Abstract
Green hydrogen derived from renewable resources is increasingly recognized as a basis for future low-carbon energy systems. This study presents a comprehensive techno-environmental comparison of two thermochemical conversion pathways utilizing biomethane: steam methane reforming (SMR) and chemical looping reforming (CLR). Through integrated process simulations, compositional analyses, energy modeling, and cost evaluation, we examine the comparative advantages of each route in terms of hydrogen yield, carbon separation efficiency, process energy intensity, and economic performance. The results demonstrate that CLR achieves a significantly higher hydrogen concentration in the raw syngas stream (62.44%) than SMR (43.14%), with reduced levels of residual methane and carbon monoxide. The energy requirements for hydrogen production are lower in the CLR system, averaging 1.2 MJ/kg, compared to 3.2 MJ/kg for SMR. Furthermore, CLR offers a lower hydrogen production cost (USD 4.3/kg) compared to SMR (USD 6.4/kg), primarily due to improved thermal integration and the absence of solvent-based CO2 capture. These insights highlight the potential of CLR as a next-generation reforming strategy for producing green hydrogen. To advance its technology readiness, it is proposed to develop a pilot-scale CLR facility to validate system performance under operational conditions and support the pathway to commercial implementation.
1. Introduction
The decarbonization of energy-intensive sectors, including transportation, power generation, and industrial manufacturing, necessitates the rapid scaling of clean hydrogen technologies [1]. Hydrogen, when produced from renewable sources, serves as a flexible, carbon-free energy carrier with applications ranging from fuel cells to synthetic fuels and various chemical processes [2].
Biomethane, an upgraded form of biogas produced through anaerobic digestion, serves as a renewable and dispatchable hydrogen feedstock [3]. Unlike fossil-derived methane, biomethane is carbon-neutral throughout its life cycle [4]. Harnessing this feedstock through efficient thermochemical conversion routes enables the production of green hydrogen, aligning with the principles of a circular economy [5]. Steam methane reforming (SMR) remains the industrial standard for hydrogen production, but is inherently carbon-intensive. Integrating a carbon capture and storage system (CCS) with SMR helps mitigate emissions, but it also adds cost and complexity to the system [6]. On the other hand, chemical looping reforming (CLR) is an emerging technology that offers inherent carbon dioxide (CO2) separation, potentially increasing energy efficiency and simplifying downstream processing [7].
Green hydrogen is commonly defined as hydrogen produced with minimal environmental impact, typically through the use of renewable energy sources such as solar, wind, hydro, or sustainably sourced biomass [8,9]. This low-carbon or carbon-neutral approach aims to reduce greenhouse gas (GHG) emissions, enhance energy security, and support global climate objectives. Various international standards and regulatory bodies define green hydrogen using criteria such as the share of renewable input energy, emission thresholds (e.g., ≤3 kg CO2eq/kg H2), or a minimum GHG reduction compared to conventional hydrogen production pathways like SMR [10,11,12,13,14,15,16,17,18,19,20].
Several production pathways exist for green hydrogen, including electrolysis powered by renewable electricity, photonic-based water splitting (e.g., photocatalysis and photoelectrochemical methods), biological processes such as dark fermentation, and thermochemical processes utilizing biomass or biogas [21,22,23,24,25,26,27]. These diverse methods offer a range of technical maturity, cost, and scalability. Thermochemical processes, including water splitting, gasification, and reforming, provide viable pathways for producing green hydrogen by utilizing thermal energy to drive chemical reactions [28,29,30,31,32]. SMR typically results in significant CO2 emissions unless coupled with carbon capture [33]. CLR utilizes an oxygen carrier to convert biomethane into hydrogen within a two-reactor system, comprising a fuel reactor for hydrogen generation and an air reactor for carrier regeneration [34].
Despite the maturity and widespread use of SMR, its continued reliance on fossil fuels and high CO2 emissions pose significant challenges for aligning with emerging green hydrogen standards, especially in the absence of carbon capture systems. While CLR offers a promising low-carbon alternative by enabling hydrogen production from renewable feedstocks, such as biomethane, and inherently integrating CO2 separation, current research on CLR remains limited in several key aspects. These include the lack of comparative evaluations against established technologies, such as SMR, under consistent feedstock and process boundary conditions, as well as limited techno-environmental benchmarking.
This comparative study aims to address these gaps by providing a side-by-side evaluation of SMR and CLR using biomethane as a common renewable feedstock. The analysis focuses on techno-environmental performance, process efficiency, utility requirements, and production cost, supported by detailed process modeling and system-level comparisons. The accompanying process diagrams illustrate unit operations and integration pathways, offering valuable insights into the readiness and strategic potential of CLR as a viable alternative to SMR in the transition to green hydrogen.
2. Process Description
2.1. Biogas Purification
Raw biogas consists of 50–70% methane and 30–50% carbon dioxide, along with trace amounts of hydrogen sulfide (H2S), moisture, and siloxanes. To be suitable for reforming, the biogas must be upgraded to at least 95% methane. In both systems, biogas is compressed and cooled before entering a diethanolamine (DEA) absorber, where CO2 and H2S are selectively removed. The rich DEA stream is regenerated through heating in a reboiler and recycled.
2.2. Steam Methane Reforming (SMR) System
The SMR route involves several stages, as illustrated in Figure 1, and details of the system description can be found in [35].
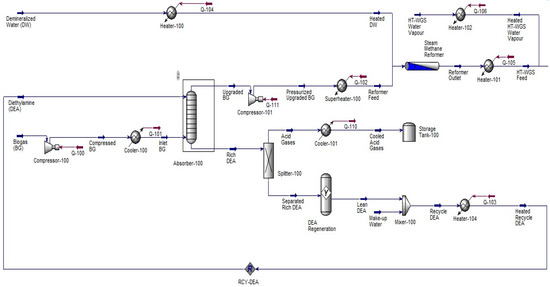
Figure 1.
The steam methane reforming process used to produce green hydrogen.
- Reformer feed preparation: Biomethane is pressurized and superheated to 30 kPa and 365.7 °C before entering the reformer. Demineralized water is vaporized and mixed with biomethane to create a steam–methane mixture.
- Steam reforming: In the reformer, the endothermic reaction CH4 + H2O → CO + 3H2 occurs over a nickel-based catalyst with the conversion rate of 85% at 1000 °C and 500 kPa. This produces synthesis gas (syngas) composed of up to 44% hydrogen, with the unconverted CH4, CO, and H2O.
- Water–gas shift (WGS) reaction: The syngas undergoes both high-temperature (HT) and low-temperature (LT) WGS reactions to convert CO and water into CO2 and H2, thereby increasing the hydrogen yield by up to 57%.
- Cooling and separation: The shifted gas is cooled before entering the pressure swing adsorption (PSA) unit, where it is purified to reach a purity of 99.99% hydrogen.
- Carbon capture and storage: CO2-rich tail gas from PSA and WGS is sent to a second DEA absorber (CCS section). CO2 is captured, compressed, and stored in a separate tank.
- Hydrogen storage: Purified hydrogen is routed to a storage tank for use.
2.3. Chemical Looping Reforming (CLR) System
CLR replaces traditional reforming with a redox cycle using solid oxygen carriers. The process is shown in Figure 2, and the details can be found in [35].
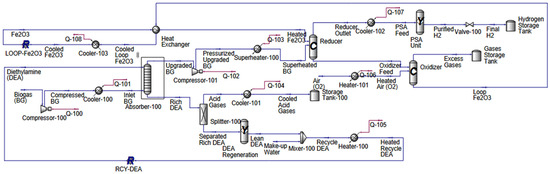
Figure 2.
The chemical looping reforming process used to produce green hydrogen.
- Reformer (reducer reactor): Biomethane is fed to a reducer reactor containing a metal oxide. Methane is partially oxidized to CO and H2, while the oxygen carrier (Fe2O3) is reduced. In the reformer, the reduction equation used is CH4 + Fe2O3 ↔ CO + 2H2 + 2FeO. The obtained hydrogen yield is up to 64%. The reactor conversion is 99%, which was determined through process simulation. Side reactions such as methane cracking or carbon deposition were not modeled explicitly, as they are known to be thermodynamically suppressed under these optimized operating conditions.
- PSA unit: The H2-rich reformer gas is purified until it reaches 99.98% purity.
- Oxidizer reactor: The reduced oxygen carrier is transferred to an oxidizer, where it is regenerated by air. This reaction is exothermic, releasing heat.
- Hydrogen storage: Purified hydrogen is routed to a storage tank for use.
The entire simulation, including process modeling, performance evaluation, and economic analysis, was conducted using Aspen HYSYS r14.0 software. Both systems were simulated under steady-state conditions with syngas composition and utility consumption derived from converged mass and energy balances.
3. Results and Discussion
A structured comparison between SMR and CLR is presented in Table 1, followed by a detailed discussion supported by compositional and performance data. Each criterion illustrates the fundamental differences in design philosophy between the two technologies. SMR relies on well-established catalytic reactions but requires complex downstream processing for CO conversion and CO2 capture. Its scalability is proven, but its dependence on water and energy-intensive solvent systems limits both its flexibility and cost efficiency. CLR introduces a paradigm shift by integrating fuel conversion and CO2 separation into a single loop. The oxygen carrier mediates oxidation in a controlled manner, eliminating the need for direct combustion or steam-driven shift reactions. This significantly reduces water demand and simplifies THE overall plant design.

Table 1.
Comparison between SMR and CLR for the production of green hydrogen from biomethane.
3.1. Environmental Impacts
From the process analysis, the syngas composition at each process is obtained and listed in Table 2. It shows that the CLR yields a substantially higher hydrogen concentration of 62.44% compared to 43.14% for SMR, demonstrating its superior hydrogen production efficiency. However, this high level of methane conversion is accompanied by the formation of a substantial amount of CO, reaching up to 31.22% in the syngas. This elevated CO concentration is an inherent outcome of the partial oxidation mechanism employed in the CLR fuel reactor, where lattice oxygen from the solid oxygen carrier facilitates the controlled oxidation of methane. Unlike complete combustion, which would produce CO2, this oxygen-limited environment favors the generation of CO alongside hydrogen as part of the syngas mixture. Therefore, it is expected that higher energy consumption by PSA in the CLR system will be required to eliminate this CO content until the required hydrogen purity is reached.

Table 2.
Molar concentration for each system.
In contrast, SMR exhibits higher levels of unconverted CH4 at 13.33% and CO formation at 14.38%, necessitating additional downstream processing, such as HT-WGS and LT-WGS reactors, as well as PSA. From an environmental standpoint, minimizing CO in the product stream is important to reduce CO2 emissions during downstream oxidation. Since CLR delivers a cleaner syngas stream, it eliminates the need for supplementary shift reactors, thereby simplifying system design and improving overall process integration.
The CO2 molar concentration after WGS in SMR remains significant and must be managed through carbon capture, contributing to overall lifecycle emissions. By contrast, the CLR process shows no net CO2 generation throughout the syngas purification stages. The CO2 content remains negligible in the syngas, indicating that complete internal utilization or avoidance of CO2-forming reactions has occurred. This outcome is attributed to CLR’s use of a solid oxygen carrier, which enables partial oxidation of the fuel without external combustion or gas-phase oxygen, fundamentally avoiding CO2 production in the reforming step. This positions CLR as a truly low-carbon or even carbon-neutral route for hydrogen production, particularly when biomethane is utilized.
3.2. Energy Consumption
Figure 3 shows the estimated energy usage by both systems for green hydrogen production. Energy consumption profiles reinforce CLR’s superior thermal efficiency in hydrogen production. This study reveals that SMR consumes approximately 3.2 MJ of energy per kilogram of hydrogen produced, whereas CLR requires only 1.2 MJ/kg, representing a significant improvement over existing literature. For comparison, Antonini et al. [36] reported energy demands exceeding 11 MJ/kg for SMR using biomethane, primarily due to the high steam-to-carbon ratios, external heating requirements, and energy-intensive CO2 capture processes. The significantly higher energy demand in their study can be attributed to the use of a cogeneration system supplying electricity to the grid, which inherently involves broader system boundaries and includes energy penalties associated with external steam generation, and high steam-to-carbon ratios. In contrast, the SMR model presented in this study focuses strictly on process-level energy consumption, excluding grid export and assuming internally optimized heat recovery, lower S/C ratios, and efficient integration of utility systems, which results in more favorable thermal performance. Therefore, while the absolute values differ due to system boundary assumptions, the comparison remains valid for highlighting CLR’s intrinsic thermal advantages under comparable process conditions.
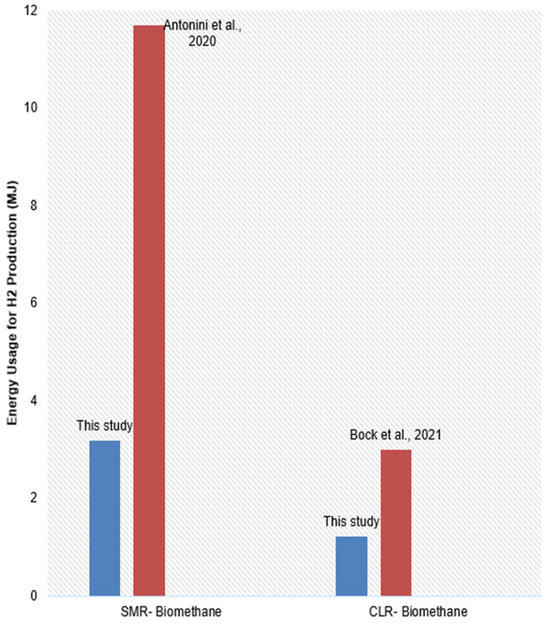
Figure 3.
Estimated energy usage for green hydrogen production [36,37].
Bock et al. [37] reported an energy consumption of approximately 3 MJ/kg of hydrogen for CLR, which already reflects substantial benefits from internal heat integration and coupling with a 3 MW biogas digester. In their system, the integration with a biogas plant enabled a steady biomethane supply and waste heat utilization, thereby improving overall process energy efficiency. However, the CLR configuration in this study demonstrates just 1.2 MJ/kg of H2 production, representing a significant advancement beyond previous benchmarks. Unlike Bock et al.’s broader energy system, which includes upstream biogas processing and auxiliary loads, the present analysis isolates the CLR hydrogen production loop, providing a focused assessment of its core process efficiency. These results not only validate the technical feasibility of CLR as a low-energy, high-yield hydrogen production pathway but also offer strong empirical support for its future scalability and industrial relevance.
From an environmental perspective, both pathways significantly outperform conventional fossil-based hydrogen production. However, CLR is designed to exhibit superior CO2 management. While SMR relies on amine-based absorption and regeneration, which is energy-intensive and sensitive to solvent degradation, CLR naturally separates CO2 in the reducer, producing a nearly pure stream with minimal parasitic losses.
3.3. Hydrogen Production Cost
Table 3 presents a comparison of utility consumption and associated hourly costs for SMR and CLR systems. The consumption rate of each utility per hour, expressed in appropriate units (e.g., kW for electricity, kJ/h for steam and cooling water), quantifies the instantaneous demand of each system during steady-state operation and forms the basis for calculating the corresponding cost per hour.

Table 3.
Utility consumption for each system.
As illustrated in Figure 4, the results compare the Total Capital Cost, Annual Operating Cost, Total Cost per Year, and Final Hydrogen Production (in million kg/year) for SMR and CLR. SMR exhibits a lower total capital cost compared to CLR. This is attributed to the mature technology ecosystem and established commercial supply chains for SMR equipment. In contrast, CLR involves newer, less standardized components, such as dual-reactor systems, solid looping material handling, and oxygen carrier synthesis, which currently elevate the upfront capital investments despite process simplification. CLR exhibits a marginally higher annual operating cost, primarily due to its requirement for an oxygen supply and the periodic replenishment of oxygen carrier materials. These contribute to additional auxiliary power consumption and consumables, resulting in a slight increase in OPEX compared to SMR. However, these costs are expected to decline with advances in carrier materials and optimization of oxygen integration [38].
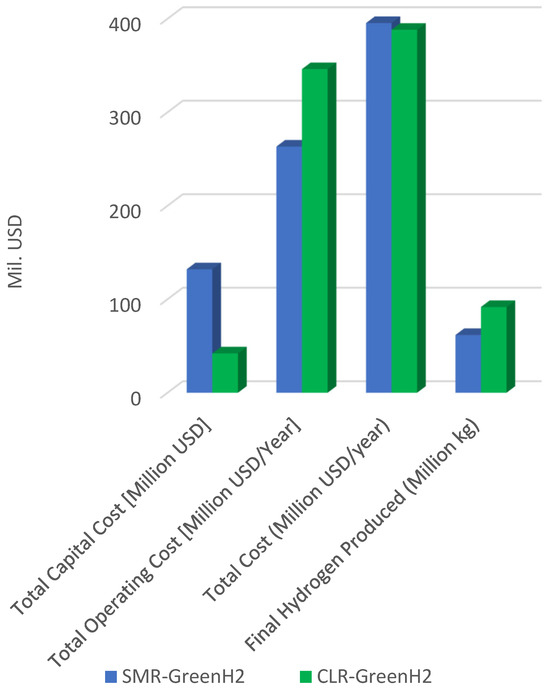
Figure 4.
Summary of the total hydrogen production cost.
While both systems show comparable total annual costs, CLR’s higher hydrogen yield improves its overall economic efficiency. It produces significantly more hydrogen over the same operational period due to its superior methane conversion, enhanced thermal integration, and minimal loss of carbon as residual CO or CH4. This higher productivity reduces the fixed and variable costs per kilogram of H2, especially over longer operational durations. The CLR system also delivers a greater hydrogen output than SMR under identical feedstock input conditions. This is a direct reflection of the process chemistry, where CLR avoids the thermodynamic penalties associated with the WGS and solvent-based CO2 removal steps, enabling higher syngas hydrogen content and lower purification loads. Additionally, CLR exhibits near-complete CH4 conversion and minimal CO/CO2 reformation losses.
Although SMR currently benefits from mature infrastructure and broader market integration, its dependency on high-purity water, natural gas infrastructure, and CCS logistics limits its scalability in distributed applications. CLR, in contrast, demonstrates clear viability for modular deployment, particularly in settings where local biogas sources and decentralized hydrogen demand coincide. Its high thermal efficiency, near-complete methane conversion, and simplified system architecture position it as a strong candidate for distributed green hydrogen production. However, it is essential to acknowledge that many of the core performance metrics presented, such as energy consumption per kilogram of hydrogen, syngas composition, and cost implications, are highly sensitive to several process parameters. Factors such as oxygen carrier reactivity and lifespan, hydrogen and CO recovery efficiency during PSA, reactor temperature stability, and internal heat recovery effectiveness can significantly influence overall performance. If, for example, the oxygen carrier exhibits premature deactivation or excessive attrition, the system may require more frequent regeneration cycles or replacement, resulting in increased operational costs and reduced thermal efficiency. While the current modeling assumes stable operating conditions and ideal equipment performance, future work should incorporate a detailed sensitivity analysis and uncertainty quantification to better capture system variability and guide risk-mitigated deployment strategies.
4. Conclusions
This study presents a comprehensive comparison between SMR integrated with CCS and CLR for the production of green hydrogen from biomethane. CLR demonstrates clear advantages in multiple performance dimensions, including a higher hydrogen yield in syngas, reduced residual carbonaceous species, lower energy intensity, and more efficient carbon capture. The cost comparison between SMR and CLR presented in this study is based on process simulations and has not yet been validated through pilot-scale experimental data; therefore, the economic estimates should be interpreted as indicative rather than definitive pending future demonstration results. While this study emphasizes CO2 separation efficiency as a key performance metric, it does not address other environmental impacts such as water usage, solid waste generation, or potential emissions from auxiliary processes, which warrant further investigation in future work.
To accelerate the deployment of CLR, it is strongly recommended that a pilot-scale demonstration plant be developed. Such a facility would enable validation of the process under realistic industrial conditions, providing a crucial bridge between laboratory-scale performance and commercial deployment. It would help address several key scale-up challenges, including oxygen carrier attrition and regeneration behavior over long durations, reactor thermal management, solid–gas contact efficiency, and robust solids handling and circulation systems, particularly under continuous or load-varying operation. Upon amplification to industrial scales, engineering complexities increase significantly. For example, maintaining uniform temperature profiles across ample fuel and air reactors becomes more challenging due to heat transfer limitations and thermal lag, which can potentially impact conversion efficiency and carrier stability. Similarly, oxygen carrier transport between reactors must be finely controlled to prevent carryover losses, agglomeration, or mechanical degradation, which can compromise redox performance and lifecycle economics. Beyond technical issues, large-scale CLR systems must also demonstrate flexibility in integration with local biogas supply chains. This will require modular, adaptable designs that can operate in distributed settings with intermittent resource availability. To ensure competitiveness with SMR and other hydrogen technologies, future studies should also evaluate lifecycle emissions, dynamic process control, and maintenance-related issues, including strategies for oxygen carrier recycling or upgrading.
Author Contributions
Conceptualization, S.M.Y. and N.M.A.; methodology, S.M.Y. and Siti Sorfina; software, S.S.J.; validation, N.M.A.; formal analysis, S.M.Y.; investigation, A.M.; resources, S.M.Y.; data curation, S.S.J.; writing—original draft preparation, S.M.Y.; writing—review and editing, S.M.Y.; visualization, Siti Sorfina; supervision, S.M.Y.; project administration, S.M.Y.; funding acquisition, S.M.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Tenaga Nasional Berhad under Research Grant R-C-TF-0434-23-003-1.
Data Availability Statement
The data presented in this study are openly available in the article.
Acknowledgments
The authors would like to thank Tenaga Nasional Berhad (TNB) for the financial support and provide special thanks to the Advanced Materials Technology and Energy Generation units of TNB Research Sdn Bhd and the Department of Mechanical Engineering, College of Engineering of Universiti Tenaga Nasional (UNITEN) for their full cooperation in this work.
Conflicts of Interest
Author Salmi Mohd Yunus and Siti Sorfina Johari were employed by the company TNB Research Sdn Bhd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CCS | Carbon capture and storage system |
| CH4 | Methane |
| CLR | Chemical looping reforming |
| CO | Carbon monoxide |
| CO2 | Carbon dioxide |
| DEA | Diethanolamine |
| GHG | Greenhouse gas |
| H2 | Hydrogen |
| H2O | Water (vapor) |
| H2S | Hydrogen sulfide |
| HT-WGS | High-temperature water–gas shift |
| LT-WGS | Low-temperature water–gas shift |
| PSA | Pressure swing adsorption |
| SMR | Steam methane reforming |
| WGS | Water–gas shift |
References
- Dally, B. The potential role of hydrogen in decarbonizing heavy industry in Saudi Arabia. In The Clean Hydrogen Economy and Saudi Arabia; Routledge: London, UK, 2024; pp. 584–605. [Google Scholar]
- Guilbert, D.; Gianpaolo, V. Hydrogen as a clean and sustainable energy vector for global transition from fossil-based to zero-carbon. Clean. Technol. 2021, 3, 881–909. [Google Scholar] [CrossRef]
- Sarkar, O.; Modestra, J.A.; Rova, U.; Christakopoulos, P.; Matsakas, L. Waste-derived renewable hydrogen and methane: Towards a potential energy transition solution. Fermentation 2023, 9, 368. [Google Scholar] [CrossRef]
- Sadhukhan, J. Net-zero action recommendations for scope 3 emission mitigation using life cycle assessment. Energies 2022, 15, 5522. [Google Scholar] [CrossRef]
- Durak, H. Comprehensive assessment of thermochemical processes for sustainable waste management and resource recovery. Processes 2023, 11, 2092. [Google Scholar] [CrossRef]
- Altaf, C.T.; Demir, O.; Colak, T.O.; Karagöz, E.; Kurt, M.; Sankir, N.D.; Sankir, M. Decarbonizing the Industry with Green Hydrogen. In Towards Green Hydrogen Generation; Wiley: Hoboken, NJ, USA, 2024; pp. 1–48. [Google Scholar]
- Wang, D.; Joshi, A.; Fan, L.S. Chemical looping technology–a manifestation of a novel fluidization and fluid-particle system for CO2 capture and clean energy conversions. Powder. Technol. 2022, 409, 117814. [Google Scholar] [CrossRef]
- Liu, W.; Wan, Y.; Xiong, Y.; Gao, P. Green hydrogen standard in China: Standard and evaluation of low-carbon hydrogen, clean hydrogen, and renewable hydrogen. Int. J. Hydrogen Energy 2022, 47, 24584–24591. [Google Scholar] [CrossRef]
- Sarker, A.K.; Azad, A.K.; Rasul, M.G.; Doppalapudi, A.T. Prospect of green hydrogen generation from hybrid renewable energy sources: A review. Energies 2023, 16, 1556. [Google Scholar] [CrossRef]
- Batra, G. Renewable energy economics: Achieving harmony between environmental protection and economic goals. Social Sci. Chron. 2023, 2, 1–32. [Google Scholar] [CrossRef]
- Mneimneh, F.; Ghazzawi, H.; Abu Hejjeh, M.; Manganelli, M.; Ramakrishna, S. Roadmap to achieving sustainable development via green hydrogen. Energies 2023, 16, 1368. [Google Scholar] [CrossRef]
- Evro, S.; Oni, B.A.; Tomomewo, O.S. Carbon neutrality and hydrogen energy systems. Int. J. Hydrogen Energy 2024, 78, 1449–1467. [Google Scholar] [CrossRef]
- Abad, A.V.; Dodds, P.E. Green hydrogen characterisation initiatives: Definitions, standards, guarantees of origin, and challenges. Energy Policy 2020, 138, 111300. [Google Scholar] [CrossRef]
- Musa, M.; Hosseini, T.; Lai, T.; Haque, N.; Giddey, S. Life cycle assessment methodology evaluation and greenhouse gas impact of hydrogen production routes in Australia. Front. Energy 2024, 1–18. [Google Scholar] [CrossRef]
- Cheng, W.; Lee, S. How green are the national hydrogen strategies? Sustainability 2022, 14, 1930. [Google Scholar] [CrossRef]
- Pistolesi, C.; Giaconia, A.; Bassano, C.; De Falco, M. Flexible Green Ammonia Production: Impact of Process Design on the Levelized Cost of Ammonia. Fuels 2025, 6, 39. [Google Scholar] [CrossRef]
- Acharya, A. Scaling-up green hydrogen development with effective policy interventions. J. Sustain. Dev. 2022, 15, 135–149. [Google Scholar] [CrossRef]
- Lee, H.; Ahn, J.; Choi, D.G.; Park, S.Y. Analysis of the role of hydrogen energy in achieving carbon neutrality by 2050: A case study of the Republic of Korea. Energy 2024, 304, 132023. [Google Scholar] [CrossRef]
- Quitzow, R.; Nunez, A.; Marian, A. Positioning Germany in an international hydrogen economy: A policy review. Energy Strategy Rev. 2024, 53, 101361. [Google Scholar] [CrossRef]
- Soliman Hunter, T.; Brent, K.; Wawryk, A.; Pettit, J.; Camatta, N. Hydrogen production in Australia from renewable energy: No doubt green and clean, but is it mean? J. Energy Nat. Resour. Law 2024, 42, 593–637. [Google Scholar] [CrossRef]
- Vidas, L.; Castro, R. Recent developments on hydrogen production technologies: State-of-the-art review with a focus on green-electrolysis. Appl. Sci. 2021, 11, 11363. [Google Scholar] [CrossRef]
- Hassan, Q.; Tabar, V.S.; Sameen, A.Z.; Salman, H.M.; Jaszczur, M. A review of green hydrogen production based on solar energy; techniques and methods. Energy Harvest. Syst. 2024, 11, 20220134. [Google Scholar] [CrossRef]
- Quaiyoom, A.; Dev, N.; Singh, B.; Yadav, A.K. Comparative assessment of hydrogen production methods from renewable energy: A review. In Highly Efficient Thermal Renewable Energy Systems; CRC Press: Boca Raton, FL, USA, 2024; pp. 194–210. [Google Scholar]
- Chavan, G.T.; Dubal, D.P.; Cho, E.C.; Patil, D.R.; Gwag, J.S.; Mishra, R.K.; Mishra, Y.K.; An, J.; Yi, J. A Roadmap of Sustainable Hydrogen Production and Storage: Innovations and Challenges. Small 2025, 21, 2411444. [Google Scholar] [CrossRef]
- Li, A.; Qiliang, Z.; Mi, Y.; Ur Rehman, H.; Shoaib, M.; Cao, X.; Wang, N. Triboelectric Nanogenerator Drives Electrochemical Water Splitting for Hydrogen Production: Fundamentals, Progress, and Challenges. Small 2025, 21, 2407043. [Google Scholar] [CrossRef]
- Qureshi, F.; Tahir, M. Photoelectrochemical water splitting with engineering aspects for hydrogen production: Recent advances, strategies and challenges. Int. J. Hydrogen Energy 2024, 69, 760–776. [Google Scholar] [CrossRef]
- Jain, R.; Panwar, N.L.; Jain, S.K.; Gupta, T.; Agarwal, C.; Meena, S.S. Bio-hydrogen production through dark fermentation: An overview. Biomass Convers. Biorefin. 2024, 14, 12699–12724. [Google Scholar] [CrossRef]
- Ahmad, A.; Rambabu, K.; Hasan, S.W.; Show, P.L.; Banat, F. Biohydrogen production through dark fermentation: Recent trends and advances in transition to a circular bioeconomy. Int. J. Hydrogen Energy 2024, 52, 335–357. [Google Scholar] [CrossRef]
- Ansari, S.A.; Alam, M.W.; Dhanda, N.; Abbasi, M.S.; Ahmed, M.E.; Alrashidi, A.B.; Al-Farhan, A.M.; Abebe, B. Sustainable Hydrogen Production, a Review of Methods, Types, Applications, Challenges, and Future Perspectives. Glob. Chall. 2025, 9, 2500086. [Google Scholar] [CrossRef]
- Alabi, O.O.; Gbadeyan, O.J.; Towoju, O.A.; Deenadayalu, N. Enhancing sustainable energy production through biomass gasification gas technology: A review. F1000Research 2024, 13, 511. [Google Scholar] [CrossRef]
- Mignogna, D.; Ceci, P.; Cafaro, C.; Corazzi, G.; Avino, P. Production of biogas and biomethane as renewable energy sources: A review. Appl. Sci. 2023, 13, 10219. [Google Scholar] [CrossRef]
- Saleem, M. Possibility of utilizing agriculture biomass as a renewable and sustainable future energy source. Heliyon 2022, 8, e08905. [Google Scholar] [CrossRef]
- Cabello, A.; Mendiara, T.; Abad, A.; Izquierdo, M.T.; García-Labiano, F. Production of hydrogen by chemical looping reforming of methane and biogas using a reactive and durable Cu-based oxygen carrier. Fuel 2022, 322, 124250. [Google Scholar] [CrossRef]
- Goel, A.; Moghaddam, E.M.; Liu, W.; He, C.; Konttinen, J. Biomass chemical looping gasification for high-quality syngas: A critical review and technological outlooks. Energy Convers. Manag. 2022, 268, 116020. [Google Scholar] [CrossRef]
- Mohd Yunus, S.; Yusup, S.; Johari, S.S.; Mohd Afandi, N.; Manap, A.; Mohamed, H. Comparative Hydrogen Production Routes via Steam Methane Reforming and Chemical Looping Reforming of Natural Gas as Feedstock. Hydrogen 2024, 5, 761–775. [Google Scholar] [CrossRef]
- Antonini, C.; Treyer, K.; Streb, A.; van der Spek, M.; Bauer, C.; Mazzotti, M. Hydrogen production from natural gas and biomethane with carbon capture and storage–A techno-environmental analysis. Sustain. Energy Fuel 2020, 4, 2967–2986. [Google Scholar] [CrossRef]
- Bock, S.; Stoppacher, B.; Malli, K.; Lammer, M.; Hacker, V. Techno-economic analysis of fixed-bed chemical looping for decentralized, fuel-cell-grade hydrogen production coupled with a 3 MWth biogas digester. Energy Convers. Manag. 2021, 250, 114801. [Google Scholar] [CrossRef]
- Argyris, P.A.; Wong, J.; Wright, A.; Pereira, L.M.; Spallina, V. Reducing the cost of low-carbon hydrogen production via emerging chemical looping process. Energy Convers. Manag. 2023, 277, 116581. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).