Effect of Partial Noble Metal (M = Pd, Rh, Ru, Pt) Substitution in La1−xSrxCo1−yMyO3 Perovskite-Derived Catalysts for Dry Reforming of Methane
Abstract
1. Introduction
2. Methodology
2.1. Catalyst Synthesis
2.2. Catalyst Characterization
2.3. DRM Activity Measurements
2.4. Transient Studies (CH4/CO2-TPSR/O2-TPO)-
3. Results and Discussion
3.1. Catalyst Characterization
3.2. Catalytic Activity for Dry Reforming Reaction
3.3. CH4/CO2 TPSR and O2-TPO
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Abbreviation | Full Form |
| DRM | Dry reforming of methane |
| TPSR | Temperature-programmed surface reaction |
| TPO | Temperature-programmed oxidation |
| TGA | Thermogravimetric analysis |
| H2-TPR | Hydrogen temperature-programmed reduction |
| XRD | X-ray diffraction |
| BET | Braeuer–Emmett–Teller (Surface Area Measurement) |
| GC | Gas chromatography |
| FTS | Fischer–Tropsch synthesis |
| RWGS | Reverse water–gas shift |
| ODH | Oxalyldihydrazide |
| GHSV | Gas hourly space velocity |
| RT | Room temperature |
References
- Royer, S.; Duprez, D. Catalytic Oxidation of Carbon Monoxide over Transition Metal Oxides. ChemCatChem 2011, 3, 24–65. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Goula, G.; Hatzisymeon, M.; Betsi-Argyropoulou, I.; Botzolaki, G.; Kousi, K.; Kondarides, D.I.; Taylor, M.J.; Parlett, C.M.A.; Osatiashtiani, A.; et al. Effect of support oxygen storage capacity on the catalytic performance of Rh nanoparticles for CO2 reforming of methane. Appl. Catal. B Environ. 2019, 243, 490–501. [Google Scholar] [CrossRef]
- Hegde, M.S.; Madras, G.; Patil, K.C. Noble Metal Ionic Catalysts. Acc. Chem. Res. 2009, 42, 704–712. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, T.; Upadhyay, A.; Sinha, A.K.; Deb, S.K.; Devi, R.N. Effect of Pt incorporation in LaBO3 (B=Mn, Fe, Co) perovskites on water gas shift activity. J. Mol. Catal. A Chem. 2014, 395, 506–513. [Google Scholar] [CrossRef]
- Villoria, J.-A.; Mota, N.; Al-Sayari, S.A.; Alvarez-Galvan, M.-C. Perovskites as Catalysts in the Reforming of Hydrocarbons: A Review. Micro Nanosyst. 2012, 4, 231–252. [Google Scholar] [CrossRef]
- Ziaei-Azad, H.; Khodadadi, A.; Esmaeilnejad-Ahranjani, P.; Mortazavi, Y. Effects of Pd on Enhancement of Oxidation Activity of LaBO3 (B = Mn, Fe, Co and Ni) Pervoskite Catalysts for Pollution Abatement from Natural Gas Fueled Vehicles. Appl. Catal. B: Environ. 2011, 102, 62. [Google Scholar] [CrossRef]
- Juhász, K.; Lévay, K.; Hegedűs, L.; Balogh-Weiser, D.; Pirault-Roy, L.; Hell, Z. Application of supported lanthanum catalysts in the hydrogenation of nitriles. React. Kinet. Mech. Catal. 2021, 133, 687–698. [Google Scholar] [CrossRef]
- Kantam, M.L.; Kishore, R.; Yadav, J.; Sudhakar, M.; Venugopal, A. Chemoselective Hydrogenation of the Olefinic Bonds Using a Palladium/Magnesium-Lanthanum Mixed Oxide Catalyst. Adv. Synth. Catal. 2012, 354, 663–669. [Google Scholar] [CrossRef]
- Royer, S.; Duprez, D.; Kaliaguine, S. Oxygen mobility in LaCoO3 perovskites. Catal. Today 2006, 112, 99–102. [Google Scholar] [CrossRef]
- Royer, S.; Van Neste, A.; Davidson, R.; McIntyre, S.; Kaliaguine, S. Methane Oxidation over Nanocrystalline LaCo1-XFeXO3: Resistance to SO2 Poisoning. ACS Catal. 2004, 43, 5670–5680. [Google Scholar] [CrossRef]
- Sarshar, Z.; Kleitz, F.; Kaliaguine, S. Novel oxygen carriers for chemical looping combustion: La1−xCexBO3 (B = Co, Mn) perovskites synthesized by reactive grinding and nanocasting. Energy Environ. Sci. 2011, 4, 4258–4269. [Google Scholar] [CrossRef]
- Keav, S.; Matam, S.K.; Ferri, D.; Weidenkaff, A. Structured Perovskite-Based Catalysts and Their Application as Three-Way Catalytic Converters—A Review. Catalysts 2014, 4, 226–255. [Google Scholar] [CrossRef]
- Lim, H.S.; Lee, M.; Kim, Y.; Kang, D.; Lee, J.W. Low-temperature CO2 hydrogenation to CO on Ni-incorporated LaCoO3 perovskite catalysts. Int. J. Hydrogen Energy 2021, 46, 15497–15506. [Google Scholar] [CrossRef]
- Sakpal, T.; Lefferts, L. Structure-dependent activity of CeO2 supported Ru catalysts for CO2 methanation. J. Catal. 2018, 367, 171–180. [Google Scholar] [CrossRef]
- García-Moncada, N.; Navarro, J.C.; Odriozola, J.A.; Lefferts, L.; Faria, J.A. Enhanced catalytic activity and stability of nanoshaped Ni/CeO2 for CO2 methanation in micro-monoliths. Catal. Today 2021, 383, 205–215. [Google Scholar] [CrossRef]
- Aneggi, E.; Llorca, J.; Boaro, M.; Trovarelli, A. Surface-structure sensitivity of CO oxidation over polycrystalline ceria powders. J. Catal. 2005, 234, 88–95. [Google Scholar] [CrossRef]
- Jin, T.; Okuhara, T.; Mains, G.J.; White, J.M. Temperature-programmed desorption of carbon monoxide and carbon dioxide from platinum/ceria: An important role for lattice oxygen in carbon monoxide oxidation. J. Phys. Chem. 1987, 91, 3310–3315. [Google Scholar] [CrossRef]
- Granger, P.; Parvulescu, V.I. Catalytic NOx Abatement Systems for Mobile Sources: From Three-Way to Lean Burn after-Treatment Technologies. Chem. Rev. 2011, 111, 3155–3207. [Google Scholar] [CrossRef]
- Lambrou, P.S.; Efstathiou, A.M. The effects of Fe on the oxygen storage and release properties of model Pd–Rh/CeO2–Al2O3 three-way catalyst. J. Catal. 2006, 240, 182–193. [Google Scholar] [CrossRef]
- Tejuca, L.G.; Fierro, J.L.G.; Tascón, J.M.D. Advance in Catalysis. In Structure and Reactivity of Perovskite-Type Oxides; Elsevier: Amsterdam, The Netherlands, 1989; Volume 36, pp. 237–328. [Google Scholar]
- Bisht, A.; Zhang, P.; Shivakumara, C.; Sharma, S. Pt-Doped and Pt-Supported La1–xSrxCoO3: Comparative Activity of Pt4+and Pt0Toward the CO Poisoning Effect in Formic Acid and Methanol Electro-oxidation. J. Phys. Chem. C 2015, 119, 14126–14134. [Google Scholar] [CrossRef]
- Forni, L.; Oliva, C.; Vatti, F.P.; Kandala, M.A.; Ezerets, A.M.; Vishiakov, A.V. La-Ce-Co perovskites as catalysts for exhaust gas depollution. Appl. Catal. A Gen. 1996, 7, 269–284. [Google Scholar] [CrossRef]
- Arandiyan, H.; Dai, H.; Deng, J.; Liu, Y.; Bai, B.; Wang, Y.; Li, X.; Xie, S.; Li, J. Three-dimensionally ordered macroporous La0.6Sr0.4MnO3 with high surface areas: Active catalysts for the combustion of methane. J. Catal. 2013, 307, 327–339. [Google Scholar] [CrossRef]
- Deng, J.; Zhang, L.; Dai, H.; He, H.; Au, C.T. Hydrothermally fabricated single-crystalline strontium-substituted lanthanum manganite microcubes for the catalytic combustion of toluene. J. Mol. Catal. A Chem. 2009, 299, 60–67. [Google Scholar] [CrossRef]
- Huang, K.; Chu, X.; Yuan, L.; Feng, W.; Wu, X.; Wang, X.; Feng, S. Engineering the surface of perovskite La0.5Sr0.5MnO3 for catalytic activity of CO oxidation. Chem. Commun. 2014, 50, 9200–9203. [Google Scholar] [CrossRef]
- Arai, H.; Yamada, T.; Eguchi, K.; Seiyama, T. Catalytic combustion of methane over various perovskite-type oxides. Appl. Catal. 1986, 26, 265–276. [Google Scholar] [CrossRef]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A Review on Bimetallic Nickel-Based Catalysts for CO2 Reforming of Methane. ChemPhysChem 2017, 18, 3117. [Google Scholar] [CrossRef]
- Das, S.; Ashok, J.; Bian, Z.; Dewangan, N.; Wai, M.H.; Du, Y.; Borgna, A.; Hidajat, K.; Kawi, S. Silica–Ceria Sandwiched Ni Core–shell Catalyst for Low Temperature Dry Reforming of Biogas: Coke Resistance and Mechanistic Insights. Appl. Catal. B Environ. 2018, 230, 220. [Google Scholar] [CrossRef]
- Wang, K.; Zhong, P. A kinetic study of Co oxidation over the perovskite-like oxide LaSrNio4. J. Serb. Chem. Soc. 2010, 75, 249–258. [Google Scholar] [CrossRef]
- Das, S.; Bhattar, S.; Liu, L.; Wang, Z.; Xi, S.; Spivey, J.J.; Kawi, S. Effect of Partial Fe Substitution in La0.9Sr0.1NiO3 Perovskite-Derived Catalysts on the Reaction Mechanism of Methane Dry Reforming. ACS Catal. 2020, 10, 12466–12486. [Google Scholar] [CrossRef]
- Jangam, A.; Das, S.; Dewangan, N.; Hongmanorom, P.; Hui, W.M.; Kawi, S. Conversion of CO2 to C1 Chemicals: Catalyst Design, Kinetics and Mechanism Aspects of the Reactions. Catal. Today 2020, 358, 3–29. [Google Scholar] [CrossRef]
- Oemar, U.; Ang, M.L.; Hee, W.F.; Hidajat, K.; Kawi, S. Perovskite LaxM1-xNi0.8Fe0.2O3 Catalyst for Steam Reforming of Toluene: Crucial Role of Alkaline Earth Metal at Low Steam Condition. Appl. Catal. B Environ. 2014, 148–149, 231. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.; Saw, E.T. Progress in Synthesis of Highly Active and Stable Nickel-Based Catalysts for Carbon Dioxide Reforming of Methane. ChemSusChem 2015, 8, 3556. [Google Scholar] [CrossRef] [PubMed]
- Pakhare, D.; Spivey, J. A review of Dry (CO2) Reforming of Methane over Noble Metal Catalysts. Chem. Soc. Rev. 2014, 43, 7813. [Google Scholar] [CrossRef] [PubMed]
- Pakhare, D.; Schwartz, V.; Abdelsayed, V.; Haynes, D.; Shekhawat, D.; Poston, J.; Spivey, J. Kinetic and Mechanistic Study of Dry (CO2) Reforming of Methane over Rh-substituted La2Zr2O7 Pyrochlores. J. Catal. 2014, 316, 78. [Google Scholar] [CrossRef]
- Bonmassar, N.; Bekheet, M.F.; Schlicker, L.; Gili, A.; Gurlo, A.; Doran, A.; Gao, Y.; Heggen, M.; Bernardi, J.; Klötzer, B.; et al. In Situ-Determined Catalytically Active State of LaNiO3 in Methane Dry Reforming. ACS Catal. 2020, 10, 1102. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Kawi, S. Promotional Effect of Alkaline Earth over Ni–La2O3 Catalyst for CO2 Reforming of CH4: Role of Surface Oxygen Species on H2 Production and Carbon Suppression. Int. J. Hydrogen Energy 2011, 36, 14435. [Google Scholar] [CrossRef]
- Sutthiumporn, K.; Maneerung, T.; Kathiraser, Y.; Kawi, S. CO2 Dry-reforming of Methane over La0.8Sr0.2Ni0.8M0.2O3 Perovskite (M = Bi, Co, Cr, Cu, Fe): Roles of Lattice Oxygen on C–H Activation and Carbon Suppression. Int. J. Hydrogen Energy 2012, 37, 11195. [Google Scholar] [CrossRef]
- Chen, S.; Yang, T.; Lu, H.; Liu, Y.; He, Y.; Li, Q.; Gao, J.; Feng, J.; Yan, H.; Miller, J.T.; et al. Increased Hydrogenation Rates in Pd/La-Al2O3 Catalysts by Hydrogen Transfer O(-La) Sites Adjacent to Pd Nanoparticles. ACS Catal. 2022, 12, 15696–15706. [Google Scholar] [CrossRef]
- Zheng, X.; Lin, H.; Zheng, J.; Duan, X.; Yuan, Y. Lanthanum Oxide-Modified Cu/SiO2 as a High-Performance Catalyst for Chemoselective Hydrogenation of Dimethyl Oxalate to Ethylene Glycol. ACS Catal. 2013, 3, 2738–2749. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Chen, Y.-W. Hydrogenation of p-Chloronitrobenzene on Lanthanum-Promoted NiB Nanometal Catalysts. Ind. Eng. Chem. Res. 2006, 45, 2973–2980. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Verykios, X.E. Kinetic Study of the Catalytic Reforming of Methane with Carbon Dioxide to Synthesis Gas over Ni/La2O3 Catalyst. Catal. Today 2001, 64, 83. [Google Scholar] [CrossRef]
- Tsipouriari, V.A.; Verykios, X.E. Carbon and Oxygen Reaction Pathways of CO2 Reforming of Methane over Ni/La2O3 and Ni/Al2O3 Catalysts Studied by Isotopic Tracing Techniques. J. Catal. 1999, 187, 85. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.J.; Zeng, L.; Zhao, J.; Tian, H.; Chen, S.; Li, K.; Sang, S.; Gong, J. On the Role of Ce in CO2 Adsorption and Activation over Lanthanum Species. Chem. Sci. 2018, 9, 3426. [Google Scholar] [CrossRef]
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-based Micro- and Mesoporous Catalysts for Dry Reforming of Methane. Catal. Sci. Technol. 2018, 8, 2763. [Google Scholar] [CrossRef]
- Wen, W.; Wang, X.; Jin, S.; Wang, R. LaCoO3 perovskite in Pt/LaCoO3/K/Al2O3 for the improvement of NOx storage and reduction performances. RSC Adv. 2016, 6, 74046–74052. [Google Scholar] [CrossRef]
- Marcos, J.A.; Buitrago, R.H.; Lombardo, E.A. Surface chemistry and catalytic activity of La1 − yMyCoO3 perovskite (M = Sr or Th): 1. Bulk and surface reduction studies. J. Catal. 1987, 105, 95–106. [Google Scholar] [CrossRef]
- Nakamura, T.; Petzow, G.; Gauckler, L.J. Stability of the Perovskite Phase LaBO3 (B = V, Cr, Mn, Fe, Co, Ni) in Reducing Atmosphere I. Experimental Results. Mater. Res. Bull. 1979, 14, 649. [Google Scholar] [CrossRef]
- Peng, Y.; Si, W.; Luo, J.; Su, W.; Chang, H.; Li, J.; Hao, J.; Crittenden, J. Surface Tuning of La0.5Sr0.5CoO3 Perovskite Catalysts by Acetic Acid for NOx Storage and Reduction. Environ. Sci. Technol. 2016, 50, 6442–6448. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.C.; Hegde, M.S.; Rattan, T.; Aruna, S.T. Chemistry of Nanocrystalline Oxide Materials; World Scientific: Singapore, 2008. [Google Scholar]
- Bisht, A.; Yadav, P.K.; Dhakar, S.; Sharma, S. Pt4+ as an Active Site for Oxygen Evolution Reaction in La1–xSrxCo1–yPtyO3. J. Phys. Chem. C 2021, 125, 25488–25496. [Google Scholar] [CrossRef]
- Bisht, A.; Sihag, A.; Satyaprasad, A.; Mallajosyala, S.S.; Sharma, S. Pt Metal Supported and Pt4+ Doped La1−xSrxCoO3: Non-performance of Pt4+ and Reactivity Differences with Pt Metal. Catal. Lett. 2018, 148, 1965–1977. [Google Scholar] [CrossRef]
- Yadav, P.K.; Kumari, S.; Naveena, U.; Deshpande, P.A.; Sharma, S. Insights into the substitutional chemistry of La1−xSrxCo1−yMyO3 (M = Pd, Ru, Rh, and Pt) probed by in situ DRIFTS and DFT analysis of CO oxidation. Appl. Catal. A Gen. 2022, 643, 118768. [Google Scholar] [CrossRef]
- Sukumar, M.; Kennedy, L.J.; Vijaya, J.J.; Al-Najar, B.; Bououdina, M. Structural, magnetic and catalytic properties of La2-xBaxCuO4 (0 ≤ x ≤ 0.5) perovskite nanoparticles. Ceram. Int. 2018, 44, 18113–18122. [Google Scholar] [CrossRef]
- Bisht, A.; Pentyala, P.; Deshpande, P.A.; Sharma, S. La0.80Sr0.20CoO3 as a noble-metal-free catalyst for the direct oxidation of formic acid under zero applied potential. Electrochem. Commun. 2019, 99, 1–4. [Google Scholar] [CrossRef]
- Chica, A.; Sayas, S. Effective and stable bioethanol steam reforming catalyst based on Ni and Co supported on all-silica delaminated ITQ-2 zeolite. Catal. Today 2009, 146, 37–43. [Google Scholar] [CrossRef]
- Futai, M.; Yonghua, C.; Louhui. Characterization of perovskite-type oxide catalysts RECoO3 by TPR. React. Kinet. Catal. Lett. 1986, 31, 47–54. [Google Scholar] [CrossRef]
- Kumar Yadav, P.; Sharma, S. Ni, Co & Cu substituted CeO2: A catalyst that improves H2/CO ratio in the dry reforming of methane. Fuel 2024, 358, 130163. [Google Scholar]
- Yadav, P.K.; Patrikar, K.; Mondal, A.; Sharma, S. Ni/Co in and on CeO2: A comparative study on the dry reforming reaction. Sustain. Energy Fuels 2023, 7, 3853–3870. [Google Scholar] [CrossRef]
- Yadav, P.K.; Verma, P.; Sharma, S. Bimetal (CuNi and CuCo) substituted CeO2: An approach for low temperature dry reforming of methane. Mol. Catal. 2024, 565, 114398. [Google Scholar] [CrossRef]
- Gangwar, B.P.; Mitra, R.; Parui, A.; Gakhad, P.; Yadav, P.K.; Singh, A.K.; Tiwary, C.S.; Biswas, K.; Sharma, S. Utilization of High Entropy Alloy (Co–Cu–Fe–Mn–Ni) and Support (CeO2) Interaction for CO2 Conversion into Syngas. Adv. Sustain. Syst. 2024, 8, 2400219. [Google Scholar] [CrossRef]
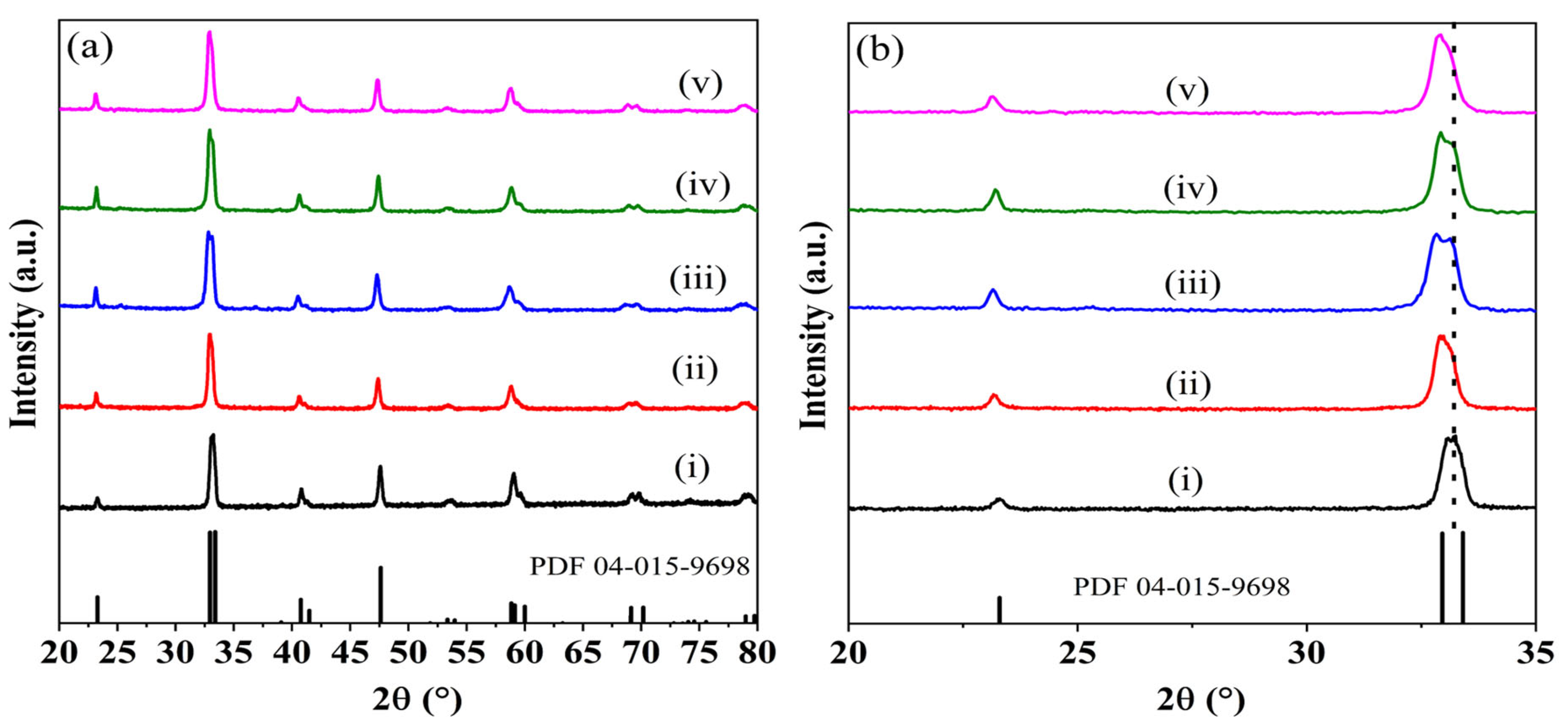
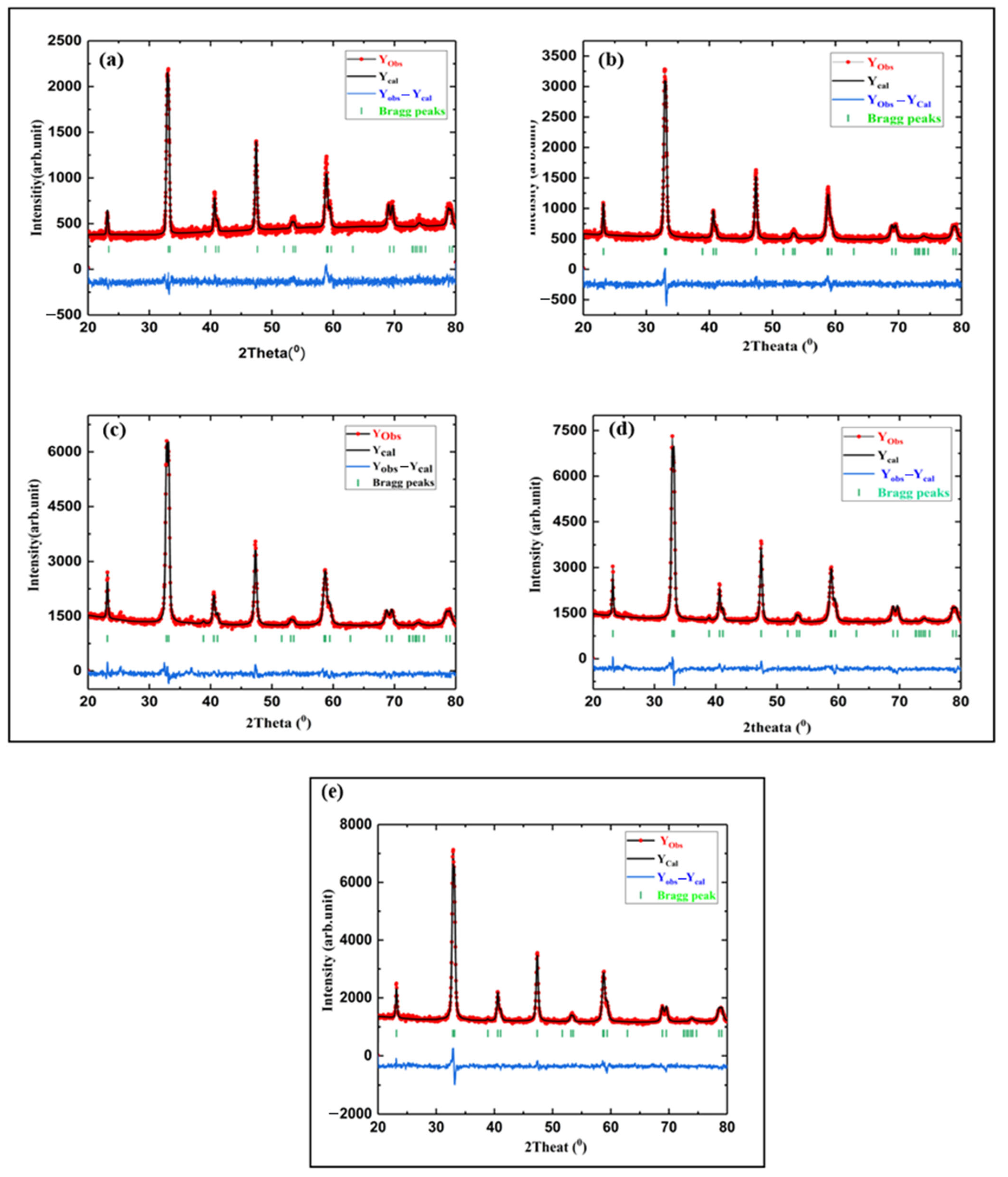
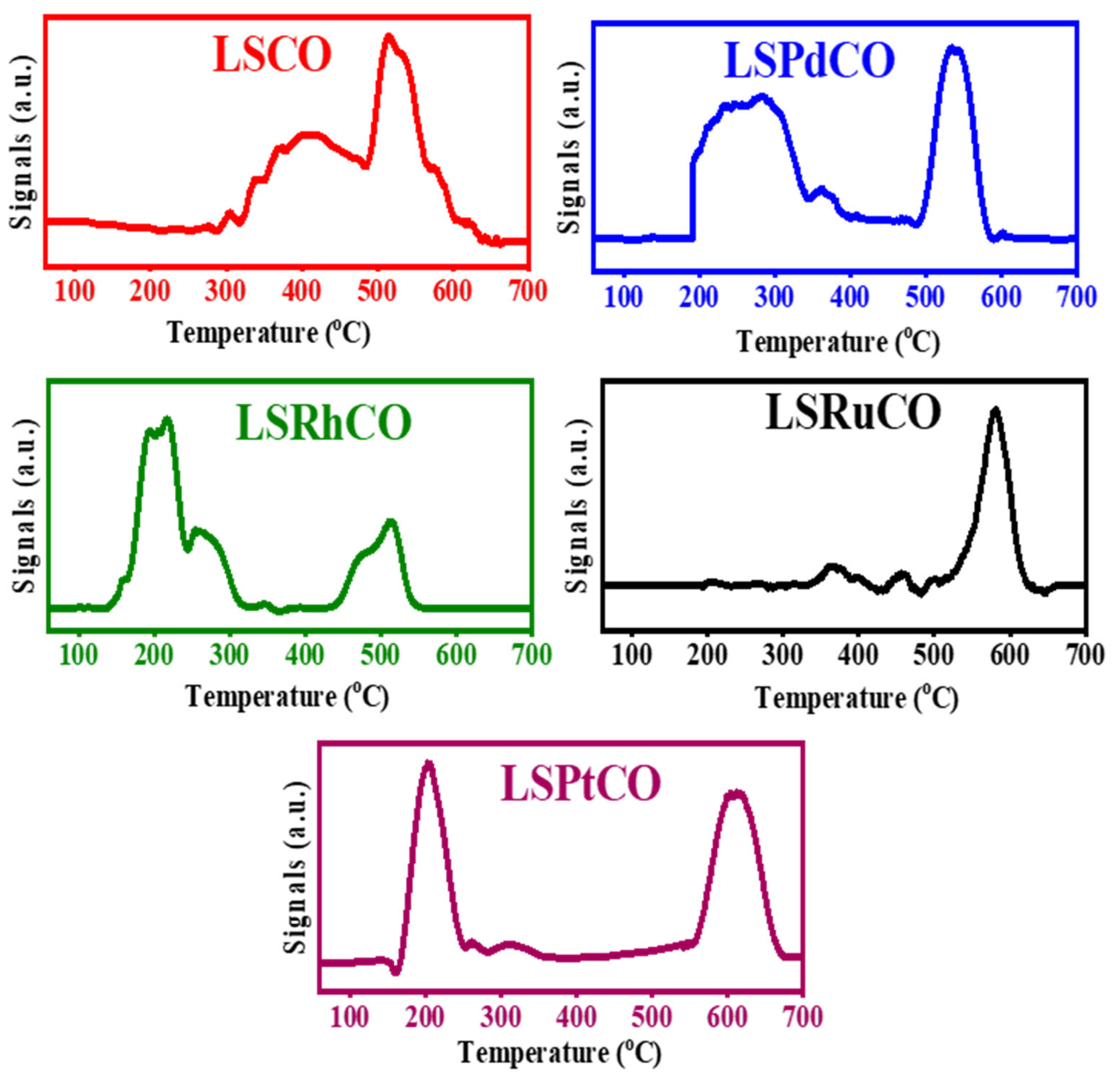
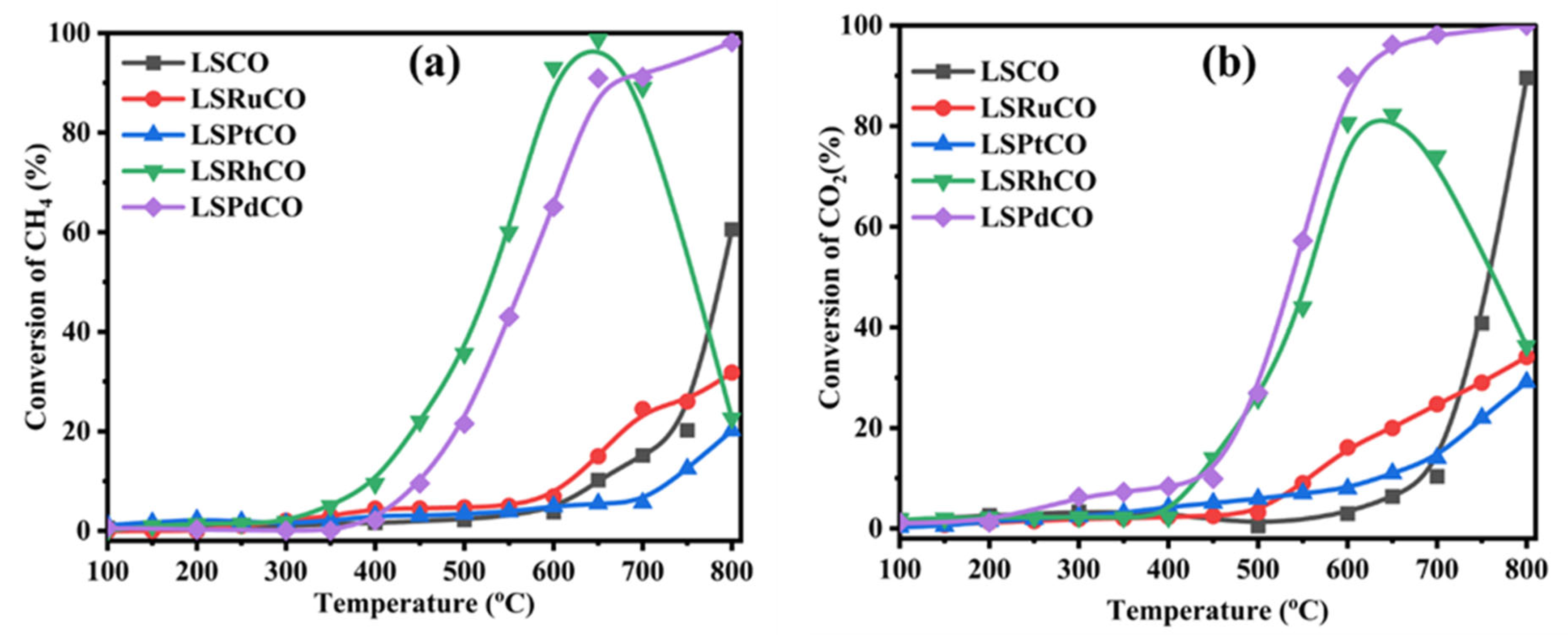
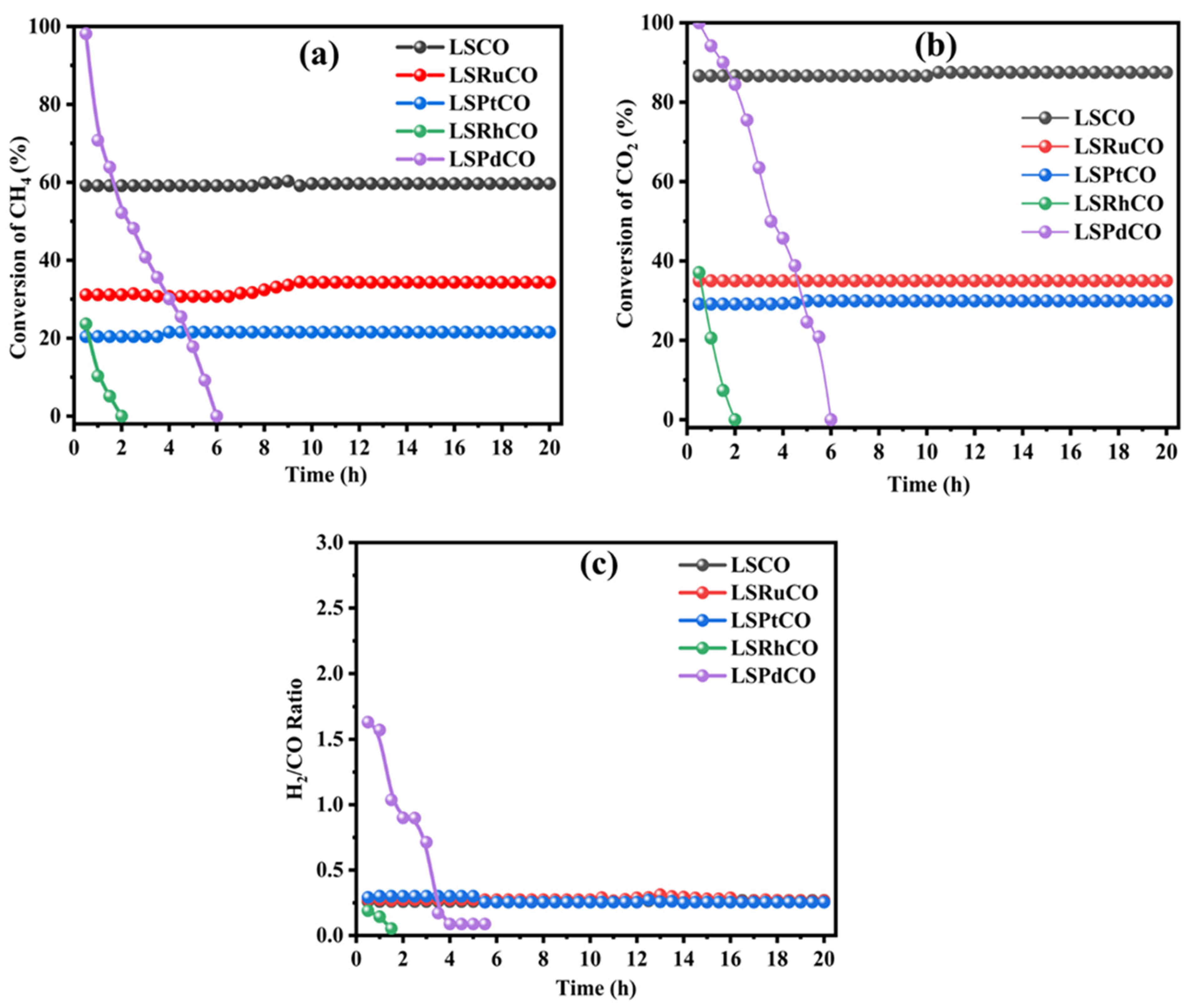
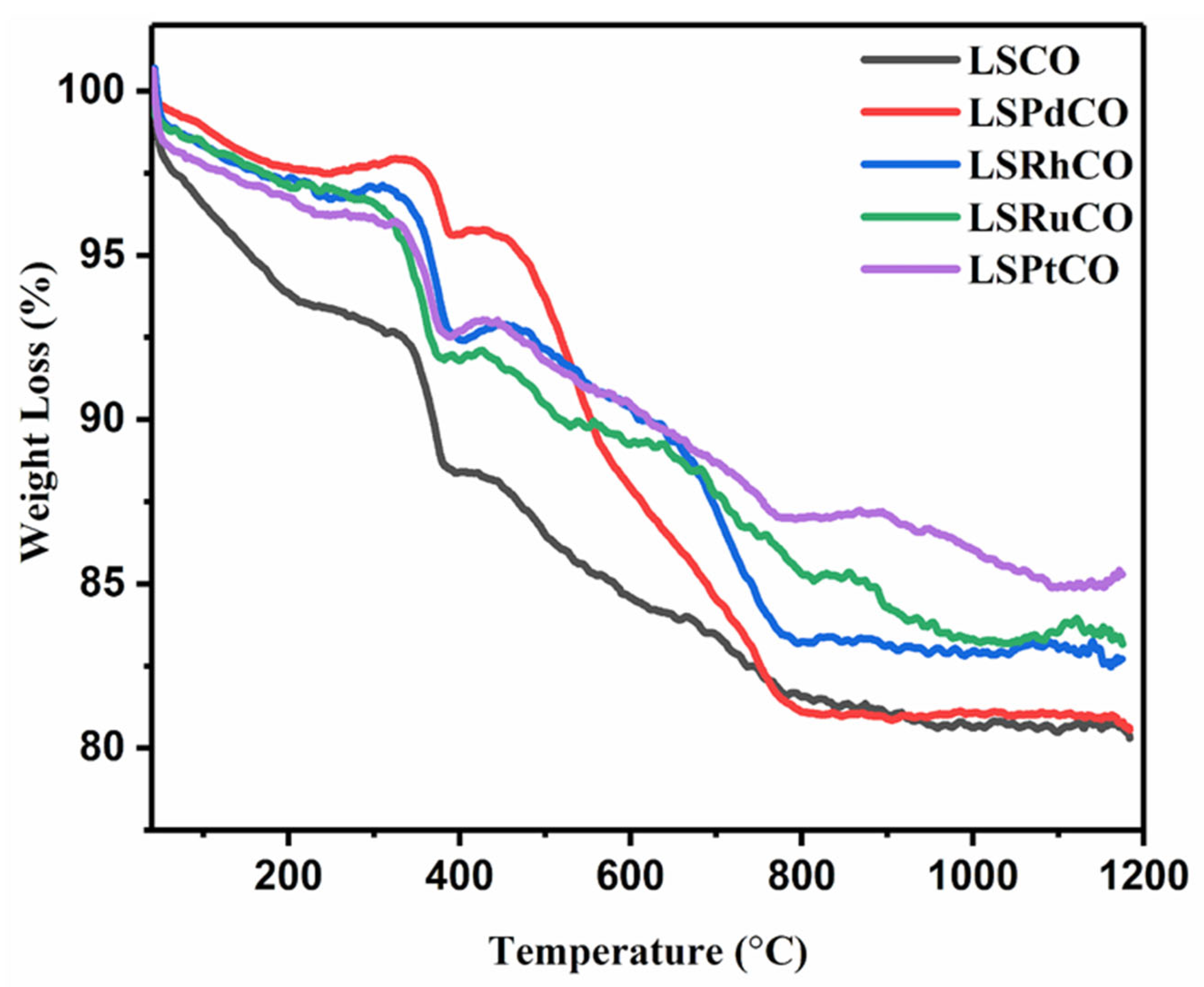
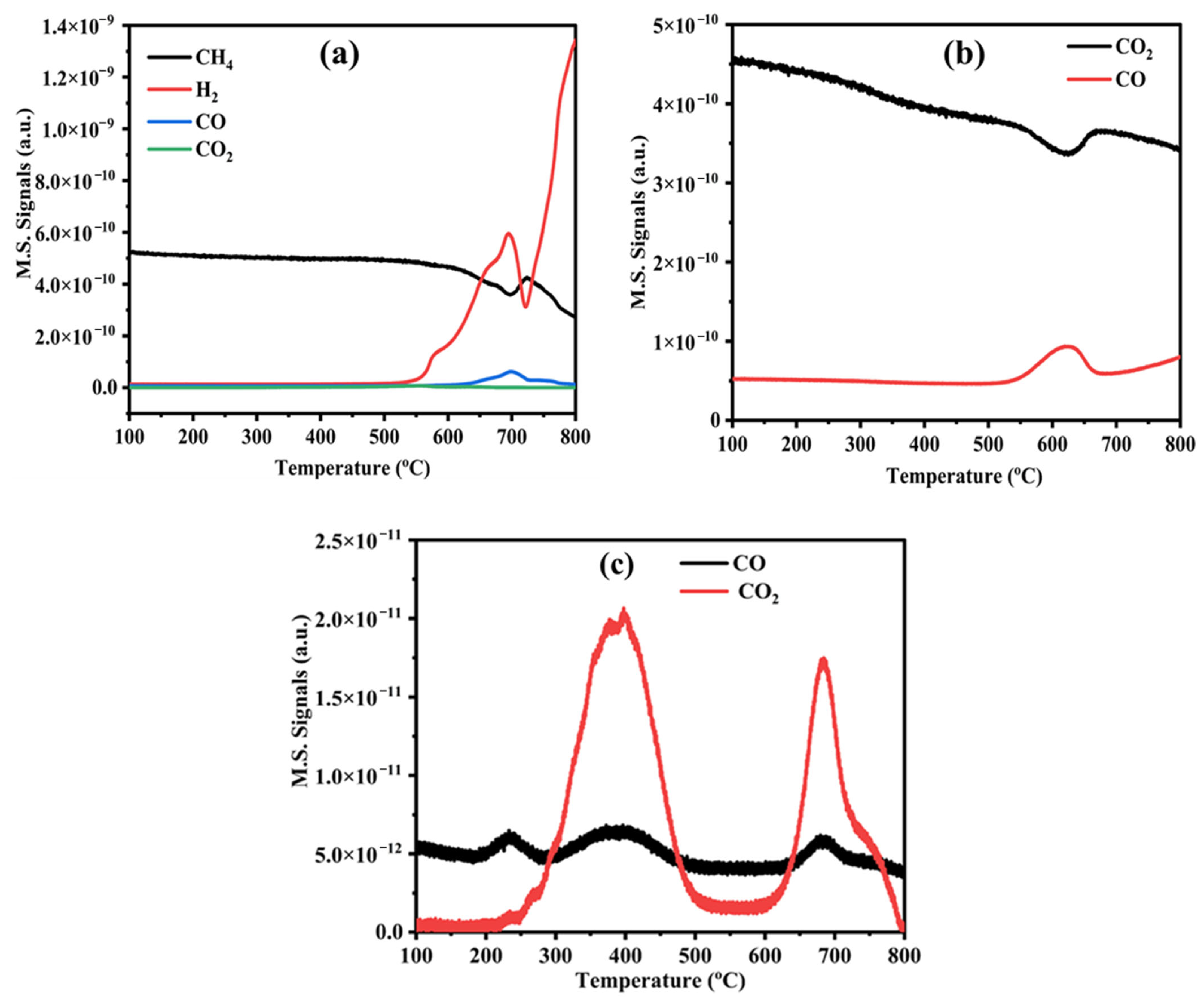
| Compound Formula | La(NO3)3·6H2O (g) | Sr(NO3)2 (g) | Co(NO3)2·6H2O (g) | Noble Metal Salt (Type) | Noble Metal Solution (mL, 1% w/v) | Noble Metal Content (%wt.) | ODH (g) |
|---|---|---|---|---|---|---|---|
| La0.85Sr0.15Co0.95 Pd0.05O3 (LSPdCO) | 5.0 | 0.4 | 3.7 | Pd(NO3)2 | 18.1 | 2.2% (Pd) | 3.9 |
| La0.85Sr0.15Co0.95 Rh0.05O3 (LSRhCO) | 5.0 | 0.4 | 3.7 | RhCl3 | 14.2 | 2.1% (Rh) | 3.8 |
| La0.85Sr0.15Co0.95 Ru0.05O3 (LSRuCO) | 5.0 | 0.4 | 3.7 | RuCl3·H2O | 15.3 | 2.1% (Ru) | 3.8 |
| La0.85Sr0.15Co0.95 Pt0.05O3 (LSPtCO) | 5.0 | 0.4 | 3.7 | H2PtCl6·6H2O | 35.1 | 4.0% (Pt) | 3.8 |
| Compounds | LSCO | LSPdCO | LSPtCO | LSRhCO | LSRuCO |
|---|---|---|---|---|---|
| Crystal System | Rhombohedral | Rhombohedral | Rhombohedral | Rhombohedral | Rhombohedral |
| Space group | R-3c (No. 167) | R-3c (No. 167) | R-3c (No. 167) | R-3c (No. 167) | R-3c (No. 167) |
| Lattice parameters (Å) | |||||
| a | 5.4330 (2) | 5.44516 (4) | 5.4582 (2) | 5.4446 (2) | 5.4499 (2) |
| c | 13.1514 (7) | 13.1915 (7) | 13.1612 (8) | 13.1582 (8) | 13.1838 (9) |
| Cell volume (Å3) | 336.19 (6) | 338.72 (2) | 339.57 (3) | 337.79 (5) | 339.11 (3) |
| R Factors | |||||
| Rp | 4.35 | 3.74 | 2.62 | 2.69 | 3.39 |
| Rwp | 5.48 | 4.70 | 3.34 | 3.43 | 2.65 |
| Robs | 5.35 | 3.61 | 4.39 | 7.39 | 5.07 |
| GOF | 1.20 | 1.12 | 1.26 | 1.29 | 1.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yadav, P.K.; Jabotra, G.; Sharma, S. Effect of Partial Noble Metal (M = Pd, Rh, Ru, Pt) Substitution in La1−xSrxCo1−yMyO3 Perovskite-Derived Catalysts for Dry Reforming of Methane. Hydrogen 2025, 6, 49. https://doi.org/10.3390/hydrogen6030049
Yadav PK, Jabotra G, Sharma S. Effect of Partial Noble Metal (M = Pd, Rh, Ru, Pt) Substitution in La1−xSrxCo1−yMyO3 Perovskite-Derived Catalysts for Dry Reforming of Methane. Hydrogen. 2025; 6(3):49. https://doi.org/10.3390/hydrogen6030049
Chicago/Turabian StyleYadav, Pradeep Kumar, Ganesh Jabotra, and Sudhanshu Sharma. 2025. "Effect of Partial Noble Metal (M = Pd, Rh, Ru, Pt) Substitution in La1−xSrxCo1−yMyO3 Perovskite-Derived Catalysts for Dry Reforming of Methane" Hydrogen 6, no. 3: 49. https://doi.org/10.3390/hydrogen6030049
APA StyleYadav, P. K., Jabotra, G., & Sharma, S. (2025). Effect of Partial Noble Metal (M = Pd, Rh, Ru, Pt) Substitution in La1−xSrxCo1−yMyO3 Perovskite-Derived Catalysts for Dry Reforming of Methane. Hydrogen, 6(3), 49. https://doi.org/10.3390/hydrogen6030049






