1. Introduction
The origin of life on Earth is likely related to geochemical and electrochemical processes occurring in the early hydrosphere. One of the most realistic scenarios involves redox reactions that provide both structural building blocks and a source of energy for the self-organization of matter. The interior of the Earth, particularly the upper mantle, provides the conditions for geochemical reactions involving magnesium and iron silicate minerals. Minerals such as olivine ((Mg,Fe)
2SiO
4) can interact with fluids containing water and sulfates (SO
42−), especially at depths of 100 to 300 km, temperatures above 1100 °C, and pressures of 2 to 7 GPa. At low oxidation potentials (reducing environments), the iron and magnesium components of olivine can reduce water or sulfates to molecular hydrogen (H
2). Such reactions are analogous to serpentinization in shallower parts of the Earth’s crust and may represent a significant source of geochemical hydrogen in the mantle [
1]. Hydration of the mantle enhances the mobility of Fe
2+ and Mg
2+ in olivine by creating cationic defects, which promote redox processes in the presence of water and sulfates. This mechanism may explain the geological generation of molecular hydrogen (H
2), a reducing agent that is potentially crucial for early life [
2]. Accumulating H
2 in the mantle could have supported a reduced atmosphere, which, according to models, may have contained 10–30% H
2 under high volcanic activity and low hydrogen escape [
3].
The first extremophile archaea and bacteria are microorganisms capable of developing in the harsh conditions that existed on early Earth [
4]. A prerequisite for their emergence is the formation of organic compounds and elementary organized structures. The first finds of biogenic graphite date back 3.8 billion years [
5]. The oldest evidence for existing organisms are stromatolites, which are 3.5 billion years old [
6].
Szostak and co-authors pointed out that geothermal energy can overcome thermodynamic barriers in chemical evolution by creating local conditions favorable for the synthesis of organic molecules [
7]. In 2010, other data showed that the probability of life arising is greater in hot freshwater mineral springs and ponds than in marine environments [
8,
9]. In formose reaction experiments conducted in conditions simulating a mineral environment, the aqueous phase was enriched with inorganic ions. This had allowed the synthesis of key molecules for life, such as ribose and uracil, the basic building blocks of RNA [
9].
Analysis of the cytoplasmic composition of contemporary cells reveals high concentrations of potassium, magnesium, zinc, and phosphate ions, which are characteristic of freshwater environments but not of modern seawater. This fact supports the hypothesis that life may have originated in continental geothermal systems rather than in the ocean [
10]. There are studies that link the origin of the cell membrane to conditions in freshwater lakes formed by geothermal springs and rainwater in the primordial hydrosphere [
11].
The study of Miller and Urey demonstrated in laboratory conditions that a mixture of H
2, CH
4, NH
3, and water vapor under the influence of electric discharge and UV radiation synthesized simple amino acids as glycine, alanine etc. [
12,
13]. In 2015, Ignatov and Mosin demonstrated a linear relationship between the mass of the sediment and the number of carbon atoms in the experiments of Miller and Wilson [
14,
15].
In hot thermal environments, the amino acid glycine can be synthesized experimentally through a redox reaction between H
2, CH
4, NH
3, and H
2O [
16,
17]. Such activation has been achieved in the laboratory using an electric corona discharge [
12,
13,
17,
18]. Under laboratory conditions similar to those of the primordial atmosphere and hydrosphere, processes of self-organization of organic molecules into protostructures have also been described [
19]. Adamatzky and co-authors [
20] include studies involving hydrogen in the context of proteinoid synthesis, such as those by Harada and Fox (1957) [
21,
22], Ignatov and Mosin (2013) [
23], and Kwon et al. (2018) [
24].
Hydrogenobacter spp. are an excellent model for studying biological processes in the primary hydrosphere.
Hydrogenobacter thermophilus is a thermophilic microorganism that utilizes molecular hydrogen as its primary energy source [
25]. This organism is autotroph and uses CO
2 as a carbon source. It does not require organic molecules from the environment, but synthesizes them from inorganic substances. It inhabits high-temperature environments (~70 °C), rich in minerals, CO
2, and H
2, and can exist in the absence of light.
H. thermophilus uses the reductive tricarboxylic acid cycle (reductive TCA cycle)—one of the most ancient autotrophic metabolic pathways, considered a possible primary mechanism for CO
2 fixation.
H. thermophilus belongs to the phylum Aquificae—one of the most ancient lineages of bacteria inhabiting hydrothermal vents and hot mineral springs. The organism represents a living model of an ancient life form, sustained entirely by geochemical resources—H
2, CO
2 and heat. Its metabolism, habitat, and evolutionary history are consistent with hypotheses of the origin of life in hot mineral environments. This microorganism requires iron–sulfur (Fe–S) clusters, which are essential for the functioning of enzymes in the reductive TCA cycle [
26,
27]. The genome of its strain, TK-6, is compact (~1.5 Mbp) and contains essential metabolic genes similar to those associated with the minimal genome scenarios of the Last Universal Common Ancestor (LUCA) [
28]. Iron–sulfur clusters can be linked to the mineral origin of metabolism. A recent study used a combined approach (homology, genomic context, and phylogenetic analysis) to identify Fe–S cluster biogenesis systems in over 10,000 archaeal and bacterial genomes [
29]. Geothermal energy, derived from volcanic activity and magma, is considered a stable and long-lasting source of heat. Due to the repetitive nature of this energy factor, the protostructures formed in hot mineral water are more resistant to destruction. This creates favorable conditions for self-organization processes [
30]. Hot mineral lakes on the early Earth’s surface were energetically active reaction environments, and spontaneous heterogeneous redox reactions can occur. One example is the interaction between magnesium metal (Mg
0) and iron ions (Fe
2+):
This reaction is thermodynamically favorable, with an electrochemical potential of +1.93 V and a negative Gibbs free energy (ΔG < 0). In this process, magnesium acts as a reducing agent, donating electrons, and the iron ions are reduced by accepting them. In an environment containing CO
2 and H
+, magnesium can also react with water, releasing molecular hydrogen:
H
2 is a key reducing agent in prebiotic chemistry, capable of participating in the reduction of CO
2 to organic compounds. Thus, the hot mineral water of the early Earth acted as a natural reaction medium, favorable for the emergence of life. In the presence of ions such as Mg
2+, Fe
2+, and SO
42−, reducing conditions are created in which electrochemical reactions can occur. Electrochemical methods for hydrogen production offer environmentally friendly alternatives that mirror redox processes believed to have occurred in early Earth’s geochemical environments [
31]. Minerals containing magnesium and iron participate in processes that lead to the formation of H
2, a key energy source in early biochemistry. In these environments, simple redox systems can form, stabilizing organic molecules and maintaining primary biostructures.
The mineral water in Rupite (Bulgaria), at a temperature above 50 °C, shows a negative oxidation–reduction potential (ORP) under experimental conditions. The water temperature is 73.4 °C. Its composition includes ions such as HS
−, HCO
3−, and possibly molecular hydrogen. These data support the hypothesis of the presence of reducing conditions suitable for the emergence of life [
31,
32].
The hot mineral springs in the Rupite area in Bulgaria represent a modern natural analogue of the primary hydrosphere. The mineral water of Rupite contains mainly HS
-, HCO
3− and Ca
2+ ions [
32]. Molecular hydrogen is the subject of analysis under specific conditions. In the hot mineral water of Rupite, hydrogen-oxidizing microorganisms of the genera
Hydrogenophilus and
Hydrogenobacter/
Aquifex form an internal hydrogen ecosystem, in which molecular hydrogen serves as a local source of energy through microaerophilic and aerobic oxidation [
33]. It creates favorable conditions for autotrophic growth and early biochemical processes in hot geothermal environments [
34]. Derekova et al. isolated a new thermophilic, strictly aerobic, Gram-positive, spore-forming chemoorganotrophic bacterium from three hot springs in the Rupite Basin region, named Anoxybacillus rupiensis [
35].
The presence of
Pseudomonas fluorescence was established at the Rupite 1 spring with a degree of certainty (99.9%). The analysis was performed with MALDI-ToF MS identification. This testifies to the ability of this type of bacteria to adapt to thermophilic and mineral-saturated environments [
36].
Species of the genus Pseudomonas are some of the most rapidly adapting bacteria to various chemical and physical factors. They can adapt to environments with warm water and potential hydrogen content, as P. fluorescence, as proved in the warm lake of Rupite. In this study, we present laboratory experiments with Pseudomonas aeruginosa for development in an anaerobic liquid environment in the presence of hydrogen, conditions characteristic of the primary hydrosphere.
This study aimed to analyze the role and mechanisms of redox processes involving Mg0 and H2 in the context of chemical evolution and the emergence of life. The emphasis is placed on their ability to support the formation of protomembranes and the stabilization of primary biomolecules, as evaluated by available experimental data and natural analogues. The aim is to conduct experiments with the bacterial species H. thermophilus and P. aeruginosa to adapt them to reducing environments, such as those found in the primary hydrosphere, where water is saturated with molecular hydrogen.
2. Materials and Methods
Hydrogen-rich water
The chemical reaction used for receiving hydrogen-rich liquid environment is [
37,
38]
Magnesium. For the formation of H2 in the culture medium mesopeptone broth, pure magnesium (Mg), EC No. 231-104-6III from “Chem Spectrum Ltd.” (Wollaston, UK), was added in two final concentrations—1 ppm and 2 ppm.
2.1. Nuclear Magnetic Resonance (NMR)
Nuclear magnetic resonance (NMR) spectra were recorded on a Bruker Avance II+ 600 MHz spectrometer (Billerica, MA, USA) with a 5 mm direct detection dual broadband probe. All experiments were conducted at a temperature of 298 K. Proton (1H) NMR spectra were acquired with 128 K domain points, a spectral width of 9600 Hz, 16 scans, and a relaxation delay of 60 s. Chemical shifts were referenced to a residual signal of dmso-d6 (2.50 ppm), which was placed in a coaxial capillary inside the NMR tube and also served as the lock solvent. The signal half-widths (Δν1/2) were determined using the dominant (commanding) peak in each spectrum.
In our research, water samples were measured with NMR [
38].
2.2. Theoretical Calculations for Water Clusters and Hydrogen Bonds
The NMR parameters of the water samples were computed using the
Gaussian 16 software package [
39]. Structural optimization of the water clusters was carried out using various quantum chemical approaches, including MP2/CBS-e [
40], M06-2X/aug-cc-pVDZ [
41], by B3LYP/6-31++G** [
42], and MP2/aug-cc-pVTZ [
43]. The M06-2X/aug-cc-pVDZ functional was explicitly applied to benchmark non-covalent interactions and hydrogen bond cooperativity, due to its known accuracy in modeling dispersion forces. In addition, the hybrid density functional MPW1PW91 was employed in conjunction with the 6-311+G(2d,p) basis set, selected for its reliable performance in predicting NMR shielding tensors and hydrogen-bond geometries in moderate-sized water clusters, while maintaining computational efficiency [
44,
45]. This combination of functionals ensures robust modeling of both geometric and spectroscopic parameters, allowing for a comprehensive assessment of water cluster structures. NMR shielding tensors were calculated using the Gauge-Including Atomic Orbital (GIAO), as implemented in
Gaussian 16, following the formalism originally introduced by Ditchfield and Hameka [
46]. Solvent effects were incorporated implicitly using the solvation model based on density (SMD) with default water parameters, allowing more realistic modeling of the aqueous environment [
47].
2.3. Microorganisms
Cultivation of H. thermophilus TK-6
A live culture of H. thermophilus strain TK-6 with chemolithoautotrophic properties was used in this study. The strain was initially cultivated by Oleg V. Mosin (1966–2016) in the Biotechnology Department at Moscow State University of Applied Biotechnology, Moscow 109316, Russian Federation. Cultivation was carried out under anaerobic conditions at 70 °C in a CO2-based mineral medium at a pH of 7.0. Two gas-phase conditions were used. The control sample had 100% CO2 at 1 atm. The experimental sample had a proportion of H2/CO2 at 1 atm total pressure. Cultures were grown in 125 mL serum bottles containing equal volume of mineral medium. After 24 h, cells were harvested by centrifugation and processed for protein analysis.
Pseudomonas aeruginosa
Pure cultures of three strains of
P. aeruginosa were tested [
48]. They are clinical strains from the Collection of Microbial Strains at the Microbiological Laboratory, Faculty of Veterinary Medicine, Forestry University, Sofia, Bulgaria, which is registered in the Global Register of Scientific Collections.
Culture media. All cultivations of H. thermophilus were performed in protein-free, mineral, CO2-based nutrient broth, pH = 7.0. Nutrient broth and agar were used, as well as the selective for Pseudomonas spp. medium Cetrimide agar (Merck-Bio Lab, Bulgaria).
2.4. Microbiological Research
Experimental conditions.
The cultivation of H. thermophilus was carried out in 125 mL serum bottles (Duran). They were sealed with rubber bottle stoppers and aluminum caps, under anaerobic conditions.
The gas phase was composed of
Control group: 100% CO2 at 1 atm.
Experimental groups:
The incubation temperature was 70 °C, maintained with a thermostatic bath (Memmert WB series). For the determination of cell density, samples were taken at certain intervals and analyzed with a counting chamber (Neubauer improved) under a light microscope (Olympus CX23, Hachioji, Tokyo, Japan) at 400× magnification. The initial inoculation was 0.1 × 106 cells/mL.
Dissolved H2 in the experimental group was achieved by gas injection and confirmed with a gas chromatograph (GC) and a thermal conductometric detector (TCD).
The tested strains of P. aeruginosa were inoculated in nutrient broth with 1 ppm H2, 2 ppm H2, and without H2 for control samples. They were cultured at 35–37 °C for 18–24 h under anaerobic conditions created with Microbiology Anaerocult® A mini, Merck KG A, Darmstadt, Germany.
The physical parameters pH, ORP, temperature and amount of H2 were measured with a YY-400 device.
2.5. Electrophoresis
Hydrogenobacter thermophilus TK-6 cultures were grown anaerobically at 70 °C for 24 h under various gas phases, including CO2 (control sample) and H2/CO2 mixture (40:60, 1.0 atm). Cells were harvested by centrifugation, washed with PBS, and lysed by sonication in SDS-containing buffer. The protein concentration was determined using the Bradford assay.
Equal protein amounts (25 µg) were separated on 12% SDS-PAGE gels and stained with Coomassie Brilliant Blue or transferred onto PVDF membranes for Western blot analysis. Membranes were blocked with 5% skim milk in TBST and probed with primary antibodies specific for [NiFe]-hydrogenase or other target proteins (1:1000 dilution), followed by HRP-conjugated antibodies (1:5000). Detection was performed using chemiluminescence (ECL, Thermo Fisher, Waltham, MA, USA).
SDS-PAGE and Western Blotting
Whole-cell protein extracts were obtained by sonication in SDS buffer, followed by quantification of proteins using the Bradford method. Samples containing 25 µg total protein were denatured in Laemmli buffer and separated via 12% SDS-PAGE at 120 V for 90 min. Gels were stained with Coomassie Brilliant Blue R-250.
For Western blotting, proteins were transferred to PVDF membranes and blocked with 5% skim milk in TBST. Membranes were probed with anti-[NiFe]-hydrogenase primary antibodies (1:1000) and HRP-conjugated secondary antibodies (1:5000). Detection was performed via enhanced chemiluminescence.
3. Results
3.1. Research with H. thermophilus
Figure 1 and
Table 1 present the results obtained in the studies carried out on the development of the extremophile species
H. thermophilus in an anaerobic environment in the presence and absence of molecular hydrogen. The graph and the data in the table show that
H. thermophilus develops better in the presence of molecular hydrogen. It also reproduces in its absence, but to a lesser extent.
The temperature of the water was 70 °C for both the three experimental and one control groups. The incubation was anaerobic. The volume of the liquid medium was 125 mL, and that of the gas phase was also 125 mL. The proportion H
2/CO
2 = 40:60 is optimal for experimental conditions similar as the primordial atmosphere [
3]. The dissolved molecular hydrogen for H
2/CO
2 = 40:60 is 0.36 ppm, for H
2/CO
2 = 60:40 is 0.544 ppm, and for H
2/CO
2 = 20:80 is 0.18 ppm.
The results show that the addition of molecular hydrogen stimulates the growth of H. thermophilus at 70 °C and pH = 7.0 under anaerobic conditions. The initial growth in the first 4–6 h was identical in both groups. The differences were reduced after that, indicating that hydrogen enhances growth and does not affect the duration of the lag phase. Obviously, dissolved hydrogen in an amount of 1 ppm is biologically active for this thermophilic chemolithotrophic species.
The growth of
H. thermophilus under different H
2/CO
2 gas-phase conditions was modeled using the following logistic growth function:
where N(t) is the cell density, K is the environmental carrying capacity, r is the specific growth rate, and t
0 is the time at which the growth rate is maximal (inflection point).
This model effectively captures the S-shaped growth pattern typical of microbiological populations in closed systems. The highest values of K ≈ 115 × 106 CFU/mL and r ≈ 0.32 h−1 were obtained H2/CO2 = 60:40, confirming hydrogen’s role as a key energy donor. In contrast, the CO2 control sample exhibited reduced growth, supporting the importance of hydrogen in early anaerobic ecosystems.
The experiments were performed by Oleg Mosin and the analyses were performed by the first author.
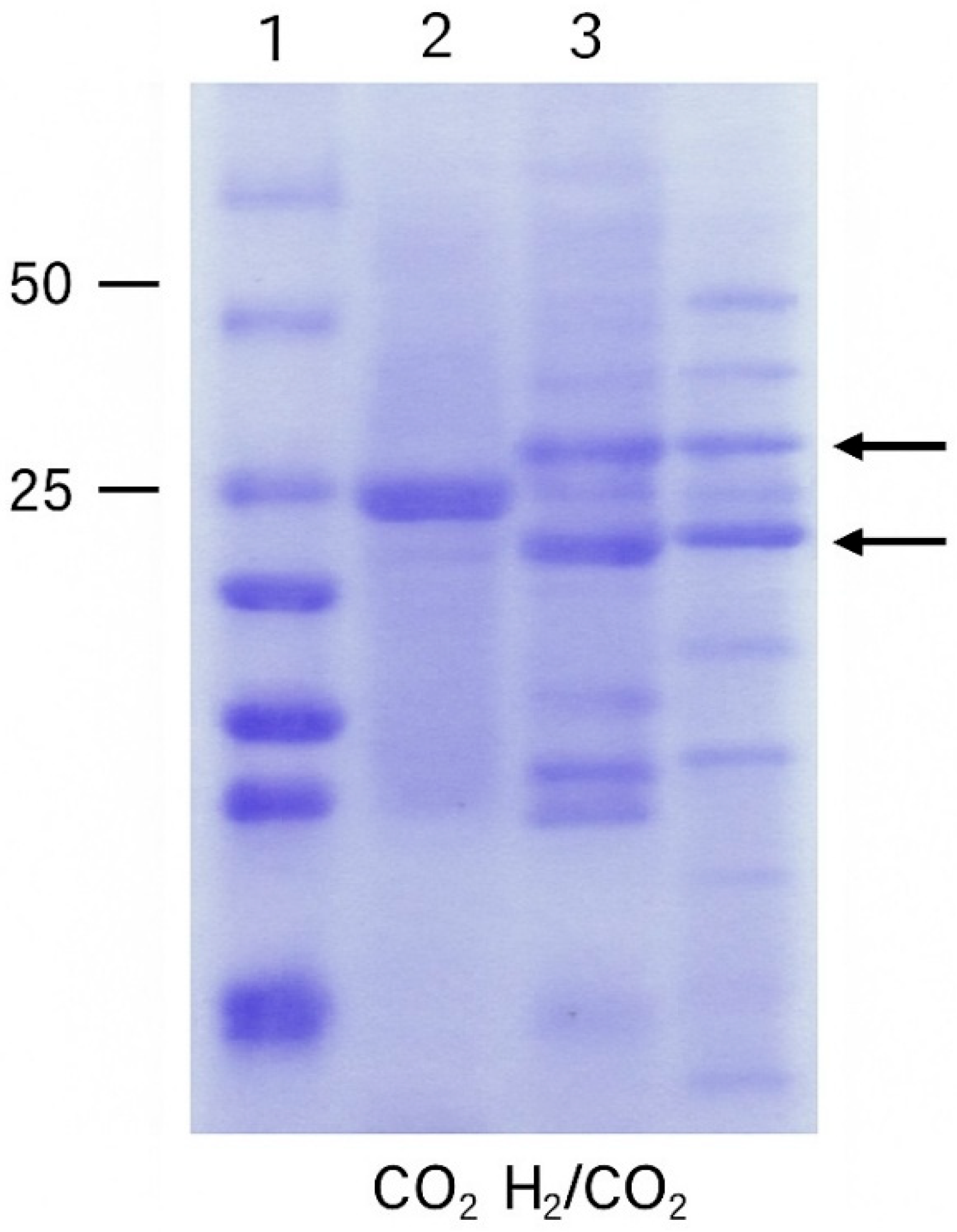
3.2. Electropherogram of H. thermophilus
Figure 2 presents the data obtained during electrophoresis of the tested
H. thermophilus strain TK-6.
The SDS-PAGE analysis revealed distinct differences in the protein expression profile of H.
H. thermophiles under hydrogen-enriched versus CO
2-only anaerobic conditions. Notably, two bands with an estimated molecular weight of approximately 25 kDa (
Figure 2, line 3) were upregulated in the presence of molecular hydrogen. These proteins are likely candidates for [NiFe]-hydrogenases or other enzymes involved in hydrogen oxidation and CO
2 fixation via the reductive TCA cycle. The observed pattern supports the analysis that
H. thermophiles adjusts its proteomic response in favor of autotrophic metabolism when molecular hydrogen is available as an electron donor.
3.3. Analysis with Pseudomonas aeruginosa
The confirmation, via MALDI-ToF MS, of
Pseudomonas fluorescence presence suggests its adaptability to warm, mineral-rich conditions in the pond of Rupite, Bulgaria [
36].
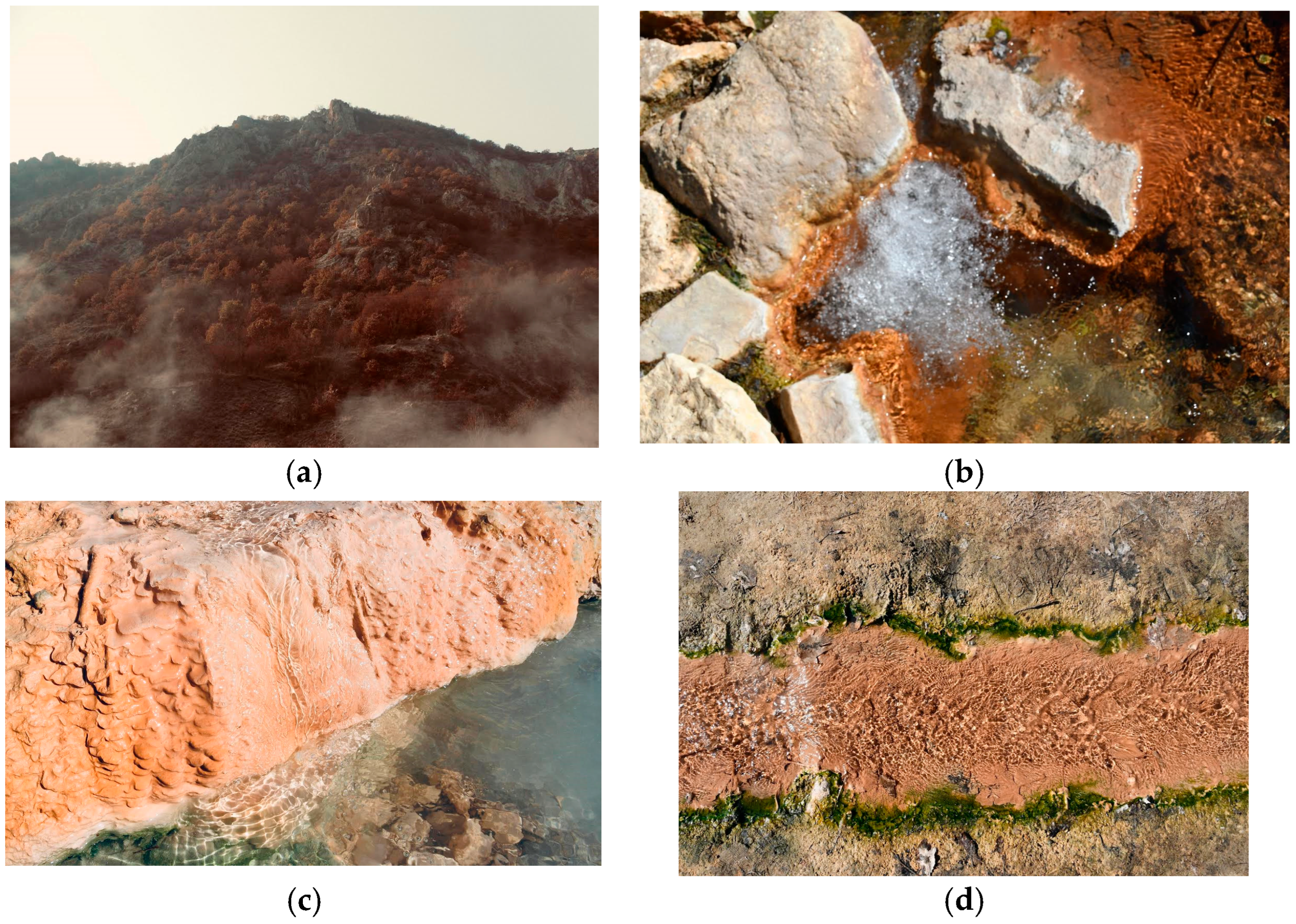
Figure 3 shows the extinct volcano Kozhuh (a), source Rupite (b), pond, and shore (c), and stream mineral water (d).
All tested P. aeruginosa strains demonstrated visible growth within 24 h of incubation under laboratory conditions. Comparative analyses of cultures grown in nutrient media supplemented with Mg0, leading to in situ generation of molecular hydrogen (H2), showed no significant difference in growth compared to control media lacking magnesium. The estimated concentrations of molecular hydrogen were approximately 1 ppm and 2 ppm, depending on the amount of metallic magnesium added. Under anaerobic conditions, the turbidity of the remaining cultures was low across all tested variants, including those with or without exposure. This indicates limited metabolic activity and biomass accumulation in the absence of oxygen, consistent with the known facultative aerobic nature of P. aeruginosa.
Under aerobic conditions, lower turbidity was observed in cultured cells exposed to molecular hydrogen, particularly at a concentration of 2 ppm. This suggests a mild inhibitory effect on the bacterial growth.
However, the observed effects were modest and within the expected physiological variability for facultative aerobic bacteria, suggesting that molecular hydrogen at these concentrations does not exert an intense selective pressure under the tested conditions.
In contrast to P. aeruginosa, which demonstrates limited sensitivity to molecular hydrogen, H. thermophilus exhibits a pronounced metabolic response to H2 due to hydrogenotrophic metabolism and the presence of active hydrogenases, making it a more suitable model for studying redox processes under conditions resembling the prebiotic hydrosphere.
3.4. Hydrogen-Bonded Water Clusters as Redox-Active Microenvironments: Insights from 1H NMR and DFT
The results obtained from the studies using the NMR method are presented in
Table 2 and
Figure 4.
Fourier Transform Infrared (FT-IR) spectroscopy and DFT calculations similar to those applied in the studies with hydrogen-rich water [
38], glacial waters and ice I
h [
49], distilled water [
50] were employed to model the structure and properties of hydrogen-bonded water clusters as a model of prebiotic conditions.
Table 3 demonstrates the average chemical shifts of modeled water clusters under fast exchange conditions calculated using the GIAO/SMD/MPW1PW91/6-311+G-(2d,p) method combined with MP2/aug-cc-pVTZ geometries [
39,
40].
Figure 5 demonstrates the schematic representation of DFT-optimized cluster geometries and electron density surfaces in hydrogen water (1 ppm H
2).
DFT analyses, presented in
Figure 4, indicate that the hydrogen water forms energetically stable clusters, especially in the range from (H
2O)
4 to (H
2O)
8, which exhibit 4 to 12 hydrogen bonds (
Table 3) and corresponding chemical shifts (δ) between 4.38 and 5.42 ppm. These δ values reflect increasing hydrogen-bond cooperativity and suggest the presence of cyclic and three-dimensional network geometries that are energetically favorable. The observed relationship between cluster size, number of hydrogen bonds, and δ follows logarithmic trends, where each additional water molecule contributes progressively less to total chemical shift, indicating saturation in hydrogen-bond network density.
The stability of larger clusters, such as (H2O)10 and (H2O)20, with δ values up to 5.60 ppm and 30+ hydrogen bonds, further supports the analysis that such structured water can create a microenvironment suitable for redox processes in prebiotic chemistry.
While the simulations are performed under low-entropy and non-oxidative conditions, the resulting structures support the plausibility of such clusters as a model of the geochemical niches that could have existed in Earth’s primordial hydrosphere.
4. Discussion
The results obtained in the present research clearly demonstrate that H. thermophilus grows well in an anaerobic environment at a temperature of 70 °C, and its multiplication is enhanced in the presence of molecular hydrogen. These experiments support the hypothesis that thermophilic organisms could have developed under conditions resembling the early Earth’s hydrosphere. The results obtained by us also show the ability of P. aeruginosa to multiply in anaerobic conditions with the presence of H2. This observation aligns with the hypothesis that some microorganisms may tolerate early Earth-like environments, though they are not necessarily ancient. This also explains the establishment of bacteria of the genus Pseudomonas in mineral water from Rupite in Bulgaria, where the conditions are similar. Probably not only the oldest microorganisms on our planet, archaebacteria are capable of development under conditions characteristic of the ancient hydrosphere. Some modern bacteria such as P. aeruginosa, which are widespread and adaptive, can also survive and reproduce under atypical and mildly adverse environmental conditions.
Experimental studies, including the analysis of mineral water from Rupite, Bulgaria support hypotheses about the origin of life in hot mineral environments. It serves as a realistic model of the primordial hydrosphere due to its high temperature and the presence of reducing ions such as HS
−, HCO
3− and Mg
2+. Protostructures with densities reaching a certain percentage of that of a living cell have been modeled by corona discharge. In such environments, spontaneous self-organization of matter and the formation of stable molecular structures are observed, including clusters around metal ions that can stabilize biomolecules such as RNA and lipids [
17]. These results support the thesis that hot mineral lakes, subjected to geochemical, electrochemical and thermal processes, could have served as reaction environments for the emergence of life on Earth.
Mg
2+ ions stabilize RNA, ATP, and phosphate structures. Ferrous ions (Fe
2+) and metallic iron (Fe
0) can form iron–sulfur clusters, the catalytic centers of enzymes, making hot mineral springs realistic biochemical precursors to cellular forms. In the anoxic primordial hydrosphere, iron ions (Fe
2+) can react with reducing agents such as Mg
0 or precipitate to form metallic iron (Fe
0). In the presence of sulfur compounds such as H
2S, Fe
2+ and Fe
0 can form iron–sulfur clusters (Fe-S). These iron sulfides, formed in hot springs on the early Earth, are capable of catalyzing the reduction of CO
2 to methanol (CH
3OH) by nonenzymatic mechanisms, thus supporting prebiotic carbon activity. The presence of manganese and sunlight greatly enhances catalytic activity. The reactions occur at temperatures between 80 and 120 °C. This further supports the hypothesis that hot lakes were realistic environments for the emergence of life. The research highlights the role of iron-containing minerals as catalysts of prebiotic redox processes that aided in the fixation of CO
2 and the formation of molecular hydrogen. The temperature range of 80–120 °C, as well as the presence of water vapor and dissolved ions in hot springs, are considered realistic conditions in the environment of the early Earth [
51].
Other studies have shown that chlorate (ClO
3−), a strong oxidant, can be abiotically reduced to chloride ion (Cl
−) by Fe(II)-containing minerals such as siderite and ferrihydrite. This process is important for understanding the chemical evolution of both Earth and Mars. Oxychlorine compounds have been found on the surface of Mars, suggesting possible abiotic reduction mechanisms there in the past. Chlorine was not widely distributed in the primordial hydrosphere of Earth, but the process of abiotic reduction by Fe(II) itself is essential. This fact highlights the potential of Fe(II)-based systems to participate in electron transport in prebiotic environments. This may have been key for the synthesis of organic molecules and for maintaining local redox gradients—important for chemical evolution [
52].
Hydrogenobacter spp. are excellent model organisms for studying the biological processes of the primary hydrosphere [
53]. They are chemolithoautotrophic, Gram-negative, non-motile, non-spore-forming microorganisms. They use H
2 as an electron donor and carbon dioxide (CO
2) as the sole carbon source. The main metabolic pathway for CO
2 fixation is the reductive tricarboxylic acid cycle (rTCA). Three molecules of CO
2 are fixed through biochemical reactions to synthesize pyruvate (C
3H
3O
3) or other precursors [
25]. Adam and Perner note that H
2 is an electron donor in hydrothermal vents. In this environment, hydrogen oxidation represents a significant source of energy, sufficient to drive ATP synthesis and autotrophic CO
2 fixation [
54]. Therefore, hydrogen-oxidizing bacteria play a crucial role in the ecosystem of deep-sea habitats. Among the major chemosynthetic reactions occurring in hydrothermal vents, the oxidation of sulfides and H
2 plays a central role [
55]. For autotrophic carbon fixation, the metabolism of hydrogen oxidation is more favorable than the oxidation of sulfides or thiosulfates, although much less energy is released.
The hydrogen reaction that occurs in water is as follows:
Zhu et al. showed that as temperature increases, the concentration of H
2 decreases, most likely due to decreased solubility and/or degassing [
56]. This is essential for assessing prebiotic conditions and the capacity of different environments to retain hydrogen as an energy and reducing resource. This is visible in
Table 4.
Temperature is a critical factor for the stability and retention of H2 in solutions or mineral phases. This indicates that cooler environments are more favorable for the accumulation/retention of molecular hydrogen, which is an important reducing agent.
Many bacteria possess genes for hydrogenases (enzymes for the oxidation or production of molecular hydrogen). Even in anoxic environments, H
2 serves as an efficient electron donor for respiration and fermentation. The oxidation of molecular hydrogen, especially by periplasmic membrane-bound [NiFe]-hydrogenases, contributes to the generation of proton-motivated force and ATP synthesis via respiratory chains. These are processes that are also applicable in anaerobic aqueous environments with the presence of hydrogen.
H. thermophilus, but also
P. aeruginosa, possesses a membrane-bound [NiFe]-hydrogenase and a proven ability to oxidize molecular hydrogen under microphilic conditions [
57,
58]. The current experiments demonstrate the potential for growth of these microorganisms in anaerobic conditions in the presence of molecular hydrogen.
5. Conclusions
The results of this study demonstrate that Hydrogenobacter thermophilus is capable of active growth in an anaerobic mineral environment enriched with hydrogen at 70 °C, with even low concentrations of dissolved H2 (0.36–0.54 ppm) sufficient to activate the metabolic pathway.
It was established that molecular hydrogen functions as an effective reducing agent, supporting cellular energetics through its involvement in electron chains, including [NiFe]-hydrogenases.
The results obtained by us also show the ability of P. aeruginosa to develop in anaerobic conditions with the presence of H2. This also confirms our hypothesis about the possibility of the development of microorganisms in the conditions of the primitive Earth hydrosphere. The results indicate that not only are the oldest microorganisms on our planet—archaebacteria—are capable of development under conditions characteristic of the ancient hydrosphere, but also some modern bacteria, such as P. aeruginosa.
Quantum chemical analyses (NMR, DFT) revealed the formation of water clusters around ions and molecules, contributing to the stabilization of redox environments favorable for biosynthesis.
These findings indicate that hydrogen-rich mineral environments could potentially act as a natural setting favorable for biomolecular synthesis. This study suggests that molecular hydrogen (H2) may have played a significant role as an electron donor during early chemical evolution.
This article was written in memory of Oleg V. Mosin (1966–2016).