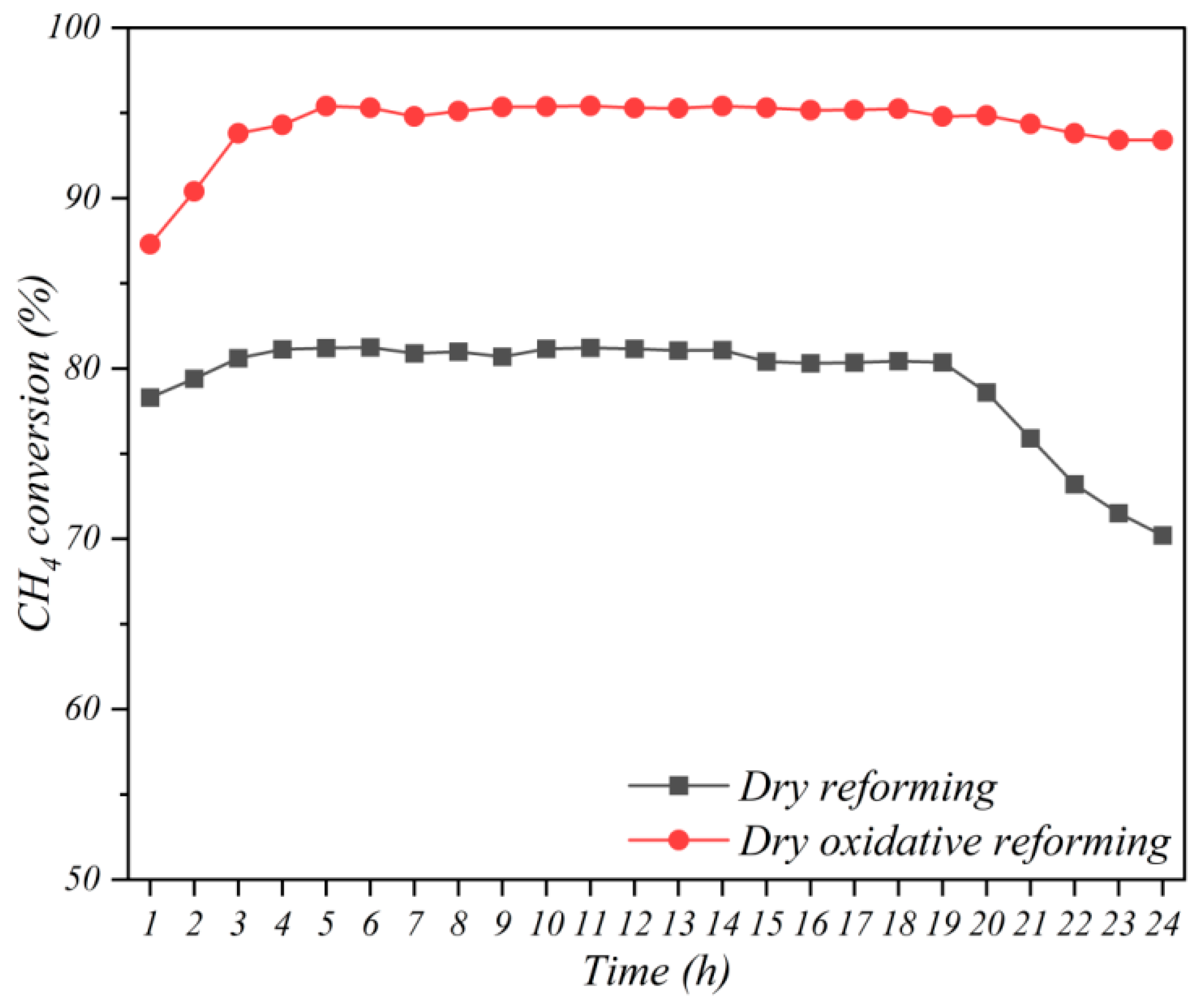
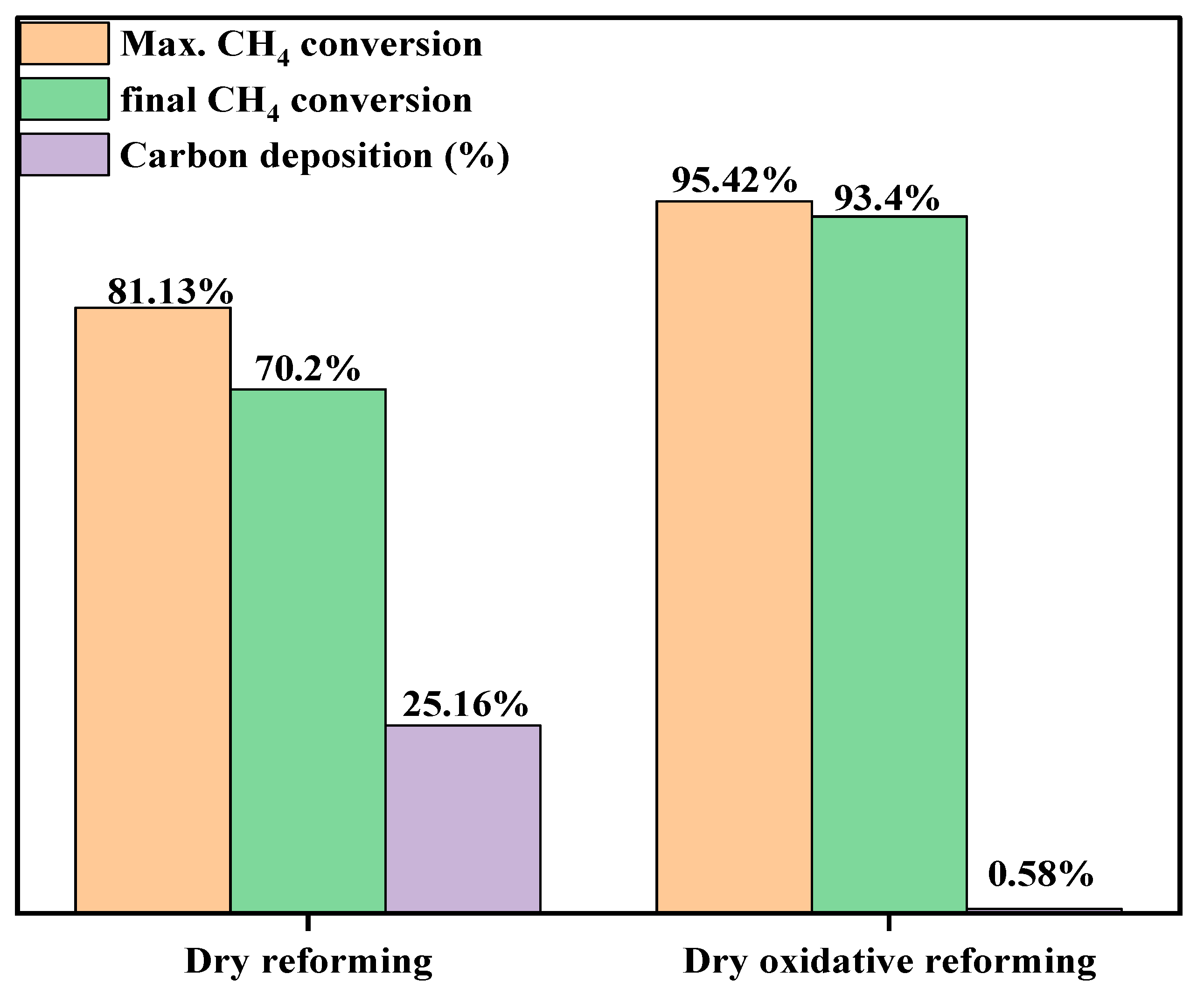
3.3. Stability Analysis of Promoted Catalyst
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Abe, H.; Mizoguchi, H.; Eguchi, R.; Hosono, H. Exploration of heterogeneous catalyst for molecular hydrogen ortho-para conversion. Exploration 2024, 4, 20230040. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H.; Dhir, A. Response surface methodology for optimization of dry oxidative reforming for hydrogen enrichment of biogas. Biomass Convers. Biorefin. 2023, 13, 2875–2883. [Google Scholar] [CrossRef]
- Zhou, C.; Li, J.; Meng, X.; Chen, Q.; Yan, Z.; Li, J.; Gao, X.; Ueda, S.; Wang, S.; Ai, L.; et al. Research progress of hydrogen production and CO2 fixation in molten slag cooling process. Carbon. Neutralization 2024, 3, 441–460. [Google Scholar] [CrossRef]
- Moogi, S.; Hyun Ko, C.; Hoon Rhee, G.; Jeon, B.H.; Ali Khan, M.; Park, Y.K. Influence of catalyst synthesis methods on anti-coking strength of perovskites derived catalysts in biogas dry reforming for syngas production. Chem. Eng. J. 2022, 437, 135348. [Google Scholar] [CrossRef]
- Gao, Y.; Jiang, J.; Meng, Y.; Yan, F.; Aihemaiti, A. A review of recent developments in hydrogen production via biogas dry reforming. Energy Convers. Manag. 2018, 171, 133–155. [Google Scholar] [CrossRef]
- Pietraszek, A.; Koubaissy, B.; Roger, A.C.; Kiennemann, A. The influence of the support modification over Ni-based catalysts for dry reforming of methane reaction. Catal. Today 2011, 176, 267–271. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.W.; Shin, C.H.; Park, M.B.; Min, H.K. Nickel silicate beta zeolite prepared by interzeolite transformation: A highly active and stable catalyst for dry reforming of methane. Chem. Eng. J. 2022, 431, 133364. [Google Scholar] [CrossRef]
- Leba, A.; Yıldırım, R. Determining most effective structural form of nickel-cobalt catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2020, 45, 4268–4283. [Google Scholar] [CrossRef]
- Tomishige, K. Syngas production from methane reforming with CO2/H2O and O2 over NiO–MgO solid solution catalyst in fluidized bed reactors. Catal. Today 2004, 89, 405–418. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Catalytic reforming of synthetic biogas for hydrogen enrichment over Ni supported on ZnO-CeO2 mixed catalyst. Biomass Bioenergy 2019, 125, 70–78. [Google Scholar] [CrossRef]
- Hamzah, A.B.; Fukuda, T.; Ookawara, S.; Yoshikawa, S.; Matsumoto, H. Process intensification of dry reforming of methane by structured catalytic wall-plate microreactor. Chem. Eng. J. 2021, 412, 128636. [Google Scholar] [CrossRef]
- Serrano-Lotina, A.; Daza, L. Long-term stability test of Ni-based catalyst in carbon dioxide reforming of methane. Appl. Catal. A Gen. 2014, 474, 107–113. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef] [PubMed]
- Cruz, P.L.; Navas-Anguita, Z.; Iribarren, D.; Dufour, J. Exergy analysis of hydrogen production via biogas dry reforming. Int. J. Hydrogen Energy 2018, 43, 11688–11695. [Google Scholar] [CrossRef]
- Calgaro, C.O.; Perez-Lopez, O.W. Biogas dry reforming for hydrogen production over Ni-M-Al catalysts (M = Mg, Li, Ca, La, Cu, Co, Zn). Int. J. Hydrogen Energy 2019, 44, 17750–17766. [Google Scholar] [CrossRef]
- Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Biogas reforming for hydrogen enrichment by ceria decorated over nickel catalyst supported on titania and alumina. Int. J. Hydrogen Energy 2018, 43, 21246–21255. [Google Scholar] [CrossRef]
- Rosha, P.; Mohapatra, S.K.; Mahla, S.K.; Dhir, A. Hydrogen enrichment of biogas via dry and autothermal-dry reforming with pure nickel (Ni) nanoparticle. Energy 2019, 172, 733–739. [Google Scholar] [CrossRef]
- Kumar Yadav, P.; Jabotra, G.; Sharma, S. Effect of Partial Noble Metal (M = Pd, Rh, Ru, Pt) Substitution in La1−xSrxCo1−yMyO3 Perovskite-Derived Catalysts for Dry Reforming of Methane. Hydrogen 2025, 6, 49. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, M.; Li, X.; Hao, Q.; Chen, H.; Ma, X. Ni-based catalysts prepared for CO2 reforming and decomposition of methane. Energy Convers. Manag. 2020, 205, 112419. [Google Scholar] [CrossRef]
- Siang, T.J.; Singh, S.; Omoregbe, O.; Bach, L.G.; Phuc, N.H.H.; Vo, D.V.N. Hydrogen production from CH4 dry reforming over bimetallic Ni–Co/Al2O3 catalyst. J. Energy Inst. 2018, 91, 683–694. [Google Scholar] [CrossRef]
- Yadav, P.K.; Verma, P.; Sharma, S. Bimetal (CuNi and CuCo) substituted CeO2: An approach for low temperature dry reforming of methane. Mol. Catal. 2024, 565, 114398. [Google Scholar] [CrossRef]
- Liu, K.; Nawaz, M.A.; Liao, G. Progress and Future Challenges in Designing High-Performance Ni/CeO2 Catalysts for CO2 Methanation: A Critical Review. Carbon. Neutralization 2025, 4, e190. [Google Scholar] [CrossRef]
- Sengupta, S.; Deo, G. Modifying alumina with CaO or MgO in supported Ni and Ni-Co catalysts and its effect on dry reforming of CH4. J. CO2 Util. 2015, 10, 67–77. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A review of CH4–CO2 reforming to synthesis gas over Ni-based catalysts in recent years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, H.Y.; Cho, J.M.; Choi, J.H.; Bae, J.W. Ni/M-Al2O3 (M = Sm, Ce or Mg) for combined steam and CO2 reforming of CH4 from coke oven gas. J. CO2 Util. 2017, 21, 211–218. [Google Scholar] [CrossRef]
- Jin, B.; Li, S.; Liu, Y.; Liang, X. Engineering metal-oxide interface by depositing ZrO2 overcoating on Ni/Al2O3 for dry reforming of methane. Chem. Eng. J. 2022, 436, 135195. [Google Scholar] [CrossRef]
- Sharma, H.; Dhir, A. Hydrogen augmentation of biogas through dry reforming over bimetallic nickel-cobalt catalysts supported on titania. Fuel 2020, 279, 118389. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Duy Nguyen, T.; Phuong, P.T.T.; Duc Truong, Q.; Abdul Jalil, A.; Ainirazali, N.; Vo, D.-V.N. Insight into the influence of rare-earth promoter (CeO2, La2O3, Y2O3, and Sm2O3) addition toward methane dry reforming over Co/mesoporous alumina catalysts. Chem. Eng. Sci. 2020, 228, 115967. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.A.; Fakeeha, A.H.; Abasaeed, A.E. Effects of Selected Promoters on Ni/Y-Al2O3 Catalyst Performance in Methane Dry Reforming. Chin. J. Catal. 2011, 32, 1604–1609. [Google Scholar] [CrossRef]
- Zhu, J.; Peng, X.; Yao, L.; Shen, J.; Tong, D.; Hu, C. The promoting effect of La, Mg, Co and Zn on the activity and stability of Ni/SiO2 catalyst for CO2 reforming of methane. Int. J. Hydrogen Energy 2011, 36, 7094–7104. [Google Scholar] [CrossRef]
- Liu, H.; Costa PDa Taief, H.B.H.; Benzina, M.; Gálvez, M.E. Mg-promotion of Ni natural clay-supported catalysts for dry reforming of methane. RSC Adv. 2018, 8, 19627–19634. [Google Scholar] [CrossRef] [PubMed]
- Sadeq Al-Fatesh, A.; Olajide Kasim, S.; Aidid Ibrahim, A.; Hamza Fakeeha, A.; Elhag Abasaeed, A.; Alrasheed, R.; Ashamari, R.; Bagabas, A. Combined magnesia, ceria and nickel catalyst supported over γ-alumina doped with titania for dry reforming of methane. Catalysts 2019, 9, 188. [Google Scholar] [CrossRef]
- Wei, Q.; Gao, X.; Liu, G.; Yang, R.; Zhang, H.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Facile one-step synthesis of mesoporous Ni-Mg-Al catalyst for syngas production using coupled methane reforming process. Fuel 2018, 211, 1–10. [Google Scholar] [CrossRef]
- Ma, Y.; Ma, Y.; Long, G.; Li, J.; Hu, X.; Ye, Z.; Wang, Z.; Buckley, C.E.; Dong, D. Synergistic promotion effect of MgO and CeO2 on nanofibrous Ni/Al2O3 catalysts for methane partial oxidation. Fuel 2019, 258, 116103. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, Q.; Wen, J.; Tang, T.; Wang, H.; Liu, M.; Ning, P.; Deng, L.; Shi, Y. Insight into the role of CaO in coke-resistant over Ni-HMS catalysts for CO2 reforming of methane. Appl. Surf. Sci. 2020, 521, 146395. [Google Scholar] [CrossRef]
- Hambali, H.U.; Jalil, A.A.; Abdulrasheed, A.A.; Siang, T.J.; Abdullah, T.A.T.; Ahmad, A.; Vo, D.-V.N. Fibrous spherical Ni-M/ZSM-5 (M: Mg, Ca, Ta, Ga) catalysts for methane dry reforming: The interplay between surface acidity-basicity and coking resistance. Int. J. Energy Res. 2020, 44, 5696–5712. [Google Scholar] [CrossRef]
- Singha, R.K.; Yadav, A.; Shukla, A.; Iqbal, Z.; Pendem, C.; Sivakumar, K.; Bal, D.R. Promoting Effect of CeO2 and MgO for CO2 Reforming of Methane over Ni-ZnO Catalyst. Phys. Theor. Chem. 2016, 1, 3075–3085. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Bagabas, A.A.; Lanre, M.S.; Osman, A.I.; Kasim, S.O.; Ibrahim, A.A.; Arasheed, R.; Alkhalifa, A.; Elnour, A.Y.; Abasaeed, A.E.; et al. Catalytic performance of metal oxides promoted nickel catalysts supported on mesoporous γ-Alumina in dry reforming of methane. Processes 2020, 8, 522. [Google Scholar] [CrossRef]
- Chaudhary, P.K.; Koshta, N.; Deo, G. Effect of O2 and temperature on the catalytic performance of Ni/Al2O3 and Ni/MgAl2O4 for the dry reforming of methane (DRM). Int. J. Hydrogen Energy 2020, 45, 4490–4500. [Google Scholar] [CrossRef]
- O’Connor, A.M.; Ross, J.R.H. The effect of O2 addition on the carbon dioxide reforming of methane over Pt/ZrO2 catalysts. Catal. Today 1998, 46, 203–210. [Google Scholar] [CrossRef]
- Bahari, M.B.; Setiabudi, H.D.; Nguyen, T.D.; Jalil, A.A.; Ainirazali, N.; Vo, D.V.N. Hydrogen production via CO2-CH4 reforming over cobalt-supported mesoporous alumina: A kinetic evaluation. Int. J. Hydrogen Energy 2021, 46, 24742–24753. [Google Scholar] [CrossRef]
- Omoregbe, O.; Danh, H.T.; Abidin, S.Z.; Setiabudi, H.D.; Abdullah, B.; Vu, K.B.; Vo, D.-V.N. Influence of Lanthanide Promoters on Ni/SBA-15 Catalysts for Syngas Production by Methane Dry Reforming. Procedia Eng. 2016, 148, 1388–1395. [Google Scholar] [CrossRef]
- Movasati, A.; Alavi, S.M.; Mazloom, G. CO2 reforming of methane over Ni/ZnAl2O4 catalysts: Influence of Ce addition on activity and stability. Int. J. Hydrogen Energy 2017, 42, 16436–16448. [Google Scholar] [CrossRef]
- Wang, H.; Mo, W.; He, X.; Fan, X.; Ma, F.; Liu, S.; Tax, D. Effect of Ca Promoter on the Structure, Performance, and Carbon Deposition of Ni-Al2O3Catalyst for CO2-CH4 Reforming. ACS Omega 2020, 5, 28955–28964. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Suryawinata, I.Y.; Kawi, S. Highly carbon resistant multicore-shell catalyst derived from Ni-Mg phyllosilicate nanotubes@silica for dry reforming of methane. Appl. Catal. B 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Ay, H.; Üner, D. Dry reforming of methane over CeO2 supported Ni, Co and Ni–Co catalysts. Appl. Catal. B 2015, 179, 128–138. [Google Scholar] [CrossRef]
- Taherian, Z.; Yousefpour, M.; Tajally, M.; Khoshandam, B. Catalytic performance of Samaria-promoted Ni and Co/SBA-15 catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2017, 42, 24811–24822. [Google Scholar] [CrossRef]
- Song, I.K.; Cho, Y.S.; Choi, G.S.; Park, J.B.; Kim, D.J. The growth mode change in carbon nanotube synthesis in plasma-enhanced chemical vapor deposition. Diam. Relat. Mater. 2004, 13, 1210–1213. [Google Scholar] [CrossRef]
- Bower, C.; Zhou, O.; Zhu, W.; Werder, D.J.; Jin, S. Nucleation and growth of carbon nanotubes by microwave plasma chemical vapor deposition. Appl. Phys. Lett. 2000, 77, 2767. [Google Scholar] [CrossRef]
- Lu, Y.; Guo, D.; Ruan, Y.; Zhao, Y.; Wang, S.; Ma, X. Facile one-pot synthesis of Ni@HSS as a novel yolk-shell structure catalyst for dry reforming of methane. J. CO2 Util. 2018, 24, 190–199. [Google Scholar] [CrossRef]
- Mo, L.; Leong, K.K.M.; Kawi, S. A highly dispersed and anti-coking Ni-La2O3/SiO2 catalyst for syngas production from dry carbon dioxide reforming of methane. Catal. Sci. Technol. 2014, 4, 2107–2114. [Google Scholar] [CrossRef]
- Abdulrasheed, A.A.; Jalil, A.A.; Hamid, M.Y.S.; Siang, T.J.; Abdullah, T.A.T. Dry reforming of CH4 over stabilized Ni-La@KCC-1 catalyst: Effects of la promoter and optimization studies using RSM. J. CO2 Util. 2020, 37, 230–239. [Google Scholar] [CrossRef]
- Park, J.H.; Heo, I.; Chang, T.S. Dry reforming of methane over Ni-substituted CaZrNiOx catalyst prepared by the homogeneous deposition method. Catal. Commun. 2019, 120, 1–5. [Google Scholar] [CrossRef]
- Huang, X.; Mo, W.; He, X.; Fan, X.; Ma, F.; Tax, D. Effects of Promoters on the Structure, Performance, and Carbon Deposition of Ni-Al2O3Catalysts for CO2-CH4 Reforming. ACS Omega 2021, 6, 16381–16390. [Google Scholar] [CrossRef]
- Naeem, M.A.; Al-Fatesh, A.S.; Khan, W.U.; Abasaeed, A.E.; Fakeeha, A.H. Syngas Production from Dry Reforming of Methane over Nano Ni Polyol Catalysts. Int. J. Chem. Eng. Appl. 2013, 4, 315–320. [Google Scholar] [CrossRef]
- Juan-Juan, J.; Román-Martínez, M.C.; Illán-Gómez, M.J. Nickel catalyst activation in the carbon dioxide reforming of methane. Effect of pretreatments. Appl. Catal. A Gen. 2009, 355, 27–32. [Google Scholar] [CrossRef]
- Lau, C.S.; Tsolakis, A.; Wyszynski, M.L. Biogas upgrade to syn-gas (H2-CO) via dry and oxidative reforming. Int. J. Hydrogen Energy 2011, 36, 397–404. [Google Scholar] [CrossRef]
- Ranjbar, A.; Rezaei, M. Dry reforming reaction over nickel catalysts supported on nanocrystalline calcium aluminates with different CaO/Al2O3 ratios. J. Nat. Gas Chem. 2012, 21, 178–183. [Google Scholar] [CrossRef]
- Matos, J.; Díaz, K.; García, V.; Cordero, T.C.; Brito, J.L. Methane transformation in presence of carbon dioxide on activated carbon supported nickel-calcium catalysts. Catal. Lett. 2006, 109, 163–169. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, H.; Yadav, P.K.; Sharma, S.; Dhir, A. Dry-Oxidative Reforming of Biogas for Hydrogen Generation over Ca and Mg-Promoted Titania-Supported Nickel Catalyst. Hydrogen 2025, 6, 64. https://doi.org/10.3390/hydrogen6030064
Sharma H, Yadav PK, Sharma S, Dhir A. Dry-Oxidative Reforming of Biogas for Hydrogen Generation over Ca and Mg-Promoted Titania-Supported Nickel Catalyst. Hydrogen. 2025; 6(3):64. https://doi.org/10.3390/hydrogen6030064
Chicago/Turabian StyleSharma, Himanshu, Pradeep Kumar Yadav, Sudhanshu Sharma, and Amit Dhir. 2025. "Dry-Oxidative Reforming of Biogas for Hydrogen Generation over Ca and Mg-Promoted Titania-Supported Nickel Catalyst" Hydrogen 6, no. 3: 64. https://doi.org/10.3390/hydrogen6030064
APA StyleSharma, H., Yadav, P. K., Sharma, S., & Dhir, A. (2025). Dry-Oxidative Reforming of Biogas for Hydrogen Generation over Ca and Mg-Promoted Titania-Supported Nickel Catalyst. Hydrogen, 6(3), 64. https://doi.org/10.3390/hydrogen6030064





