1. Introduction
The raw material industry is an energy-intensive industry that is responsible for significant greenhouse gas (GHG) emissions. Energy reduction and decarbonization in this sector are very important. The production of primary metal typically includes ore mining and concentrating, smelting or separation, and refining to obtain the element in its metallic form. The most significant environmental impacts of the production of the majority of metals are generated in the smelting, purification, and refining stages, in which metals are transformed from a concentrate into their metallic form. Up to now, the metallurgical industry has used carbon in the form of coal, a cheap and easily mined raw material, as a reducing agent to remove oxygen from oxide ores to produce the majority of metals. During the metal extraction process, carbon is oxidized to CO
2, resulting in considerable CO
2 emissions that have a negative impact on climate change. According to the International Energy Agency [
1], the final energy consumption for metal and mineral production has almost doubled over 20 years, from 25.5 EJ in 2002 to 47.5 EJ in 2019. This surge parallels the broader rise in global final energy consumption. After a long, steady trend of around 9% before 2000, metal and mineral production increased to represent 11.4% of global final energy consumption in 2019, after a peak of more than 12% in the mid-2010s [
2].
A radical change to replace carbon in ore processes for metal production is essential to achieve the EU target to reduce the carbon emissions from the industrial sector by 80–95%, which is related to the emissions generated in 1990. The heavy economic burden added to the critical metal operation cost due to the CO
2 tax, the necessity to reach the 2050 targets for a climate-neutral society, as well as the need to respond to the environmental concerns of local communities dictate the replacement of carbon and the minimization of the related to the production processes CO
2 emissions. Accelerated climate changes are expected to have a high impact on the CO
2 tax. Joint recommendations for the next European Mandate (2024–2029) prepared by the Sectoral Social Dialogue Committee for Extractive Industries pointed out that “a strong focus on the circular economy is key to open strategic autonomy and the preservation of natural resources” [
3].
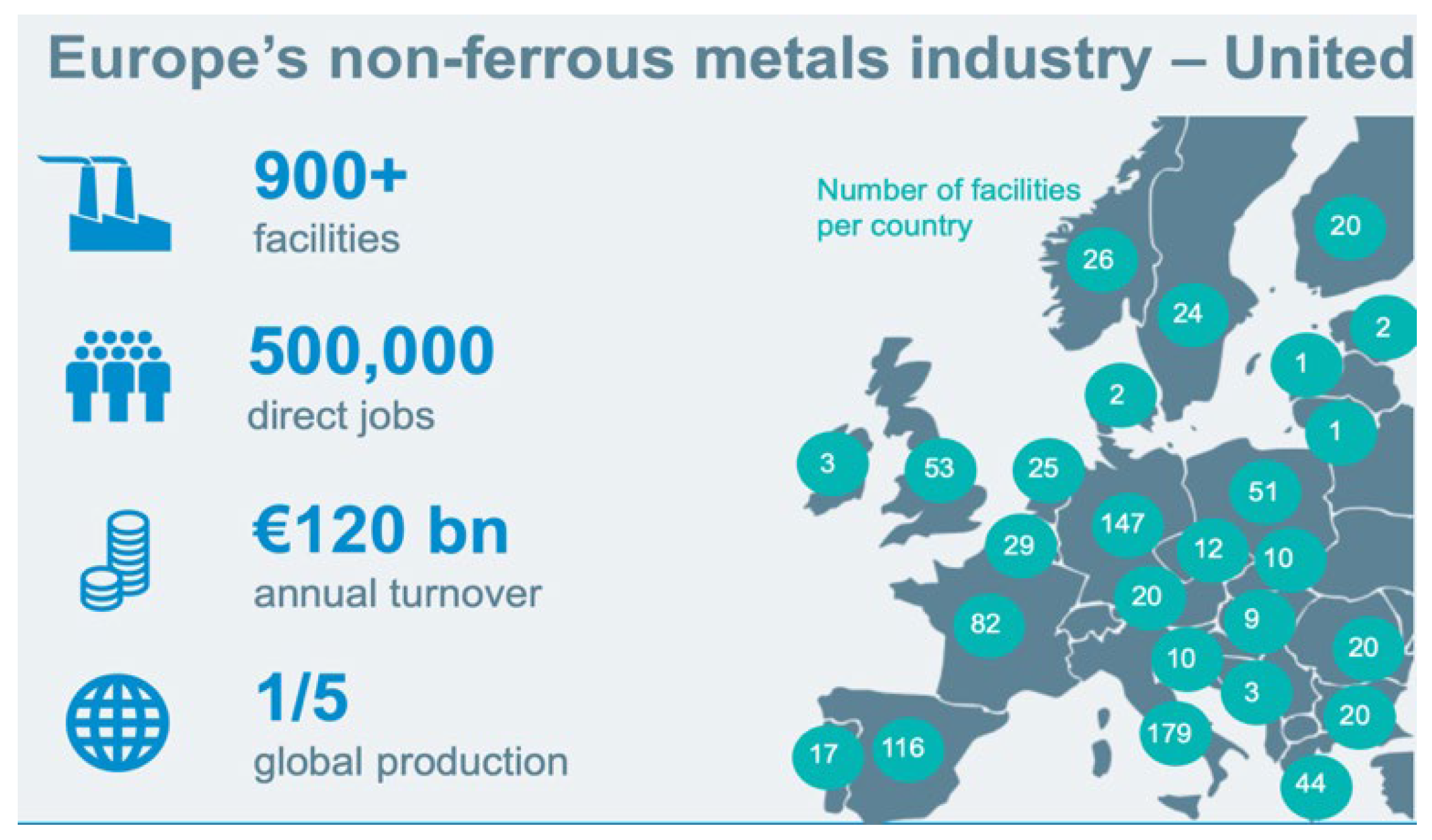
It is worth mentioning that the non-ferrous metals sector provides 500,000 direct jobs and 3 million indirect jobs in the EU, with thousands more across Europe more broadly (see
Figure 1). These jobs are often high-quality, well-paid jobs, and trade unions have come together to fight to keep these jobs in Europe. European trade unions call for urgent action to level the global playing field to ensure Europe’s competitiveness in producing high-quality green products while preserving and creating jobs [
4].
2. Hydrogen as an Eco-Friendly Reducing Agent in Metal Production
Hydrogen is expected to play a key role in the decarbonization of the energy system. As of December 2023, more than 42 hydrogen strategies and roadmaps have been published by governments around the world [
5]. In this direction, as the process industries push toward decarbonization, a decisive shift from carbon has been enabled by the adoption of hydrogen as (a) hydrogen serves as both a sustainable energy source and an eco-friendly reducing agent in metal production and (b) hydrogen can enhance performance due to many factors [
6]. These include the lack of contaminants found in coal and coke, which simplifies metal purification. Moreover, the reduction temperature is lower in H
2-based processes, while simpler, more efficient, and controllable continuous reactors can be utilized. Finally, consistent hydrogen supply through Renewable Energy Sources (RES) is possible while simultaneously reducing reliance on coal mining and imports.
The development of new processes using hydrogen gas rather than solid materials like coal and coke can improve the performance of metal production and lead to the application of cleaner, less polluting and energy-consuming processes as (i) H2 gas is cleaner and does not contain critical contaminants, like coal and coke, and, therefore, the purification of the produced metal is easier; (ii) H2 gas metal reduction can be carried out at much lower temperatures; (iii) simpler, higher-performance, easy-to-control, continuous reactors can potentially be used; (iv) uninterrupted H2 supply can be secured with the development of a RES-to-H2 integrated system coupling H2 production with the metal production process; and (v) coal mining and dependency on coal imports would not be required at its present extent.
However, the application of hydrogen in the metallurgy of critical metals is limited to only a small number of refractory metals like molybdenum and tungsten. For oxide minerals, using hydrogen instead of coal and assuming the achievement of 90% metal reduction, hydrogen will reduce the relevant emissions from 5.80 to 0.47 t of CO
2 per ton of FeNi [
7,
8], 2.75 to 0.22 t of CO
2 per ton of Sb [
9], and 3.66 to 0.29 t of CO
2 per ton of Sn [
10], accounting for a reduction of 114 Mt CO
2 emissions globally. Severe energy reductions are also expected compared to the currently applied processes due to the 400 to 600 °C lower reaction temperature required for the metal oxide–hydrogen reduction process, depending on the type of metal oxide [
11,
12,
13,
14,
15].
Besides oxide minerals, sulfide ores represent a major source of critical metals. However, the treatment of sulfide minerals is more complicated, as it results in the production of H2S gas and not of water, as in the case of oxide ores. Hydrogen sulfide is a toxic gas and cannot be emitted into the atmosphere as is. Therefore, the development of a sustainable process for the direct hydrogen reduction of sulfide minerals should involve, as an integral part, an efficient and economical process of the transformation of hydrogen sulfide to inert, easily manageable, harmless compounds. Thermodynamic analysis shows that there are several critical metals, like Ni, Co, Cu, and Sb, and other technically important metals, like Sn, associated with the production of critical metals, like Ta and Nb, that can be produced through the hydrogen reduction of their oxides and sulfide mineral resources.
Proposed alternatives to convert H
2S into other value-added products to substitute the Claus process with comparatively higher Technology Readiness Level (TRL), such as partial oxidation, catalytic thermal, and reformation, are still reported to be energy-consuming, although demonstrating good technical feasibility. Therefore, new technologies for more sustainable and energy-efficient H
2S conversion, without requiring high energy consumption at large scales, such as non-thermal plasma and electrochemistry, is an ongoing and continuing research process. These emerging low-TRL technologies have considerable potential to be low-carbon sustainable processes when coupled with renewable energy. However, appropriate performance optimization strategies, such as the proper selection of catalysts, design of reactor systems, and operating conditions, are needed to enable economical and feasible scale-up. This enables the produced H
2 to be recycled in the sulfide reaction process and the unused H
2 and produced sulfur to be sold. The proposed process directly addresses all the inherent disadvantages of the conventional production of Ni, Co, and Cu from sulfide ores, like complexity and many high-temperature production stages and unusually high CO
2 emissions, successfully addressing the environmental problems and rendering the production process from H
2 consumer to H
2 producer. It is important to note that the energy required to produce hydrogen from H
2S is (381 kJ/mole), 23% less than that required for hydrogen production through water electrolysis (498 kJ/mole), providing a significant opportunity for low-cost H
2 production and use [
16].
3. Potential Applications of Green H2 as a Reducing Agent
The utilization of hydrogen as a reducing agent has been extensively studied at lab and pilot scales and has started to find industrial applications in the reduction of iron ores to produce sponge iron [
17]. Replacing coal with hydrogen seems a promising option for decarbonizing the steel production process. In the following paragraphs, metallurgical sectors where H
2 could play a transformative role in a low-carbon future for the extractive industry are presented.
3.1. Nickel–Cobalt Ferroalloys
Nickel is a commercially important metal used in lithium-ion batteries (7%), and in the form of ferroalloys, it is mainly used for the production of stainless steel (~81%). Cobalt is primarily used in the manufacturing of rechargeable lithium-ion batteries (57%) for portable electronic devices, energy storage systems, electric vehicles, and magnets for electric motors (4%), and also in the form of Ni-based alloys mainly used in turbine engine components (13%). Nickel–cobalt ferroalloys are currently produced via carbothermic pre-reduction processing of laterite ores in rotary kilns, achieving only a 40–45% reduction of nickel and iron oxides in the ore at this stage [
7,
8]. This process is followed by the reduction of the pre-reduced material using carbon electrodes in a submerged arc furnace (SAF), which yields Ni-Co ferroalloys containing 13–29% nickel metal. This process is energy-intensive, consumes considerable amounts of coal and lignite (2.85 Kg/t FeNi), and results in CO
2 emissions of 5.8 kg CO
2/t FeNi [
12]. The main innovation proposed in this case is the hydrogen reduction of nickeliferous laterite in a fluidized bed reactor to reduce iron, nickel, and cobalt oxides, followed by the concentration of the metallic iron, nickel, and cobalt by magnetic separation of the formed metal phase and, finally, production of refined ferroalloy with high nickel content in SAF. This process will eliminate the use of coal in the reduction process, produce high nickel content ferroalloy, significantly reduce the electric energy consumed in the SAF, and eliminate the use of fossil fuels as all the processes are fully electrified.
3.2. Sulfide Nickel–Cobalt Ores
Nickel produced from sulfide ores constitutes about 30% of the nickel production worldwide [
13,
14]. The conventional process for nickel extraction from sulfide ores involves smelting Ni sulfide concentrates to produce a nickel sulfide melt, known as Bessemer matte; matte roasting to remove sulfur; the reduction to crude nickel; carbonylation and purification to nickel metal; leaching and electrowinning of nickel matte; or electrorefining of crude nickel metal. The whole process is complicated and expensive and generates a substantial amount of SO
2 gas, which is captured to produce sulfuric acid at a significant cost and without avoiding a certain quantity of SO
2 being released into the atmosphere. The high cost of SO
2 management alone has, in some instances, resulted in the shutdown of smelters. This is a major drawback of the conventional industrial process for producing sulfuric acid from the generated SO
2 gas.
A breakthrough process for the treatment of nickel sulfide concentrates may involve (i) hydrogen reduction of the concentrate in a fluidized bed to produce an alloy of metallic iron, nickel, and cobalt and hydrogen sulfide as a by-product of the reaction between hydrogen introduced as reductant and sulfur present in the concentrate; (ii) magnetic separation of iron, nickel, and cobalt metals and further refining to produce high-quality ferronickel alloy; and (iii) decomposition of hydrogen sulfide to hydrogen gas and solid sulfur in a plasma reactor achieving hydrogen recycling in the reduction process and producing inert, marketable sulfur material. The main advantages of the proposed process include the (i) simplification of the highly complex treatment process of nickel–cobalt sulfide concentrates, (ii) recovery of the used hydrogen positively affecting the cost and sustainability of the overall sulfide mineral reduction process, and (iii) effectively addressing the conventional sulfide ore processes environmental problem of SO
2(g) generation through the efficient processing of H
2S and the consecutive production of H
2 and sulfur [
15,
16,
18]. In both cases, the ferronickel produced can be either sold to the stainless steel industry or further processed by hydro-electrometallurgical processing to produce nickel and cobalt metal.
3.3. Tin Production from Low-Grade Cassiterite Ores
The most important tin mineral is cassiterite (SnO2), which is commonly concentrated by gravity separation methods due to the differences between the density of cassiterite and the gangue materials (usually silicates), as well as flotation techniques. Various low-grade tin deposits are found in Europe, and they contain various critical metals like W, Nb, and Ta. The currently applied carbothermic reduction used to extract Sn from gangue minerals is not green or environmentally friendly, as it typically produces CO2 and slag at high temperatures. Limited research on the reduction of cassiterite using methane, carbonaceous reductants, and reduction by gaseous mixtures of CO and H2 has been performed.
The reduction of cassiterite using hydrogen as a reducing agent, besides the elimination of CO
2 emissions, has two decisive advantages compared to carbon and carbon monoxide processing: (i) the rate of H
2 diffusion in the solid particles pores is 4 times higher, and its thermal conductivity is 7 times higher than that of CO, and (ii) the reduction with appropriate hydrogen–steam mixtures is expected to reduce cassiterite and produce metal with a low iron content in a one-step process with a high reaction yield, as indicated by the relevant H
2-Fe-Fe
2O
3-Fe
3O
4-phase equilibrium diagram [
9].
3.4. Direct Smelting of Low-Grade Antimony Ores to Produce Metallic Antimony
Currently, pyrometallurgical processes are used to produce antimony from stibnite (Sb
2S
3) sulfide ores. This involves roasting low-grade stibnite ores at 1000 °C to produce antimony oxide (Sb
2O
3) and then reducing Sb
2O
3 to antimony metal using charcoal in a reverberatory furnace (1200 °C) with the formation of slag [
10]. The main disadvantages of the above processes involve (i) the production of SO
2(g) during sulfide roasting, (ii) CO
2 emissions due to coal reduction, and (iii) increased antimony losses due to high-temperature processing. Direct smelting of stibnite replacing coal by hydrogen to produce metallic antimony could be a process in a considerably lower temperature, around 625 °C (instead of 1000 °C), and the H
2S(g) produced can be decomposed in a plasma reactor to produce H
2(g) to be recycled in the process and marketable S(s).
3.5. Low-Grade Copper Sulfide Ores and Tailings to Produce Metallic Copper
Most of the currently exploited copper ores are in the form of sulfides, especially chalcopyrite (CuFeS
2) and, to a lesser extent, chalcocite (Cu
2S). The conventional pyrometallurgical method of copper extraction from these ores involves several steps, including roasting, smelting, converting, fire refining, and electrorefining. The oxidation of the contained sulfur results in the generation of highly polluting sulfur dioxide gas [
19]. An alternative process could be the reduction of copper sulfides by hydrogen to produce metallic copper. Although this alternative seems to be attractive for its simplicity, there are some technical problems that should be addressed, including the unfavorable thermodynamics, the low equilibrium constant of the H
2 reduction reaction, and the SO
2(g) generation. An efficient method to overcome the thermodynamic barrier and increase the copper yield is to shift the reaction equilibrium continuously to the right by introducing sufficiently large quantities of H
2 in the system and continuously removing the generated H
2S as soon as it is formed. The coupling of the sulfide reduction process with the continuous decomposition of the formed H
2S(g) to H
2(g) and S(s) will allow the continuous recycling and feed of the produced H
2(g) in sufficient quantities into the system and secure the economic performance of the process without adding excess cost.
4. Thermal Processing Electrification
Thermal processing reactors like rotary kilns have become the backbone of many industrial processes, such as in roasting and calcination, particularly in recent years, as the pressure to create a circular economy has continued to rise. Currently, in thermal processing, direct-fired rotary kilns (DFRKs) are mainly used, which rely on direct contact between the process gas and the material in order to heat the material to the specified temperature. Direct-fired kilns can be either the co-current design or counter-current design, referring to the direction in which the process gas flows through the drum in relation to the material. While DFRKs are relatively simple in design, operation, and maintenance, and they are efficient in terms of heat transfer due to the direct contact between the material and the combustion gases, they also have some major drawbacks, which turn out to be crucial in many applications. By adapting DFRK technology, off-gases flowing with an inherent velocity are produced, and particles that are too fine can become entrained in the process of gas flow and be carried out through the exhaust gas system. This results in a high rate of reprocessing and can significantly reduce the overall process efficiency. Dealing with this problem requires the design of the kiln to be centered around permissible gas velocities as opposed to heat transfer requirements. Additionally, DFRKs provide limited atmosphere control as the atmosphere inside the kiln is essentially the same as the combustion atmosphere, where oxygen is also present. As a result, DFRKs cannot maintain an inert or non-reactive atmosphere, and the inevitable oxidizing environment can affect the quality of materials that are sensitive to oxidation. Finally, the direct exposure of the material to the heat leads to a gradient in temperature, with parts of the material closer to the flame being hotter than those further away. This uneven temperature distribution can affect the consistency and quality of the process’s material, leading to a non-uniform final product.
Replacement of DFRKs with electrified indirect-fired rotary kilns (IFRΚs) and microwave rotary kilns (MRKs) removes the need for process gasses (see
Figure 2), solving the issue of fine and ultrafine material loss through drag, reducing emissions and improving the controllability and flexibility of the calcination and roasting processes. The capacity to process without losses of fine and ultrafine material is the major contributor to process efficiency, as losses can exceed 25% in many industries, as previously described. Additionally, IFRΚs can maintain an inert or non-reactive atmosphere, avoiding an inevitable oxidizing environment, which can affect the quality of materials that are sensitive to oxidation. Finally, the direct exposure of the material to the heat leads to a gradient in temperature, with parts of the material closer to the flame being hotter than those further away. This uneven temperature distribution can affect the consistency and quality of the process’s material, leading to a non-uniform final product.
It is worth mentioning that in the cement industry, the amount of cement kiln dust (CKD) generated typically ranges from 15 to 20% of the produced cement, leading to an annual CKD production of approximately 12.9 metric tons. Similarly, the lime industry generates Lime Kiln Dust (LKD), with an annual production of approximately 1.8 to 3.6 million metric tons in the United States alone [
21]. CKD and LKD are usually stockpiled, and it is estimated that the total amount of kiln dust currently stockpiled throughout the country exceeds close to 90 million metric tons (100 million tons), creating substantial issues in their management.
5. Discussion
The global direct and indirect GHG emissions of the metal production industry are estimated at 3400 million tons CO
2-eq/y, from which 71% are attributed to iron and steel production, 11% to aluminum, 8% to non-metallic minerals, and the remaining 10% to non-ferrous metals. The cradle-to-gate GWP per kilogram of each of the metals production routes analyzed is 9, 13, 8.3, 3.1, 13, and 6.6 kg CO
2-eq per kg for FeNi (20% in Ni), Ni (class 1), Co, Cu, Sb, and Sn, respectively [
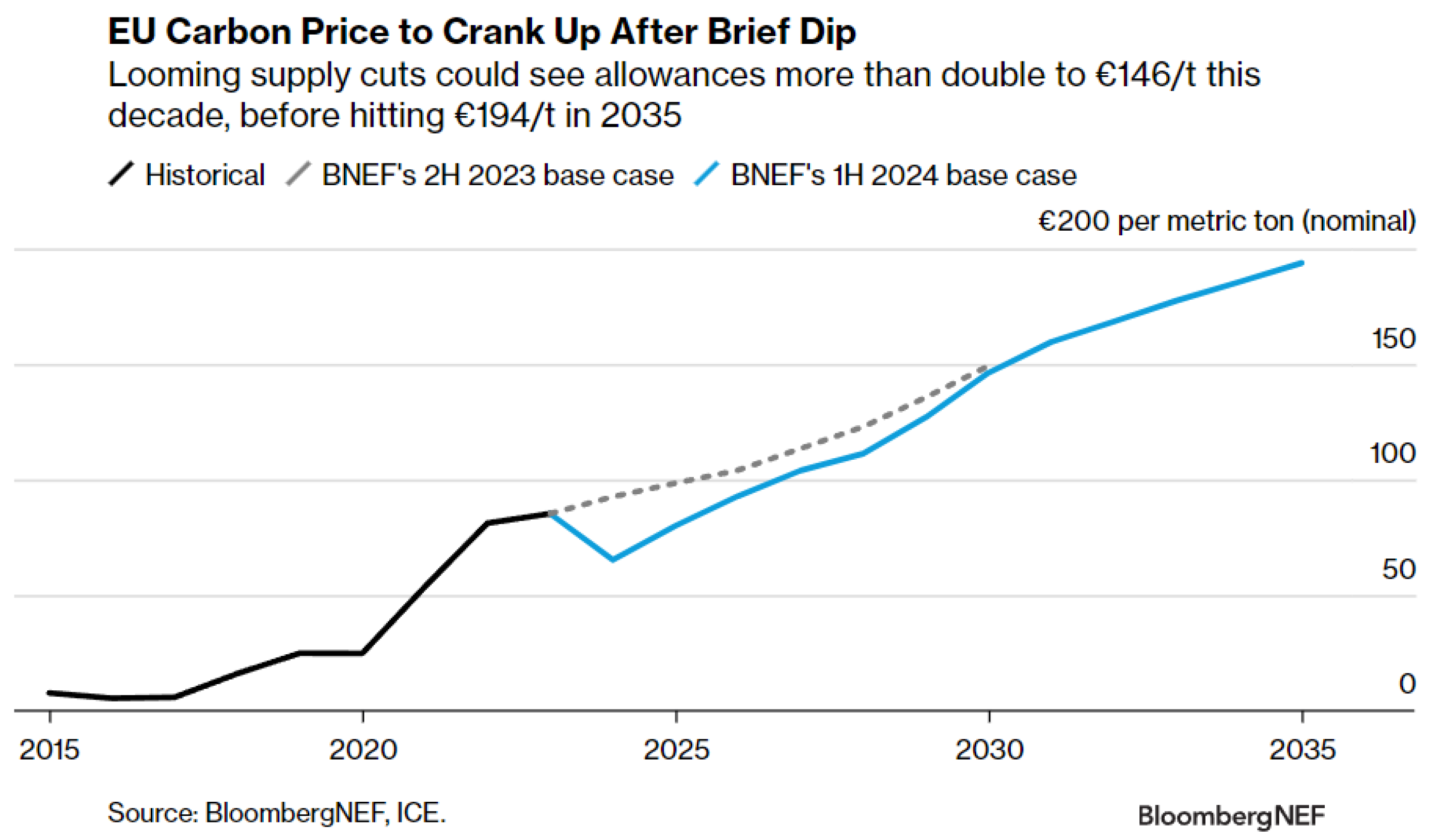
22]. It is worth mentioning that the price of EU emission allowances is expected to dip to an average of EUR 65 per metric ton (USD 70/t) this year before more than doubling to EUR 146/t by the end of the decade. Prices could approach the EUR 200/t milestone in 2035 if policy parameters remain unchanged (see
Figure 3) [
23].
The European non-ferrous metals sector is facing global challenges on many fronts: unbalanced international competition, unfair access to raw materials, huge pressures to decarbonize, and high energy costs. Trade unions call on stakeholders to take urgent action to enable the industry and its workers to continue to develop world-leading, high-quality, and low-carbon products. In parallel with the new European high-energy-price environment, imposing new trade barriers on energy-intensive products, like critical metals, could have unwanted consequences for European value chains.
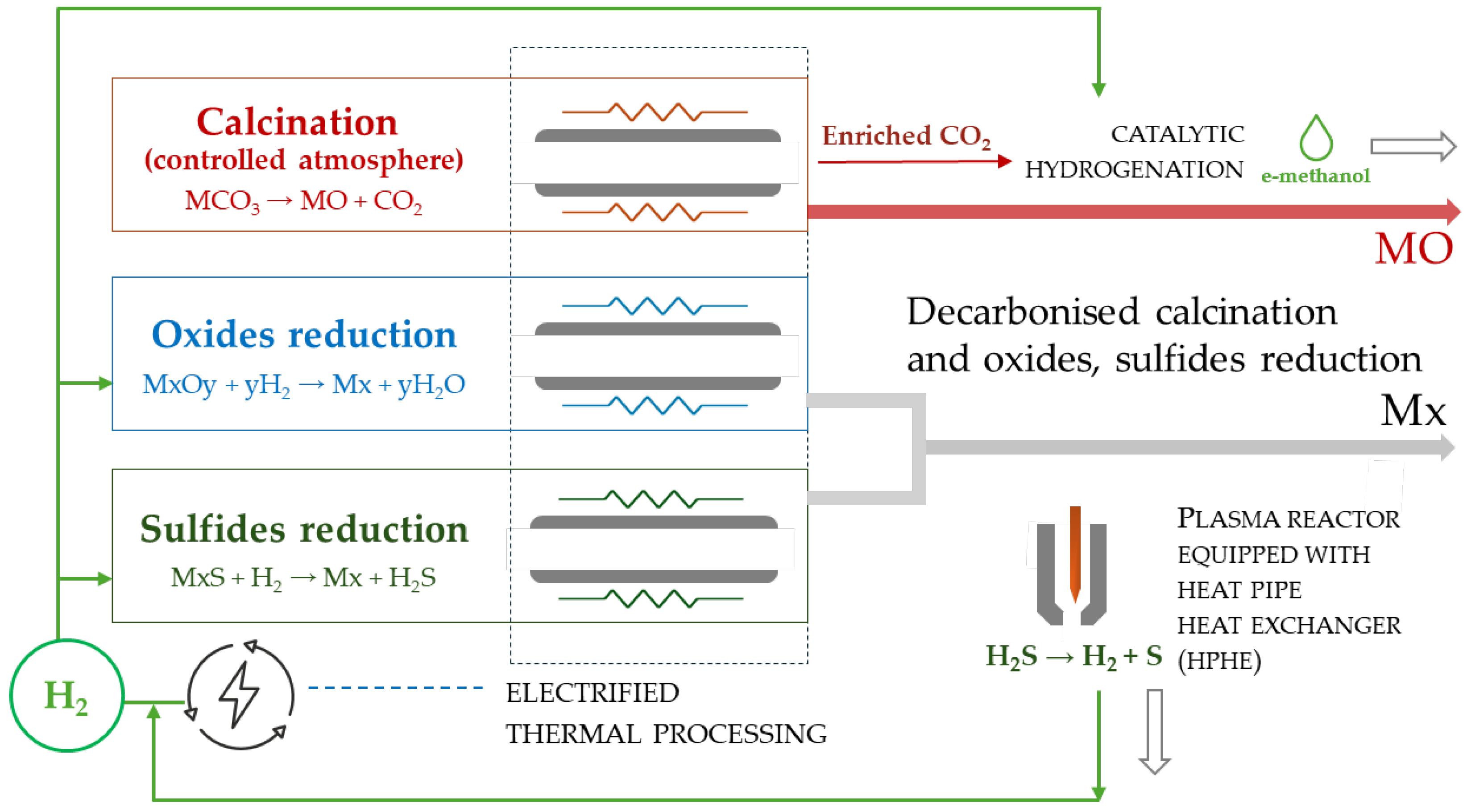
The integration of innovative electrification technologies for the indirect heating of metallurgical processes like calcination and the utilization of H
2 as an alternative reductant for both oxides and sulfide ores can significantly assist in providing environmentally and technically sustainable solutions for extractive industrial decarbonization, as shown in
Figure 4.
Oxide reduction of iron (Fe
2O
3), nickel (NiO), and cobalt (CoO) to produce FeNi can replace 1.5 tons of carbon currently consumed to produce 1 ton of FeNi with 0.5 tons of H
2 per ton of FeNi. This results in the reduction of 5.6 tons of CO
2 emissions per ton of produced FeNi. Moreover, FeNi production from laterite will be 32% more energy efficient due to the higher pre-reduction degree of the laterite ore (70–80% reduction efficiency compared to 40–50% of the current process), lower reduction temperature (600–700 °C compared to 900–1000 °C of the current process), and use of a more efficient and productive fluidized bed reactor compared to the rotary kilns currently used [
25,
26]. A carbon amount of about 0.75 t (or 0.93t of metallurgical coke)/t Sb
m can also substituted by H
2, requiring a significantly lower reduction temperature (<600 °C) in comparison to the current industrial processing temperature (1200–400 °C) [
10]. In the case of sulfide ores, the main gains in energy consumption, process efficiency, cost, and reduction in CO
2 emissions originate from (i) the application of less and much simpler unit operations, (ii) the use of fluidized bed and plasma reactors operating at lower temperatures, (iii) capability to recycle the produced hydrogen in the reduction process, and (iv) secure additional income from selling the hydrogen surplus and the produced sulfur. Electrified calcination kilns operating in a controlled atmosphere will minimize flue gas velocities and fine particle loss on exhaust gas systems, which can be enriched in CO
2 and effectively utilized for synthetic fuel production [
27].
The raw materials sector is sustainable in a low-carbon economy. The potential for growth exists and is compatible with the targets of a low-carbon economy. In the future, innovation, increasing energy costs and CO2 prices, and investment support schemes might make these measures more financially attractive. In order to reach the goals of this transition, there is a need to step forward in specific areas of expertise as prerequisites for technology development, which will involve the most important participants maintaining and continuing to develop their competence in metallurgical and process technology disciplines.
As the process industries push towards decarbonization, a decisive shift from carbon is enabled by the adoption of hydrogen as (a) hydrogen serves as both a sustainable energy source and an eco-friendly reducing agent in metal production and (b) hydrogen can enhance performance due to many factors. These include the lack of contaminants found in coal and coke, which simplifies metal purification. Moreover, the reduction temperature is lower in H2-based processes, while simpler, more efficient, and controllable continuous reactors can be utilized. Finally, consistent hydrogen supply through RES is possible while simultaneously reducing reliance on coal mining and imports.
6. Conclusions
To achieve sustainable and uninterrupted growth in the next decades and become climate-neutral, the world will need to produce more critical metals from indigenous raw material resources to supply strategic sectors, such as renewable energy, electric cars, and digital technologies. However, the heavy economic burden added to the critical metal’s operation cost due to the carbon tax, the necessity to reach the 2050 targets for a climate-neutral society, as well as the need to respond to the environmental concerns of local communities dictate the replacement of carbon and the minimization of the related to the production processes CO2 emissions. In this framework, H2 can have a vital role not only as a source of clean fuel energy but also as a climate-neutral reducing agent to produce metals, which is aligned with the decarbonization policies of the metallurgical industry. Even though it already has a fundamental role in sustainable steelmaking, industrial-scale application in non-ferrous metallurgy is still in its early stages, necessitating the further development of fundamental knowledge and resolution of key technical challenges. The present paper presented authors’ perspectives for consideration in relation to H2 application as a reducing agent for nickel–cobalt ferroalloy production; the treatment of cassiterite, antimony, and copper sulfide ores; and also analyzed the carbon emission earning potential. The decarbonization of the metallurgical sector offers transformative opportunities through H2-based technologies and sustainable practices, but addressing its challenges—such as scalability, cost, and infrastructure—requires a strong academic–industry synergy to drive innovation, knowledge sharing, and practical implementation.