Abstract
Hydrogen atom transfer (HAT), a concerted charge transfer involving two elementary particles, a proton and an electron, plays a key role in many areas of chemistry and biochemistry. A molecular dynamics study based on density functional theory was performed to investigate the reaction mechanism of hydrogen atom transfer from quercetin anions to the hydroxyl radical in a neutral aqueous media. Intrinsic bond orbital (IBO) analysis of a series of structures obtained from trajectories was performed in simulations in which the reaction occurred, and the electron flow along the reaction coordinate was determined and applied to investigate the reaction mechanism. The reaction in the simulations proceeded rapidly as proton-coupled electron transfer (PCET) or electron transfer–proton transfer (ET-PT) depending on the initial position and solvation of the reactants.
1. Introduction
Reactions involving the transfer of a net hydrogen atom from a donor to an acceptor are important in many areas of chemistry and biochemistry [1,2]. For example, a reaction step involving the transfer of this simple species is an important part of the process in photosystem II [3], a range of biochemical processes [4], oxidative damage to biomolecules [5], oxidation of polymers [6], and chemical catalysis [7,8]. Furthermore, the hydrogen atom transfer reaction step is a fundamental part of radical scavenging reactions where reactive and harmful free radical species are converted into less toxic ones [5]. Reactive oxygen species (ROS) and oxygen-centered free radicals, such as superoxide radical anions (O2•ˉ) and hydroxyl (•OH), alkoxyl (RO•), or hydroperoxyl radicals (•OOH), are responsible for a number of illnesses including cardiovascular diseases, diabetes, cancer, DNA damage and mutations, and cell aging [9,10]. Therefore, special attention is paid to the prevention of oxidative stress, in which the properties of natural polyphenols for radical trapping or radical scavenging play a significant role [11,12]. Polyphenols and flavonoids are secondary plant metabolites found in fruits, vegetables, herbs, olive oil, nuts, and red wine [13,14]. More than 7000 flavonoids have been identified so far, which have become attractive due to their exceptional biological activity and benefits for human health [15]. Flavonoids are divided into flavonoles, flavanoles, flavanones, flavones, anthocyanidins, and isoflavones, and their diverse antioxidant efficiency is related to the nature and position of the substituents in the ring system [14]. One of the most important dietary flavonoids found in food is the flavone quercetin, 3,3′,4′,5,7-pentahydroxyflavone [16]. Furthermore, the quercetin skeleton (Figure 1) is the basis of more complex bioflavonoids (glycosides) such as rutin and quercitrin.
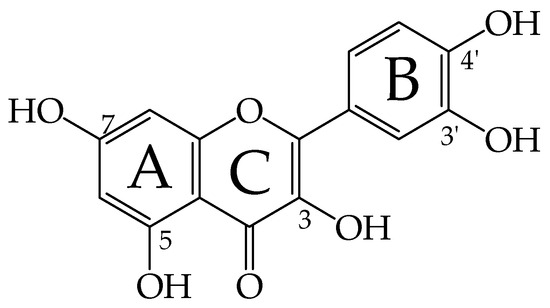
Figure 1.
Structure of the quercetin molecule and labels of OH groups and rings. The quercetin Qˉ(4′) and Qˉ(7) anions are obtained by the dissociation of the H atom from the 4′-OH and 7-OH group, respectively.
Quercetin has significant antioxidant, anti-inflammatory, antibacterial, antiviral, and anticancer properties [16,17,18,19,20,21]. Under physiological conditions, quercetin dissociates and forms the corresponding 4′-OH or 7-OH monoanions (Figure 1) [22,23,24,25,26,27,28,29]. Given the dissociation constants for deprotonation [22,23,24,25,26,27,28,29], these species predominate in neutral aqueous solutions and in body fluids or the intestinal tract as the main physiologically reactive form of quercetin. The radical-trapping antioxidative reactions of quercetin and other flavonoids have been extensively studied experimentally [5,30,31,32,33,34] and theoretically [34,35,36,37,38,39,40,41,42]. Overall, the first reaction step in the reactions of flavonoid and polyphenolic antioxidants A(OH)n with ROS involves hydrogen atom transfer and can be written as
A(OH)n + R-O• → A(OH)n−1-O• + R-OH
In the first reaction step of these radical scavenging reactions that interrupt ROS-induced oxidative damage, flavonoids can transfer a hydrogen atom to a reactive radical [5,30,31] to produce the corresponding resonance-stabilized, relatively unreactive flavonoid radicals. One-step hydrogen atom transfer (HAT) and proton-coupled electron transfer (PCET) or consecutive sequential proton-loss electron transfer (SPLET), stepwise electron transfer–proton transfer (ET-PT), and sequential proton-loss electron transfer (PT-ET) reaction mechanisms have been proposed for a number of these flavonoid charge transfer reactions based on experimental and theoretical evidence [5,30,31]. In HAT, a hydrogen atom is transferred as an entity to an acceptor in one reaction step. The HAT process does not involve significant charge separation and has been found to be the main reaction channel in non-polar solvents. In PCET, a proton and an electron can be transferred in a single reaction step from different sets of orbitals to the same acceptor or, in the case of bidirectional PCET, to different proton and electron acceptors or donors [1,2,3,43,44]. The PCET reaction may involve charge transfer with simultaneous proton tunneling [1,2,3,43,44,45]. In distinguishing HAT and PCET processes, the intrinsic bond orbital (IBO) analysis proposed by Knizia [46,47,48] proved to be unequivocal. IBOs are a form of localized molecular orbitals that represent computed self-consistent field wave functions designed to minimize the number of atoms on which the orbitals are centered. IBOs allow visualization of molecular orbitals in an intuitive way as well as changes in the electronic structure along the reaction paths [46,47,48]. For intrinsic reaction coordinate (IRC) analysis or trajectories obtained from molecular dynamics (MD), continuous rearrangement of the IBOs along the reaction path reveals electron flow and bond transformations. By following the IBOs for these obtained structures, reaction mechanisms can be studied [46,47,48]. In this paper, we present intrinsic bond orbital (IBO) analysis on trajectories obtained from a DFT molecular dynamics study for hydrogen atom transfer from quercetin 4′-OH and 7-OH anions to the hydroxyl radical in an aqueous medium model system.
2. Computational Methods
The model system consists of a quercetin anion, dissociated at 4′-OH (Qˉ(4′)) or 7-OH (Qˉ(7)), in a ~22.7 Å × 22.7 Å × 22.1 Å box solvated with 405 water molecules. Initial cell size optimization and geometry optimization of the molecules in the box was performed using the CP2K code [49]. The PBE functional [50], GTH pseudopotential [51], Grimme’s D3 dispersion correction [52], and TZVP-MOLOPT and DZVP-MOLOPT base sets for quercetin and water, respectively [53], were used with a 260 Ry cutoff for the grids and periodic boundary condition (PBC) applied. The systems were thermally equilibrated with an NVT Nose–Hoover thermostat at 300 K and 1 bar with a step of 0.5 fs. In the obtained thermally equilibrated systems, the hydroxyl radical was produced by the deletion of a H atom from one water molecule around the quercetin anions, a total of 26 positions for Qˉ(4′) and Qˉ(7) each. The local spin density approximation was applied, and the charge and multiplicities were −1 and 2, respectively. Molecular dynamics simulations were performed with an NVT Nose–Hoover thermostat at 300 K and 1 bar with a 0.5 fs step. For simulations in which hydrogen atom transfer from the quercetin anion was observed, the structures of the quercetin anion, hydroxyl radical, and surrounding waters were extracted from the trajectories. Single-point calculations with GAUSSIAN 16 software [54] at the unrestricted ωB97X-D/def2-TZVP level were performed on these structures and trajectories to obtain wave functions. Intrinsic bond orbital analysis and the IBO localization procedure on these wave functions were performed with the IBOVIEW program [47].
3. Results and Discussion
In the MD simulations performed, the hydrogen atom transfer reaction occurred when the hydroxyl radical was initially located in the hydrophilic regions around the quercetin anion [55]. The reaction occurred at 4′-OH and 3′-OH in the case of Qˉ(7′) and at 7-OH and 3′-OH in the case of Qˉ(4). These hydroxyl groups of the quercetin anions were also shown to be reactive in the Car–Parrinello molecular dynamics study of the reaction of quercetin anions with the superoxide radical [56]. The reaction proceeded rapidly when the hydroxyl radical was initially located close to the quercetin anion, and when it was located further away, the reaction occurred if the surrounding water supported efficient hopping in a mechanism similar to Grotthuss’s. A similar pattern was also observed in molecular dynamics simulations of quercetin anions with the superoxide radical [56], hydroxyl radical with gallic acid [57], ascorbate [58], and dopamine [59]. Figure 2 shows changes in bond distances, charge, and spin in an example simulation of the reaction of the quercetin Qˉ(7) anion at 4′-OH with a nearby hydroxyl radical. The reaction took place quickly, and the proton was located in the middle between the 4′-O donor and the hydroxyl radical acceptor in 11.5 fs. During this time, the IBO spin of the O atom of the hydroxyl radical changed from about 1 to 0 and the IBO charge changed from about −0.35 to −0.92, indicating electron acceptance. The changes in IBOs involved in this process are shown in Figure 3 and Figure 4. In the figures, the IBOs of α and β electrons are shown in green and blue, respectively, and the columns correspond to times of 1.5, 8.0, and 11.5 fs. At the beginning of the simulation, α and β electron IBOs can be identified, corresponding to the aromatic group of the Qˉ(7) anion, (a) and (d), the unpaired electron (g), and the lone pair of the hydroxyl radical, (j) and (m) (Figure 3). During the simulation, the change in β IBO follows the electron flow from the aromatic β IBO (d) to the lone pair of the hydroxyl radical (f), while α IBO remains on the aromatic group representing the unpaired electron of the formed quercetin radical (c).
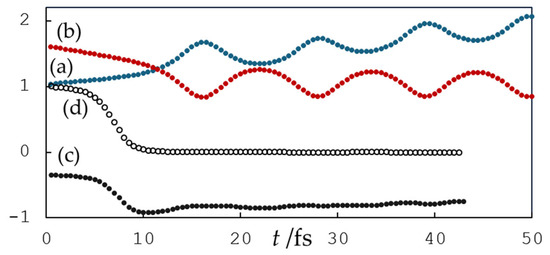
Figure 2.
Changes in (a) proton donor and H atom distance (in Å), (b) proton acceptor and H atom distance (in Å), (c) localized charge, and (d) spin of the hydroxyl radical O atom in an example simulation of the reaction of the quercetin Qˉ(7) anion at 4′-OH with a nearby hydroxyl radical.
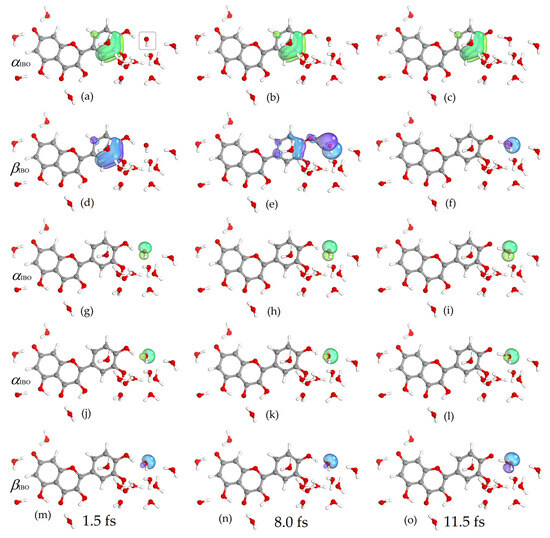
Figure 3.
Changes in α (green) and β (blue) IBOs involved in electron transfer for the reaction of the quercetin Qˉ(7) anion at 4′-OH with a nearby hydroxyl radical (marked) at 1.5, 8.0, and 11.5 fs. See also Video S1 in Supplementary Materials.
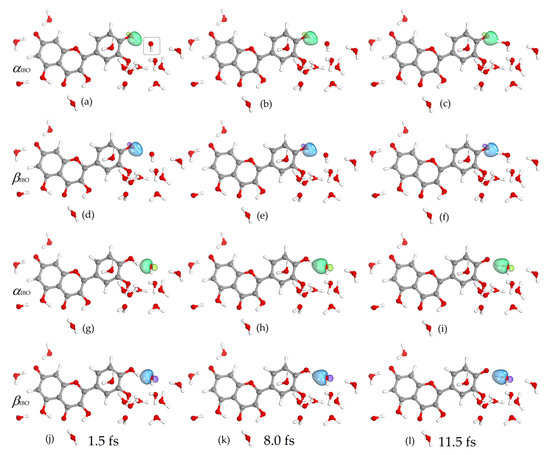
Figure 4.
Changes in α (green) and β (blue) IBOs involved in proton transfer for the reaction of the quercetin Qˉ(7) anion at 4′-OH with a nearby hydroxyl radical (marked) at 1.5, 8.0, and 11.5 fs. See also Video S2 in Supplementary Materials.
The lone pair, (j) and (m), and the free electron (g) of the hydroxyl radical are reorganized with the transferred electron into two lone pairs, (f,l) and (i,o), of the formed hydroxide anion (Figure 3). The α and β IBOs involved in proton transfer are shown in Figure 4. The σ bonding orbital of the quercetin Qˉ(7) anion 4′-OH group, α (a) and β (d) IBOs, is the proton donor, while the lone pair of the hydroxyl radical, α (g) and β (j) IBOs, is the proton acceptor that becomes the bonding σ orbital of the formed water molecule, α (i) and β (l) IBOs (Figure 3). The above analysis shows that the proton and electron do not travel together but follow different IBOs, which is a characteristic of the PCET process [46,60].
Figure 5 shows the changes in the OH bond distances of the proton donor and acceptor in the simulation of the reaction of the quercetin Qˉ(4) 7-OH with a distant hydroxyl radical separated by three water molecules. The IBO analysis shows that the electron transfer occurred rapidly in 167 fs, while the proton transfer occurred later. The proton was located between the Qˉ(4) 7-OH donor and the acceptor water molecule in 515 fs. The α and β IBOs involved in electron and proton transfer are shown in Figure 6 and Figure 7, respectively. For electron transfer, a β electron from the π aromatic bonding orbital, α (a) and β (d) IBOs in Figure 6, jumps into the unpaired electron orbital of the hydroxyl radical, α (g) IBO, resulting in the formation of a lone electron pair of the hydroxide anion, as indicated by the change in spin and charge, α (e) and β (h) IBOs (Figure 6). The formed quercetin radical slowly transfers a proton from the 7-OH group to a nearby water molecule and is further transferred to the hydroxide anion by reorganization of hydrogen bonds in a Grotthuss-like mechanism, as shown by IBO analysis (Figure 7). In this case, the simulation shows that the hydrogen atom transfer occurs by a stepwise electron transfer–proton transfer (ET-PT) reaction mechanism. It is worth noting that quercetin radicals, the products in these simulations, experimentally generated by photoionization and spectroscopically characterized by transient absorption spectroscopy, were found to be stable for several milliseconds within a pH range between 2 and 9 [61].
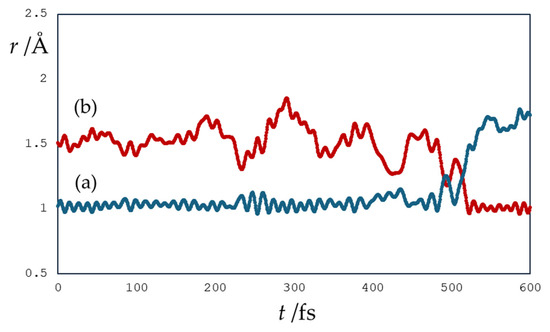
Figure 5.
Changes in (a) proton donor and H atom distance (in Å) and (b) proton acceptor and H atom distance (in Å) in an example simulation of the reaction of the quercetin Qˉ(4′) anion at 7-OH with a distant hydroxyl radical separated by 3 water molecules.
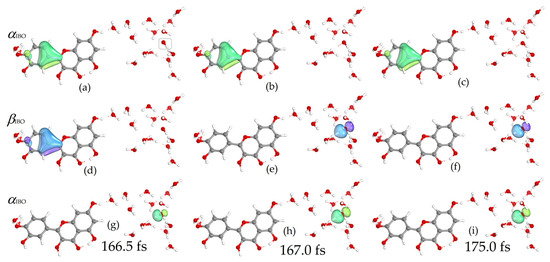
Figure 6.
Changes in α (green) and β (blue) IBOs involved in electron transfer for the reaction of the quercetin Qˉ(4′) anion at 7-OH with a distant hydroxyl radical (marked) at 166.5, 167, and 175 fs. See also Video S3 in Supplementary Materials.
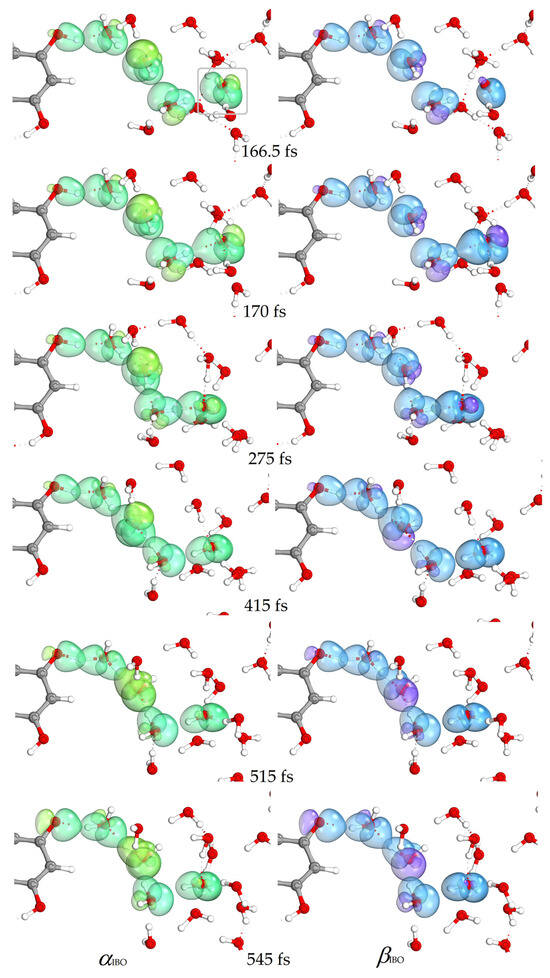
Figure 7.
Changes in α (green) and β (blue) IBOs involved in proton transfer for the reaction of the quercetin Qˉ(4′) anion at 7-OH with a distant hydroxyl radical (marked) at 166.5, 170, 275, 415, 515, and 545 fs. At 170 fs, β (blue) IBOs appear on the hydroxyl radical by electron transfer from the quercetin Qˉ(4′) anion. See also Video S4 in Supplementary Materials.
4. Conclusions
Intrinsic bond orbital (IBO) analysis was performed on structures obtained from trajectories of molecular dynamics simulations for hydrogen atom transfer reactions from quercetin 4′-OH and 7-OH anions to the hydroxyl radical in a model aqueous medium system. The reorganization of chemical bonds in the simulations proceeded rapidly depending on the initial position, orientation, and solvation of the reactants. In all performed simulations where hydrogen atom transfer occurred, the electron that paired with the unpaired electron in the α IBO of the hydroxyl radical came from the β IBO of the π aromatic system of the quercetin anions. The proton was released from the OH group of quercetin, and both electrons that formed the σ bonding orbital in the α and β IBOs, after the proton was released, remained on the quercetin O atom as a lone pair. The proton acceptor was the lone electron pair of the hydroxyl radical. IBO analysis revealed that the net hydrogen atom transfer from one of the OH groups of the quercetin anions to the hydroxyl radical utilizes different sets of IBOs for proton and electron transfer. In the case where these two processes take place simultaneously, a proton-coupled electron transfer (PCET) reaction mechanism can be proposed, and in the case where the hydroxyl radical is initially at a greater distance, stepwise electron transfer–proton transfer (ET-PT) occurs.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/hydrogen6020039/s1: Videos S1–S4: Animations of changes in α (green) and β (blue) IBOs from Figure 3, Figure 4, Figure 6 and Figure 7 during simulations, respectively.
Author Contributions
Conceptualization, V.P.; methodology, V.P. and D.V.; software, D.V. and V.P.; formal analysis, D.V. and V.P.; investigation, D.V. and V.P.; writing—original draft preparation, V.P.; writing—review and editing, V.P.; visualization, D.V. and V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the project FarmInova (KK.01.1.1.02.0021) funded by the European Regional Development Fund.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This research was performed using the advanced computing service provided by University of Zagreb University Computing Centre—SRCE.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| DFT | Density functional theory |
| IBO | Intrinsic bond orbital |
| HAT | Hydrogen atom transfer |
| PCET | Proton-coupled electron transfer |
| ET | Electron transfer |
| PT | Proton transfer |
| SPLET | Sequential proton-loss electron transfer |
| IRC | Intrinsic reaction coordinate |
| MD | Molecular dynamics |
References
- Mayer, J.M. Understanding Hydrogen Atom Transfer: From Bond Strengths to Marcus Theory. Acc. Chem. Res. 2011, 44, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.M.; Hrovat, D.A.; Thomas, J.L.; Borden, W.T. Proton-Coupled Electron Transfer versus Hydrogen Atom Transfer in Benzyl/Toluene, Methoxyl/Methanol, and Phenoxyl/Phenol Self-Exchange Reactions. J. Am. Chem. Soc. 2002, 124, 11142–11147. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.J.; Huynh, M.H.V.; Thorp, H.H. The Possible Role of Proton-Coupled Electron Transfer (PCET) in Water Oxidation by Photosystem II. Angew. Chem. Int. Ed. 2007, 46, 5284–5304. [Google Scholar] [CrossRef] [PubMed]
- Klinman, J.P.; Offenbacher, A.R. Understanding Biological Hydrogen Transfer Through the Lens of Temperature Dependent Kinetic Isotope Effects. Acc. Chem. Res. 2018, 51, 1966–1974. [Google Scholar] [CrossRef]
- Ingold, K.U.; Pratt, D.A. Advances in Radical-Trapping Antioxidant Chemistry in the 21st Century: A Kinetics and Mechanisms Perspective. Chem. Rev. 2014, 114, 9022–9046. [Google Scholar] [CrossRef]
- Smith, L.M.; Aitken, H.M.; Coote, M.L. The Fate of the Peroxyl Radical in Autoxidation: How Does Polymer Degradation Really Occur? Acc. Chem. Res. 2018, 51, 2006–2013. [Google Scholar] [CrossRef]
- Capaldo, L.; Ravelli, D. Hydrogen Atom Transfer (HAT): A Versatile Strategy for Substrate Activation in Photocatalyzed Organic Synthesis. Eur. J. Org. Chem. 2017, 2017, 2056–2071. [Google Scholar] [CrossRef]
- Poon, J.-F.; Pratt, D.A. Recent Insights on Hydrogen Atom Transfer in the Inhibition of Hydrocarbon Autoxidation. Acc. Chem. Res. 2018, 51, 1996–2005. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free Radicals, Reactive Oxygen Species, Oxidative Stress and Its Classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive Oxygen Species, Toxicity, Oxidative Stress, and Antioxidants: Chronic Diseases and Aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Sezgin-Bayindir, Z.; Paiva-Martins, F.; Bravo-Díaz, C. Biochemistry of Antioxidants: Mechanisms and Pharmaceutical Applications. Biomedicines 2022, 10, 3051. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, C.; Di Ferdinando, M.; Fini, A.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants and Developmental Regulators: Relative Significance in Plants and Humans. Int. J. Mol. Sci. 2013, 14, 3540–3555. [Google Scholar] [CrossRef] [PubMed]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Romano, B.; Pagano, E.; Montanaro, V.; Fortunato, A.L.; Milic, N.; Borrelli, F. Novel Insights into the Pharmacology of Flavonoids. Phytother. Res. 2013, 27, 1588–1596. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as Antioxidants in Plants: Location and Functional Significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Wang, W.; Sun, C.; Mao, L.; Ma, P.; Liu, F.; Yang, J.; Gao, Y. The Biological Activities, Chemical Stability, Metabolism and Delivery Systems of Quercetin: A Review. Trends Food Sci. Technol. 2016, 56, 21–38. [Google Scholar] [CrossRef]
- Zou, H.; Ye, H.; Kamaraj, R.; Zhang, T.; Zhang, J.; Pavek, P. A Review on Pharmacological Activities and Synergistic Effect of Quercetin with Small Molecule Agents. Phytomedicine 2021, 92, 153736. [Google Scholar] [CrossRef]
- Xiong, F.; Zhang, Y.; Li, T.; Tang, Y.; Song, S.-Y.; Zhou, Q.; Wang, Y. A Detailed Overview of Quercetin: Implications for Cell Death and Liver Fibrosis Mechanisms. Front. Pharmacol. 2024, 15, 1389179. [Google Scholar] [CrossRef]
- Ishizawa, K.; Yoshizumi, M.; Kawai, Y.; Terao, J.; Kihira, Y.; Ikeda, Y.; Tomita, S.; Minakuchi, K.; Tsuchiya, K.; Tamaki, T. Pharmacology in Health Food: Metabolism of Quercetin In Vivo and Its Protective Effect Against Arteriosclerosis. J. Pharmacol. Sci. 2011, 115, 466–470. [Google Scholar] [CrossRef]
- Kee, J.-Y.; Han, Y.-H.; Kim, D.-S.; Mun, J.-G.; Park, J.; Jeong, M.-Y.; Um, J.-Y.; Hong, S.-H. Inhibitory Effect of Quercetin on Colorectal Lung Metastasis through Inducing Apoptosis, and Suppression of Metastatic Ability. Phytomedicine 2016, 23, 1680–1690. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of Quercetin and Resveratrol Co-Incorporated in Liposomes against Inflammatory/Oxidative Response Associated with Skin Cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ramešová, Š.; Sokolová, R.; Degano, I.; Bulíčková, J.; Žabka, J.; Gál, M. On the Stability of the Bioactive Flavonoids Quercetin and Luteolin under Oxygen-Free Conditions. Anal. Bioanal. Chem. 2012, 402, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Escandar, G.M.; Sala, L.F. Complexing Behavior of Rutin and Quercetin. Can. J. Chem. 1991, 69, 1994–2001. [Google Scholar] [CrossRef]
- Sokolová, R.; Ramešová, Š.; Degano, I.; Hromadová, M.; Gál, M.; Žabka, J. The Oxidation of Natural Flavonoid Quercetin. Chem. Commun. 2012, 48, 3433–3435. [Google Scholar] [CrossRef]
- Lemańska, K.; Szymusiak, H.; Tyrakowska, B.; Zieliński, R.; Soffers, A.E.M.F.; Rietjens, I.M.C.M. The Influence of pH on Antioxidant Properties and the Mechanism of Antioxidant Action of Hydroxyflavones. Free. Radic. Biol. Med. 2001, 31, 869–881. [Google Scholar] [CrossRef]
- Musialik, M.; Kuzmicz, R.; Pawłowski, T.S.; Litwinienko, G. Acidity of Hydroxyl Groups: An Overlooked Influence on Antiradical Properties of Flavonoids. J. Org. Chem. 2009, 74, 2699–2709. [Google Scholar] [CrossRef]
- Álvarez-Diduk, R.; Ramírez-Silva, M.T.; Galano, A.; Merkoçi, A. Deprotonation Mechanism and Acidity Constants in Aqueous Solution of Flavonols: A Combined Experimental and Theoretical Study. J. Phys. Chem. B 2013, 117, 12347–12359. [Google Scholar] [CrossRef]
- Zenkevich, I.G.; Guschina, S.V. Determination of Dissociation Constants of Species Oxidizable in Aqueous Solution by Air Oxygen on an Example of Quercetin. J. Anal. Chem. 2010, 65, 371–375. [Google Scholar] [CrossRef]
- Yazdanshenas, R.; Gharib, F. Protonation Equilibria Studies of Quercetin in Aqueous Solutions of Ethanol and Dimethyl Sulphoxide. J. Mol. Liq. 2016, 224, 1227–1232. [Google Scholar] [CrossRef]
- Litwinienko, G.; Ingold, K.U. Solvent Effects on the Rates and Mechanisms of Reaction of Phenols with Free Radicals. Acc. Chem. Res. 2007, 40, 222–230. [Google Scholar] [CrossRef]
- Foti, M.C.; Daquino, C.; Mackie, I.D.; DiLabio, G.A.; Ingold, K.U. Reaction of Phenols with the 2,2-Diphenyl-1-Picrylhydrazyl Radical. Kinetics and DFT Calculations Applied To Determine ArO-H Bond Dissociation Enthalpies and Reaction Mechanism. J. Org. Chem. 2008, 73, 9270–9282. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as Antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Hara, Y.; Simic, M.G. Reduction Potentials of Flavonoid and Model Phenoxyl Radicals. Which Ring in Flavonoids Is Responsible for Antioxidant Activity? J. Chem. Soc. Perkin Trans. 2 1996, 11, 2497–2504. [Google Scholar] [CrossRef]
- Torić, J.; Karković Marković, A.; Mustać, S.; Pulitika, A.; Jakobušić Brala, C.; Pilepić, V. Proton-Coupled Electron Transfer and Hydrogen Tunneling in Olive Oil Phenol Reactions. Int. J. Mol. Sci. 2024, 25, 6341. [Google Scholar] [CrossRef]
- La Rocca, M.V.; Rutkowski, M.; Ringeissen, S.; Gomar, J.; Frantz, M.-C.; Ngom, S.; Adamo, C. Benchmarking the DFT Methodology for Assessing Antioxidant-Related Properties: Quercetin and Edaravone as Case Studies. J. Mol. Model. 2016, 22, 250. [Google Scholar] [CrossRef]
- Zhang, D.; Liu, Y.; Chu, L.; Wei, Y.; Wang, D.; Cai, S.; Zhou, F.; Ji, B. Relationship Between the Structures of Flavonoids and Oxygen Radical Absorbance Capacity Values: A Quantum Chemical Analysis. J. Phys. Chem. A 2013, 117, 1784–1794. [Google Scholar] [CrossRef]
- Klein, E.; Rimarčík, J.; Senajová, E.; Vagánek, A.; Lengyel, J. Deprotonation of Flavonoids Severely Alters the Thermodynamics of the Hydrogen Atom Transfer. Comput. Theor. Chem. 2016, 1085, 7–17. [Google Scholar] [CrossRef]
- Chiodo, S.G.; Leopoldini, M.; Russo, N.; Toscano, M. The Inactivation of Lipid Peroxide Radical by Quercetin. A Theoretical Insight. Phys. Chem. Chem. Phys. 2010, 12, 7662–7670. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A 2004, 108, 4916–4922. [Google Scholar] [CrossRef]
- Leopoldini, M.; Marino, T.; Russo, N.; Toscano, M. Density Functional Computations of the Energetic and Spectroscopic Parameters of Quercetin and Its Radicals in the Gas Phase and in Solvent. Theor. Chem. Acc. 2004, 111, 210–216. [Google Scholar] [CrossRef]
- Dimić, D.; Milenković, D.; Dimitrić Marković, J.; Marković, Z. Antiradical Activity of Catecholamines and Metabolites of Dopamine: Theoretical and Experimental Study. Phys. Chem. Chem. Phys. 2017, 19, 12970–12980. [Google Scholar] [CrossRef] [PubMed]
- Amić, A.; Marković, Z.; Dimitrić Marković, J.M.; Stepanić, V.; Lučić, B.; Amić, D. Towards an Improved Prediction of the Free Radical Scavenging Potency of Flavonoids: The Significance of Double PCET Mechanisms. Food Chem. 2014, 152, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Mayer, J.M. Proton-Coupled Electron Transfer: A Reaction Chemist’s View. Annu. Rev. Phys. Chem. 2004, 55, 363–390. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, D.R.; Gagliardi, C.J.; Hull, J.F.; Murphy, C.F.; Kent, C.A.; Westlake, B.C.; Paul, A.; Ess, D.H.; McCafferty, D.G.; Meyer, T.J. Proton-Coupled Electron Transfer. Chem. Rev. 2012, 112, 4016–4093. [Google Scholar] [CrossRef]
- Truhlar, D.G. Tunneling in Enzymatic and Nonenzymatic Hydrogen Transfer Reactions. J. Phys. Org. Chem. 2010, 23, 660–676. [Google Scholar] [CrossRef]
- Klein, J.E.M.N.; Knizia, G. cPCET versus HAT: A Direct Theoretical Method for Distinguishing X–H Bond-Activation Mechanisms. Angew. Chem. Int. Ed. 2018, 57, 11913–11917. [Google Scholar] [CrossRef]
- Knizia, G. Intrinsic Atomic Orbitals: An Unbiased Bridge between Quantum Theory and Chemical Concepts. J. Chem. Theory Comput. 2013, 9, 4834–4843. [Google Scholar] [CrossRef]
- Knizia, G.; Klein, J.E.M.N. Electron Flow in Reaction Mechanisms—Revealed from First Principles. Angew. Chem. Int. Ed. 2015, 54, 5518–5522. [Google Scholar] [CrossRef]
- Kühne, T.D.; Iannuzzi, M.; Del Ben, M.; Rybkin, V.V.; Seewald, P.; Stein, F.; Laino, T.; Khaliullin, R.Z.; Schütt, O.; Schiffmann, F.; et al. CP2K: An Electronic Structure and Molecular Dynamics Software Package—Quickstep: Efficient and Accurate Electronic Structure Calculations. J. Chem. Phys. 2020, 152, 194103. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Goedecker, S.; Teter, M.; Hutter, J. Separable Dual-Space Gaussian Pseudopotentials. Phys. Rev. B 1996, 54, 1703–1710. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Hansen, A.; Brandenburg, J.G.; Bannwarth, C. Dispersion-Corrected Mean-Field Electronic Structure Methods. Chem. Rev. 2016, 116, 5105–5154. [Google Scholar] [CrossRef] [PubMed]
- VandeVondele, J.; Hutter, J. Gaussian Basis Sets for Accurate Calculations on Molecular Systems in Gas and Condensed Phases. J. Chem. Phys. 2007, 127, 114105. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Campo, M.G.; Corral, G.M. Structural, Dynamic, and Hydration Properties of Quercetin and Its Aggregates in Solution. J. Phys. Condens. Matter 2022, 34, 294001. [Google Scholar] [CrossRef]
- Lespade, L. Ab Initio Molecular Dynamics of the Reaction of Quercetin with Superoxide Radical. Chem. Phys. 2016, 475, 32–38. [Google Scholar] [CrossRef]
- Lespade, L. Ab Initio Molecular Dynamics of Electron Transfer from Gallic Acid to Small Radicals: A Comparative Study between Hydroxyl and Nitrogen Dioxide Radicals. Comput. Theor. Chem. 2018, 1135, 6–10. [Google Scholar] [CrossRef]
- Lespade, L. Ab Initio Molecular Dynamics of the Reactivity of Vitamin C toward Hydroxyl and HO2/O2− radicals. J. Mol. Model. 2017, 23, 347. [Google Scholar] [CrossRef]
- Milovanović, B.; Ilić, J.; Stanković, I.M.; Popara, M.; Petković, M.; Etinski, M. A Simulation of Free Radicals Induced Oxidation of Dopamine in Aqueous Solution. Chem. Phys. 2019, 524, 26–30. [Google Scholar] [CrossRef]
- Zeppilli, D.; Orian, L. Concerted Proton Electron Transfer or Hydrogen Atom Transfer? An Unequivocal Strategy to Discriminate These Mechanisms in Model Systems. Phys. Chem. Chem. Phys. 2025, 27, 6312–6324. [Google Scholar] [CrossRef]
- Kohlmann, T.; Goez, M. Laser Access to Quercetin Radicals and Their Repair by Co-Antioxidants. Chem. A Eur. J. 2020, 26, 17428–17436. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).