Use of Hydrogen Energy and Fuel Cells in Marine and Industrial Applications—Current Status
Abstract
1. Introduction
- −
- Technologies for hydrogen production, storage, and transport.
- −
- Hybrid fuel cell technologies used in industrial and marine applications.
2. Technologies Used in Maritime Applications for the Production, Storage, and Maritime Transport of Hydrogen
2.1. Hydrogen Production Technologies
2.1.1. Hydrogen Produced via the Electrolysis of Water
2.1.2. Hydrogen as an Industrial By-Product
2.2. Hydrogen Storage in Marine Applications
2.2.1. Hydrogen Storage Under Pressure
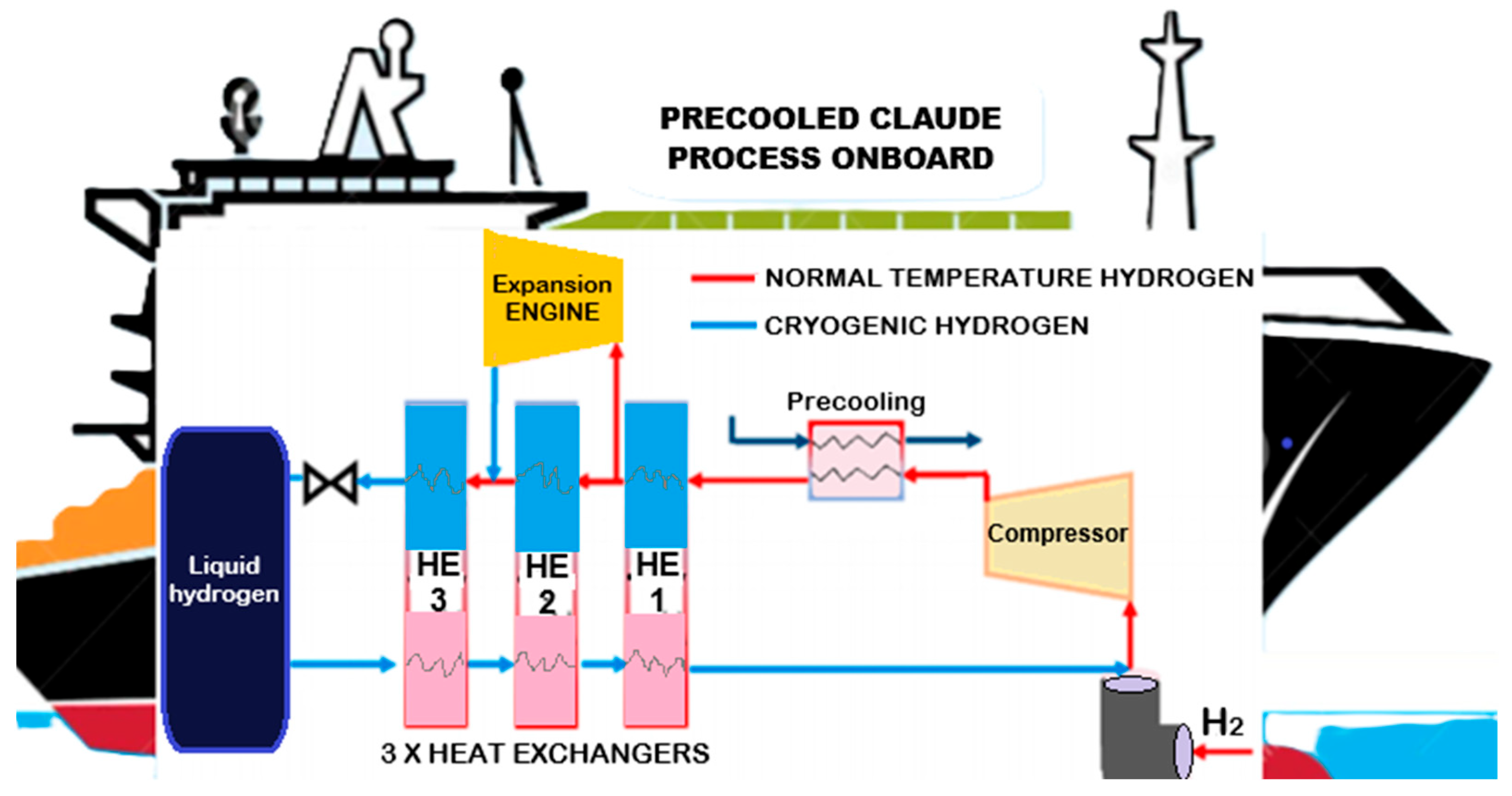
2.2.2. Liquid Hydrogen Storage
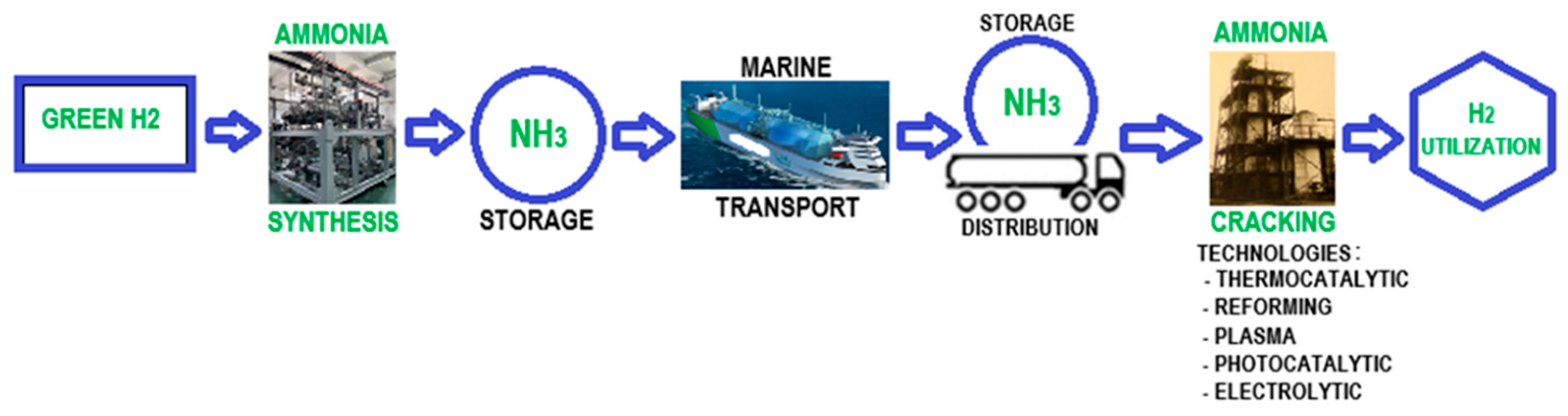
2.2.3. Hydrogen Storage Using Ammonia and Methanol
2.2.4. Solid Hydrogen Storage
2.3. Hydrogen Transportation in Marine Applications
2.3.1. Transport of Hydrogen Gas
2.3.2. Liquid Hydrogen Transportation
- o
- Report no. 1 (March 2023), which addresses the demand and potential of the hydrogen market, hydrogen supply, storage and distribution and hydrogen business models in ports,
- o
- Report no. 2 (September 2023), which in addition to gap analyses and recommendations on priority areas for research and innovation projects, safety-related regulations, codes and standards and non-technical provisions (policy, regulatory, strategic, governance, investment, etc.) addresses the governance of hydrogen-related activities and infrastructure and hydrogen carriers in port areas, hydrogen and hydrogen carriers in ports, in the vicinity of ports and in the broader context of the port area,
- o
- Report no. 3 (December 2023) presenting case studies on hydrogen production, port equipment for hydrogen consumption, maritime transport for hydrogen consumption and hydrogen import.
- −
- Green hydrogen fuels, in the medium and long term, will be the foundation for the decarbonization of the international maritime sector.
- −
- Ethanol and ammonia are the most promising (ammonia is more attractive due to its zero carbon content).
- −
- Hydrogen could be an option for short distances, but for longer distances, its role is quite limited due to the ample space required.
- −
- The production costs of renewable fuels are currently high, but will become competitive in the coming decades.
- −
- The choice of fuel depends largely on fuel price and availability, supply chain, costs of adapting ship and port infrastructure, technological maturity, sustainability issues, net environmental performance and economic viability. The adoption of climate-neutral sources could come sooner with government intervention.
- −
- The energy density of different fuels and the implications for onboard storage are elements that require further analysis (ample storage space means less cargo capacity and lower revenues).
- −
- For domestic shipping, batteries could also be an option [55].
3. Fuel Cell Technologies for Marine Applications
3.1. Fuel Cells for Marine Applications
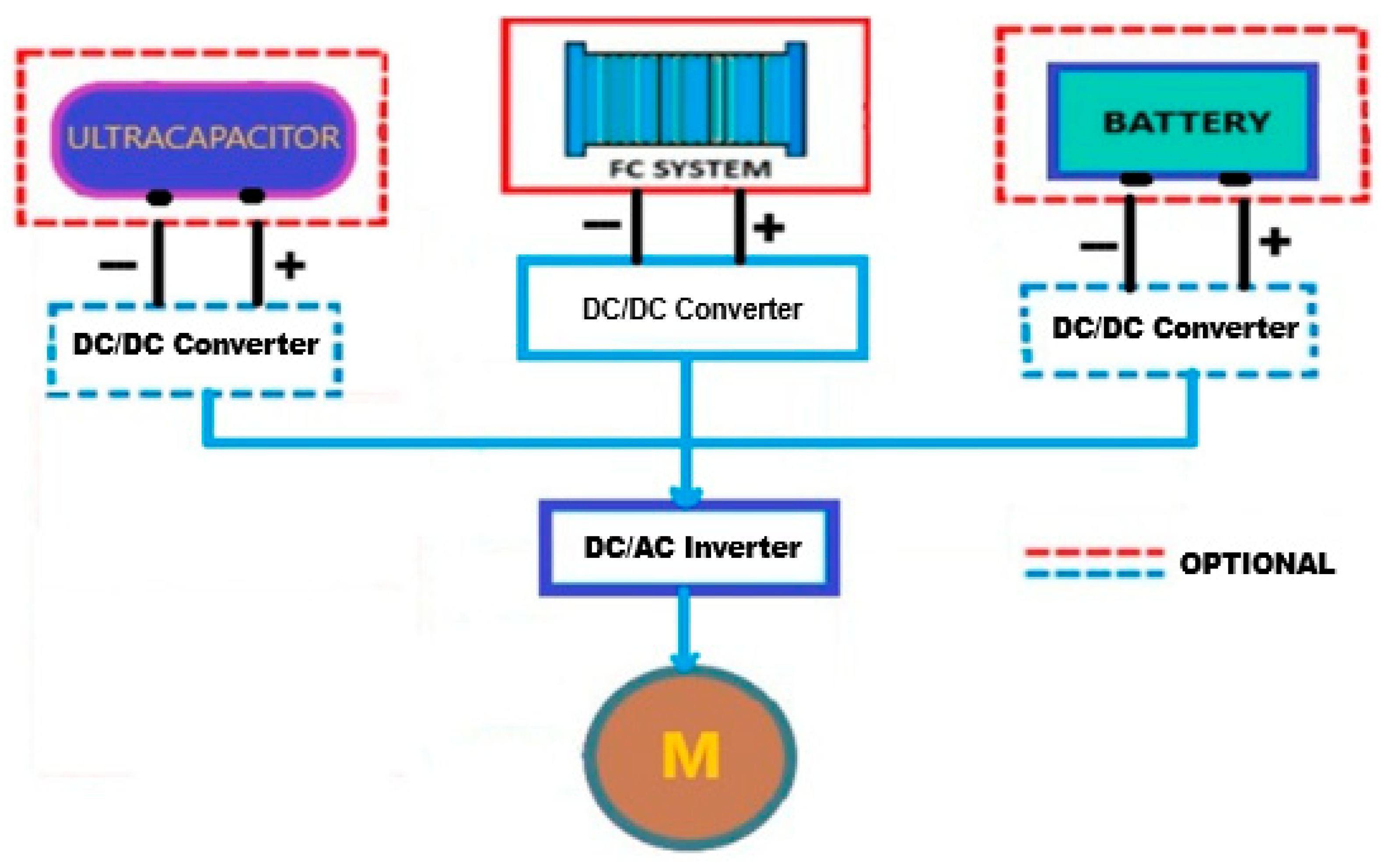
3.2. Hybrid Fuel Cell Power Systems. Energy Storage
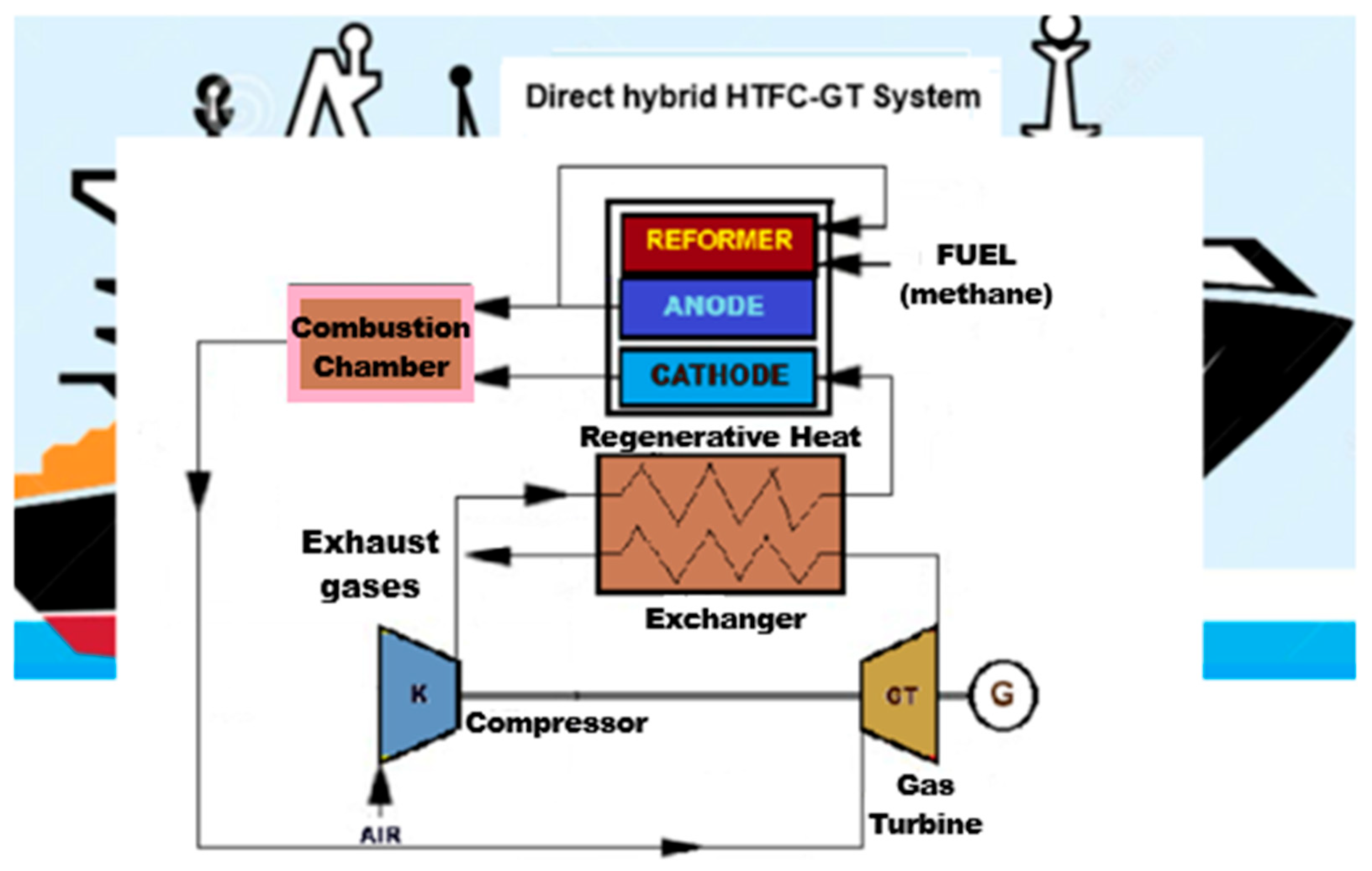
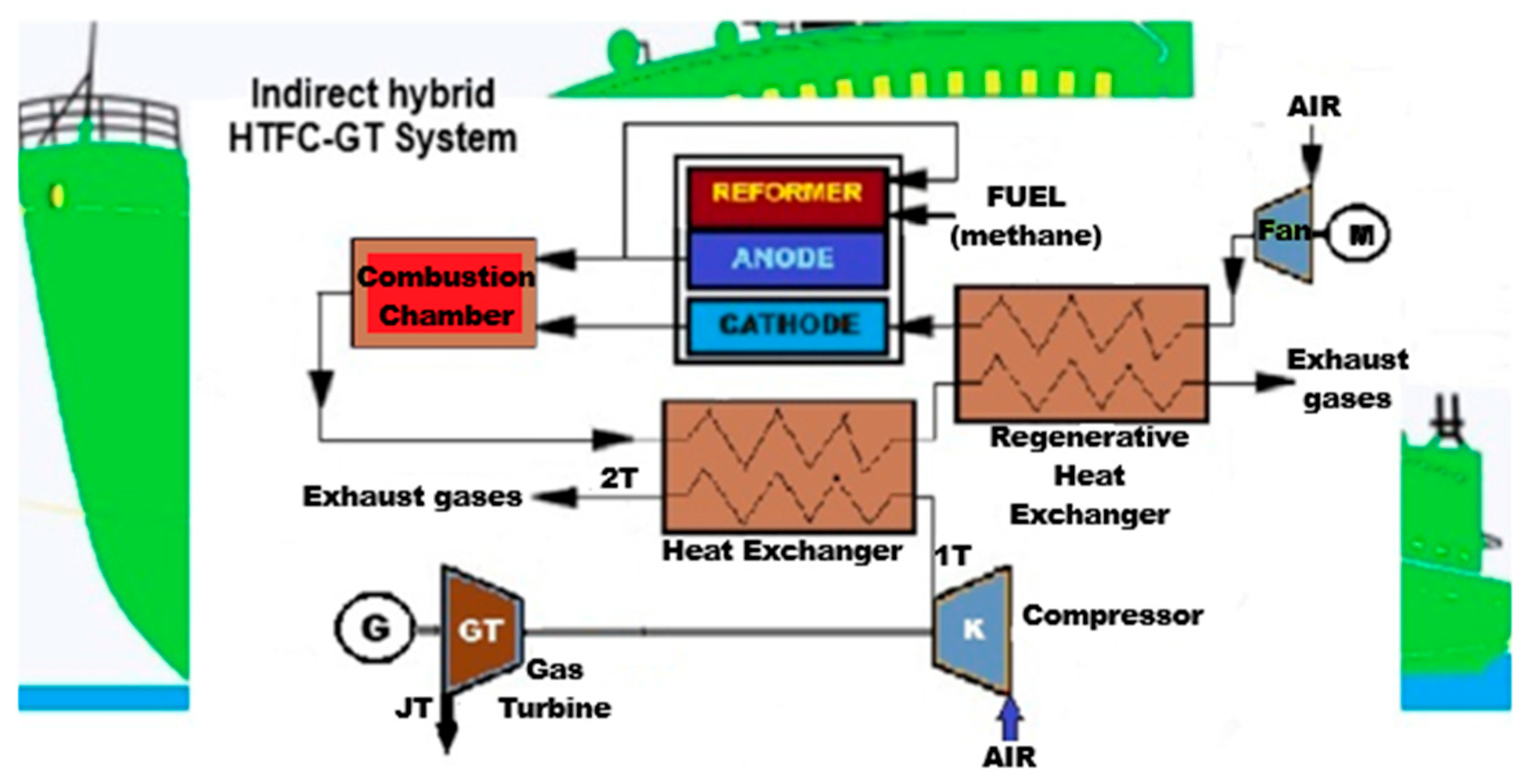
3.3. Hybrid Energy Supply Systems with Fuel Cells and Gas Turbines
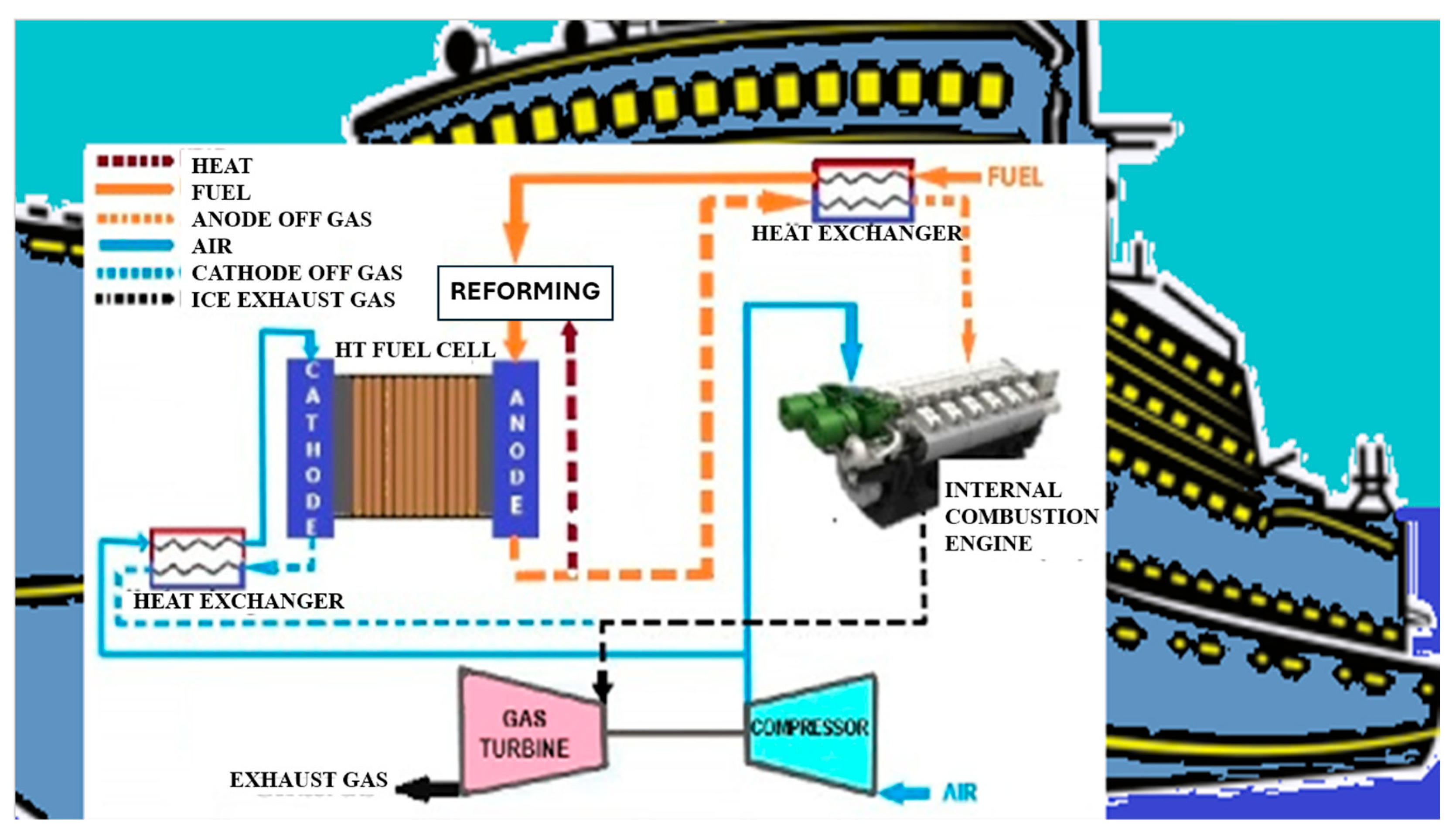
3.4. Hybrid Power Systems with Fuel Cells (FCs) and Internal Combustion Engine (ICE)
4. Challenges of Maritime Safety Regulations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AEM | Alkaline Anion Exchange Membrane |
| ARP | Anode Recycle Percentage |
| ATR | Autothermal Reforming |
| AWE | Alkaline Water Electrolysis |
| CCS | Carbon Capture and Storage |
| CCUS | Carbon Capture, Utilisation, and Storage |
| CFRP | Carbon Fibre-Reinforced Plastic |
| CGH2 | Compressed Gaseous Hydrogen |
| CHE | Cargo Handling Equipment |
| FC | Fuel Cell |
| FCMHPPs | Hybrid Marine Fuel Cell Power Plants |
| GHG | Global Greenhouse Gas |
| GT | Gas Turbine |
| HDV | Heavy-Duty Vehicle |
| HES | Hydrogen Energy Storage |
| HT | High Temperature |
| ICE | Internal Combustion Engine |
| LIB | Lithium-Ion Battery |
| LNG | Liquefied natural gas |
| LOHC | Liquid Organic Hydrogen Carrier |
| LT | Low Temperature |
| MCFC | Molten Carbonate Fuel Cell |
| OGV | Ocean-Going Vessel |
| PEM | Proton Exchange Membrane |
| PEMFC | Proton Exchange Membrane Fuel Cell |
| POX | Partial Oxidation |
| SMR | Steam Methane Reforming |
| SOE | Solid Oxide Water Electrolysis |
| SOFC | Solid Oxide Fuel Cell |
References
- Reducing Barriers to Maritime Fuel Projects Is Key to Decarbonising Shipping. 2024. Available online: https://www.weforum.org/stories/2024/04/why-reducing-barriers-for-maritime-fuel-projects-is-key-to-progressing-on-decarbonization (accessed on 15 June 2025).
- Echim, S.; Budea, S. Applications of hydrogen energy in the field of transport. Int. Multidiscip. Sci. GeoConference SGEM 2024, 24, 39–46. [Google Scholar] [CrossRef]
- International Maritime Organisation (IMO). IMO Strategy on Reduction of GHG Emissions from Ships. 2023. Available online: https://www.imo.org/en/ourwork/environment/pages/2023-imo-strategy-on-reduction-of-ghg-emissions-from-ships.aspx (accessed on 15 June 2025).
- Fourth IMO Greenhouse Gas Study. International Maritime Organisation 2020. Published in 2021 by the IMO, London SE1 7SR. Available online: www.imo.org (accessed on 13 December 2024).
- Li, J.; Xu, H.; Zhou, K.; Li, J.-Q. A review on the research progress and application of compressed hydrogen in the marine hydrogen fuel cell power system. Helion 2024, 10, e25304. [Google Scholar] [CrossRef] [PubMed]
- DNV Hydrogen Forecast to 2050, Energy Transition Outlook 2022. Available online: https://aben.com.br/wpcontent/uploads/2022/06/DNV_Hydrogen_Report_2022_Highres_single1.pdf (accessed on 15 May 2025).
- Decarbonising Maritime: Overcoming Challenges with Innovation and Ingenuity, DNV Maritime Impact. 2024. Available online: https://www.dnv.com/expert-story/maritime-impact/decarbonizing-maritime-overcoming-challenges-with-innovation-and-ingenuity/ (accessed on 15 June 2025).
- Hora, C.; Dan, F.C.; Rancov, N.; Badea, G.E.; Secui, C. Main Trends and Research Directions in Hydrogen Generation Using Low Temperature Electrolysis: A Systematic Literature Review. Energies 2022, 15, 6076. [Google Scholar] [CrossRef]
- Boretti, A.; Banik, B.K. Advances in Hydrogen Production from Natural Gas Reforming. Adv. Energy Sustain. Res. 2021, 2, 2100097. [Google Scholar] [CrossRef]
- Ozcan, H.; El-Emam, R.S.; Horri, B.A. Thermochemical looping technologies for clean hydrogen production –Current status and recent advances. J. Clean. Prod. 2023, 362, 135295. [Google Scholar] [CrossRef]
- Badawi, E.Y.; Elkharsa, R.A.; Abdelfattah, E.A. Value proposition of bio-hydrogen production from different biomass sources. Energy Nexus 2023, 10, 100194. [Google Scholar] [CrossRef]
- Jiao, H.; Tsigkou, K.; Elsamahy, T.; Pispas, K.; Sun, J.; Manthos, G.; Schagerl, M.; Sventzouri, E.; Al-Tohamy, R.; Kornaros, M.; et al. Recent advances in sustainable hydrogen production from microalgae: Mechanisms, challenges, and future perspectives. Ecotoxicol. Environ. Saf. 2024, 270, 115908. [Google Scholar] [CrossRef] [PubMed]
- Shanmugaratnam, S.; Ravirajan, P.; Shivatharsiny, Y.; Velauthapillai, D. Green hydrogen production through photocatalytic seawater splitting on MS2/TiO2 nanocomposites over simulated solar irradiation. Int. J. Hydrogen Energy 2024, 91, 673–682. [Google Scholar] [CrossRef]
- Oh, V.B.Y.; Ng, S.F.; Ong, W.J. Is photocatalytic hydrogen production sustainable? Assessing the potential environmental enhancement of photocatalytic technology against steam methane reforming and electrocatalysis. J. Clean Prod. 2022, 379, 134673. [Google Scholar] [CrossRef]
- Franco, A.; Giovannini, C. Recent and Future Advances in Water Electrolysis for Green Hydrogen Generation: Critical Analysis and Perspectives. Sustainability 2023, 15, 16917. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Alnazha, A.A.; Ahmed, U.; El-Adawy, M.; Habib, M.A. Review on techno-economics of hydrogen production using current and emerging processes: Status and perspectives. Result Eng. 2024, 21, 101890. [Google Scholar] [CrossRef]
- IEA. Electrolysers. 2023. Available online: https://www.iea.org/energy-system/low-emission-fuels/electrolysers#overview (accessed on 10 July 2025).
- Chi, J.; Yu, H.M. Water electrolysis based on renewable energy for hydrogen production. Chin. J. Catal. 2018, 39, 390–394. [Google Scholar] [CrossRef]
- Erdemir, D.; Dincer, I. A new solar energy-based system integrated with hydrogen storage and heat recovery for sustainable community. Sustain. Energy Technol Assess 2022, 52, 102355. [Google Scholar] [CrossRef]
- About AEM Electrolysis. 2025. Available online: https://www.enapter.com/aem-electrolysis/ (accessed on 18 June 2025).
- Horizon’s New AEM Electrolyser Technology Brings the World Closer to $1/kg Green Hydrogen. Available online: https://www.einnews.com/pr_news/691547793/horizon-s-new-aem-electrolyser-technology-brings-the-world-closer-to-1-kg-green-hydrogen (accessed on 18 June 2025).
- Hygreen Energy Launches New AEM Electrolyser Systems. Available online: https://www.hygreenenergy.com/hygreen-energy-launches-new-aem-electrolyzer-systems/2024 (accessed on 18 June 2025).
- Dere, C.; Inal, O.B.; Zincir, B. Utilisation of waste heat for on-board hydrogen production in ships. Int. J. Hydrogen Energy 2024, 75, 271–283. [Google Scholar] [CrossRef]
- Fu, Z.; Lu, L.; Zhang, C.; Xu, Q.; Zhang, X.; Gao, Z.; Li, J. Fuel cell and hydrogen in maritime application: A review on aspects of technology, cost and regulations. Sustain. Energy Technol. Assess. 2023, 57, 103181. [Google Scholar] [CrossRef]
- Marefati, M.; Mehrpooya, M. Introducing a Hybrid Photovoltaic Solar, Proton Exchange Membrane Fuel Cell and Thermoelectric Device System. Sustain. Energy Technol Assess 2019, 36, 100550. [Google Scholar] [CrossRef]
- International Energy Agency. Global Hydrogen Review 2023. Available online: https://www.iea.org/reports/global-hydrogen-review-2023 (accessed on 15 May 2025).
- Alavi-Borazjani, A.S.; Adeel, S.; Chkoniya, V. Hydrogen as a Sustainable Fuel: Transforming Maritime Logistics. Energies 2024, 18, 1231. [Google Scholar] [CrossRef]
- Scarpati, G.; Frasci, E.; Ilio, G.D.; Jannelli, E. A comprehensive review on metal hydrides-based hydrogen storage systems for mobile applications. J. Energy Storage 2024, 102, 113934. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Laffineur, L.; Campe, R.; Perreault, P.; Verbruggen, S.W.; Lenaerts, S. Challenges in the use of hydrogen for maritime applications. Energy Environ. Sci. 2021, 14, 815–843. [Google Scholar] [CrossRef]
- Samoilov, V.; Sultanova, M.; Borisov, R.; Lavrent’EV, V.; Ramazanov, D.; Egazar’YAnts, S.; Maximov, A. The production of a liquid organic hydrogen carrier (LOHC) and jet fuel fractions by hydroprocessing of pyrolysis fuel oil (PFO). Int. J. Hydrogen Energy 2024, 49, 1386–1400. [Google Scholar] [CrossRef]
- Makaryan, I.A.; Sedov, I.V.; Maksimov, A.L. Hydrogen Storage Using Liquid Organic Carriers. Russ. J. Appl. Chem. 2020, 93, 1815–1830. [Google Scholar] [CrossRef]
- Feki, I.; Shirinbayan, M.; Nouira, S.; Bi, R.T.; Maeso, J.-B.; Thomas, C.; Fitoussi, J. Composites in high-pressure hydrogen storage: A review of multiscale characterisation and mechanical behaviour. J. Pre-Proof 2024, 16, 100555. [Google Scholar] [CrossRef]
- Durbin, D.J.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on-board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Hafner, T.; Macher, J.; Brandstätter, S.; Trattner, A. Advancing hydrogen storage: Development and verification of a high-pressure permeation test setup for polymeric barrier materials. Int. J. Hydrogen Energy 2024, 96, 882–891. [Google Scholar] [CrossRef]
- Xing, H.; Stuart, C.; Spence, S.; Chen, H. Fuel Cell Power Systems for Maritime Applications: Progress and Perspectives. Sustainability 2021, 13, 1213. [Google Scholar] [CrossRef]
- Aziz, M. Liquid Hydrogen: A Review on Liquefaction, Storage, Transportation, and Safety. Energies 2021, 14, 5917. [Google Scholar] [CrossRef]
- Xu, X.; Xu, H.; Zheng, J.; Chen, L.; Wang, J. A high-efficiency liquid hydrogen storage system cooled by a fuel-cell-driven refrigerator for hydrogen combustion heat recovery. Energy Convers. Manag. 2020, 226, 113496. [Google Scholar] [CrossRef]
- Makepeace, J.W.; He, T.; Weidenthaler, C.; Jensen, T.R.; Chang, F.; Vegge, T.; Ngene, P.; Kojima, Y.; de Jongh, P.E.; Chen, P.; et al. Reversible ammonia-based and liquid organic hydrogen carriers for high-density hydrogen storage: Recent progress. Int. J. Hydrogen Energy 2019, 44, 7746–7767. [Google Scholar] [CrossRef]
- Spatolisano, E.; Pellegrini, L.A.; de Angelis, A.R.; Cattaneo, S.; Roccaro, E. Ammonia as a Carbon-Free Energy Carrier: NH3 Cracking to H2. Ind. Eng. Chem. Res. 2023, 62, 10813–10827. [Google Scholar] [CrossRef]
- Iulianelli, A.; Ribeirinha, P.; Mendes, A.; Basile, A. Methanol steam reforming for hydrogen generation via conventional and membrane reactors: A review. Renew Sustain. Energy Rev. 2014, 29, 355–368. [Google Scholar] [CrossRef]
- Svanberg, M.; Ellis, J.; Lundgren, J.; Landalv, I. Renewable methanol as a fuel for the shipping industry. Ren. Sust. Energy Rev. 2018, 94, 1217–1228. [Google Scholar] [CrossRef]
- Sánchez, A.; Blanco, E.C.; Martín, M. Comparative assessment of methanol and ammonia: Green fuels vs. hydrogen carriers in fuel cell power generation. Appl. Energy 2024, 374, 124009. [Google Scholar] [CrossRef]
- Nivedhitha, K.S.; Beena, T.; Banapurmath, N.R.; Umarfarooq, M.A.; Ramasamy, V.; Elahi, M.; Soudagar, M.; Agbulut, Ü. Advances in hydrogen storage with metal hydrides: Mechanisms, materials, and challenges. Int. J. Hydrogen Energy 2024, 61, 1259–1273. [Google Scholar] [CrossRef]
- Jayabal, R. Hydrogen energy storage in maritime operations: A pathway to decarbonization and sustainability. Int. J. Hydrogen Energy 2025, 109, 1133–1144. [Google Scholar] [CrossRef]
- Benet, Á.; Villalba-Herreros, A.; D’aMore-Domenech, R.; Leo, T.J. Knowledge gaps in fuel cell-based maritime hybrid power plants and alternative fuels. J. Power Sources 2022, 548, 232066. [Google Scholar] [CrossRef]
- Lipiäinen, S.; Sillman, J.; Vakkilainen, E.; Soukka, R.; Tuomaala, M. Hydrogen transport options for a large industrial user: Analysis on costs, efficiency, and GHG emissions in steel mills. Sustain. Prod. Consum. 2024, 44, 1–13. [Google Scholar] [CrossRef]
- Hunt, J.D.; Zakeri, B.; Nascimento, A.; Vasconcelos de Freitas, M.A.; Amorim Fdo, C.; Guo, F.; Witkamp, G.-J.; van Ruijven, B.; Wada, Y. Hydrogen balloon transportation: A cheap and efficient mode to transport hydrogen. Int. J. Hydrogen Energy 2023, 53, 875–884. [Google Scholar] [CrossRef]
- Hora, C.; Dan, F.C.; Secui, D.-C.; Hora, H.N. Systematic Literature Review on Pipeline Transport Losses of Hydrogen, Methane, and Their Mixture, Hythane. Energies 2024, 17, 4709. [Google Scholar] [CrossRef]
- Wang, Z. An Overview of the Key Technology of Renewable Energy Multi-Energy Complementary Hydrogen-Storage-Transport. Appl. Comput. Eng. 2025, 128, 53–58. [Google Scholar] [CrossRef]
- Kim, K.; Roh, G.; Kwag, K.; Hwang, P.-I. Economic study of hybrid power system using boil-off hydrogen for liquid hydrogen carriers. Int. J. Hydrogen Energy 2024, 61, 1107–1119. [Google Scholar] [CrossRef]
- Kawasaki Heavy Industries—2024 Report—On the Front Lines of Value Creation. Available online: https://global.kawasaki.com/en/corp/sustainability/report/2024/pdf/24_houkokusyo.pdf (accessed on 1 May 2025).
- Lanni, D.; Di Cicco, G.; Minutillo, M.; Cigolotti, V.; Perna, A. Techno-economic assessment of a green liquid hydrogen supply chain for ship refuelling. Int. J. Hydrogen Energy 2025, 97, 104–116. [Google Scholar] [CrossRef]
- Le, T.-H.; Tran, N.; Lee, H.-J. Development of Liquid Organic Hydrogen Carriers for Hydrogen Storage and Transport. Int. J. Mol. Sci. 2024, 25, 1359. [Google Scholar] [CrossRef] [PubMed]
- Semchukova, V.; Topolski, K.; Abdin, Z. Hydrogen technology for maritime applications: A review of challenges, opportunities, and lessons from the port authority of New York and New Jersey. Renew. Sustain. Energy Rev. 2025, 216, 115641. [Google Scholar] [CrossRef]
- Clean Hydrogen Partnership. Study on Hydrogen in Ports and Industrial Coastal Areas—Reports. 2023. Available online: https://www.clean-hydrogen.europa.eu/media/publications/study-hydrogen-ports-and-industrial-coastal-areas-reports_en (accessed on 1 May 2025).
- Elammas, T. Hydrogen fuel cells for marine applications: Challenges and opportunities. Int. J. Res. Adv. Eng. Technol. 2023, 9, 38–43. Available online: www.allengineeringjournal.in (accessed on 1 March 2025).
- Xing, L.; Xuan, J.; Das, P.K. Fuel Cell Fundamentals. In Fuel Cells for Transportation: Fundamental Principles and Applications; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Tronstad, T.; Åstrand, H.H.; Haugom, G.P.; Langfeldt, L. Study on the Use of Fuel Cells in Shipping. EMSA 2017. Available online: https://www.emsa.europa.eu/publications/item/2921-emsa-study-on-the-use-of-fuel-cells-in-shipping.html (accessed on 1 January 2025).
- FCW. Technologies Molten Carbonate Fuel Cell. Available online: https://fuelcellsworks.com/knowledge/technologies/mcfc (accessed on 13 September 2024).
- Baldi, F.; Moret, S.; Tammi, K.; Marechal, F. The role of solid oxide fuel cells in future ship energy systems. Energy 2020, 194, 116811. [Google Scholar] [CrossRef]
- Rubri-Hydrogen Fuel Cell Ship. 2024. Available online: https://www.hfsinopower.com/hydrogen-fuel-cell-ship-1?_gl=1*965899*_up*MQ..*_gs*MQ..&gclid=CjwKCAjwx8nCBhAwEiwA_z__02Gq7rGEvmqQqBcdRuX4LvRJoXlI2TJgdCzLoc8gW1qDf20K5deg5RoCNx0QAvD_BwE&gbraid=0AAAAA9l8QxnbRYsxJlYdUwBlBLVpnsnTg (accessed on 15 June 2025).
- Van Sickle, E.; Ralli, P.; Pratt, J.W.; Klebanoff, L.E. MV Sea Change: The first commercial 100% hydrogen fuel cell passenger ferry in the world. Int. J. Hydrogen Energy 2025, 105, 389–404. [Google Scholar] [CrossRef]
- Wang, Z.; Dong, B.; Wang, Y.; Li, M.; Liu, H.; Han, F. Analysis and evaluation of fuel cell technologies for sustainable ship power: Energy efficiency and environmental impact. Energy Convers. Manag. 2024, 21, 100482. [Google Scholar] [CrossRef]
- Fuel Cell Power Systems for Marine and Offshore Applications © 2023; American Bureau of Shipping: Spring, TX, USA, 2023.
- Broer, A.; Polinder, H.; van Biert, L. Polymer electrolyte membrane fuel cell degradation in ships—Review of degradation mechanisms and research gaps. J. Power Sources 2025, 640, 236678. [Google Scholar] [CrossRef]
- Sorlei, I.-S.; Bizon, N.; Thounthong, P.; Varlam, M.; Carcadea, E.; Culcer, M.; Iliescu, M.; Raceanu, M. Fuel Cell Electric Vehicles—A Brief Review of Current Topologies and Energy Management Strategies. Energies 2021, 14, 252. [Google Scholar] [CrossRef]
- RiverCell3, R&D Project Gets Underway. Available online: https://www.now-gmbh.de/en/news/pressreleases/rivercell3-rd-project-gets-underway/2024 (accessed on 21 March 2025).
- Kwaśniewski, T.; Piwowarski, M. Design analysis of hybrid Gas Turbine-Fuel Cell power plant in stationary and marine applications. Pol. Marit. Res. 2020, 27, 107–119. [Google Scholar] [CrossRef]
- Loreti, G.; Facci, A.L.; Ubertini, S. High-Efficiency Combined Heat and Power through a High-Temperature Polymer Electrolyte Membrane Fuel Cell and Gas Turbine Hybrid System. Sustainability 2021, 13, 12515. [Google Scholar] [CrossRef]
- Chuahy, F.D.F.; Kokjohn, S.L. Solid oxide fuel cell and advanced combustion engine combined cycle: A pathway to 70% electrical efficiency. Appl. Energy 2019, 235, 391–408. [Google Scholar] [CrossRef]
- Sapra, H.; Stam, J.; Reurings, J.; van Biert, L.; van Sluijs, W.; de Vos, P.; Visser, K.; Vellayani, A.P.; Hopman, H. Integration of solid oxide fuel cell and internal combustion engine for maritime applications. Appl. Energy 2021, 281, 115854. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Liu, H.; Guo, F.; Li, C.; Xiu, X.; Wang, C.; Qin, J.; Wei, L. Exergetic and exergoeconomic evaluation of an SOFC-Engine-ORC hybrid power generation system with methanol for ship application. Fuel 2024, 357, 129944. [Google Scholar] [CrossRef]
- Zhou, Z.; Tao, J. Hydrogen-powered vessels in green maritime decarbonisation: Policy drivers, technological frontiers and challenges. Front. Mar. Sci. 2025, 12, 1601617. [Google Scholar] [CrossRef]
- ISO/TR 15916:2015; Basic Considerations for the Safety of Hydrogen Systems. International Organization for Standardization: Geneva, Switzerland, 2015. Available online: www.iso.org/standard/56546.html (accessed on 19 June 2025).
- ISO 13984:1999; Liquid Hydrogen—Land Vehicle Fuelling System Interface. International Organization for Standardization: Geneva, Switzerland, 1999. Available online: www.iso.org/standard/23570.html (accessed on 19 June 2025).
- ISO 13985:2006; Liquid Hydrogen—Land Vehicle Fuel Tanks. International Organization for Standardization: Geneva, Switzerland, 2006. Available online: www.iso.org/standard/39892.html (accessed on 19 June 2025).
- DNV White Paper Guides Shipowners on Safe and Scalable Adoption of Ammonia and Hydrogen Fuels. 2025. Available online: https://www.dnv.com/news/dnv-white-paper-guides-shipowners-on-scalable-adoption-of-ammonia-and-hydrogen-fuels/ (accessed on 19 June 2025).
- Georgopoulou, C.; Di Maria, C.; Di Ilio, G.; Cigolotti, V.; Minutillo, M.; Rossi, M.; Sullivan, B.P.; Bionda, A.; Rautanen, M.; Ponzini, R.; et al. On the identification of regulatory gaps for hydrogen as maritime fuel. Sustain. Energy Technol. Assess. 2025, 75, 104224. [Google Scholar] [CrossRef]
- Zhaka, V.; Samuelsson, B. Hydrogen as fuel in the maritime sector: From production to propulsion. Energy Rep. 2024, 12, 5249–5267. [Google Scholar] [CrossRef]
- Brouzas, S.; Zadeh, M.; Lagemann, B. Essentials of hydrogen storage and power systems for green shipping. Int. J. Hydrogen Energy 2025, 100, 1543–1560. [Google Scholar] [CrossRef]
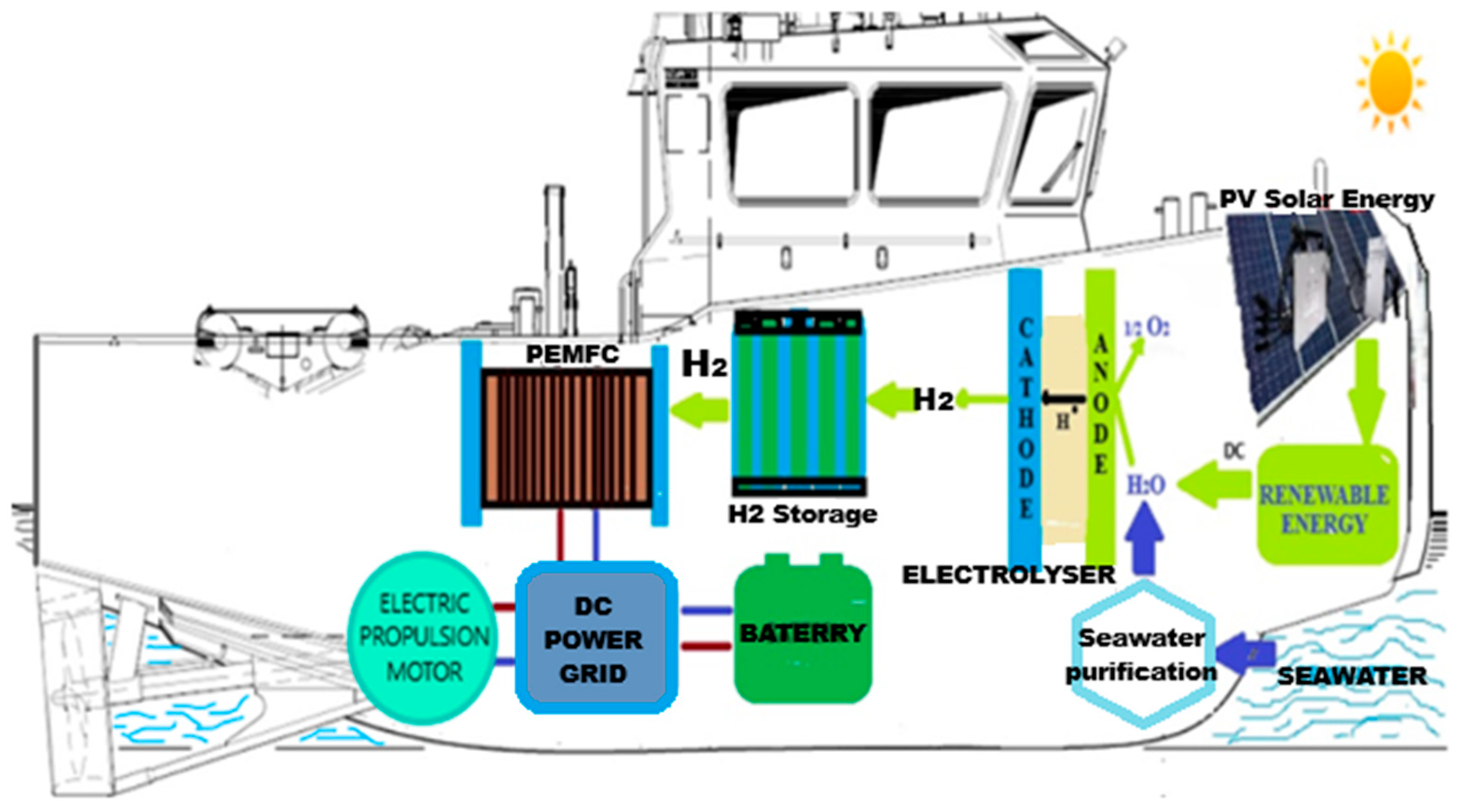
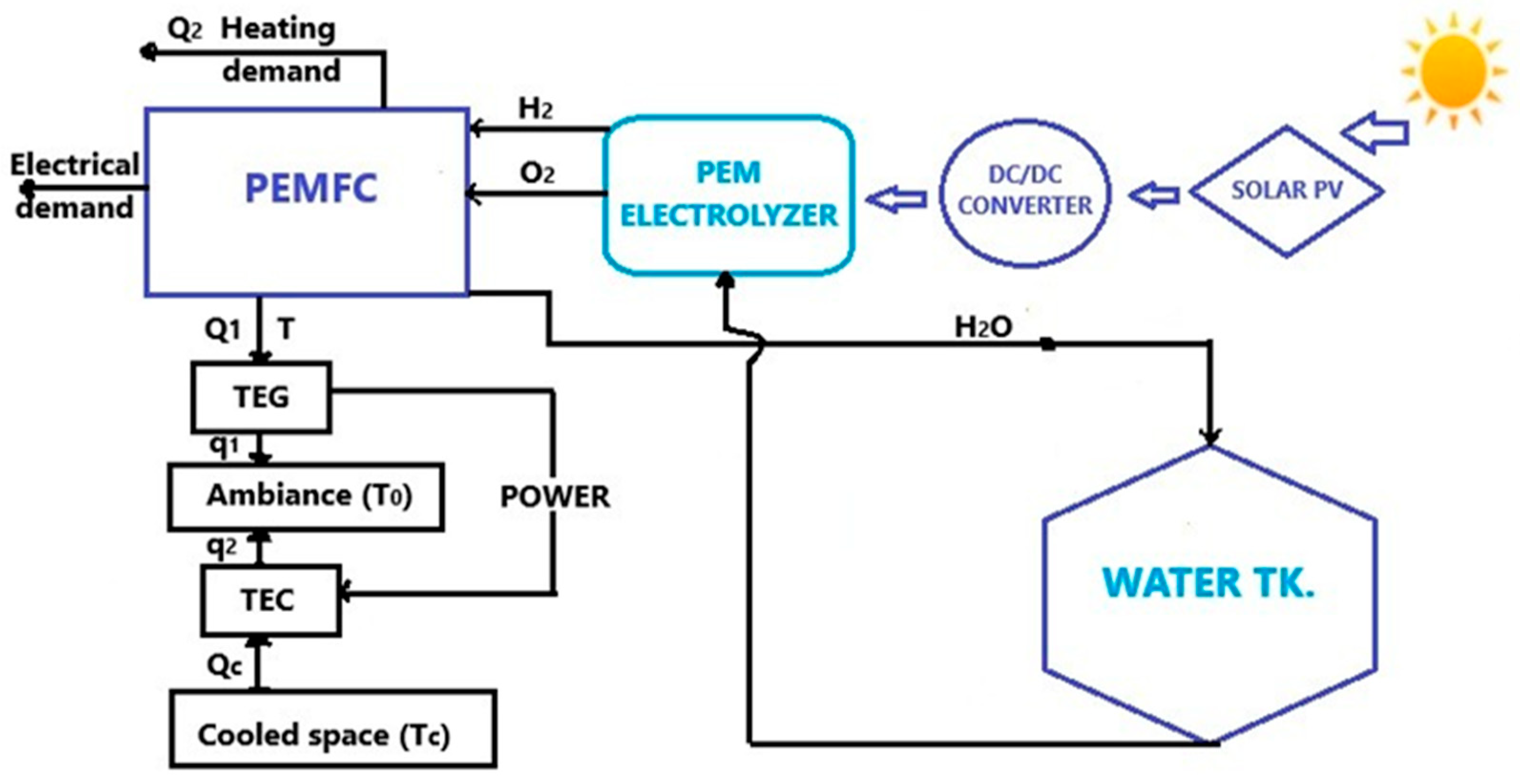
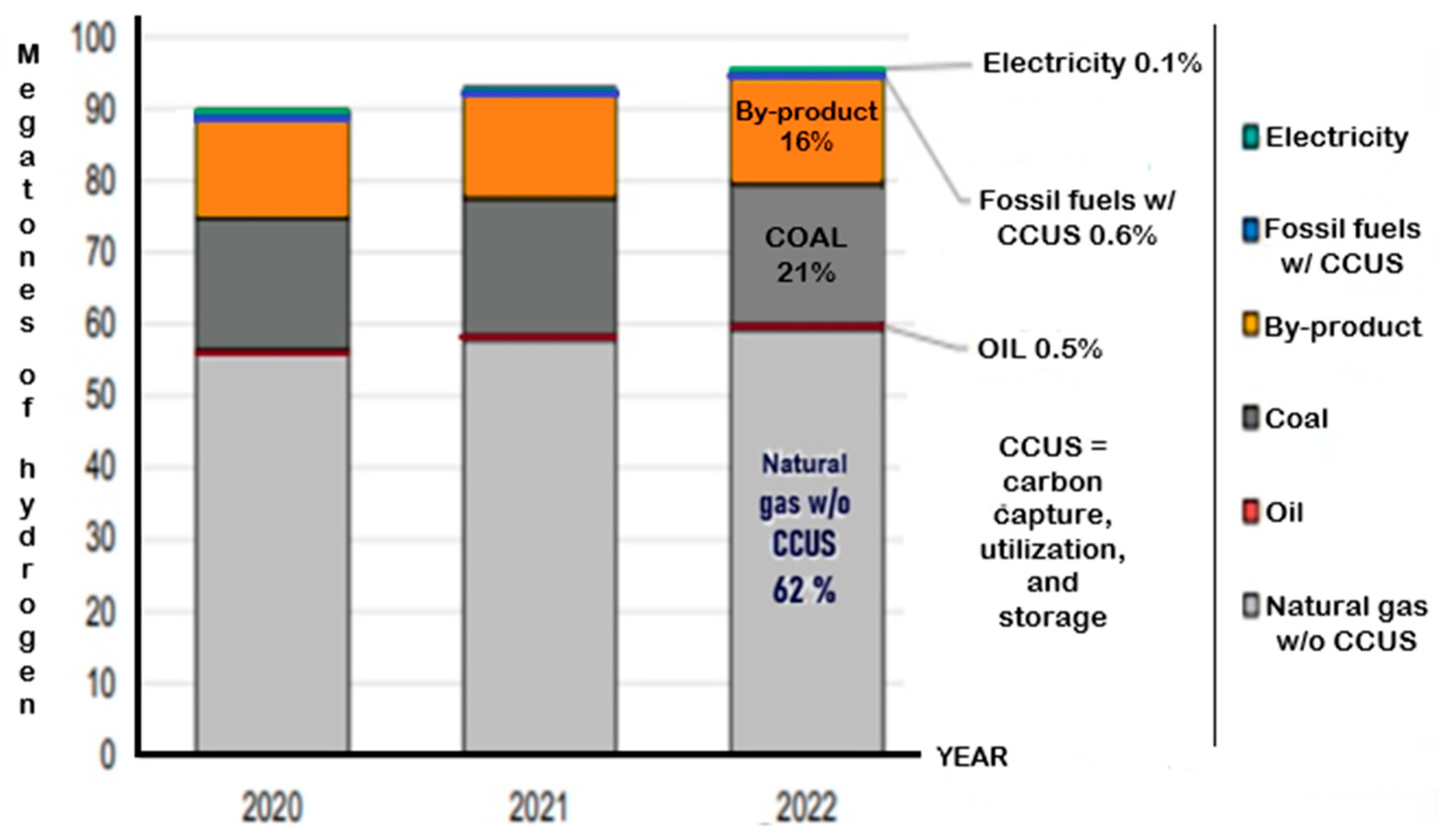
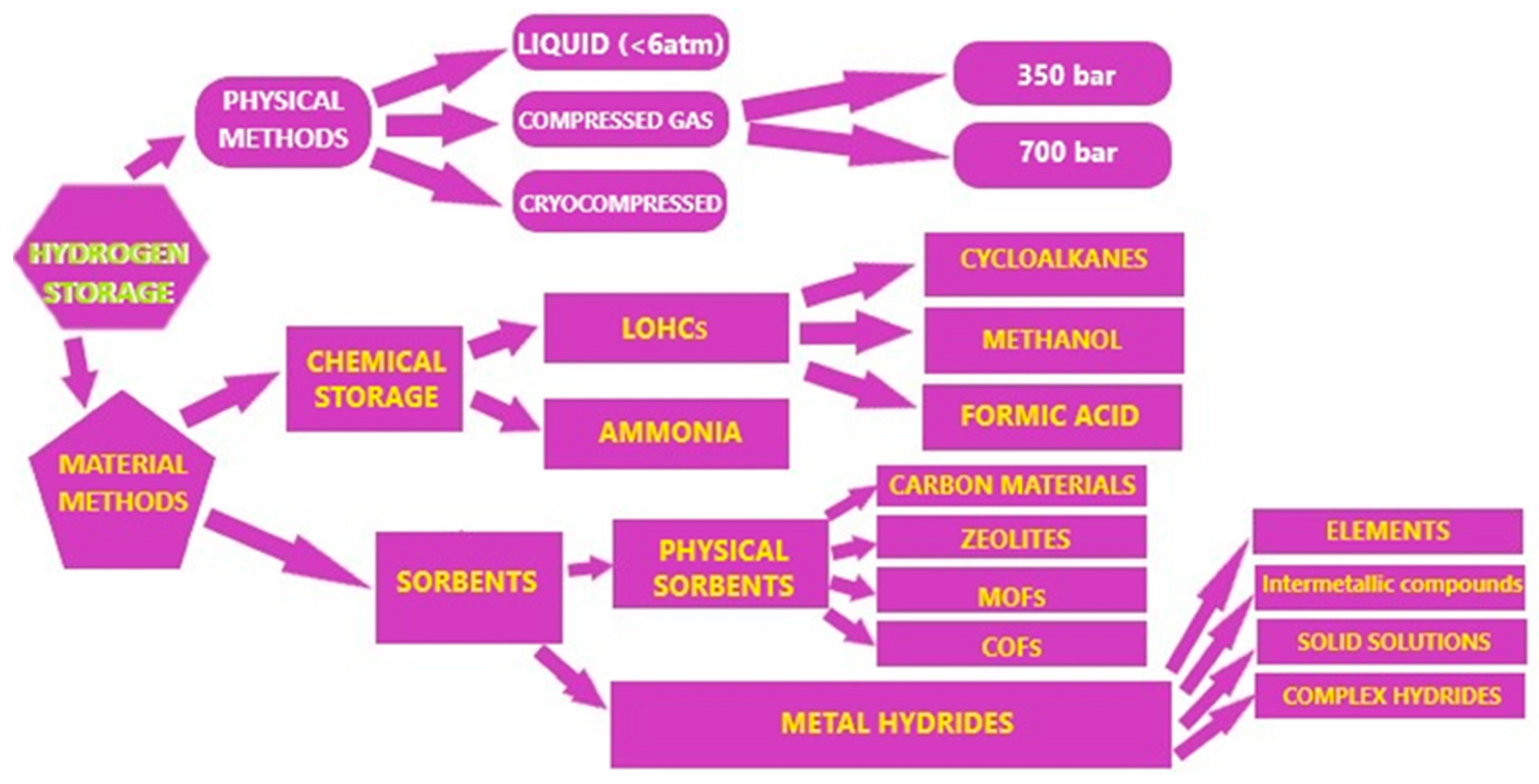
| Source | Method | Process | Efficiency (%) | Cost ($/kg) |
|---|---|---|---|---|
| Fossil fuel use | Hydrocarbon reforming | Steam reforming | 74–85 | 2.27 |
| Partial oxidation | 60–75 | 1.48 | ||
| Autothermal reforming | 60–75 | 1.48 | ||
| Use of renewable sources | Biological | Bio-photolysis of biomass | 10–11 | 2.13 |
| Anaerobic fermentation of biomass | 60–80 | 2.57 | ||
| Photofermentation of biomass | 0.1 | 2.83 | ||
| Thermochemistry | Biomass gasification | 30–40 | 2.05 | |
| Biomass pyrolysis | 35–50 | 1.7 | ||
| Water decomposition | Water thermolysis | 20–45 | 8.4 | |
| Water photolysis | 0.06 | 10 | ||
| Proton exchange membrane (PEM) water electrolysis | 65–80 | 4.96–8.12 | ||
| Alkaline electrolyte (AE) water electrolysis | 70–80 | 4.8 | ||
| Solid oxide electrolyte (SOE) water electrolysis | 90 | 3.63 | ||
| Technology | Strengths | Constraints | H2 Yield (g/kg Feedstock) | ProductionCost ($/kg H2) | TRL |
|---|---|---|---|---|---|
| Gasification | Reusing industrial leftovers and forest waste efficiently converts a lot of plant material without needing expensive oxygen for steam gasification. | CO2 release, tar and char formation deactivate catalysts, and H2 is varied due to diverse biomass, high temperatures, catalyst regeneration needed, and costly reactors. | 40–190 | 1.7–2.2 | 9 |
| Steam Methane Reforming | Current industrial design does not need costly oxygen sources. | CO2 release, high temperatures, and catalyst regeneration are required. | 40–130 | 0.8 | 9 |
| Electrolysis | Electrolysis yields pure hydrogen from water using electricity, typically from renewables, ensuring a clean energy output. Its scalability allows diverse applications, while adaptability to intermittent renewables supports grid stability. Moreover, on-site production minimises transportation needs, promising for decentralised energy solutions. AWE and PEM technologies are commercially available. SOEC and AEM technologies are maturing. | Electrolysis demands substantial energy input, potentially impacting its overall carbon footprint, especially when renewable electricity is not readily available. High initial equipment costs, less-than-optimal efficiencies, and maintenance requirements may hinder cost-effectiveness. Scaling up electrolysis for large-scale hydrogen production might pose engineering and logistical challenges, affecting its widespread adoption. | - | 3.5–10 | 7–9 |
| Photo Fermentation | Recycling organic and biological waste thoroughly, with almost complete conversion of materials, at low temperature and pressure levels. | Limited production and slow rate of H2, demanding a large surface area, requiring bacterial control, high energy needed for enzymes, and inefficient solar energy conversion. | 9–49 | 3.5 | 4 |
| Dark Fermentation | Recycling organic and biological waste, utilising fast-growing algal biomass, operating at low temperatures and pressures, and accommodating appropriate carbon sources. | Limited production and slow rate of H2, high generation of by-products, requiring pre-treatment. | 4–44 | 2.3 | 5 |
| Pyrolysis | Current industrial setup recycles forest residue and industrial waste, converting biomass into gas, bio-oil, and biochar in a versatile and straightforward process. | CO2 release, tar and char formation, H2 variability from complex biomass, catalyst regeneration needed, and a costly reactor. | 25–65 | 2.1–3.1 | 7 |
| Technology | Economic Obstacles | Technical Obstacles | Way Forward |
|---|---|---|---|
| Gasification | Due to the elevated temperature required, a large investment and operating cost are required. | Corrosion, plugging, and catalyst deactivation. Inadequate commercialisation and product standardisation | Membrane reactors need to integrate H2 production methods to increase the efficiency. |
| Steam Methane Reforming | Expenses incurred during the process of the Catalyst companies | - | The lower consumption cost and the catalyst’s lifetime offset the higher unit catalyst cost. |
| Electrolysis | The charge of electricity accounts for up to 40–57% of the levelized cost of hydrogen. | Combining the energy system and business operations is a significant barrier to large-scale technology deployment. | To achieve minimal CO2 emissions, consider the electricity source’s carbon footprint. Various geographic areas and clever operation tactics can also cut costs. |
| Photo Fermentation | Increased yield at a high energy cost | - | Metabolic engineering has the potential to compensate for the breakthrough in the biohydrogen process. The effects of nutrient limitation and substrate utilisation were studied to identify the chromosomal genes in microalgae responsible for increased hydrogen production. Photobioreactor development requires optimal design. |
| Dark Fermentation | The cost of the substrate is the primary factor influencing the cost of biohydrogen. | Proper bioreactor development, construction, operation, and regulation | The combination of dark and photo fermentation reduces feedback inhibition. |
| Production Method | Hydrogen Type | Key Implications |
|---|---|---|
| Gasification of brown coal (lignite) or black coal (bituminous) | Brown and Black Hydrogen | Carbon-intensive processes with high CO2 emissions. |
| Steam methane reforming (SMR) | Grey Hydrogen | High CO2 emissions; widely used but environmentally harmful. |
| Natural gas reforming with Carbon Capture and Storage (CCS) | Blue Hydrogen | Reduces CO2 emissions but still has pre-chain emissions; a transitional solution. |
| Methane pyrolysis | Turquoise Hydrogen | Low-carbon, produces solid carbon; significant CO2 reduction compared to grey hydrogen. |
| Water electrolysis by renewable energy | Green Hydrogen | Minimal CO2 emissions, sustainable; key solution for decarbonization. |
| Solar-powered electrolysis | Yellow Hydrogen | Relies exclusively on sunlight; minimal emissions. |
| Nuclear-powered electrolysis | Pink Hydrogen | Low-carbon alternative without weather dependency; with nuclear waste concerns. |
| Oil sands and water–gas shift reaction | Aqua Hydrogen | Emission-free, avoids CO2 release; low-cost. |
| Biological processes (dark and photo-fermentation) | Biohydrogen | Low emissions; renewable when feedstock is sustainable. |
| Materials | Hydrogen Storage Capacity |
|---|---|
| MgH2 | 7.7 wt% |
| MgV | 4.4 wt% |
| MgCo | 3.9 wt% |
| TiCr1.2V0.8 | 6.7 wt% |
| MgNb2O5 | 6.9 wt% |
| MgCr2O3 | 5.9 wt% |
| MgFe3O4 | 2.5 wt% |
| MgPd | 3.0 wt% |
| 0.65MgH2/0.35ScH2 | 4.2 wt% |
| Mg5Ni3La | 5.50 wt% |
| Mg10Ni3La | 5.16 wt% |
| Mg15Ni3La | 4.60 wt% |
| Mg20Ni3La | 4.51 wt% |
| Ag/TiO2/CNT | 10.94 wt% |
| MgF2+SrH2 | 5.30 wt% |
| (MgF2+SrH2)/Gr | 6.1 wt% |
| FeCoNi/GS | 6.24 wt% |
| Zr0.6Y0.4Fe2 | 1.77 wt% |
| Mg0.55Ti0.20Si0.25 | 234 mAhg−1 |
| Mg45Zr5Co5 | 425 mAhg−1 |
| Mg50CO50 | 372 mAhg−1 |
| Mg45Pd5CO50 | 379 mAhg−1 |
| Mg2Ni | 450 mAhg−1 |
| Mg67Ni27Nb4 | 273 mAhg−1 |
| Mg0.8Al0.2Ni | 350 mAhg−1 |
| Mg67Ni27Nb1Al5 | 339 mAhg−1 |
| Mg1.75Nb0.25Ni | 600 mAhg−1 |
| Storage Technology | Compressed Gas Storage | Cryogenic Liquid Storage | Solid-State Storage | Adsorption-Based Storage | Ammonia-Based Storage |
|---|---|---|---|---|---|
| Volumetric Capacity (g/L) | 10–15 g/L | ~8 MJ/L | 100–130 g/L | 20–50 g/L | 50–60 g/L |
| Gravimetric Capacity (wt%) | 1–2% | 2–3% | 1–1.5% | 0.5–1.5% | 10–12% |
| Pressure/Temperature Requirements | Up to 700 bar | −253 °C | 0.1–5 MPa, ambient temperature | Low pressure, cryogenic temperatures | Ambient pressure, mild temperatures |
| Energy Density | Low (~10 MJ/m3) | High | Medium | Medium | Medium |
| Safety Features | High-pressure vessel needed | Advanced cryogenic insulation | Chemically stable materials | Special adsorbent materials | Requires controlled ammonia handling |
| Approximate Cost | $500–$1000/kg H2 | $1500–$3000/kg H2 | $2000–$5000/kg H2 | $1000–$2000/kg H2 | $700–$1500/ kg H2 |
| Application Suitability | Short-distance transport | Long-distance transport | Compact storage for vehicles | Portable energy systems | Energy carriers for transport and storage |
| Type of FC | Fuels | Electrode | Temperature (°C) | Life Span (h) | Power Density (W/cm2) | Specific Power (W/kg) | Fuel Economy ($/nm) | Possibilities Applications |
|---|---|---|---|---|---|---|---|---|
| AFC | H2 | Pt/Ag Pt/Ni | 60–200 | 10,000 | 0.5–1.0 | 35–105 | 3.04 | Employ potassium hydroxide as the electrolyte to facilitate the conversion of hydrogen and oxygen into electrical energy and water, historically used in space applications |
| LT-PEMFC | H2 | Pt/C Pt/C | 50–100 | 6000 | 1.0–2.0 | 300–1000 | 3.71 | Utilising hydrogen and oxygen to generate electrical energy and water |
| PAFC | H2, LNG, MeOH | Pt/C Pt/C | 140–200 | 10,000 | 0.1–0.5 | 100–220 | 3.30 | Employ phosphoric acid electrolyte to convert hydrogen and oxygen into electrical energy and water, commonly found in stationary power generation setups |
| DMFC | MeOH | Pt/C Pt-Ru/C | 75–120 | 6000 | 0.5–1.0 | 10–30 | 6.35 | Efficiently convert methanol fuel and oxygen into electricity and water without requiring hydrogen gas, commonly used in portable electronic devices |
| MCFC | H2, MeOH, Hydroxide | Li/NiO Ni/Cr | 650–700 | 15,000 | 0.2–0.4 | 30–40 | 5.03 | Utilise molten carbonate electrolyte to transform hydrogen and carbon dioxide into electrical energy and water, suited for high-temperature applications such as large-scale power generation |
| SOFC | H2, MeOH, Oxicarbide | Sr/LaMnO3 Ni/YSZ | 500–1000 | 20,000 | 0.3–1.0 | 15–20 | 4.37 | Utilise a solid oxide electrolyte, functioning at temperatures above 600 ◦C to convert hydrogen and oxygen into electricity and water, noted for their efficiency and versatility |
| LNGFC | LNG | Pt/C Pt | 160–200 | 10,000 | 0.3–06 | 15–20 | 3.97 | Harness liquefied natural gas as a fuel source, converting LNG and oxygen into electricity and water, with potential applications in clean transportation and power generation |
| HT-PEMFC | H2 | Pt/C Pt/C | 150–200 | 6000 | 0.9–1.8 | 200–1000 | 4.10 | Employ proton exchange membrane and operate between 150 ◦C and 200 ◦C, effectively converting hydrogen and oxidant into electrical energy and water, offering specific advantages |
| BFC | Biomass | Pt/Pd PB | 80–1000 | 15,000 | 0.5–1.0 | 10–30 | 4.66 | Utilise biomass-derived organic materials as fuel, converting biomass and oxidant into electricity and water, holding potential in renewable energy systems |
| Application | Hydrogen Fuel Cells | Hybrid Energy Systems | Port Operations | Auxiliary Power Units | Cargo Handling Equipment | Hydrogen-Powered Tugboats |
|---|---|---|---|---|---|---|
| Efficiency (%) | Up to 60% | 50–80% | N/A | 40–70% | N/A | Up to 55% |
| Emissions Reduction (%) | 90% (GHGs) | 35% (efficiency gain) | Up to 70% | 30–50% | Up to 70% | 85% |
| Key Challenges | Fuel supply and storage | System complexity | Limited infrastructure | Integration with existing systems | High initial investment | Limited refuelling facilities |
| Example | MF hydra ferry, Norway | Offshore wind platforms | Hydrogen-powered cranes | Hydrogen backup power for cruise ships | Hydrogen forklifts in ports | Hydrogen-powered harbour tugboats |
| Operational Feasibility | High for short-haul vessels | Moderate, requires renewable integration | High but infrastructure-dependent | Moderate, dependent on ship type | High for large ports | Moderate for pilot-scale projects |
| Hazards Onboard | Safety Measures |
|---|---|
| Potential hydrogen leaks in the Fuel Cell Room | Designate Fuel Cell Room as a hazardous area. Install hydrogen detectors in areas prone to hydrogen leaks to trigger emergency shutdown systems. Implement air locks for access to the Fuel Cell Room. Implement continuous ventilation systems to prevent hydrogen accumulation in closed spaces. Ensure independent air ventilation for the Fuel Cell Room and fuel cells within it. Equip with hydrogen detectors for leak detection. Implement emergency shutdown (ESD) systems for automatic shutdown hydrogen supply. Validate hazardous area modifications through gas dispersion analyses. Adhere to the International Code of Safety for Ships (IGF Code) requirements. Incorporate additional structural fire protection fire detection and alarm capabilities. |
| Collision risks due to hydrogen storage tanks | Locate hydrogen storage tanks at a safe distance from vessel sides as per IGF Code requirements. Consider special requirements for cryogenic LH2 storage. |
| Lack of specific and comprehensive rules governing hydrogen | Meticulously design hazardous areas to avoid impacting critical vessel areas. Monitor regulatory updates and adapt installation practices and safety measures accordingly. |
| Installation of various electrical equipment on ships | Adhere to strict safety protocols for electrical equipment installations in hazardous zones. Ensure continuous validation of safety measures. Monitor regulatory updates and adapt safety measures accordingly. |
| Risk of spontaneous ignition from hydrogen release from the high-pressure manifold (CH2) | Install protective steel plates between the hydrogen manifold and composite tanks. |
| Potential over-pressurisation of hydrogen tanks (CH2) | Utilise pressure relief devices to prevent over-pressurisation in case of fire. |
| Risks associated with bunkering operations | Implement safety measures during bunkering operations, including pressure testing of the bunkering system. Follow best practices and guidelines for the safe transfer of hydrogen fuel during bunkering operations. |
| Standard Name | Standard Code | Issued by |
|---|---|---|
| International Code of Safety for Ship Using Gases or Other Low-flashpointFuels | IGF Code | International Maritime Organisation |
| Hydrogen-fuelled ships | BV NR678 | Bureau Verittas |
| ABS Requirements for Liquefied Hydrogen Carriers | - | American Bureau of Shipping |
| ABS Requirements for Hydrogen Fuelled Vessels | - | American Bureau of Shipping |
| RINA Rules of Safety For Ships Using Hydrogen as a Fuel | - | RINA |
| LR Classification of Ships using Gases or other Low-flashpoint Fuels | - | Lloyd’s Register |
| Basic Considerations for the Safety of Hydrogen Systems | ISO/TR 15916 | International Standardisation Organisation |
| Fuel cell road vehicles, Safety specifications, Protection against hydrogenhazards for vehicles fuelled with compressed hydrogen | ISO 23273 | International Standardisation Organisation |
| Road vehicles, Compressed gaseous hydrogen (CGH2) and hydrogen/naturalgas blends fuel system components | ISO 12619 | International Standardisation Organisation |
| Gaseous Hydrogen, Fuelling Station | ISO 19880 | International Standardisation Organisation |
| Hydrogen fuel quality | ISO 14687 | International Standardisation Organisation |
| Hydrogen detection apparatus | ISO 26142 | International Standardisation Organisation |
| Fuel Cell Technologies | ISO IEC/TC 105 | International Standardisation Organisation |
| Safety Standard For Hydrogen And Hydrogen Systems Guidelines for HydrogenSystem Design, Materials, Selection, Operations, Storage and Transportation | NASA | |
| Standard for Cryogenic Hydrogen Storage | CGA H-3 | Compressed Gas Association |
| Hydrogen Pipeline Systems | CGA G-5.6 | Compressed Gas Association/European IndustrialGases Association |
| Hydrogen Vent Systems | CGA G-5.5 | Compressed Gas Association |
| Hydrogen Piping and Pipelines | ASME B31.12 | American Society of Mechanical Engineers |
| Gaseous Hydrogen–Fuelling Stations—Valves | CSA HGV4.4 | CSA Group |
| Fuelling System Guideline | CSA HGV4.9 | CSA Group |
| Fuel cell technologies | BS EN 62282 | British Standards/European Standards |
| Proton Exchange Membrane Fuel Cell | SAC GB/T 20042 | Standardisation Administration of China |
| Proton exchange membrane fuel cell module for road vehicles | GB/T 33978 | China Fuel Cell Standards |
| Stationary fuel cell power systems | JIS C 62282 | Japanese Industrial Standards |
| Compressed Gases and Cryogenic Fluids Code | NFPA 55 | National Fire Protection Association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Echim, S.-M.; Budea, S. Use of Hydrogen Energy and Fuel Cells in Marine and Industrial Applications—Current Status. Hydrogen 2025, 6, 50. https://doi.org/10.3390/hydrogen6030050
Echim S-M, Budea S. Use of Hydrogen Energy and Fuel Cells in Marine and Industrial Applications—Current Status. Hydrogen. 2025; 6(3):50. https://doi.org/10.3390/hydrogen6030050
Chicago/Turabian StyleEchim, Sorin-Marcel, and Sanda Budea. 2025. "Use of Hydrogen Energy and Fuel Cells in Marine and Industrial Applications—Current Status" Hydrogen 6, no. 3: 50. https://doi.org/10.3390/hydrogen6030050
APA StyleEchim, S.-M., & Budea, S. (2025). Use of Hydrogen Energy and Fuel Cells in Marine and Industrial Applications—Current Status. Hydrogen, 6(3), 50. https://doi.org/10.3390/hydrogen6030050







