Hydrogen from Waste Gasification
Abstract
1. Introduction
2. Gasification to produce Hydrogen from Waste
2.1. Gasification Basics
2.2. Gasification Research on Waste to Hydrogen
2.2.1. Municipal Solid Waste
2.2.2. Waste Tires (WTs)
2.2.3. Waste Plastics (WPs)
| Reactor Type | Agent | ER | T (°C) | H2 (vol%) | CO (vol%) | H2 (g/kg MSW) | LHV (MJ/Nm3) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Fluidized bed/Fixed bed | Air | 0.1 | 600/750 | 36.85 | 35.91 | 65 | - | [81] |
| Dual fluidized bed | Steam | - | 850 | 51.2 | 23.3 | 86.94 | - | [82] |
| Fluidized bed/Tar cracker | Steam | - | 800/850 | 66.02 | 9.78 | 150 | 12.23 | [83] |
| Fluidized bed/Tar cracker | Air | 0.27 | 778/844 | 22.2 b | 25.3 b | 46 | 7.27 | [85] |
| Bubbling fluidized bed | Air | - | 850 | 9.72 b | 8.6 b | 49.16 | 5.8 | [86] |
| Fluidized bed/Tar cracker | Air | 0.32 | 796/805 | 29.8 b | 15.5 b | - | 7.8 | [87] |
| Two-stage fixed bed | - | - | 550/750 | 53.0 | 11.5 | 73 | - | [110] |
| Fixed bed | Air | 0.2 | 700 | 36.25 | 24.7 b | - | - | [89] |
| Two-stage fixed bed | Steam | - | 500/850 | 66.18 | 28.6 | 241 | - | [90] |
| Two-stage fixed bed | Steam | - | 850 | 60.0 | 33.18 | 196 | - | [90] |
| Two-stage fixed bed | Steam | - | 850 | 65.45 | 25.19 | 303 | - | [90] |
| Bubbling fluidized bed | Air | 0.248 | 850 | 6.3 b | 6 b | - | 6.6 | [91] |
| Fluidized bed | Air | 0.2 | 850 | 8.63 b | 5.2 b | 16.12 | 7.78 | [92] |
| Fluidized bed | Steam/CO2 | - | 900 | 55.73 | 24.21 | - | 11 | [93] |
| Bubbling fluidized bed | Air/Steam | 0.25 | 800 | 36.4 | 34.8 | 11.5 | 16.3 | [94] |
| Bubbling fluidized bed | Air/Steam | 0.25 | 900 | 7.0 b | 16.5 b | 13.5 | - | [95] |
| Two-stage fixed bed | Steam | - | 500/700 | 68 | 22 | 258 | - | [90] |
| Bubbling fluidized bed | Air/Steam | 0.28 | 800 | 27.25 b | 7.15 b | - | 6.11 | [98] |
| Fixed bed | - | - | 800 | 86.8 | 5.1 | - | - | [109] |
| Bubbling fluidized bed | Air | 0.25 | 750 | 3.29 | 7.53 | 126 | 5.61 | [111] |
| Two-stage fixed bed | Steam | - | 600/800 | 53.8 | 26.4 | 90 | - | [112] |
| Spouted bed | Steam | - | 900 | 61.6 | 27.8 | 184 | 15.5 | [113] |
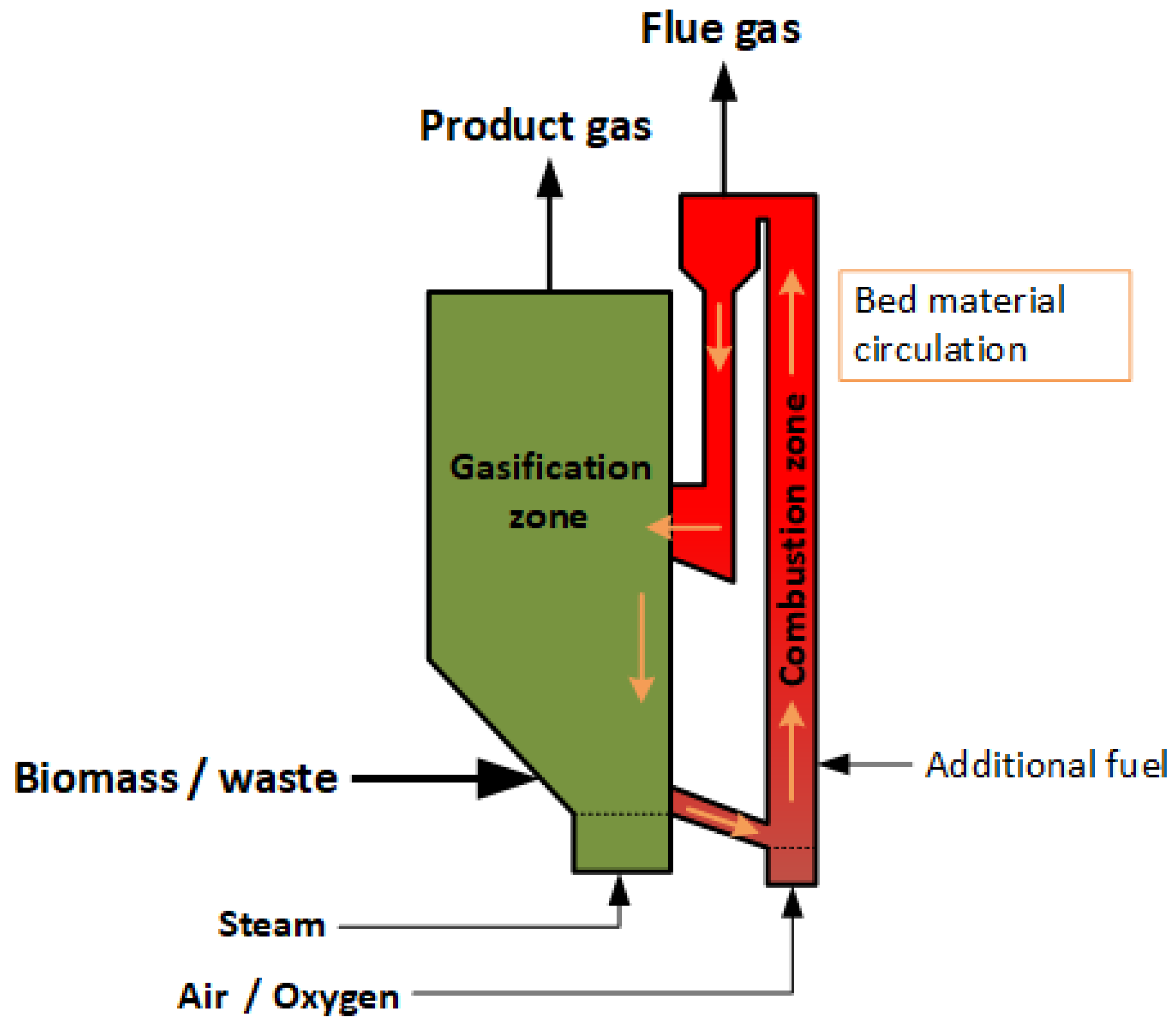
2.3. Gasification Processes
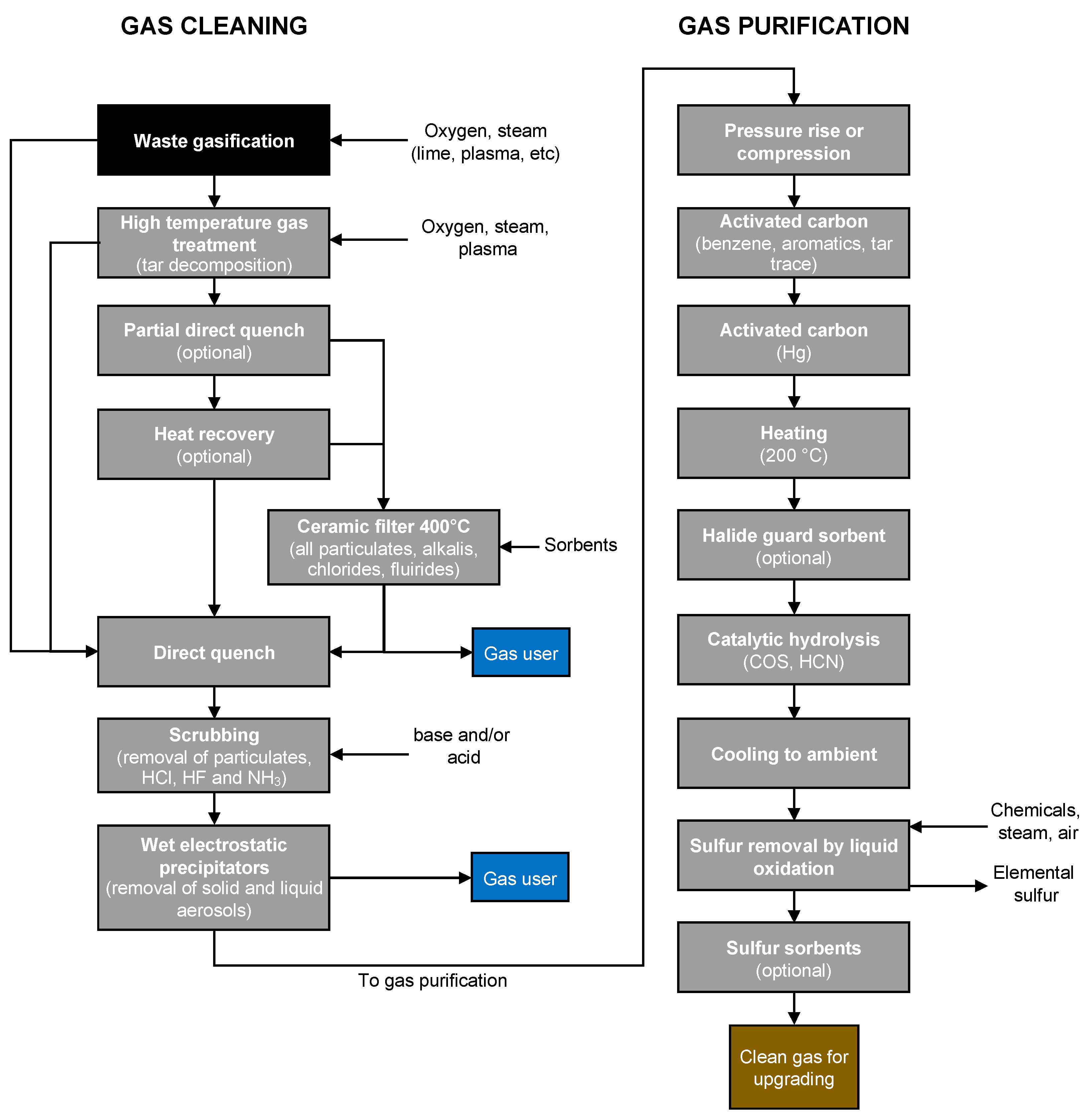
Gasifiers
3. Opportunities and Risks
3.1. Potential of Hydrogen from Waste
3.2. Alternative Technologies
4. Regulations, Mandatory Recycling Quotas
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Contreras, A.; Carpio, J.; Molero, M.; Veziroglu, T.N. Solar–Hydrogen: An Energy System for Sustainable Development in Spain. Int. J. Hydrogen Energy 1999, 24, 1041–1052. [Google Scholar] [CrossRef]
- Remón, J.; Arcelus-Arrillaga, P.; García, L.; Arauzo, J. Simultaneous Production of Gaseous and Liquid Biofuels from the Synergetic Co-Valorisation of Bio-Oil and Crude Glycerol in Supercritical Water. Appl. Energy 2018, 228, 2275–2287. [Google Scholar] [CrossRef]
- Kothari, R.; Buddhi, D.; Sawhney, R.L. Comparison of Environmental and Economic Aspects of Various Hydrogen Production Methods. Renew. Sustain. Energy Rev. 2008, 12, 553–563. [Google Scholar] [CrossRef]
- Lui, J.; Chen, W.-H.; Tsang, D.C.W.; You, S. A Critical Review on the Principles, Applications, and Challenges of Waste-to-Hydrogen Technologies. Renew. Sustain. Energy Rev. 2020, 134, 110365. [Google Scholar] [CrossRef]
- IEA. Global Hydrogen Review 2022; IEA: Paris, France, 2022. [Google Scholar]
- Kaza, S.; Yao, L.C.; Bhada-Tata, P.; Van Woerden, F. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0. [Google Scholar]
- OECD. Global Plastics Outlook: Policy Scenarios to 2060; Organisation for Economic Co-operation and Development: Paris, France, 2022; ISBN 978-92-64-66764-8. [Google Scholar]
- Toledo, M.; Ripoll, N.; Céspedes, J.; Zbogar-Rasic, A.; Fedorova, N.; Jovicic, V.; Delgado, A. Syngas Production from Waste Tires Using a Hybrid Filtration Reactor under Different Gasifier Agents. Energy Convers. Manag. 2018, 172, 381–390. [Google Scholar] [CrossRef]
- Cheng, H.; Hu, Y. Municipal Solid Waste (MSW) as a Renewable Source of Energy: Current and Future Practices in China. Bioresour. Technol. 2010, 101, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yin, L.; Wang, H.; He, P. Reprint of: Pyrolysis Technologies for Municipal Solid Waste: A Review. Waste Manag. 2015, 37, 116–136. [Google Scholar] [CrossRef] [PubMed]
- Couto, N.; Monteiro, E.; Silva, V.; Rouboa, A. Hydrogen-Rich Gas from Gasification of Portuguese Municipal Solid Wastes. Int. J. Hydrogen Energy 2016, 41, 10619–10630. [Google Scholar] [CrossRef]
- Jafri, Y.; Waldheim, L.; Lundgren, J. Emerging Gasification Technologies for Waste & Biomas; IEA Bioenergy: Paris, France, 2020. [Google Scholar]
- Belgiorno, V.; De Feo, G.; Della Rocca, C.; Napoli, R.M.A. Energy from Gasification of Solid Wastes. Waste Manag. 2003, 23, 1–15. [Google Scholar] [CrossRef]
- Sajid, M.; Raheem, A.; Ullah, N.; Asim, M.; Ur Rehman, M.S.; Ali, N. Gasification of Municipal Solid Waste: Progress, Challenges, and Prospects. Renew. Sustain. Energy Rev. 2022, 168, 112815. [Google Scholar] [CrossRef]
- Dimitriadis, A.; Bezergianni, S. Hydrothermal Liquefaction of Various Biomass and Waste Feedstocks for Biocrude Production: A State of the Art Review. Renew. Sustain. Energy Rev. 2017, 68, 113–125. [Google Scholar] [CrossRef]
- Nanda, S.; Berruti, F. Municipal Solid Waste Management and Landfilling Technologies: A Review. Environ. Chem. Lett. 2021, 19, 1433–1456. [Google Scholar] [CrossRef]
- Lombardi, L.; Carnevale, E.; Corti, A. Review of Technologies and Performances of Thermal Treatment Systems for Energy Recovery from Waste. Waste Manag. 2015, 37, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Arena, U. Process and Technological Aspects of Municipal Solid Waste Gasification. A Review. Waste Manag. 2012, 32, 625–639. [Google Scholar] [CrossRef] [PubMed]
- El-Rub, Z.A.; Bramer, E.A.; Brem, G. Review of Catalysts for Tar Elimination in Biomass Gasification Processes. Ind. Eng. Chem. Res. 2004, 43, 6911–6919. [Google Scholar] [CrossRef]
- Milne, T.A.; Elam, C.C.; Evans, R.J. Hydrogen from Biomass: State of the Art and Research Challenges. In International Energy Agency, Agreement on the Production and Utilization of Hydrogen; IEA/H2-TR-02/001; United States Department of Energy: Washington, DC, USA, 2002. [Google Scholar] [CrossRef]
- Nguyen, V.G.; Nguyen-Thi, T.X.; Phong Nguyen, P.Q.; Tran, V.D.; Ağbulut, Ü.; Nguyen, L.H.; Balasubramanian, D.; Tarelko, W.; Bandh, S.A.; Khoa Pham, N.D.K. Recent Advances in Hydrogen Production from Biomass Waste with a Focus on Pyrolysis and Gasification. Int. J. Hydrogen Energy 2024, 54, 127–160. [Google Scholar] [CrossRef]
- Munir, M.T.; Mardon, I.; Al-Zuhair, S.; Shawabkeh, A.; Saqib, N.U. Plasma Gasification of Municipal Solid Waste for Waste-to-Value Processing. Renew. Sustain. Energy Rev. 2019, 116, 109461. [Google Scholar] [CrossRef]
- Klinghoffer, N.; Castaldi, M. Gasification and Pyrolysis of Municipal Solid Waste (MSW). In Waste to Energy Conversion Technology; Woodhead Publishing: Cambridge, UK, 2013; pp. 146–176. ISBN 978-0-85709-011-9. [Google Scholar]
- Butterman, H.C.; Castaldi, M.J. CO2 as a Carbon Neutral Fuel Source via Enhanced Biomass Gasification. Environ. Sci. Technol. 2009, 43, 9030–9037. [Google Scholar] [CrossRef]
- Sansaniwal, S.K.; Pal, K.; Rosen, M.A.; Tyagi, S.K. Recent Advances in the Development of Biomass Gasification Technology: A Comprehensive Review. Renew. Sustain. Energy Rev. 2017, 72, 363–384. [Google Scholar] [CrossRef]
- Parthasarathy, P.; Narayanan, K.S. Hydrogen Production from Steam Gasification of Biomass: Influence of Process Parameters on Hydrogen Yield—A Review. Renew. Energy 2014, 66, 570–579. [Google Scholar] [CrossRef]
- Park, S.-W.; Lee, J.-S.; Yang, W.S.; Alam, T.; Seo, Y.-C. A Comparative Study of the Gasification of Solid Refuse Fuel in Downdraft Fixed Bed and Bubbling Fluidized Bed Reactors. Waste Biomass Valorization 2020, 11, 2345–2356. [Google Scholar] [CrossRef]
- Velis, C.A.; Longhurst, P.J.; Drew, G.H.; Smith, R.; Pollard, S.J.T. Production and Quality Assurance of Solid Recovered Fuels Using Mechanical—Biological Treatment (MBT) of Waste: A Comprehensive Assessment. Crit. Rev. Environ. Sci. Technol. 2010, 40, 979–1105. [Google Scholar] [CrossRef]
- Jamro, I.; Chen, G.; Baloch, H.; Wenga, T.; Ma, W. Optimization of Municipal Solid Waste Air Gasification for Higher H2 Production along with the Validation via Kinetics and Statistical Approaches. Fuel 2022, 322, 124137. [Google Scholar] [CrossRef]
- Zheng, X.; Ying, Z.; Wang, B.; Chen, C. Hydrogen and Syngas Production from Municipal Solid Waste (MSW) Gasification via Reusing CO2. Appl. Therm. Eng. 2018, 144, 242–247. [Google Scholar] [CrossRef]
- He, M.; Hu, Z.; Xiao, B.; Li, J.; Guo, X.; Luo, S.; Yang, F.; Feng, Y.; Yang, G.; Liu, S. Hydrogen-Rich Gas from Catalytic Steam Gasification of Municipal Solid Waste (MSW): Influence of Catalyst and Temperature on Yield and Product Composition. Int. J. Hydrogen Energy 2009, 34, 195–203. [Google Scholar] [CrossRef]
- Chen, G.; Jamro, I.A.; Samo, S.R.; Wenga, T.; Baloch, H.A.; Yan, B.; Ma, W. Hydrogen-Rich Syngas Production from Municipal Solid Waste Gasification through the Application of Central Composite Design: An Optimization Study. Int. J. Hydrogen Energy 2020, 45, 33260–33273. [Google Scholar] [CrossRef]
- Zhao, J.; Xie, D.; Wang, S.; Zhang, R.; Wu, Z.; Meng, H.; Chen, L.; Wang, T.; Guo, Y. Hydrogen-Rich Syngas Produced from Co-Gasification of Municipal Solid Waste and Wheat Straw in an Oxygen-Enriched Air Fluidized Bed. Int. J. Hydrogen Energy 2021, 46, 18051–18063. [Google Scholar] [CrossRef]
- Zhou, C.; Stuermer, T.; Gunarathne, R.; Yang, W.; Blasiak, W. Effect of Calcium Oxide on High-Temperature Steam Gasification of Municipal Solid Waste. Fuel 2014, 122, 36–46. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, G.; You, Y.; Xiao, B.; Liu, S.; He, P.; Guo, D.; Guo, X.; Zhang, G. Hydrogen-Rich Gas Production by Steam Gasification of Municipal Solid Waste (MSW) Using NiO Supported on Modified Dolomite. Int. J. Hydrogen Energy 2012, 37, 6503–6510. [Google Scholar] [CrossRef]
- Campoy, M.; Gomez-Barea, A.; Ollero, P.; Nilsson, S. Gasification of Wastes in a Pilot Fluidized Bed Gasifier. Fuel Process. Technol. 2014, 121, 63–69. [Google Scholar] [CrossRef]
- Domínguez, A.; Menéndez, J.A.; Pis, J.J. Hydrogen Rich Fuel Gas Production from the Pyrolysis of Wet Sewage Sludge at High Temperature. J. Anal. Appl. Pyrolysis 2006, 77, 127–132. [Google Scholar] [CrossRef]
- Hu, M.; Guo, D.; Ma, C.; Hu, Z.; Zhang, B.; Xiao, B.; Luo, S.; Wang, J. Hydrogen-Rich Gas Production by the Gasification of Wet MSW (Municipal Solid Waste) Coupled with Carbon Dioxide Capture. Energy 2015, 90, 857–863. [Google Scholar] [CrossRef]
- Kumar, U.; Paul, M.C. CFD Modelling of Biomass Gasification with a Volatile Break-up Approach. Chem. Eng. Sci. 2019, 195, 413–422. [Google Scholar] [CrossRef]
- Gohlke, O. Efficiency of Energy Recovery from Municipal Solid Waste and the Resultant Effect on the Greenhouse Gas Balance. Waste Manag. Res. J. Int. Solid Wastes Public Clean. Assoc. ISWA 2009, 27, 894–906. [Google Scholar] [CrossRef]
- Scala, F.; Solimene, R.; Montagnaro, F. 7—Conversion of Solid Fuels and Sorbents in Fluidized Bed Combustion and Gasification. In Fluidized Bed Technologies for Near-Zero Emission Combustion and Gasification; Scala, F., Ed.; Woodhead Publishing Series in Energy; Woodhead Publishing: Cambridge, UK, 2013; pp. 319–387. ISBN 978-0-85709-541-1. [Google Scholar]
- Cao, L.; Yu, I.K.M.; Xiong, X.; Tsang, D.C.W.; Zhang, S.; Clark, J.H.; Hu, C.; Ng, Y.H.; Shang, J.; Ok, Y.S. Biorenewable Hydrogen Production through Biomass Gasification: A Review and Future Prospects. Environ. Res. 2020, 186, 109547. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; Wu, C.; Ma, L.; Yuan, Z. A Study on the Economic Efficiency of Hydrogen Production from Biomass Residues in China. Renew. Energy 2008, 33, 1874–1879. [Google Scholar] [CrossRef]
- Luo, S.; Xiao, B.; Guo, X.; Hu, Z.; Liu, S.; He, M. Hydrogen-Rich Gas from Catalytic Steam Gasification of Biomass in a Fixed Bed Reactor: Influence of Particle Size on Gasification Performance. Int. J. Hydrogen Energy 2009, 34, 1260–1264. [Google Scholar] [CrossRef]
- Bhoi, P.R.; Huhnke, R.L.; Kumar, A.; Indrawan, N.; Thapa, S. Co-Gasification of Municipal Solid Waste and Biomass in a Commercial Scale Downdraft Gasifier. Energy 2018, 163, 513–518. [Google Scholar] [CrossRef]
- Li, J.; Liao, S.; Dan, W.; Jia, K.; Zhou, X. Experimental Study on Catalytic Steam Gasification of Municipal Solid Waste for Bioenergy Production in a Combined Fixed Bed Reactor. Biomass Bioenergy 2012, 46, 174–180. [Google Scholar] [CrossRef]
- Gao, W.; Farahani, M.R.; Rezaei, M.; Hosamani, S.M.; Jamil, M.K.; Imran, M.; Baig, A.Q. Experimental Study of Steam-Gasification of Municipal Solid Wastes (MSW) Using Ni-Cu/Γ-Al 2O3 Nano Catalysts. Energy Sources Part Recovery Util. Environ. Eff. 2017, 39, 693–697. [Google Scholar] [CrossRef]
- Couto, N.; Silva, V.; Monteiro, E.; Rouboa, A. Exergy Analysis of Portuguese Municipal Solid Waste Treatment via Steam Gasification. Energy Convers. Manag. 2017, 134, 235–246. [Google Scholar] [CrossRef]
- Mallick, D.; Mahanta, P.; Moholkar, V.S. Co-Gasification of Coal and Biomass Blends: Chemistry and Engineering. Fuel 2017, 204, 106–128. [Google Scholar] [CrossRef]
- Luo, S.; Zhou, Y.; Yi, C. Syngas Production by Catalytic Steam Gasification of Municipal Solid Waste in Fixed-Bed Reactor. Energy 2012, 44, 391–395. [Google Scholar] [CrossRef]
- Machin, E.B.; Pedroso, D.T.; de Carvalho, J.A. Energetic Valorization of Waste Tires. Renew. Sustain. Energy Rev. 2017, 68, 306–315. [Google Scholar] [CrossRef]
- Leung, D.Y.C.; Wang, C.L. Fluidized-Bed Gasification of Waste Tire Powders. Fuel Process. Technol. 2003, 84, 175–196. [Google Scholar] [CrossRef]
- Mastral, A.M.; Murillo, R.; Garcia, T.; Navarro, M.; Callén, M.; Lopez, J. Study of the Viability of the Process for Hydrogen Recovery from Old Tyre Oils. Fuel Process. Technol. 2002, 75, 185–199. [Google Scholar] [CrossRef]
- Home—ETRMA. Available online: https://www.etrma.org/ (accessed on 25 November 2023).
- Torretta, V.; Rada, E.C.; Ragazzi, M.; Trulli, E.; Istrate, I.A.; Cioca, L.I. Treatment and Disposal of Tyres: Two EU Approaches. A Review. Waste Manag. 2015, 45, 152–160. [Google Scholar] [CrossRef]
- Yin, C.-Y. Prediction of Higher Heating Values of Biomass from Proximate and Ultimate Analyses. Fuel 2011, 90, 1128–1132. [Google Scholar] [CrossRef]
- Tan, P.; Zhang, C.; Xia, J.; Fang, Q.-Y.; Chen, G. Estimation of Higher Heating Value of Coal Based on Proximate Analysis Using Support Vector Regression. Fuel Process. Technol. 2015, 138, 298–304. [Google Scholar] [CrossRef]
- Li, W.; Huang, C.; Li, D.; Huo, P.; Wang, M.; Han, L.; Chen, G.; Li, H.; Li, X.; Wang, Y.; et al. Derived Oil Production by Catalytic Pyrolysis of Scrap Tires. Chin. J. Catal. 2016, 37, 526–532. [Google Scholar] [CrossRef]
- Ayanoglu, A.; Yumrutas, R. Rotary Kiln and Batch Pyrolysis of Waste Tire to Produce Gasoline and Diesel like Fuels. Energy Convers. Manag. 2016, 111, 261–270. [Google Scholar] [CrossRef]
- Portofino, S.; Donatelli, A.; Iovane, P.; Innella, C.; Civita, R.; Martino, M.; Matera, D.A.; Russo, A.; Cornacchia, G.; Galvagno, S. Steam Gasification of Waste Tyre: Influence of Process Temperature on Yield and Product Composition. Waste Manag. 2013, 33, 672–678. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.; Sivamohan Reddy, N.; Hunter, H.; Vo, D.-V.; Kozinski, J.; Gökalp, I. Catalytic Subcritical and Supercritical Water Gasification as a Resource Recovery Approach from Waste Tires for Hydrogen-Rich Syngas Production. J. Supercrit. Fluids 2019, 154, 104627. [Google Scholar] [CrossRef]
- Karatas, H.; Olgun, H.; Engin, B.; Akgun, F. Experimental Results of Gasification of Waste Tire with Air in a Bubbling Fluidized Bed Gasifier. Fuel 2013, 105, 566–571. [Google Scholar] [CrossRef]
- Portofino, S.; Casu, S.; Iovane, P.; Russo, A.; Martino, M.; Donatelli, A.; Galvagno, S. Optimizing H2 Production from Waste Tires via Combined Steam Gasification and Catalytic Reforming. Energy Fuels 2011, 25, 2232–2241. [Google Scholar] [CrossRef]
- Zhang, Y.; Chunfei, W.; Nahil, M.; Williams, P. Pyrolysis-Catalytic Steam Reforming/Gasification of Waste Tires for Production of Carbon Nanotubes and Hydrogen. Energy Fuels 2015, 29, 150415125425001. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, T.; Ma, L.; Chang, J. Vacuum Pyrolysis of Waste Tires with Basic Additives. Waste Manag. 2008, 28, 2301–2310. [Google Scholar] [CrossRef]
- Han, W.; Han, D.; Chen, H. Pyrolysis of Waste Tires: A Review. Polymers 2023, 15, 1604. [Google Scholar] [CrossRef]
- Singh, R.K.; Ruj, B.; Jana, A.; Mondal, S.; Jana, B.; Sadhukhan, A.K.; Gupta, P. Pyrolysis of Three Different Categories of Automotive Tyre Wastes: Product Yield Analysis and Characterization. J. Anal. Appl. Pyrolysis 2018, 135, 379–389. [Google Scholar] [CrossRef]
- Akkouche, N.; Balistrou, M.; Loubar, K.; Awad, S.; Tazerout, M. Heating Rate Effects on Pyrolytic Vapors from Scrap Truck Tires. J. Anal. Appl. Pyrolysis 2016, 123, 419–429. [Google Scholar] [CrossRef]
- Sathiskumar, C.; Karthikeyan, S. Recycling of Waste Tires and Its Energy Storage Application of By-Products—A Review. Sustain. Mater. Technol. 2019, 22, e00125. [Google Scholar] [CrossRef]
- Pattabhi Raman, K.; Walawender, W.P.; Fan, L.T. Gasification of Waste Tires in a Fluid Bed Reactor. Conserv. Recycl. 1981, 4, 79–88. [Google Scholar] [CrossRef]
- Elbaba, I.; Chunfei, W.; Williams, P. Hydrogen Production from the Pyrolysis–Gasification of Waste Tyres with a Nickel/Cerium Catalyst. Energy Fuels 2011, 36, 6628–6637. [Google Scholar] [CrossRef]
- Elbaba, I.; Williams, P. High Yield Hydrogen from the Pyrolysis–Catalytic Gasification of Waste Tyres with a Nickel/Dolomite Catalyst. Fuel 2013, 106, 528–536. [Google Scholar] [CrossRef]
- Elbaba, I.; Williams, P. Two Stage Pyrolysis-Catalytic Gasification of Waste Tyres: Influence of Process Parameters. Appl. Catal. B Environ. 2012, 125, 136–143. [Google Scholar] [CrossRef]
- Donatelli, A.; Iovane, P.; Molino, A. High Energy Syngas Production by Waste Tyres Steam Gasification in a Rotary Kiln Pilot Plant. Experimental and Numerical Investigations. Fuel 2010, 89, 2721–2728. [Google Scholar] [CrossRef]
- Karatas, H.; Olgun, H.; Akgun, F. Experimental Results of Gasification of Waste Tire with air&CO2, Air&steam and Steam in a Bubbling Fluidized Bed Gasifier. Fuel Process. Technol. 2012, 102, 166–174. [Google Scholar] [CrossRef]
- Xiao, G.; Ni, M.-J.; Chi, Y.; Cen, K.-F. Low-Temperature Gasification of Waste Tire in a Fluidized Bed. Energy Convers. Manag. 2008, 49, 2078–2082. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.; Ahmed, U.; Abdul Jameel, A.G.; Zahid, U.; Ahmad, N.; Shahbaz, M.; Nemitallah, M.A. Technoeconomic Feasibility of Hydrogen Production from Waste Tires with the Control of CO2 Emissions. ACS Omega 2022, 7, 48075–48086. [Google Scholar] [CrossRef]
- Fox, J.A.; Stacey, N.T. Process Targeting: An Energy Based Comparison of Waste Plastic Processing Technologies. Energy 2019, 170, 273–283. [Google Scholar] [CrossRef]
- Jambeck, J.R.; Geyer, R.; Wilcox, C.; Siegler, T.R.; Perryman, M.; Andrady, A.; Narayan, R.; Law, K.L. Plastic Waste Inputs from Land into the Ocean. Science 2015, 347, 768–771. [Google Scholar] [CrossRef]
- Yong, C.Q.Y.; Valiyaveettil, S.; Tang, B.L. Toxicity of Microplastics and Nanoplastics in Mammalian Systems. Int. J. Environ. Res. Public Health 2020, 17, 1509. [Google Scholar] [CrossRef]
- Yang, R.-X.; Chuang, K.-H.; Wey, M.-Y. Effects of Nickel Species on Ni/Al2O3 Catalysts in CNTs and Hydrogen Production by Waste Plastics Gasification: Bench-Scale and Pilot-Scale Tests. Energy Fuels 2015, 29, 8178–8187. [Google Scholar] [CrossRef]
- Wilk, V.; Hofbauer, H. Conversion of Mixed Plastic Wastes in a Dual Fluidized Bed Steam Gasifier. Fuel 2013, 107, 787–799. [Google Scholar] [CrossRef]
- Jeong, Y.-S.; Park, K.-B.; Kim, J.-S. Hydrogen Production from Steam Gasification of Polyethylene Using a Two-Stage Gasifier and Active Carbon. Appl. Energy 2020, 262, 114495. [Google Scholar] [CrossRef]
- Burra, K.G.; Gupta, A.K. Synergistic Effects in Steam Gasification of Combined Biomass and Plastic Waste Mixtures. Appl. Energy 2018, 211, 230–236. [Google Scholar] [CrossRef]
- Choi, M.-J.; Jeong, Y.-S.; Kim, J.-S. Air Gasification of Polyethylene Terephthalate Using a Two-Stage Gasifier with Active Carbon for the Production of H2 and CO. Energy 2021, 223, 120122. [Google Scholar] [CrossRef]
- Sancho, J.A.; Aznar, M.P.; Toledo, J.M. Catalytic Air Gasification of Plastic Waste (Polypropylene) in Fluidized Bed. Part I: Use of in-Gasifier Bed Additives. Ind. Eng. Chem. Res. 2008, 47, 1005–1010. [Google Scholar] [CrossRef]
- Cho, M.-H.; Choi, Y.-K.; Kim, J.-S. Air Gasification of PVC (Polyvinyl Chloride)-Containing Plastic Waste in a Two-Stage Gasifier Using Ca-Based Additives and Ni-Loaded Activated Carbon for the Production of Clean and Hydrogen-Rich Producer Gas. Energy 2015, 87, 586–593. [Google Scholar] [CrossRef]
- Chai, Y.; Gao, N.; Wang, M.; Wu, C. H2 Production from Co-Pyrolysis/Gasification of Waste Plastics and Biomass under Novel Catalyst Ni-CaO-C. Chem. Eng. J. 2020, 382, 122947. [Google Scholar] [CrossRef]
- Farooq, A.; Song, H.; Park, Y.K.; Rhee, G.H. Effects of Different Al2O3 Support on HDPE Gasification for Enhanced Hydrogen Generation Using Ni-Based Catalysts. Int. J. Hydrogen Energy 2021, 46, 18085–18092. [Google Scholar] [CrossRef]
- Chunfei, W.; Williams, P. Pyrolysis–Gasification of Plastics, Mixed Plastics and Real-World Plastic Waste with and Without Ni–Mg–Al Catalyst. Energy Fuels 2010, 89, 3022–3032. [Google Scholar] [CrossRef]
- Arena, U.; Di Gregorio, F. Energy Generation by Air Gasification of Two Industrial Plastic Wastes in a Pilot Scale Fluidized Bed Reactor. Energy 2014, 68, 735–743. [Google Scholar] [CrossRef]
- Mastellone, M.L.; Zaccariello, L.; Arena, U. Co-Gasification of Coal, Plastic Waste and Wood in a Bubbling Fluidized Bed Reactor. Fuel 2010, 89, 2991–3000. [Google Scholar] [CrossRef]
- Straka, P.; Bičáková, O. Hydrogen-Rich Gas as a Product of Two-Stage Co-Gasification of Lignite/Waste Plastics Mixtures. Int. J. Hydrogen Energy 2014, 39, 10987–10995. [Google Scholar] [CrossRef]
- Han, S.W.; Tokmurzin, D.; Lee, J.J.; Park, S.J.; Ra, H.W.; Yoon, S.J.; Mun, T.-Y.; Yoon, S.M.; Moon, J.H.; Lee, J.G.; et al. Gasification Characteristics of Waste Plastics (SRF) in a Bubbling Fluidized Bed: Use of Activated Carbon and Olivine for Tar Removal and the Effect of Steam/Carbon Ratio. Fuel 2022, 314, 123102. [Google Scholar] [CrossRef]
- Han, S.W.; Lee, J.J.; Tokmurzin, D.; Lee, S.H.; Nam, J.Y.; Park, S.J.; Ra, H.W.; Mun, T.-Y.; Yoon, S.J.; Yoon, S.M. Gasification Characteristics of Waste Plastics (SRF) in a Bubbling Fluidized Bed: Effects of Temperature and Equivalence Ratio. Energy 2022, 238, 121944. [Google Scholar] [CrossRef]
- Dou, B.; Wang, K.; Jiang, B.; Song, Y.; Zhang, C.; Chen, H.; Xu, Y. Fluidized-Bed Gasification Combined Continuous Sorption-Enhanced Steam Reforming System to Continuous Hydrogen Production from Waste Plastic. Int. J. Hydrogen Energy 2016, 41, 3803–3810. [Google Scholar] [CrossRef]
- Lazzarotto, I.P.; Ferreira, S.D.; Junges, J.; Bassanesi, G.R.; Manera, C.; Perondi, D.; Godinho, M. The Role of CaO in the Steam Gasification of Plastic Wastes Recovered from the Municipal Solid Waste in a Fluidized Bed Reactor. Process Saf. Environ. Prot. 2020, 140, 60–67. [Google Scholar] [CrossRef]
- Cho, M.-H.; Mun, T.-Y.; Choi, Y.-K.; Kim, J.-S. Two-Stage Air Gasification of Mixed Plastic Waste: Olivine as the Bed Material and Effects of Various Additives and a Nickel-Plated Distributor on the Tar Removal. Energy 2014, 70, 128–134. [Google Scholar] [CrossRef]
- Plastic Waste and Recycling in the EU: Facts and Figures|News|European Parliament. Available online: https://www.europarl.europa.eu/news/en/headlines/society/20181212STO21610/plastic-waste-and-recycling-in-the-eu-facts-and-figures (accessed on 25 November 2023).
- Anuar Sharuddin, S.D.; Abnisa, F.; Wan Daud, W.M.A.; Aroua, M.K. A Review on Pyrolysis of Plastic Wastes. Energy Convers. Manag. 2016, 115, 308–326. [Google Scholar] [CrossRef]
- Qureshi, M.S.; Oasmaa, A.; Pihkola, H.; Deviatkin, I.; Tenhunen, A.; Mannila, J.; Minkkinen, H.; Pohjakallio, M.; Laine-Ylijoki, J. Pyrolysis of Plastic Waste: Opportunities and Challenges. J. Anal. Appl. Pyrolysis 2020, 152, 104804. [Google Scholar] [CrossRef]
- Kunwar, B.; Cheng, H.N.; Chandrashekaran, S.R.; Sharma, B.K. Plastics to Fuel: A Review. Renew. Sustain. Energy Rev. 2016, 54, 421–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, D.; Lei, H.; Villota, E.; Ruan, R. Jet Fuel Production from Waste Plastics via Catalytic Pyrolysis with Activated Carbons. Appl. Energy 2019, 251, 113337. [Google Scholar] [CrossRef]
- Aishwarya, K.N.; Nangarthody, S. Microwave Assisted Pyrolysis of Plastic Waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef]
- Zhang, X.; Lei, H.; Yadavalli, G.; Zhu, L.; Wei, Y.; Liu, Y. Gasoline-Range Hydrocarbons Produced from Microwave-Induced Pyrolysis of Low-Density Polyethylene over ZSM-5. Fuel 2015, 144, 33–42. [Google Scholar] [CrossRef]
- Lopez, G.; Artetxe, M.; Amutio, M.; Alvarez, J.; Bilbao, J.; Olazar, M. Recent Advances in the Gasification of Waste Plastics. A Critical Overview. Renew. Sustain. Energy Rev. 2018, 82, 576–596. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Haciosmanoglu, M.; Akbulut, U.; Dincer, I. A Review on Converting Plastic Wastes into Clean Hydrogen via Gasification for Better Sustainability. Int. J. Energy Res. 2022, 46, 4001–4032. [Google Scholar] [CrossRef]
- He, M.; Xiao, B.; Hu, Z.; Liu, S.; Guo, X.; Luo, S. Syngas Production from Catalytic Gasification of Waste Polyethylene: Influence of Temperature on Gas Yield and Composition. Int. J. Hydrogen Energy 2009, 34, 1342–1348. [Google Scholar] [CrossRef]
- Huang, J.; Veksha, A.; Jun, T.; Lisak, G. Upgrading Waste Plastic Derived Pyrolysis Gas via Chemical Looping Cracking–Gasification Using Ni–Fe–Al Redox Catalysts. Chem. Eng. J. 2022, 438, 135580. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, S.; Zhang, H.; Liu, X.; Xiong, Y. High Quality H2-Rich Syngas Production from Pyrolysis-Gasification of Biomass and Plastic Wastes by Ni–Fe@Nanofibers/Porous Carbon Catalyst. Int. J. Hydrogen Energy 2019, 44, 26193–26203. [Google Scholar] [CrossRef]
- Martínez-Lera, S.; Torrico, J.; Pallarés, J.; Gil, A. Thermal Valorization of Post-Consumer Film Waste in a Bubbling Bed Gasifier. Waste Manag. 2013, 33, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Acomb, J.C.; Wu, C.; Williams, P.T. Control of Steam Input to the Pyrolysis-Gasification of Waste Plastics for Improved Production of Hydrogen or Carbon Nanotubes. Appl. Catal. B Environ. 2014, 147, 571–584. [Google Scholar] [CrossRef]
- Erkiaga, A.; Lopez, G.; Amutio, M.; Bilbao, J.; Olazar, M. Syngas from Steam Gasification of Polyethylene in a Conical Spouted Bed Reactor. Fuel 2013, 109, 461–469. [Google Scholar] [CrossRef]
- Gündüz Han, D.; Erdem, K.; Midilli, A. Investigation of Hydrogen Production via Waste Plastic Gasification in a Fluidized Bed Reactor Using Aspen Plus. Int. J. Hydrogen Energy 2023, 48, 39315–39329. [Google Scholar] [CrossRef]
- Meng, X.M.; Jong, W.; Pal, R.; Verkooijen, A.H.M. In Bed and Downstream Hot Gas Desulfurization during Solid Fuel Gasification: A Review. Fuel Process. Technol. 2010, 91, 964–981. [Google Scholar] [CrossRef]
- Sikarwar, V.; Zhao, M.; Clough, P.; Yao, J.; Zhong, X.; Memon, M.Z.; Shah, N.; Anthony, E.; Fennell, P. An Overview of Advances in Biomass Gasification. Energy Environ. Sci. 2016, 9, 2927–3304. [Google Scholar] [CrossRef]
- Siedlecki, M. On the Gasification of Biomass in a Steam-Oxygen Blown CFB Gasifier with the Focus on Gas Quality Upgrading: Technology Background, Experiments and Mathematical Modeling; Technical University of Delft: Delft, The Netherlands, 2011; ISBN 9789085707905. [Google Scholar]
- Di Gregorio, F. Fuel Gas Technology for Biomass and Waste. Environmental and Techno-Economic Assessments; Second University of Naples: Caserta, Italy, 2012. [Google Scholar]
- Leckner, B. Process Aspects in Combustion and Gasification Waste-to-Energy (WtE) Units. Waste Manag. 2015, 37, 13–25. [Google Scholar] [CrossRef]
- Waldheim, L. Gasification of Waste for Energy Carriers—A Review; IEA Bioenergy: Paris, France, 2019; ISBN 978-1-910154-56-4. [Google Scholar]
- Hacker, V.; Fankhauser, R.; Faleschini, G.; Fuchs, H.; Friedrich, K.; Muhr, M.; Kordesch, K. Hydrogen Production by Steam–Iron Process. J. Power Sources 2000, 86, 531–535. [Google Scholar] [CrossRef]
- Hsieh, T.-L.; Xu, D.; Zhang, Y.; Nadgouda, S.; Wang, D.; Chung, C.; Pottimurthy, Y.; Guo, M.; Chen, Y.-Y.; Xu, M.; et al. 250 kWth High Pressure Pilot Demonstration of the Syngas Chemical Looping System for High Purity H2 Production with CO2 Capture. Appl. Energy 2018, 230, 1660–1672. [Google Scholar] [CrossRef]
- Sridhar, D.; Tong, A.; Kim, H.; Zeng, L.; Li, F.; Fan, L.-S. Syngas Chemical Looping Process: Design and Construction of a 25 kWth Subpilot Unit. Energy Fuels 2012, 26, 2292–2302. [Google Scholar] [CrossRef]
- Messerschmitt, A. Verfahren zur Erzeugung von Wasserstoff durch abwechselnde Oxidation und Reduktion von Eisen in von außen beheizten, in Den Heizräumen Angeordneten Zersetzern. German Patent DE266863, 09 July 1911. [Google Scholar]
- Nestl, S.; Voitic, G.; Zacharias, R.; Bock, S.; Hacker, V. High-Purity Hydrogen Production with the Reformer Steam Iron Cycle. Energy Technol. 2018, 6, 563–569. [Google Scholar] [CrossRef]
- Dahlquist, E. (Ed.) Technologies for Converting Biomass to Useful Energy: Combustion, Gasification, Pyrolysis, Torrefaction and Fermentation; CRC Press: London, UK, 2013; ISBN 978-0-429-21713-5. [Google Scholar]
- Speight, J.G. Handbook of Gasification Technology: Science, Processes, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020; ISBN 978-1-118-77353-6. [Google Scholar]
- Hrbek, J. Status Report on Thermal Biomass Gasification in Countries Participating in IEA Bioenergy Task 33; IEA Bioenergy: Paris, France, 2016. [Google Scholar]
- Santos, S.M.; Assis, A.C.; Gomes, L.; Nobre, C.; Brito, P. Waste Gasification Technologies: A Brief Overview. Waste 2023, 1, 140–165. [Google Scholar] [CrossRef]
- Vaasa Bio-Gasification Plant—Power Technology. Available online: https://www.power-technology.com/projects/vaasa-plant/ (accessed on 25 November 2023).
- Thapa, R.; Pfeifer, C.; Halvorsen, B.M. Modelling of Reaction Kinetics in Bubbling Fluidized Bed Biomass Gasification Reactor. Int. J. Energy Environ. 2014, 5, 35–44. [Google Scholar]
- Tremel, A.; Becherer, D.; Fendt, S.; Gaderer, M.; Hartmut, S. Performance of Entrained Flow and Fluidised Bed Biomass Gasifiers on Different Scales. Energy Convers. Manag. 2013, 69, 95–106. [Google Scholar] [CrossRef]
- Cai, J.; Zheng, W.; Luo, M.; Tang, X. Gasification of Biomass Waste in the Moving-Grate Gasifier with the Addition of All Air into the Oxidizing Stage: Experimental and Numerical Investigation. Process Saf. Environ. Prot. 2021, 147, 985–992. [Google Scholar] [CrossRef]
- Wilson, B.; Wilson, B.; Liss, B. Renewable Energy from Gasification of Refuse Derived Fuel; EnviroPower Renewable, Inc.: Boca Raton, FL, USA, 2020. [Google Scholar]
- Nzihou, A.; Stanmore, B. The Fate of Heavy Metals during Combustion and Gasification of Contaminated Biomass—A Brief Review. J. Hazard. Mater. 2013, 256–257, 56–66. [Google Scholar] [CrossRef]
- Pareek, A.; Dom, R.; Gupta, J.; Chandran, J.; Adepu, V.; Borse, P.H. Insights into Renewable Hydrogen Energy: Recent Advances and Prospects. Mater. Sci. Energy Technol. 2020, 3, 319–327. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Senthil Kumar, P.; Kapoor, A.; Xiao, L. Progress in the Production of Hydrogen Energy from Food Waste: A Bibliometric Analysis. Int. J. Hydrogen Energy 2022, 47, 26326–26354. [Google Scholar] [CrossRef]
- Murugesan, K.; Ramachandran, K.K.; Aravind, J. Biohydrogen Production from Waste Materials: Benefits and Challenges. Int. J. Environ. Sci. Technol. 2019, 17, 559–576. [Google Scholar] [CrossRef]
- Ferreira, A.P.R.A.; Oliveira, R.C.P.; Mateus, M.M.; Santos, D.M.F. A Review of the Use of Electrolytic Cells for Energy and Environmental Applications. Energies 2023, 16, 1593. [Google Scholar] [CrossRef]
- Collins, L. It’s Much Cheaper to Produce Green Hydrogen from Waste than Renewables. Available online: https://www.rechargenews.com/transition/its-much-cheaper-to-produce-green-hydrogen-from-waste-than-renewables/2-1-801160 (accessed on 25 November 2023).
- Torky, M.; Dahy, G.; Hassanein, A.E. GH2_MobileNet: Deep Learning Approach for Predicting Green Hydrogen Production from Organic Waste Mixtures. Appl. Soft Comput. 2023, 138, 110215. [Google Scholar] [CrossRef]
- Kapdan, I.K.; Kargi, F. Bio-Hydrogen Production from Waste Materials. Enzyme Microb. Technol. 2006, 38, 569–582. [Google Scholar] [CrossRef]
- Nipattummakul, N.; Ahmed, I.; Kerdsuwan, S.; Gupta, A.K. High Temperature Steam Gasification of Wastewater Sludge. Appl. Energy 2010, 87, 3729–3734. [Google Scholar] [CrossRef]
- Gao, N.; Li, A.; Quan, C. A Novel Reforming Method for Hydrogen Production from Biomass Steam Gasification. Bioresour. Technol. 2009, 100, 4271–4277. [Google Scholar] [CrossRef]
- Yao, J.; Kraussler, M.; Benedikt, F.; Hofbauer, H. Techno-Economic Assessment of Hydrogen Production Based on Dual Fluidized Bed Biomass Steam Gasification, Biogas Steam Reforming, and Alkaline Water Electrolysis Processes. Energy Convers. Manag. 2017, 145, 278–292. [Google Scholar] [CrossRef]
- Rudra, S.; Tesfagaber, Y.K. Future District Heating Plant Integrated with Municipal Solid Waste (MSW) Gasification for Hydrogen Production. Energy 2019, 180, 881–892. [Google Scholar] [CrossRef]
- Kiminaitė, I.; González-Arias, J.; Striūgas, N.; Eimontas, J.; Seemann, M. Syngas Production from Protective Face Masks through Pyrolysis/Steam Gasification. Energies 2023, 16, 5417. [Google Scholar] [CrossRef]
- Ganza, P.E.; Lee, B. A Novel Method for Industrial Production of Clean Hydrogen (H2) from Mixed Plastic Waste. Int. J. Hydrogen Energy 2023, 48, 15037–15052. [Google Scholar] [CrossRef]
- Khan, Z.; Suzana, Y.; Ahmad, M.; Rashidi, N.A. Integrated Catalytic Adsorption (ICA) Steam Gasification System for Enhanced Hydrogen Production Using Palm Kernel Shell. Int. J. Hydrogen Energy 2014, 39, 3286–3293. [Google Scholar] [CrossRef]
- Lamas, G.C.; Chaves, B.S.; de Oliveira Rodrigues, P.P.; da Silva Gonzales, T.; Barbosa, T.; Rousset, P.; Ghesti, G.F.; Silveira, E.A. Effect of Torrefaction on Steam-Enhanced Co-Gasification of an Urban Forest and Landfill Waste Blend: H2 Production and CO2 Emissions Mitigation. Int. J. Hydrog. Energy. 2023, 48, 27151–27169. [Google Scholar] [CrossRef]
- Seraj, S.; Azargohar, R.; Borugadda, V.; Dalai, A. Energy Recovery from Agro-Forest Wastes through Hydrothermal Carbonization Coupled with Hydrothermal Co-Gasification: Effects of Succinic Acid on Hydrochars and H2 Production. Chemosphere 2023, 337, 139390. [Google Scholar] [CrossRef]
- Arcos, J.; Santos, D. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Tunay, D.; Yildirim, O.; Ozkaya, B.; Demir, A. Effect of Organic Fraction of Municipal Solid Waste Addition to High Rate Activated Sludge System for Hydrogen Production from Carbon Rich Waste Sludge. Int. J. Hydrogen Energy 2022, 47, 26284–26293. [Google Scholar] [CrossRef]
- Lepage, T.; Kammoun, M.; Schmetz, Q.; Richel, A. Biomass-to-Hydrogen: A Review of Main Routes Production, Processes Evaluation and Techno-Economical Assessment. Biomass Bioenergy 2021, 144, 105920. [Google Scholar] [CrossRef]
- Aziz, M.; Darmawan, A.; Juangsa, F.B. Hydrogen Production from Biomasses and Wastes: A Technological Review. Int. J. Hydrogen Energy 2021, 46, 33756–33781. [Google Scholar] [CrossRef]
- Wijayasekera, C.; Hewage, K.; Siddiqui, O.; Hettiaratchi, J.; Sadiq, R. Waste-to-Hydrogen Technologies: A Critical Review of Techno-Economic and Socio-Environmental Sustainability. Int. J. Hydrogen Energy 2021, 47, 5842–5870. [Google Scholar] [CrossRef]
- Banco de Portugal. Available online: https://www.bportugal.pt/ (accessed on 25 November 2023).
- Singh, H.; Tomar, S.; Qureshi, K.; Jaremko, M.; Rai, P.K. Recent Advances in Biomass Pretreatment Technologies for Biohydrogen Production. Energies 2022, 15, 999. [Google Scholar] [CrossRef]
- Chu, C.-F.; Xu, K.-Q.; Li, Y.-Y.; Inamori, Y. Hydrogen and Methane Potential Based on the Nature of Food Waste Materials in a Two-Stage Thermophilic Fermentation Process. Int. J. Hydrogen Energy 2012, 37, 10611–10618. [Google Scholar] [CrossRef]
- Santos, M.A.V. Avaliação do Potencial de Produção de Biohidrogénio na ETVO da Valorsul, Faculdade de Ciências e Tecnologia; Universidade Nova de Lisboa: Lisbon, Portugal, 2020. [Google Scholar]
- Gonzales, R.R.; Kim, J.S.; Kim, S.-H. Optimization of Dilute Acid and Enzymatic Hydrolysis for Dark Fermentative Hydrogen Production from the Empty Fruit Bunch of Oil Palm. Int. J. Hydrogen Energy 2019, 44, 2191–2202. [Google Scholar] [CrossRef]
- Ayodele, T.R.; Alao, M.A.; Ogunjuyigbe, A.S.O.; Munda, J.L. Electricity Generation Prospective of Hydrogen Derived from Biogas Using Food Waste in South-Western Nigeria. Biomass Bioenergy 2019, 127, 105291. [Google Scholar] [CrossRef]
- Seglah, P.A.; Wang, Y.; Wang, H.; Wobuibe Neglo, K.A.; Zhou, K.; Sun, N.; Shao, J.; Xie, J.; Bi, Y.; Gao, C. Utilization of Food Waste for Hydrogen-Based Power Generation: Evidence from Four Cities in Ghana. Heliyon 2023, 9, e14373. [Google Scholar] [CrossRef]
- Cudjoe, D.; Chen, W.; Zhu, B. Valorization of Food Waste into Hydrogen: Energy Potential, Economic Feasibility and Environmental Impact Analysis. Fuel 2022, 324, 124476. [Google Scholar] [CrossRef]
- Brentner, L.; Peccia, J.; Zimmerman, J. Challenges in Developing Biohydrogen as a Sustainable Energy Source: Implications for a Research Agenda. Environ. Sci. Technol. 2010, 44, 2243–2254. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, A.; Frunzo, L.; Pirozzi, F.; Trably, E.; Escudie, R.; Lens, P.N.L.; Esposito, G. A Review on Dark Fermentative Biohydrogen Production from Organic Biomass: Process Parameters and Use of by-Products. Appl. Energy 2015, 144, 73–95. [Google Scholar] [CrossRef]
- Mernit, J.L. As Plastics Keep Piling Up, Can ‘Advanced’ Recycling Cut the Waste? Available online: https://e360.yale.edu/features/advanced-plastics-recycling-pyrolysis (accessed on 25 November 2023).
- Rijo, B.; Alves, O.; Garcia, B.; Lourinho, G.; Brito, P.; Nobre, C. Technical and Market Analysis of Biomass Gasification: Case Study in Alentejo, Portugal. J. Clean. Prod. 2023, 417, 138007. [Google Scholar] [CrossRef]
- IEA. Net Zero by 2050—A Roadmap for the Global Energy Sector; IEA: Paris, France, 2021. [Google Scholar]
- IRENA. Geopolitics of the Energy Transformation: The Hydrogen Factor; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022; ISBN 978-92-9260-370-0. [Google Scholar]
- European Commission IPCEI. Available online: https://competition-policy.ec.europa.eu/state-aid/legislation/modernisation/ipcei_en (accessed on 25 November 2023).
- IRENA. Green Hydrogen for Industry: A Guide to Policy Making; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2022; ISBN 978-92-9260-422-6. [Google Scholar]
- Clean Hydrogen Joint Undertaking Repowering the EU with Hydrogen Valleys: Clean Hydrogen Partnership Invests EUR 105.4 Million for Funding 9 Hydrogen Valleys across Europe. Available online: https://www.clean-hydrogen.europa.eu/media/news/repowering-eu-hydrogen-valleys-clean-hydrogen-partnership-invests-eur-1054-million-funding-9-2023-01-31_en (accessed on 25 November 2023).



| Color | Production Pathway | Byproducts |
|---|---|---|
| black | Gasification of coal | CO2 |
| grey | Steam reforming of natural gas without CCS * | CO2 |
| blue | Steam reforming of natural gas with CCS * | - |
| turquoise | Pyrolysis of natural gas | Elemental carbon |
| white | Naturally occurring | - |
| pink | Electrolysis of nuclear power | O2 |
| green | Electrolysis of renewable energy Conversion of biomass | O2 Carbon negative with CCS * |
| Reaction | ΔHR (kJ/mol) | Equation |
|---|---|---|
| C + ½ O2 → CO | −111 | (1) |
| C + O2 → CO2 | −394 | (2) |
| C + 2 H2 ⇌ CH4 | −85 | (3) |
| CO + ½ O2 → CO2 | −283 | (4) |
| CO + H2O ⇌ H2 + CO2 | −41 | (5) |
| CO + 3 H2 ⇌ CH4 + H2O | −206 | (6) |
| C + CO2 ⇌ 2 CO | +173 | (7) |
| H2 + ½ O2 → H2O | −242 | (8) |
| CH4 + ½O2 → 2 H2+ CO | −110 | (9) |
| C + H2O → H2 + CO | +131 | (10) |
| CH4 + CO2 ⇌ 2 CO + 2 H2 | +247 | (11) |
| CxHy + x H2O → (x + y/2) H2 + x CO | Endothermic | (12) |
| CxHy + x CO2 → (y/2) H2 + 2x CO | Endothermic | (13) |
| primary and secondary tars → CO, H2, CO2, C, CxHy * | Endothermic | (14) |
| P-U/Ref. | [27] | [29] | [30] | [31] | [32] | [33] | [36] | [34] | [35] |
|---|---|---|---|---|---|---|---|---|---|
| Moisture content (wt% wb) | 20.86 | 5.46 | 5.06 | - | 4.69 | 3.82 | 14.3 | 1.5 | - |
| Volatile matter (wt% db) | 69.03 | 64.76 | 59.34 | 82.28 | 60.28 | 83.31 | 47.0 | 86.6 | 77.52 |
| Fixed carbon (wt% db) | 3.27 | 6.44 | 8.36 | 11.79 | 8.5 | 8.64 | 8.5 | - | - |
| Ash (wt% db) | 6.87 | 23.34 | 27.24 | 5,93 | 26.53 | 4.23 | 44.6 | 8.3 | 7.12 |
| C (wt% db) | 40.49 | 61.13 | 48.61 | 51.81 | 57.86 | 65.25 | 46.3 | 69.7 | 49.51 |
| H (wt% db) | 5.81 | 6.78 | 6.01 | 5.76 | 6.70 | 9.1 | 5.2 | 10.1 | 6.42 |
| O (wt% db) | 31.53 | 30.28 | 12.49 | 30.22 | 33.62 | 20.74 | 44.8 | 10.0 | 31.86 |
| N (wt% db) | 0.61 | 0.89 | 3.87 | 0.26 | 0.81 | 0.44 | 2.9 | 0.57 | 0.78 |
| S (wt% db) | - | 0.92 | 0.33 | 0.36 | 1.01 | 0.16 | 0.86 | 0.054 | 0.48 |
| LHV (MJ/kg) | 16.02 | - | 18.59 | 21.306 | - | - | 10.0 | - | 19.99 |
| Reactor Type | Agent | ER | T (°C) | H2 (vol%) | CO (vol%) | H2 (g/kg MSW) | LHV (MJ/Nm3) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Downdraft fixed bed | Air | 0.4 | 900 | 36.51 | 26.23 | 21.6 | 14.59 | [27] |
| Bubbling fluidized bed | Air | 0.4 | 900 | 35.7 | 36.79 | 24.1 | 12.57 | [27] |
| Tube | Air | - | 703.5 | 45.79 | 33.33 | 27.69 | 9.96 | [29] |
| Drop tube | CO2/steam | - | 1000 | 43.44 | 28.85 | 37.69 | 12.41 | [30] |
| Fixed bed | Steam | - | 950 | 53.29 | 16.92 | 77.28 | 9.36 | [31] |
| Tube | Air | 0.241 | 758 | 41.36 | 28.46 | 32.0 | 12.48 | [32] |
| Fluidized bed | Air/O2 | 0.2 | 900 | 9.57 * | 31.56 * | 19.24 | [33] | |
| Bubbling fluidized bed | Air | 0.34 | 770 | 10.2 * | 12.4 * | 11.03 | 3.70 | [36] |
| Fixed bed | Steam | - | 800 | 70.0 | 7.0 | [34] | ||
| Fixed bed | Steam | - | 850 | 59.0 | 15.0 | 98.15 | 9.34 | [35] |
| Fixed bed | Steam | - | 750 | 49.42 | 13.0 | 24.8 | [38] | |
| Fixed bed | Steam | - | 900 | 53.22 | 25.72 | 86.05 | 9.0 | [31] |
| Downdraft fixed bed | Air | 0.2 | 800 | 10.2 * | 14.1 * | 6.8 | [45] | |
| Fixed bed | Steam | - | 900 | 58.2 | 13.7 | [46] | ||
| Fixed bed | Steam | - | 900 | 54.22 | 22.72 | 84.72 | [44] | |
| Fixed bed | O2 | - | 600 | 50.65 | 34.0 | [47] | ||
| Fluidized bed | Steam | - | 900 | 49.6 | 22.0 | 80.0 | [48] | |
| Fluidized bed | Air/CO2 | 0.25/0.4 | 900 | 39.8 | 50.0 | 81.7 | [11] |
| P-U/Reference | [58] | [59] | [60] | [61] | [62] | [63] | [8] | [64] | [65] |
|---|---|---|---|---|---|---|---|---|---|
| Moisture content (wt% wb) | 0.76 | 1.62 | ns | 1.21 | 0.68 | ns | 0.8 | 0.82 | 0.75 |
| Volatile matter (wt% db) | 63.04 | 63.41 | 67.7 | 65.1 | 65.1 | 61.8 | 62.4 | 62.7 | 64.97 |
| Fixed carbon (wt%) | 32 | 21.86 | 25.5 | 8.8 | 28.95 | 33.8 | 29.4 | 32.31 | 30.08 |
| Ash (wt% db) | 4.2 | 13.21 | 6.8 | 4.9 | 46.63 | 4.4 | 7.4 | 4.17 | 4.16 |
| C (wt% db) | 83.34 | 80.46 | 77.3 | 67.9 | 79.55 | 85.2 | 87.1 | 81.2 | 83.92 |
| H (wt% db) | 6.56 | 8.14 | 6.2 | 6.6 | 7.99 | 7.3 | 7.7 | 7.2 | 6.83 |
| O (wt% db) | 2.78 | 6.9 | 7.1 | 17.4 | 2.53 | 0.5 | 3.1 | 8.7 | 7.55 |
| N (wt% db) | 0.79 | 0.82 | 0.6 | 1.9 | 0.15 | 0.4 | 0.4 | 0.8 | 0.78 |
| S (wt% db) | 1.33 | 3.68 | 1.8 | 1.28 | 2.47 | 2.3 | 1.7 | 2.1 | 0.92 |
| LHV (MJ/kg) | - | 36.34 | - | 29.5 | 37.4 | 37.1 | 35.8 | - | 38.6 |
| Reactor Type | Agent | ER | T (°C) | H2 (vol%) | CO (vol%) | H2 (g/kg MSW) | LHV (MJ/Nm3) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Rotary kiln | Steam | - | 1000 | 65.0 | 17.25 | 100.1 | 25.0 | [60] |
| Tube | Steam | - | 625 | 46.7 | 5.2 | 39.44 | - | [61] |
| Bubbling fluidized bed | Air | 0.15 | 805 | 20.0 b | 3.52 b | - | 7.03 | [62] |
| Rotary kiln | Steam | - | 750 | 73.3 | 21.5 | - | 17.74 | [63] |
| Hybrid filtration | Air | - | 1294 | 3.3 b | 4.1 b | - | 10.26 | [8] |
| Rotary kiln | Steam | - | 850 | 57.0 | 17.7 | - | 22.56 a | [74] |
| Fixed bed | Air | 0.07 | 500 | 30.4 | 8.2 | 32.0 | 6.0 | [52] |
| Fixed bed | Steam | 787 | 50.0 | 9.8 | 30.37 | 22.0 | [70] | |
| Fixed bed | Steam | 800 | 48.81 | 3.89 | - | 15.21 | [75] | |
| Fixed bed | Air/Steam | 800 | 22.63 | 4.88 | - | 7.34 | [75] | |
| Two-staged fixed bed | - | 600/800 | 57.47 | 16.06 | 36.32 | - | [64] | |
| Two-staged fixed bed | - | 500/800 | 60.8 | 9.7 | - | - | [72] | |
| Two-staged fixed bed | Steam | 500/900 | 56.8 | 15.0 | - | - | [73] | |
| Two-staged fixed bed | - | 500/800 | 56.0 | 11.0 | - | - | [71] | |
| Fixed bed | Air | 700 | 2.8 b | 4.0 b | - | 7.5 | [76] |
| Type | MC (wt% wb) | VC (wt% db) | FC (wt% db) | Ash (wt% db) | C (wt% db) | H (wt% db) | O (wt% db) | S (wt% db) | N (wt% db) | Cl (wt% db) | LHV (MJ/kg) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PE | 0.02 | 99.85 | 0 | 0.15 | 85.81 | 13.86 | 0 | 0.06 | 0.12 | - | - | [31] |
| 0.01 | 99.9 | 0 | 0 | 85.60 | 13.72 | 0.68 | 0 | - | - | - | [81] | |
| 0 | >99 | - | <0.01 | 85.84 | 14.07 | <0.01 | 0 | 0.09 | - | 43.38 | [82] | |
| 0.08 | 99.92 | 0 | 0 | 85.29 | 14.51 | 0.20 | 0 | 0 | - | 43.30 | [83] | |
| PET | 0 | 87.96 | 12 | 0.04 | 62.8 | 4.3 | 32.79 | 0.04 | 0.07 | - | 22.77 | [84] |
| 0.21 | 94.69 | 5.1 | 0 | 60.97 | 4.25 | 32.82 | 0 | 0.03 | - | 21.97 | [85] | |
| PP | 0 | 100 | 0 | 0 | 86 | 14.0 | 0 | 0 | 0 | - | - | [84] |
| 0.4 | 99.6 | 0 | 0 | 85.18 | 14.39 | 0.43 | 0 | 0 | - | - | [85] | |
| - | >99 | - | <0.1 | 85.86 | 13.91 | 0.13 | 0 | 0.10 | - | 43.42 | [82] | |
| 0 | 99.0 | - | 0.94 | 85.25 | 14.71 | 0 | 0 | 0.04 | - | - | [86] | |
| 0.1 | 96.7 | 3.2 | 0.03 | 84.3 | 13.8 | 1.7 | - | 0.6 | - | 45.1 | [87] | |
| PS | 0 | 84.6 | 14.4 | 0.97 | 46.87 | 6.06 | 45.48 | 0 | 0.62 | - | 18.09 | [64] |
| LDPE | 0.1 | 99.9 | 0 | 0 | 92.3 | 7.4 | 0.2 | 0 | 0 | - | 40.3 | [88] |
| - | - | - | - | 85.38 | 14.62 | 0 | 0 | 0 | - | - | [88] | |
| HDPE | 0 | 99.7 | 0.3 | 0 | 85.71 | 14.29 | 0 | 0 | 0 | - | 43.10 | [64] |
| 0 | 99.1 | 0.01 | 0 | 85.4 | 14.6 | 0 | 0 | 0 | - | - | [89] | |
| PVC | 0.1 | 66.6 | 29.0 | 4.3 | 50.1 | 6.2 | 22.3 | 0 | 0 | 20.5 | - | [87] |
| MP | - | 96.3 | 1.1 | 2.6 | 77.1 | 11.5 | 11.2 | - | 0.2 | - | - | [90] |
| 0.47 | - | - | 2.04 | 76.54 | 12.84 | 7.91 | - | 0.2 | - | 39.7 | [91] | |
| 0.3 | - | - | 1.3 | 84.4 | 14.0 | 0 | 0 | 0 | - | 45.5 | [92] | |
| 0.4 | 98.9 | - | 2.7 | 82.3 | 12.3 | 5.0 | 0.1 | 0.3 | - | 42.2 | [93] | |
| 4.4 | 86.73 | 4.1 | 9.2 | 68.85 | 8.89 | 21.3 | 0.14 | 0.4 | 0.32 | 24.68 | [94] | |
| 4.4 | 82.91 | 3.9 | 8.8 | 62.52 | 8.07 | 19.34 | 0.13 | 0.36 | 0.37 | 24.68 | [95] | |
| 0.51 | 62.35 | 18 | 19.1 | 65.18 | 15.61 | 9.15 | 0.11 | 0.16 | 2.98 | - | [96] | |
| - | - | - | - | 77.1 | 11.5 | 11.2 | - | 0.2 | - | - | [90] | |
| - | 93.2 | 3.5 | 3.3 | 68.9 | 12.8 | 14.3 | 0.2 | 0.5 | - | 35.2 | [97] | |
| 0.38 | 94.71 | 4.37 | 0.54 | 82.41 | 13.42 | 2.86 | 0 | 0.18 | 1.13 | 43.17 | [98] |
| Contaminants/Applications | Raw Product Gas | Gas Engine | Gas Turbine | Fuel Cell | Syngas |
|---|---|---|---|---|---|
| Particles (mg/Nm3) | 100–50,000 | <50 | <10 | <0.1 | <1 |
| Tar (mg/Nm3) | 20–100,000 | <50 | <10 | n.a. | 0.1–1 |
| Sulfur species (ppmv) | 50–5000 | <20–50 | <20 | SOFC < 0.06–10 PAFC < 50 MC < 0.5 | <0.01–1 |
| Nitrogen species (ppmv) | 100–20,000 | <80 | <50 a | Not limited | <0.02–1 |
| Alkali species (ppmv) | 10–1000 | <0.025–0.1 | <0.025–0.1 | Not specified | <0.01 |
| Halides (Cl + F + Br) (ppmv) | 5–1000 | <100 | <1 | <1 | <0.01 |
| Autothermal Gasifiers | Allothermal Gasifiers |
|---|---|
| One reactor | Two reactors or a separate heating system |
| Simpler design | More complex design |
| One gas stream to clean | Cleaning of two streams often needed. |
| Oxygen or steam/oxygen as gasifying agent for syngas production | Gasification by steam/CO2, so air separation unit is not necessary |
| Generally lower tar content in the produced gas due to higher temperature. | High tar content in the produced gas due to lower temperature. |
| Characteristic/Gasifier Type | Fixed Bed | BFB | CFB | Entrained Flow |
|---|---|---|---|---|
| Capacity range MW thermal (Typical operation pressure) | Downdraft < 1 Updraft < 10 (mainly atmospheric) | 10–50 (atmospheric) <200 (<3 MPa) | 20–150 (<3 MPa) | 100–400 (1–8 MPa) |
| Bed particle size (mm) | none | 0.1–1 | 0.1–0.4 | None |
| Fuel particle size (mm) | 10–100 | 1–100 | 1–100 | <1 |
| Operating temperature (°C) | 800 | 800–950 | 800–950 | 1400–1500 |
| Gas superficial velocity (m/s) | 0.1–0.5 | 0.5–1.5 | 4–8 | 15–25 |
| Solids mixing | Very low | High, complex | High complex | Low |
| Tar content in gas (g/Nm3) | Downdraft < 1 Updraft >> 10 | >10 | >10 | <<1 |
| Particles in gas (g/Nm3) | 0.1–0.2 | 0.1–1 | 2–20 | 1 |
| Biomass Type | Reactor | Gasifying Agent | H2 Production | Reference |
|---|---|---|---|---|
| Sewage sludge | Semi-batch | Steam | 0.07 g H2/g | [143] |
| Pine sawdust | Fixed bed (updraft) | Steam | 79.91 g H2/kg | [144] |
| Wood chips | Dual fluidized bed | Steam | 39.7 g H2/kg | [145] |
| Municipal solid waste | Tubular batch reactor | Air | 26.53 g H2/kg | [29] |
| Municipal solid waste | Allothermal fixed bed downdraft gasifier | Air | 157 g H2/kg | [146] |
| Mixture of air and steam | 165.3 g H2/kg | |||
| Steam | 199.6 g H2/kg | |||
| 3-ply surgical face masks | Bubbling fluidized bed reactor | Steam | 29.27 g H2/kg | [147] |
| Mixed plastic waste | Hybrid Microwave plasma Enhanced gasification | Steam | 150.9 g H2/kg | [148] |
| Palm kernel shell | Fluidized bed gasifier | Steam | 150 g H2/kg | [149] |
| Urban forest waste | Simulation (stoichiometric thermodynamic equilibrium model) | Steam | 84.25 g H2/Kg | [150] |
| Canola meal hydrochar | Tubular batch reactor | Hydrothermal co-gasification | 11.03 g H2/kg | [151] |
| Technology | TRL | Process Scale | CAPEX (M€) | Hydrogen Production Cost (€/kg) | Considerations |
|---|---|---|---|---|---|
| Gasification (dry thermal and hydrothermal) | 7 | Mid-scale/Available | 11.0–197.5 | 1.628–1.886 | Dry thermal gasification: Formation of tar and char that deposits on the catalysts (shorter catalyst life) and need for catalyst regeneration; High temperature and complex reaction leading to challenging operation control; High cost for reactor and apparatus; Fluctuating and varying gas composition, CO2 generation |
| Hydrothermal gasification: Limited to the material with high moisture content; High energy input for pressure elevation, and high reactor cost; Problems related to corrosion and plugging, difficulty to recover the catalyst, and CO2 generation. | |||||
| Pyrolysis | 7 | Mid-scale/Available | 100.9–263.3 | 1.15–2.024 | Lower production cost of H2; Formation of char and tar, CO2 generation, fluctuating and varying gas composition; Tar and char generation poisons catalysts; Cost of catalyst regeneration; Energy intensive due to feedstock drying, and elevated temperatures and pressures; Lower capital cost than gasification. |
| Dark fermentation | 5 | Pilot-scale | - | 2.364 | Higher productivity than photo-fermentation; Simple design of fermenters; Requirement for pre-treatment; Formation of undesired by-products; Low production rate, low hydrogen yield, low removal of chemical oxygen demand. |
| Photo-fermentation | 4 | Laboratory-scale | 106.0 | 2.604 | More sophisticated design and lower productivity than dark fermentation; Illumination source required; Low production rate, low energy conversion efficiency, requirement of large surface area; Higher production cost of H2; Lower capital costs expected. |
| Microbial electrolysis | 2–4 | Laboratory-scale | 2.8 | - | External voltage source needed; The requirement for external electrical supply, the economic feasibility of scaling up due to expensive construction materials, the long-term stability is questionable; High capital cost of equipment components and higher production cost of H2. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rauch, R.; Kiros, Y.; Engvall, K.; Kantarelis, E.; Brito, P.; Nobre, C.; Santos, S.M.; Graefe, P.A. Hydrogen from Waste Gasification. Hydrogen 2024, 5, 70-101. https://doi.org/10.3390/hydrogen5010006
Rauch R, Kiros Y, Engvall K, Kantarelis E, Brito P, Nobre C, Santos SM, Graefe PA. Hydrogen from Waste Gasification. Hydrogen. 2024; 5(1):70-101. https://doi.org/10.3390/hydrogen5010006
Chicago/Turabian StyleRauch, Reinhard, Yohannes Kiros, Klas Engvall, Efthymios Kantarelis, Paulo Brito, Catarina Nobre, Santa Margarida Santos, and Philipp A. Graefe. 2024. "Hydrogen from Waste Gasification" Hydrogen 5, no. 1: 70-101. https://doi.org/10.3390/hydrogen5010006
APA StyleRauch, R., Kiros, Y., Engvall, K., Kantarelis, E., Brito, P., Nobre, C., Santos, S. M., & Graefe, P. A. (2024). Hydrogen from Waste Gasification. Hydrogen, 5(1), 70-101. https://doi.org/10.3390/hydrogen5010006









