The Use of Copper-Based Delafossite to Improve Hydrogen Production Performance: A Review
Abstract
1. Introduction
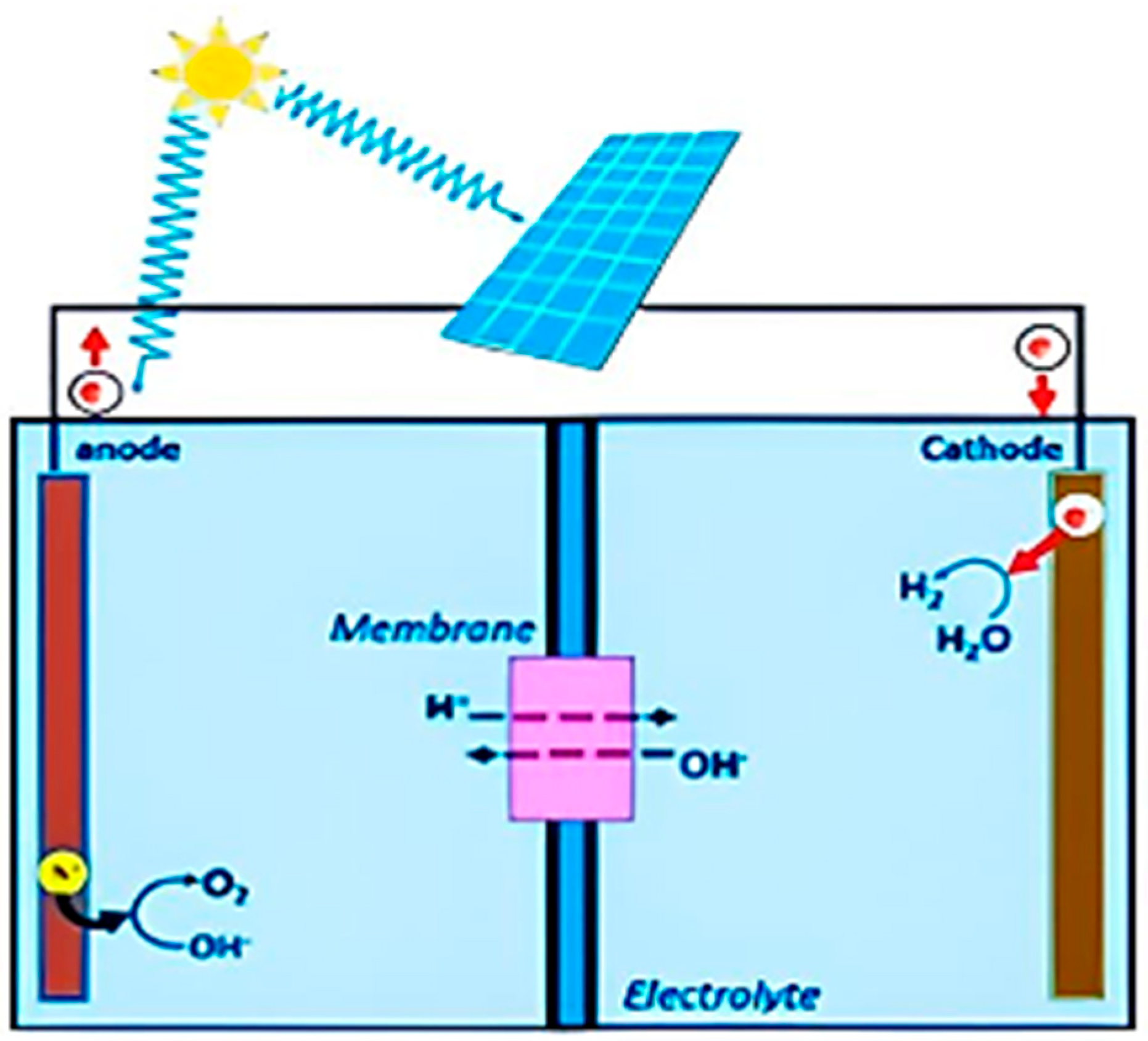
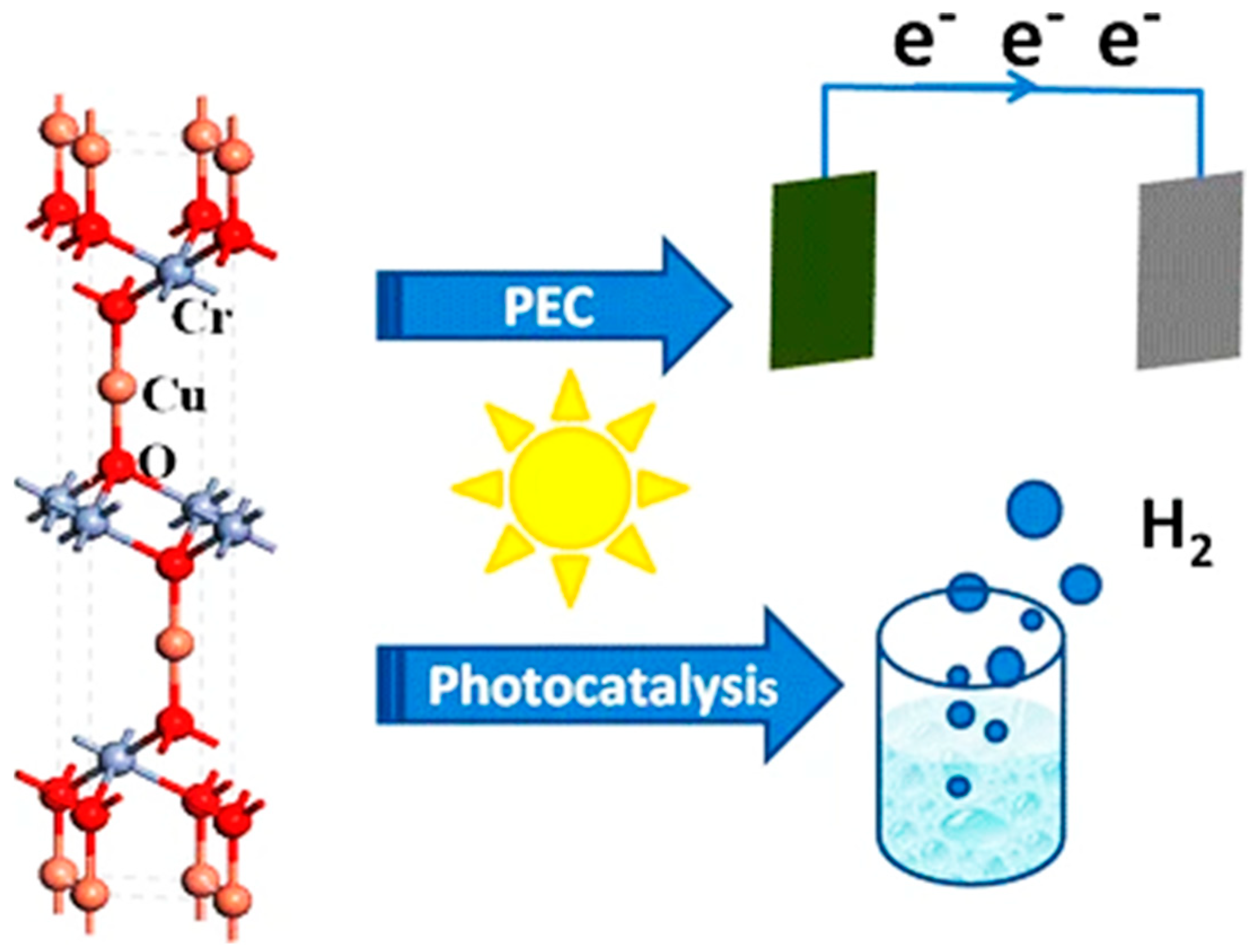
2. Delafossite Used in Water-Splitting Systems for Hydrogen Production
- -
- Reduction Reaction:
- -
- Oxidation Reaction:
- -
- Photoelectrochemical (PEC) System:
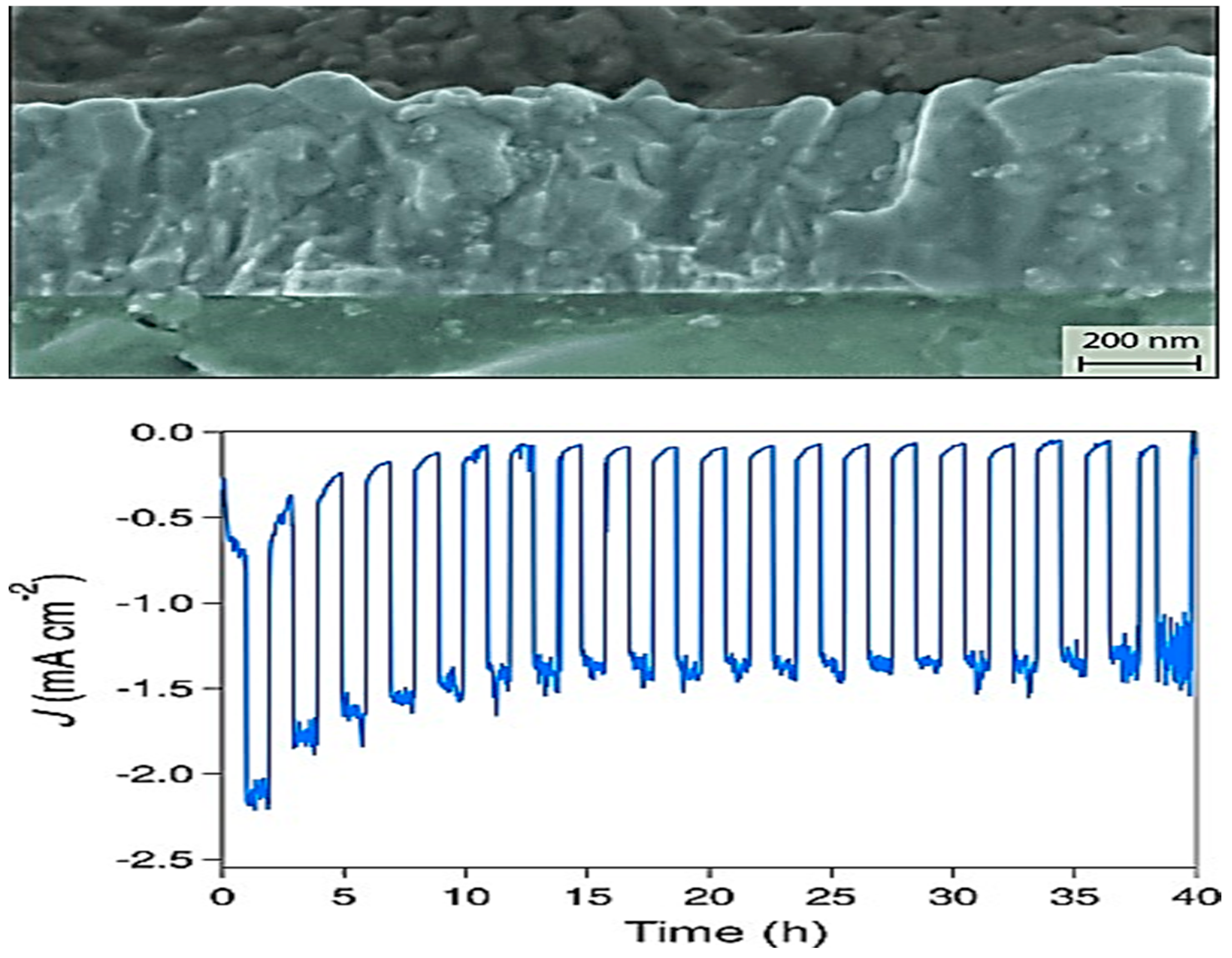
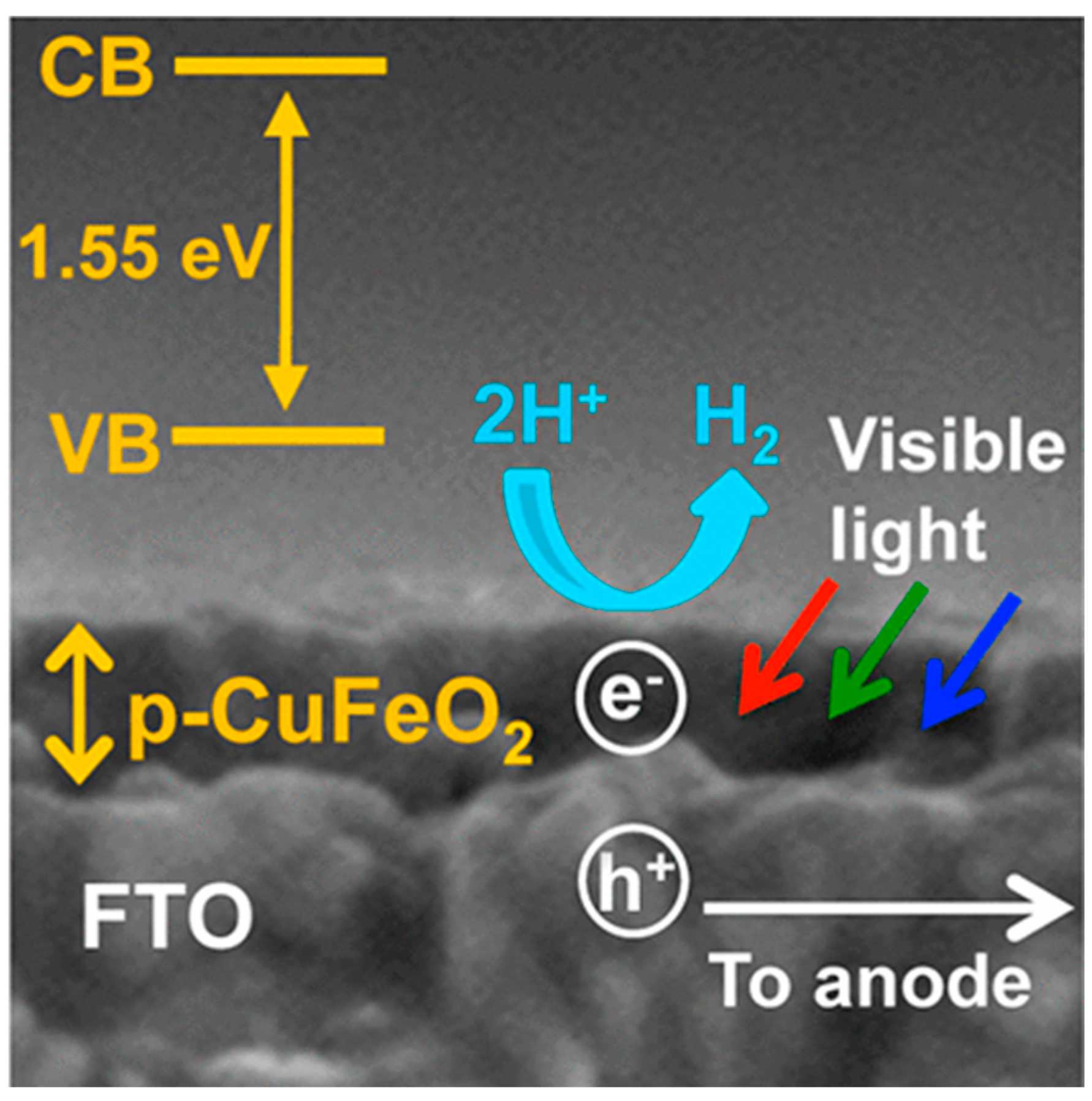
3. CuFeO2 Delafossite Materials
4. CuCrO2 Delafossite Materials
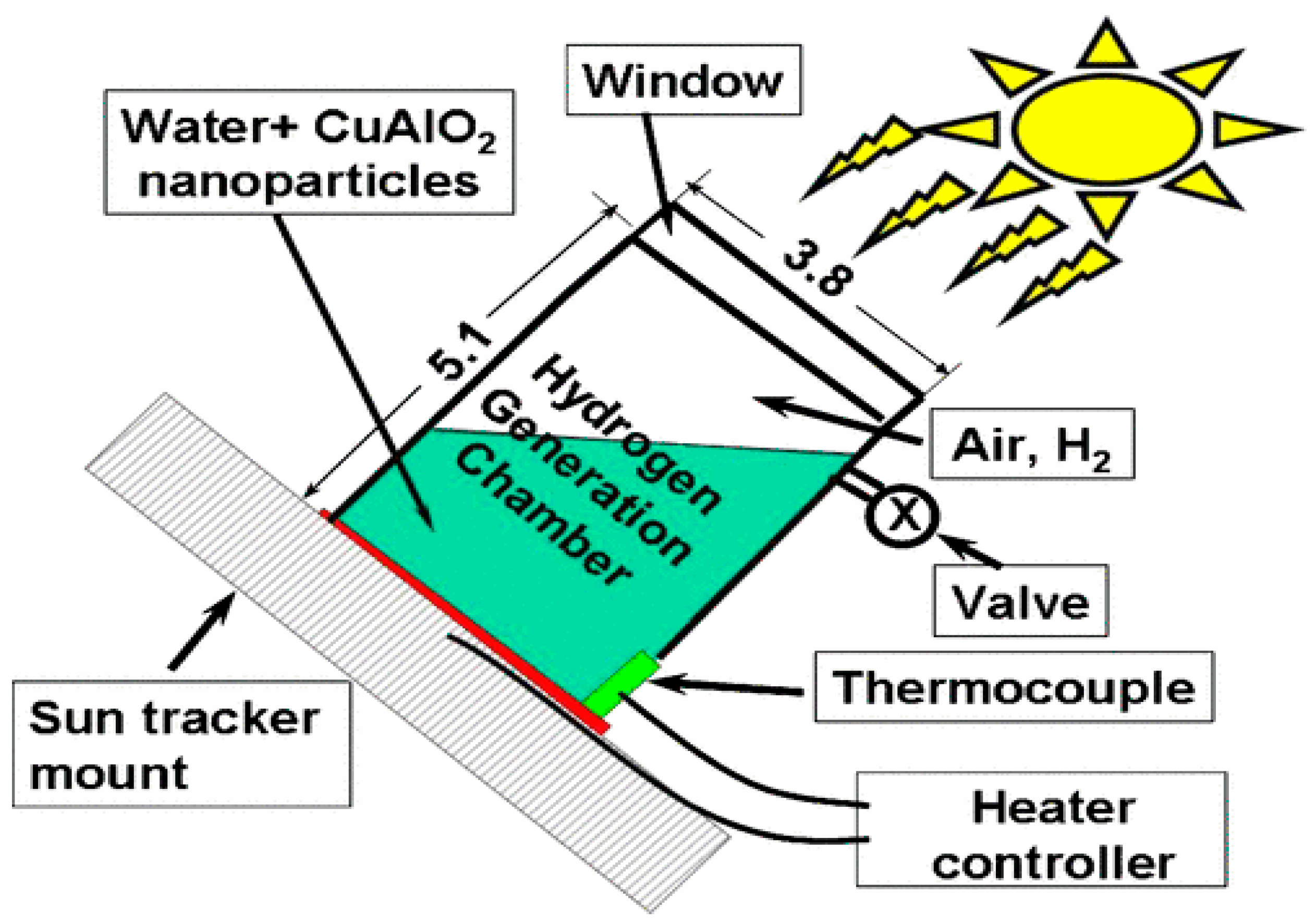
5. CuAlO2 Delafossite Materials
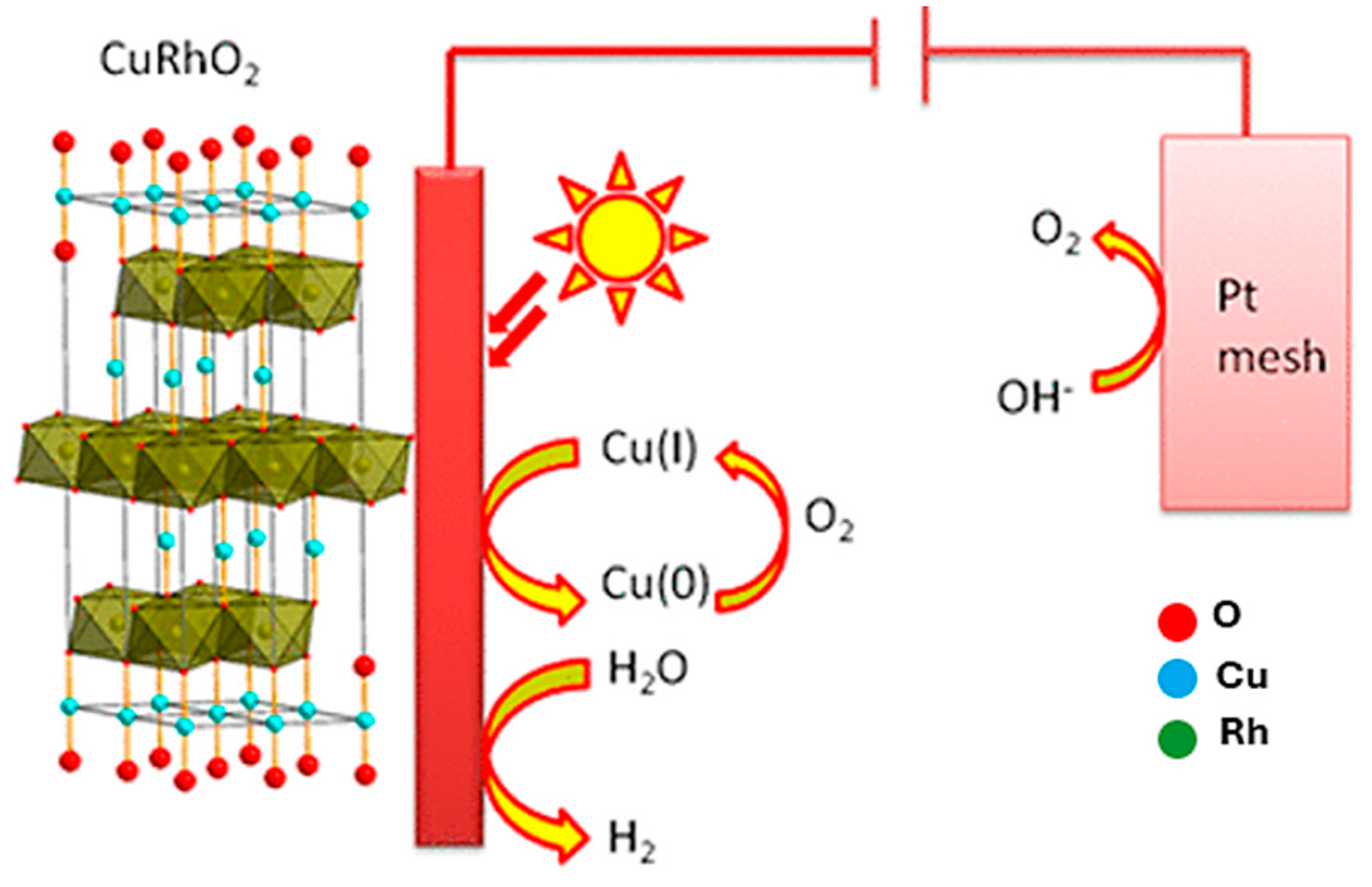
6. CuRhO2 Delafossite Materials
7. CuMnO2 Delafossite Materials
8. CuYO2 Delafossite Materials
- -
- Performance table:
| Delafossite Material | Photocatalytic Application | Key Performance Metrics | Notable Features |
| CuFeO2 | Solar water reduction | Band-edge location, stability, CO2 reduction potential | Sol–gel-based method, excellent band edge location, stability, and CO2 reduction capability |
| CuCrO2 | Photochemical H2 evolution | Band gap, stability visible light responsiveness | Efficient absorption of visible light, stable H2 production, potential for co-catalyst integration |
| CuAlO2 | Hydrogen Production | Band gap, catalytic aptitude for visible light induced H2 generation | Effective H2 generation under visible light, dependence on S2− as a reducing agent |
| CuRhO2 | Visible light water splitting | Band edge locations, photostability, self-healing interface | Unique self-healing semiconductor/electrolyte interface, stable H2 production under visible light |
| CuMnO2 | Oxygen and hydrogen evolution | Electrocatalytic activity, stability | Bifunctional electrocatalysis for OER and HER, potential for further research |
| CuYO2 | Methane steam reformation | H2 production rate, thermal stability, catalyst efficiency | Superior electrical conductivity, great stability, potential for hydrogen generation applications |
- -
- Active Sites:
- CuFeO2—Active sites are likely associated with the delafossite structure, with an emphasis on the optimized semiconductor interface using suitable overlayers or catalysts.
- CuCrO2—Active sites include the CuCrO2 surface where H2 is primarily liberated, and S2 is oxidized on the Cu2O surface when coupled with in situ created n-Cu2O.
- CuAlO2—Active sites involve the conduction band’s potential, allowing spontaneous H2 evolution, particularly in the presence of S2− as a reducing agent.
- CuRhO2—Active sites are attributed to the polycrystalline CuRhO2 surface, with photogenerated band conduction electrons reducing water and valence band holes oxygenating water.
- CuMnO2—Active sites for CuMnO2 are not explicitly mentioned in the provided text, but further research may focus on its electrocatalytic activity for oxygen and hydrogen evolution.
- CuYO2—Active sites are likely associated with the pseudo-delafossite structure, where Cu+ ions with d-d transitions and Y3+ ions interact, facilitating hydrogen generation during methane steam reformation.
- -
- Summary and Comparison:
- CuFeO2—stands out for its sol–gel-based production method, excellent band-edge location, stability, and potential for CO2 reduction;
- CuCrO2—noteworthy for efficient absorption of visible light, stable H2 production, and potential for co-catalyst integration;
- CuAlO2—effective in H2 generation under visible light, particularly with S2− as a reducing agent;
- CuRhO2—unique for its self-healing semiconductor/electrolyte interface, providing stability for sustained photo electrolysis of water;
- CuMnO2—limited details provided, emphasizing further research opportunities;
- CuYO2—superior electrical conductivity, great stability, and potential for hydrogen generation applications during methane steam reforming.
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Younsi, M.; Saadi, S.; Bouguelia, A.; Aider, A.; Trari, M. Synthesis and characterization of oxygen-rich delafossite CuYO2+x—Application to H2-photo production. Sol. Energy Mater. Sol. Cells 2007, 91, 1102–1109. [Google Scholar] [CrossRef]
- Sazali, N. Emerging technologies by hydrogen: A review. Int. J. Hydrogen Energy 2020, 45, 18753–18771. [Google Scholar] [CrossRef]
- Aravindan, M.; Madhan, K.V.; Hariharan, V.S.; Narahari, T.; Arun, K.P.; Madhesh, K.; Praveen, K.J.G.; Prabakaran, R. Fuelling the future: A review of non-renewable hydrogen production and storage techniques. Renew. Sustain. Energy Rev. 2023, 188, 113791. [Google Scholar] [CrossRef]
- Aravindan, M.; Kumar, P. Hydrogen towards sustainable transition: A review of production, economic, environmental impact and scaling factors. Results Eng. 2023, 20, 101456. [Google Scholar]
- Pahwa, P.K.; Pahwa, G.K. Hydrogen Economy; The Energy and Resources Institute (TERI): New Delhi, India, 2014. [Google Scholar]
- Sohni, S.; Nidaullah, H.; Gul, K.; Ahmad, I.; Omar, A.M. Nanotechnology for Safe and Sustainable Environment: Realm of Wonders. In Nanomaterials and Their Fascinating Attributes–Development and Prospective Applications of Nanoscience and Nanotechnology; Khan, S.B., Asiri, A.M., Akhtar, K., Eds.; Bentham Science Publishers: Sharjah, United Arab Emirates, 2018; Volume 2, pp. 37–117. [Google Scholar]
- Sonthalia, A.; Kumar, N.; Tomar, M.; Geo, V.E.; Thiyagarajan, S.; Pugazhendhi, A. Moving ahead from hydrogen to methanol economy: Scope and challenges. Clean Technol. Environ. Policy 2021, 25, 1–25. [Google Scholar] [CrossRef]
- Mitra, S.; Maiti, D.K. Nanotechnology for green energy and sustainable future. In Nano Tools and Devices for Enhanced Renewable Energy; Elsevier: Amsterdam, The Netherlands, 2021; pp. 521–533. [Google Scholar]
- Rolo, I.; Costa, V.A.F.; Brito, F.P. Hydrogen-Based Energy Systems: Current Technology Development Status, Opportunities and Challenges. Energies 2023, 17, 180. [Google Scholar] [CrossRef]
- Koriche, N.; Bouguelia, A.; Aider, A.; Trari, M. Photocatalytic hydrogen evolution over delafossite CuAlO2. Int. J. Hydrogen Energy 2005, 30, 693–699. [Google Scholar] [CrossRef]
- Qazi, U.Y. Future of hydrogen as an alternative fuel for next-generation industrial applications; challenges and expected opportunities. Energies 2022, 15, 4741. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Mofijur, M.; Nuzhat, S.; Rafa, N.; Musharrat, A.; Lam, S.S.; Boretti, A. Sustainable hydrogen production: Technological advancements and economic analysis. Int. J. Hydrogen Energy 2022, 47, 37227–37255. [Google Scholar] [CrossRef]
- Gholami, F.; Tomas, M.; Gholami, Z.; Vakili, M. Technologies for the nitrogen oxides reduction from flue gas: A review. Sci. Total Environ. 2020, 714, 136712. [Google Scholar] [CrossRef]
- Larki, I.; Zahedi, A.; Asadi, M.; Forootan, M.M.; Farajollahi, M.; Ahmadi, R.; Ahmadi, A. Mitigation approaches and techniques for combustion power plants flue gas emissions: A comprehensive review. Sci. Total Environ. 2023, 903, 166108. [Google Scholar] [CrossRef] [PubMed]
- Panić, I.; Cuculić, A.; Ćelić, J. Color-coded hydrogen: Production and storage in maritime sector. J. Mar. Sci. Eng. 2022, 10, 1995. [Google Scholar] [CrossRef]
- Asghar, U.; Rafiq, S.; Anwar, A.; Iqbal, T.; Ahmed, A.; Jamil, F.; Khurram, M.S.; Akbar, M.M.; Farooq, A.; Shah, N.S.; et al. Review on the progress in emission control technologies for the abatement of CO2, SOx and NOx from fuel combustion. J. Environ. Chem. Eng. 2021, 9, 106064. [Google Scholar] [CrossRef]
- Farghali, M.; Osman, A.I.; Umetsu, K.; Rooney, D.W. Integration of biogas systems into a carbon zero and hydrogen economy: A review. Environ. Chem. Lett. 2022, 20, 2853–2927. [Google Scholar] [CrossRef]
- Yu, C.-L.; Sakthinathan, S.; Chen, S.-Y.; Yu, B.-S.; Chiu, T.-W.; Dong, C. Hydrogen generation by methanol steam reforming process by delafossite-type CuYO2 nanopowder catalyst. Microporous Mesoporous Mater. 2021, 324, 111305. [Google Scholar] [CrossRef]
- Tzimas, E.; Filiou, C.; Peteves, S.D.; Veyret, J.B. Hydrogen Storage: State-of-the-Art and Future Perspective; EUR 20995EN; EU Commission, JRC Petten: Petten, The Netherlands, 2003. [Google Scholar]
- Ren, J.; Musyoka, N.M.; Langmi, H.W.; Mathe, M.; Liao, S. Current research trends and perspectives on materials-based hydrogen storage solutions: A critical review. Int. J. Hydrogen Energy 2017, 42, 289–311. [Google Scholar] [CrossRef]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen storage technologies for future energy systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef] [PubMed]
- Bérubé, V.; Radtke, G.; Dresselhaus, M.; Chen, G. Size effects on the hydrogen storage properties of nanostructured metal hydrides: A review. Int. J. Energy Res. 2007, 31, 637–663. [Google Scholar] [CrossRef]
- Gary, S. State-of-the-art review of hydrogen storage in reversible metal hydrides for military fuel cell applications. Final. Rep. Contract 1997, 14, 1–159. [Google Scholar]
- Yang, J.; Sudik, A.; Wolverton, C.; Siegel, D.J. High capacity hydrogen storage materials: Attributes for automotive applications and techniques for materials discovery. Chem. Soc. Rev. 2010, 39, 656–675. [Google Scholar] [CrossRef]
- Liu, Q.-L.; Zhao, Z.-Y.; Zhao, R.-D.; Yi, J.-H. Fundamental properties of delafossite CuFeO2 as photocatalyst for solar energy conversion. J. Alloys Compd. 2020, 819, 153032. [Google Scholar] [CrossRef]
- Sorensen, B. Hydrogen and Fuel Cells: Emerging Technologies and Applications; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Cavaliere, P. Hydrogen and Energy Transition. In Water Electrolysis for Hydrogen Production; Springer International Publishing: Cham, Switzerland, 2023; pp. 61–104. [Google Scholar]
- National Research Council. The Hydrogen Economy: Opportunities, Costs, Barriers, and R&D Needs; National Academies Press: Washington, DC, USA, 2004.
- Greene, D.L. Transportation and energy. In The Geography of Urban Transportation; The Guileford Press: New York, NY, USA, 2004; pp. 274–294. [Google Scholar]
- Kroyan, Y.; Wojcieszyk, M. End-Use Performance of Alternative Fuels in Variou s Modes of Transportation; ADVANCEFUEL project Deliverable D; Aalto University: Espoo, Finland, 2020; Volume 5, p. 5. [Google Scholar]
- Das, P.K.; Jiao, K.; Wang, Y.; Barbir, F.; Li, X. (Eds.) Fuel Cells for Transportation: Fundamental Principles and Applications; Woodhead Publishing: Sawston, UK, 2023. [Google Scholar]
- Benghanem, M.; Mellit, A.; Almohamadi, H.; Haddad, S.; Chettibi, N.; Alanazi, A.M.; Dasalla, D.; Alzahrani, A. Hydrogen production methods based on solar and wind energy: A review. Energies 2023, 16, 757. [Google Scholar] [CrossRef]
- Paumo, H.K.; Dalhatou, S.; Katata-Seru, L.M.; Kamdem, B.P.; Tijani, J.O.; Vishwanathan, V.; Kane, A.; Bahadur, I. TiO2 assisted photocatalysts for degradation of emerging organic pollutants in water and wastewater. J. Mol. Liq. 2021, 331, 115458. [Google Scholar] [CrossRef]
- Li, T. Synthesis, Characterisation and Photocatalytic Activity of Porous Silicon-Based Materials. Doctoral Dissertation, University of East Anglia, Norwich, UK, 2017. [Google Scholar]
- Serrà, A.; Philippe, L.; Perreault, F.; Garcia-Segura, S. Photocatalytic treatment of natural waters. Reality or hype? The case of cyanotoxins remediation. Water Res. 2020, 188, 116543. [Google Scholar] [CrossRef]
- Guan, D.; Wang, B.; Zhang, J.; Shi, R.; Jiao, K.; Li, L.; Wang, Y.; Xie, B.; Zhang, Q.; Yu, J.; et al. Hydrogen society: From present to future. Energy Environ. Sci. 2023, 16, 4926–4943. [Google Scholar] [CrossRef]
- Bellal, B.; Saadi, S.; Koriche, N.; Bouguelia, A.; Trari, M. Physical properties of the delafossite LaCuO2. J. Phys. Chem. Solids 2009, 70, 1132–1136. [Google Scholar] [CrossRef]
- He, H. Solution Processed Metal Oxide Thin Films for Electronic Applications; School of Materials Science and Engineering, Zhejiang University: Hangzhou, China, 2020; p. 7. [Google Scholar]
- Lee, L.C. Vapour-Based Growth of Inorganic Compounds for Next-Generation, Stable Photovoltaics. Doctoral Dissertation, University of Cambridge, Cambridge, UK, 2020. [Google Scholar]
- Wildsmith, T. Low Temperature Precursors for SnOx Thin Films. Doctoral Dissertation, University of Bath, Bath, UK, 2014. [Google Scholar]
- Sheets, W.C. Synthetic Methods for Multifunctional Materials. Doctoral Dissertation, Northwestern University, Evanston, IL, USA, 2006. [Google Scholar]
- Karataş, Y.; Zengin, A.; Gülcan, M. Preparation and characterization of amine-terminated delafossite type oxide, CuMnO2–NH2, supported Pd (0) nanoparticles for the H2 generation from the methanolysis of ammonia-borane. Int. J. Hydrogen Energy 2022, 47, 16036–16046. [Google Scholar] [CrossRef]
- Sivagurunathan, A.T.; Adhikari, S.; Kim, D.-H. Strategies and implications of atomic layer deposition in photoelectrochemical water splitting: Recent advances and prospects. Nano Energy 2021, 83, 105802. [Google Scholar] [CrossRef]
- Chatterjee, P.; Ambati, M.S.K.; Chakraborty, A.K.; Chakrabortty, S.; Biring, S.; Ramakrishna, S.; Wong, T.K.S.; Kumar, A.; Lawaniya, R.; Dalapati, G.K. Photovoltaic/photo-electrocatalysis integration for green hydrogen: A review. Energy Convers. Manag. 2022, 261, 115648. [Google Scholar] [CrossRef]
- Prévot, M.S.; Sivula, K. Photoelectrochemical tandem cells for solar water splitting. J. Phys. Chem. C 2013, 117, 17879–17893. [Google Scholar] [CrossRef]
- Desai, M.A.; Vyas, A.N.; Saratale, G.D.; Sartale, S.D. Zinc oxide superstructures: Recent synthesis approaches and application for hydrogen production via photoelectrochemical water splitting. Int. J. Hydrogen Energy 2019, 44, 2091–2127. [Google Scholar] [CrossRef]
- Kalanur, S.S.; Duy, L.T.; Seo, H. Recent Progress in Photoelectrochemical Water Splitting Activity of WO3 Photoanodes. Top. Catal. 2018, 61, 1043–1076. [Google Scholar] [CrossRef]
- Wang, K.; Huang, D.; Li, X.; Feng, K.; Shao, M.; Yi, J.; He, W.; Qiao, L. Unconventional strategies to break through the efficiency of light-driven water splitting: A review. Electron 2023, 1, e4. [Google Scholar] [CrossRef]
- Gurunathan, K.; Baeg, J.-O.; Lee, S.M.; Subramanian, E.; Moon, S.-J.; Kong, K.-J. Visible light assisted highly efficient hydrogen production from H2S decomposition by CuGaO2 and CuGa1−xInxO2 delafossite oxides bearing nanostructured co-catalysts. Catal. Commun. 2008, 9, 395–402. [Google Scholar] [CrossRef]
- Ke, J. Artificial photosynthesis and solar fuels. In Solar-to-Chemical Conversion: Photocatalytic and Photoelectrochemcial Processes; Wiley: Hoboken, NJ, USA, 2021; pp. 7–39. [Google Scholar]
- Shi, Q.; Duan, H. Recent progress in photoelectrocatalysis beyond water oxidation. Chem Catal. 2022, 2, 3471–3496. [Google Scholar] [CrossRef]
- Qi, S.; Guo, R.; Bi, Z.; Zhang, Z.; Li, C.; Pan, W. Recent Progress of Covalent Organic Frameworks-Based Materials in Photocatalytic Applications: A Review. Small 2023, 19, e2303632. [Google Scholar] [CrossRef]
- Aboagye, D.; Djellabi, R.; Medina, F.; Contreras, S. Radical-mediated photocatalysis for lignocellulosic biomass conversion into value-added chemicals and hydrogen: Facts, opportunities and challenges. Angew. Chem. Int. Ed. 2023, 62, e202301909. [Google Scholar] [CrossRef]
- Curti, M.; AlSalka, Y.; Al-Madanat, O.; Bahnemann, D.W. Isotopic Substitution to Unravel the Mechanisms of Photocatalytic Hydrogen Production. In Photocatalytic Hydrogen Production for Sustainable Energy; Wiley: Hoboken, NJ, USA, 2023; pp. 35–61. [Google Scholar]
- Bagtache, R.; Brahimi, R.; Mahroua, O.; Boudjellal, L.; Abdmeziem, K.; Trari, M. Photoelectrochemical study of the delafossite agnio2 nanostructure: Application to hydrogen production. J. Electrochem. Energy Convers. Storage 2020, 17, 031008. [Google Scholar] [CrossRef]
- Qiu, W.-T.; Huang, Y.-C.; Wang, Z.-L.; Xiao, S.; Ji, H.-B.; Tong, Y.-X. Effective strategies towards high-performance photoanodes for photoelectrochemical water splitting. Acta Phys. Chim. Sin. 2017, 33, 80–102. [Google Scholar] [CrossRef]
- Bagnall, A.J. Novel Electrode and Photoelectrode Materials for Hydrogen Production Based on Molecular Catalysts. Doctoral Dissertation, Université Grenoble Alpes, Saint-Martin-d’Hères, France, Uppsala Universitet, Uppsala, Sweden, 2022. [Google Scholar]
- Minero, C.; Maurino, V. 16 Solar Photocatalysis for Hydrogen Production and CO2 Conversion. In Catalysis for Renewables: From Feedstock to Energy Production; Wiley: Hoboken, NJ, USA, 2007; p. 351. [Google Scholar]
- Knöppel, J. On the Stability of Anode (Photo-) Electrocatalysts in the Solar to Hydrogen Nexus. Doctoral Dissertation, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2022. [Google Scholar]
- Liu, Q.L.; Zhao, Z.Y.; Yi, J.H. Excess oxygen in delafossite CuFeO2+ δ: Synthesis, characterization, and applications in solar energy conversion. Chem. Eng. J. 2020, 396, 125290. [Google Scholar] [CrossRef]
- Mazloomi, K.; Sulaiman, N.B.; Moayedi, H. Electrical efficiency of electrolytic hydrogen production. Int. J. Electrochem. Sci. 2012, 7, 3314–3326. [Google Scholar] [CrossRef]
- Sato, M.; Mooney, H.M. The electrochemical mechanism of sulfide self-potentials. Geophysics 1960, 25, 226–249. [Google Scholar] [CrossRef]
- Peled, E.; Golodnitsky, D.; Ardel, G. Advanced model for solid electrolyte interphase electrodes in liquid and polymer electrolytes. J. Electrochem. Soc. 1997, 144, L208–L210. [Google Scholar] [CrossRef]
- Zaban, A.; Zinigrad, E.; Aurbach, D. Impedance spectroscopy of Li electrodes. 4. A general simple model of the Li− solution interphase in polar aprotic systems. J. Phys. Chem. 1996, 100, 3089–3101. [Google Scholar] [CrossRef]
- Huda, M.N.; Yan, Y.; Walsh, A.; Wei, S.-H.; Turner, J.A.; Al-Jassim, M.M. Delafossite-alloy photoelectrodes for PEC hydrogen production: A density functional theory study. In Proceedings of the SPIE—The International Society for Optical Engineering 7770, Solar Hydrogen and Nanotechnology V, San Diego, CA, USA, 24 August 2010; Volume 7770, pp. 45–54. [Google Scholar]
- Götzendörfer, S. Synthesis of Copper-Based Transparent Conductive Oxides with Delafossite Structure Via Sol-Gel Processing. Doctoral Dissertation, Universität Würzburg, Würzburg, Germany, 2010. [Google Scholar]
- Liu, Y. P-Type Transparent Conducting Oxides Synthesized by Electrohydrodynamic Process. Doctoral Dissertation, Alfred University, Alfred, NY, USA, 2017. [Google Scholar]
- Manoj, R.; Jayaraj, M.K. Characterisation of Transparent Conducting Thin Films Grown by Pulsed Laser Deposition and RF Magnetron Sputtering. Ph.D. Thesis, Department of Physics, Kerala, India, 2006. [Google Scholar]
- Bishop, P.T.; Sutton, P.A.; Gardener, M.; Wakefield, G. Novel Transparent Conducting Oxide Technology for Solar Cells. 2005. Available online: https://www.osti.gov/etdeweb/biblio/20714899 (accessed on 23 January 2024).
- Prévot, M.S.; Guijarro, N.; Sivula, K. Enhancing the performance of a robust sol–gel-processed p-type delafossite CuFeO2 photocathode for solar water reduction. ChemSusChem 2015, 8, 1359–1367. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Tang, F.Q.; Wang, L.Z. Visible light responsive metal oxide photoanodes for photoelectrochemical water splitting: A comprehensive review on rational materials design. J. Inorg. Mater. 2018, 33, 173–196. [Google Scholar]
- Gan, J.; Lu, X.; Tong, Y. Towards highly efficient photoanodes: Boosting sunlight-driven semiconductor nanomaterials for water oxidation. Nanoscale 2014, 6, 7142–7164. [Google Scholar] [CrossRef]
- Liu, J.; Luo, Z.; Mao, X.; Dong, Y.; Peng, L.; Sun-Waterhouse, D.; Kennedy, J.V.; Waterhouse, G.I.N. Recent Advances in Self-Supported Semiconductor Heterojunction Nanoarrays as Efficient Photoanodes for Photoelectrochemical Water Splitting. Small 2022, 18, e2204553. [Google Scholar] [CrossRef]
- Luan, P. Regulating Bulk and Surface Charge Behaviour of Photoanodes for Enhanced Solar Water Oxidation Efficiency; Monash University: Mulgrave, Australia, 2019. [Google Scholar]
- Prasad, U. BiVO4-Based Photoanodes for Photoelectrochemical Water Splitting. In Clean Energy Materials; American Chemical Society: Washington, DC, USA, 2020; pp. 137–167. [Google Scholar]
- Zhao, Q.M.; Zhao, Z.Y.; Liu, Q.L.; Yao, G.Y.; Dong, X.D. Delafossite CuGaO2 as promising visible-light-driven photocatalyst: Synthesize, properties, and performances. J. Phys. D Appl. Phys. 2020, 53, 135102. [Google Scholar] [CrossRef]
- Pawar, R.C.; Lee, C.S. Heterogeneous Nanocomposite-Photocatalysis for Water Purification; William Andrew: Norwich, NY, USA, 2015; ISBN 9780323393102. [Google Scholar]
- Calbo, J. Dye-Sensitized Solar Cells: Past, Present and Future. In Photoenergy and Thin Film Materials; Wiley: Hoboken, NJ, USA, 2019; pp. 49–119. [Google Scholar]
- Prévot, M.S. Investigating and Controlling Charge Carrier Behavior in p-Type Delafossite CuFeO2 Photocathodes for Solar Fuel Production; EPFL: Lausanne, Switzerland, 2017. [Google Scholar]
- Li, Z.; Luo, W.; Zhang, M.; Feng, J.; Zou, Z. Photoelectrochemical cells for solar hydrogen production: Current state of promising photoelectrodes, methods to improve their properties, and outlook. Energy Environ. Sci. 2013, 6, 347–370. [Google Scholar] [CrossRef]
- Fabre, B.; Loget, G. Silicon Photoelectrodes Prepared by Low-Cost Wet Methods for Solar Photoelectrocatalysis. Accounts Mater. Res. 2023, 4, 133–142. [Google Scholar] [CrossRef]
- Moss, B.; Babacan, O.; Kafizas, A.; Hankin, A. A review of inorganic photoelectrode developments and reactor scale-up challenges for solar hydrogen production. Adv. Energy Mater. 2021, 11, 2003286. [Google Scholar] [CrossRef]
- Woodhouse, M.; Parkinson, B.A. Combinatorial approaches for the identification and optimization of oxide semiconductors for efficient solar photoelectrolysis. Chem. Soc. Rev. 2009, 38, 197–210. [Google Scholar] [CrossRef] [PubMed]
- Esposito, D.V.; Baxter, J.B.; John, J.; Lewis, N.S.; Moffat, T.P.; Ogitsu, T.; O’Neil, G.D.; Pham, T.A.; Talin, A.A.; Velazquez, J.M.; et al. Methods of photoelectrode characterization with high spatial and temporal resolution. Energy Environ. Sci. 2015, 8, 2863–2885. [Google Scholar] [CrossRef]
- Ahmed, J.; Mao, Y. Delafossite CuAlO2 nanoparticles with electrocatalytic activity toward oxygen and hydrogen evolution reactions. In Nanomaterials for Sustainable Energy; American Chemical Society: Washington, DC, USA, 2015; pp. 57–72. [Google Scholar]
- Li, C.; He, J.; Xiao, Y.; Li, Y.; Delaunay, J.-J. Earth-abundant Cu-based metal oxide photocathodes for photoelectrochemical water splitting. Energy Environ. Sci. 2020, 13, 3269–3306. [Google Scholar] [CrossRef]
- Díez-García, M.I.; Gómez, R. Progress in ternary metal oxides as photocathodes for water splitting cells: Optimization strategies. Sol. RRL 2022, 6, 2100871. [Google Scholar] [CrossRef]
- Monllor-Satoca, D.; Díez-García, M.I.; Lana-Villarreal, T.; Gómez, R. Photoelectrocatalytic production of solar fuels with semiconductor oxides: Materials, activity and modeling. Chem. Commun. 2020, 56, 12272–12289. [Google Scholar] [CrossRef] [PubMed]
- Axel, F. Synthesis and Characterisation of Delafossite CuFeO2 for Solar Energy Applications. 2016. Available online: http://urn.kb.se/resolve?urn=urn:nbn:se:uu:diva-297710 (accessed on 23 January 2024).
- Sullivan, I.; Zoellner, B.; Maggard, P.A. Copper(I)-based p-type oxides for photoelectrochemical and photovoltaic solar energy conversion. Chem. Mater. 2016, 28, 5999–6016. [Google Scholar] [CrossRef]
- Maggard, P.A. Discovery and Development of Semiconductors and Structures for Photoelectrochemical Energy Conversion. In Springer Handbook of Inorganic Photochemistry; Springer International Publishing: Cham, Switzerland, 2022; pp. 805–850. [Google Scholar]
- Shan, B.-F.; Chen, X.-L.; Zhao, Z.-Y. Band engineering of delafossite CuB1−xFexO2 (B = Al, Cr, Sc) solid solutions as photocathodes: Visible-light response and performance enhancement. Int. J. Hydrogen Energy 2023, 51, 499–510. [Google Scholar] [CrossRef]
- Beatriceveena, T.; Murthy, A.S.R.; Prabhu, E.; Gnanasekar, K. Wide range hydrogen sensing behavior of a silver delafossite: Performance towards long term stability, repeatability and selectivity. Int. J. Hydrogen Energy 2021, 46, 2824–2834. [Google Scholar] [CrossRef]
- Tang, B.; Xiao, F.-X. An overview of solar-driven photoelectrochemical CO2 conversion to chemical fuels. ACS Catal. 2022, 12, 9023–9057. [Google Scholar] [CrossRef]
- Hsu, K.-C.; Keyan, A.K.; Hung, C.-W.; Sakthinathan, S.; Yu, C.-L.; Chiu, T.-W.; Nagaraj, K.; Fan, F.-Y.; Shan, Y.-K.; Chen, P.-C. Fabrication of CuYO2 Nanofibers by Electrospinning and Applied to Hydrogen Harvest. Materials 2022, 15, 8957. [Google Scholar] [CrossRef]
- Son, H.; Uthirakumar, P.; Park, J.H.; Park, J.-H.; Woo, S.; Kim, C.Y. Investigation of non-stoichiometric delafossite photoelectrodes composed of cuprous oxide and iron oxide for enhanced water-splitting. J. Alloy. Compd. 2023, 948, 169781. [Google Scholar] [CrossRef]
- Derbal, A.; Omeiri, S.; Bouguelia, A.; Trari, M. Characterization of new heterosystem CuFeO2/SnO2 application to visible-light induced hydrogen evolution. Int. J. Hydrogen Energy 2008, 33, 4274–4282. [Google Scholar] [CrossRef]
- Li, G.; Khim, S.; Chang, C.S.; Fu, C.; Nandi, N.; Li, F.; Yang, Q.; Blake, G.R.; Parkin, S.; Auffermann, G.; et al. In situ modification of a delafossite-type PdCoO2 bulk single crystal for reversible hydrogen sorption and fast hydrogen evolution. ACS Energy Lett. 2019, 4, 2185–2191. [Google Scholar] [CrossRef] [PubMed]
- Bouich, A.; Torres, J.C.; Chfii, H.; Marí-Guaita, J.; Khattak, Y.H.; Baig, F.; Soucase, B.M.; Palacios, P. Delafossite as hole transport layer a new pathway for efficient perovskite-based solar sells: Insight from experimental, DFT and numerical analysis. Sol. Energy 2023, 250, 18–32. [Google Scholar] [CrossRef]
- Chfii, H.; Bouich, A.; Soucase, B.M.; Abdlefdil, M. A new approach for growing high-quality delafossite CuCoO2 films by spray pyrolysis through the optimization of the Cu/Co ratio. Opt. Mater. 2023, 135, 113229. [Google Scholar] [CrossRef]
- Chfii, H.; Bouich, A.; Andrio, A.; Torres, J.C.; Soucase, B.M.; Palacios, P.; Abd Lefdil, M.; Compañ, V. The Structural and Electrochemical Properties of CuCoO2 Crystalline Nanopowders and Thin Films: Conductivity Experimental Analysis and Insights from Density Functional Theory Calculations. Nanomaterials 2023, 13, 2312. [Google Scholar] [CrossRef] [PubMed]
- Chfii, H.; Bouich, A.; Soucase, B.M.; Abd-Lefdil, M. Structural and physical properties of Mg-doped CuCoO2 delafossite thin films. Mater. Chem. Phys. 2023, 306, 128006. [Google Scholar] [CrossRef]
- Yengantiwar, A.; Shinde, P.S.; Pan, S.; Gupta, A. Delafossite CuFeO2 photocathodes grown by direct liquid injection chemical vapor deposition for efficient photoelectrochemical water reduction. J. Electrochem. Soc. 2018, 165, H831–H837. [Google Scholar] [CrossRef]
- Díaz-García, A.K.; Lana-Villarreal, T.; Gómez, R. Sol–gel copper chromium delafossite thin films as stable oxide photocathodes for water splitting. J. Mater. Chem. A 2015, 3, 19683–19687. [Google Scholar] [CrossRef]
- Brahimi, R.; Bessekhouad, Y.; Bouguelia, A.; Trari, M. CuAlO2/TiO2 heterojunction applied to visible light H2 production. J. Photochem. Photobiol. A Chem. 2007, 186, 242–247. [Google Scholar] [CrossRef]
- Beatriceveena, T.V.; Agarwal, L. Highly Selective Behavior of a Silver Delafossite: Response Towards Wide Range of Hydrogen, Repeatability and Long Term Performance. In Proceedings of the 239th ECS Meeting, Chicago, IL, USA, 30 May–3 June 2021; The Electrochemical Society, Inc.: Pennington, NJ, USA, 2021; p. 1443. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, X.; Ma, Q.; Litke, A.; Liu, P.; Zhang, Y.; Li, C.; Hensen, E.J.M. Photoelectrochemical Properties of CuCrO2: Characterization of Light Absorption and Photocatalytic H2 Production Performance. Catal. Lett. 2014, 144, 1487–1493. [Google Scholar] [CrossRef]
- Crespo, C.T. Potentiality of CuFeO2-delafossite as a solar energy converter. Sol. Energy 2018, 163, 162–166. [Google Scholar] [CrossRef]
- Benreguia, N.; Abdi, A.; Mahroua, O.; Trari, M. Photoelectrochemical properties of the crednerite CuMnO2 and its application to hydrogen production and Mn+ reduction (Mn+ = Cd2+, Pd2+, Zn2+, Ni2+, and Ag+). J. Mater. Sci. Mater. Electron. 2021, 32, 10498–10509. [Google Scholar] [CrossRef]
- Mao, L.; Mohan, S.; Gupta, S.K.; Mao, Y. Multifunctional delafossite CuFeO2 as water splitting catalyst and rhodamine B sensor. Mater. Chem. Phys. 2022, 278, 125643. [Google Scholar] [CrossRef]
- Hadia, N.M.A.; Shaban, M.; Ahmed, A.M.; Mohamed, W.S.; Alzaid, M.; Ezzeldien, M.; Hasaneen, M.F.; Malti, W.E.; Abdelazeez, A.A.A.; Rabia, M. Photoelectrochemical Conversion of Sewage Water into H2 Fuel over the CuFeO2/CuO/Cu Composite Electrode. Catalysts 2023, 13, 456. [Google Scholar] [CrossRef]
- Toyoda, K.; Hinogami, R.; Miyata, N.; Aizawa, M. Calculated descriptors of catalytic activity for water electrolysis anode: Application to delafossite oxides. J. Phys. Chem. C 2015, 119, 6495–6501. [Google Scholar] [CrossRef]
- Trari, M.; Bouguelia, A.; Bessekhouad, Y. p-Type CuYO2 as hydrogen photocathode. Sol. Energy Mater. Sol. Cells 2006, 90, 190–202. [Google Scholar] [CrossRef]
- Read, C.G.; Park, Y.; Choi, K.-S. Electrochemical synthesis of p-type CuFeO2 electrodes for use in a photoelectrochemical cell. J. Phys. Chem. Lett. 2012, 3, 1872–1876. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Ferri, M.; Piccinin, S.; Selloni, A. Structure, Electronic Properties, and Defect Chemistry of Delafossite CuRhO2 Bulk and Surfaces. Chem. Mater. 2022, 34, 1567–1577. [Google Scholar] [CrossRef]
- Huda, M.N.; Yan, Y.; Deutsch, T.; Al-Jassim, M.M.; Turner, A.J.A. II. F. 7 Photoelectrochemical Materials: Theory and Modeling; University of Texas at Arlington: Arlington, TX, USA, 2012. [Google Scholar]
- Gu, J.; Yan, Y.; Krizan, J.W.; Gibson, Q.D.; Detweiler, Z.M.; Cava, R.J.; Bocarsly, A.B. p-Type CuRhO2 as a Self-Healing Photoelectrode for Water Reduction under Visible Light. J. Am. Chem. Soc. 2014, 136, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Samu, G.F.; Janáky, C. Rapid synthesis of interconnected CuCrO2 nanostructures: A promising electrode material for photoelectrochemical fuel generation. Electrochimica Acta 2018, 272, 22–32. [Google Scholar] [CrossRef]
- Ali, A.H.; Ahmed, A.M.; Abdelhamied, M.M.; Abdel-Khaliek, A.A.; Abd El Khalik, S.; Abass, S.M.; Shaban, M.; Hasan, F.; Rabia, M. Synthesis of lead-free Cu/CuFeO2/CZTS thin film as a novel photocatalytic hydrogen generator from wastewater and solar cell applications. Res. Sq. 2023. [CrossRef]
- Liu, J.; Hu, Q.; Wang, Y.; Yang, Z.; Fan, X.; Liu, L.-M.; Guo, L. Achieving delafossite analog by in situ electrochemical self-reconstruction as an oxygen-evolving catalyst. Proc. Natl. Acad. Sci. USA 2020, 117, 21906–21913. [Google Scholar] [CrossRef]
- Gu, J.; Wuttig, A.; Krizan, J.W.; Hu, Y.; Detweiler, Z.M.; Cava, R.J.; Bocarsly, A.B. Mg-doped CuFeO2 photocathodes for photoelectrochemical reduction of carbon dioxide. J. Phys. Chem. C 2013, 117, 12415–12422. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, C.-D.; Han, D.S.; Park, H. Facilitating hole transfer on electrochemically synthesized p-type CuAlO2 films for efficient solar hydrogen production from water. J. Mater. Chem. A 2017, 5, 10165–10172. [Google Scholar] [CrossRef]
- Prévot, M.S.; Jeanbourquin, X.A.; Bourée, W.S.; Abdi, F.; Friedrich, D.; van de Krol, R.; Guijarro, N.; Le Formal, F.; Sivula, K. Evaluating charge carrier transport and surface states in CuFeO2 photocathodes. Chem. Mater. 2017, 29, 4952–4962. [Google Scholar] [CrossRef]
- Schmachtenberg, N.; Silvestri, S.; Salla, J.d.S.; Dotto, G.L.; Hotza, D.; Jahn, S.L.; Foletto, E.L. Preparation of delafossite–type CuFeO2 powders by conventional and microwave–assisted hydrothermal routes for use as photo–Fenton catalysts. J. Environ. Chem. Eng. 2019, 7, 102954. [Google Scholar] [CrossRef]
- Yu, C.-L.; Sakthinathan, S.; Hwang, B.-Y.; Lin, S.-Y.; Chiu, T.-W.; Yu, B.-S.; Fan, Y.-J.; Chuang, C. CuFeO2–CeO2 nanopowder catalyst prepared by self-combustion glycine nitrate process and applied for hydrogen production from methanol steam reforming. Int. J. Hydrogen Energy 2020, 45, 15752–15762. [Google Scholar] [CrossRef]
- Chiu, T.-W.; Hong, R.-T.; Yu, B.-S.; Huang, Y.-H.; Kameoka, S.; Tsai, A.-P. Improving steam-reforming performance by nanopowdering CuCrO2. Int. J. Hydrogen Energy 2014, 39, 14222–14226. [Google Scholar] [CrossRef]
- Guan, D.; Xu, H.; Zhang, Q.; Huang, Y.; Shi, C.; Chang, Y.; Xu, X.; Tang, J.; Gu, Y.; Pao, C.; et al. Identifying a universal activity descriptor and a unifying mechanism concept on perovskite oxides for green hydrogen production. Adv. Mater. 2023, 35, e2305074. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.; Ahn, K.-S.; Shet, S.; Huda, M.N.; Deutsch, T.G.; Wang, H.; Turner, J.A.; Wei, S.-H.; Yan, Y.; Al-Jassim, M.M. Ternary cobalt spinel oxides for solar driven hydrogen production: Theory and experiment. Energy Environ. Sci. 2009, 2, 774–782. [Google Scholar] [CrossRef]
- Zhao, Z.-Y.; Liu, Q.-L. Rational Design of a Two-Dimensional Janus CuFeO2+δ Single Layer as a Photocatalyst and Photoelectrode. J. Phys. Chem. Lett. 2021, 12, 10863–10873. [Google Scholar] [CrossRef] [PubMed]
- Keyan Arjunan, K.; Rajakumaran, R.; Sakthinathan, S.; Chen, S.M.; Chiu, T.W.; Vinothini, S. Efficient electrocatalyst for hydrogen evolution reaction based on N-rGO-MWCNT/CuAlO2 nanocomposite in acidic media. ECS J. Solid State Sci. Technol. 2021, 10, 045011. [Google Scholar] [CrossRef]
- Bouich, A.; Pradas, I.G.; Khan, M.A.; Khattak, Y.H. Opportunities, Challenges, and Future Prospects of the Solar Cell Market. Sustainability 2023, 15, 15445. [Google Scholar] [CrossRef]
- Bouich, A.; Torres, J.C.; Khattak, Y.H.; Baig, F.; Marí-Guaita, J.; Soucase, B.M.; Mendez-Blas, A.; Palacios, P. Bright future by controlling α/δ phase junction of formamidinium lead iodide doped by imidazolium for solar cells: Insight from experimental, DFT calculations and SCAPS simulation. Surf. Interfaces 2023, 40, 103159. [Google Scholar] [CrossRef]
- Bouich, A.; Marí-Guaita, J.; Soucase, B.M.; Palacios, P. Bright future by enhancing the stability of methylammonium lead triiodide perovskites thin films through Rb, Cs and Li as dopants. Mater. Res. Bull. 2023, 163, 112213. [Google Scholar] [CrossRef]
- Bouich, A.; Marí-Guaita, J.; Baig, F.; Khattak, Y.H.; Soucase, B.M.; Palacios, P. Investigation of the surface coating, humidity degradation, and recovery of perovskite film phase for solar-cell applications. Nanomaterials 2022, 12, 3027. [Google Scholar] [CrossRef]

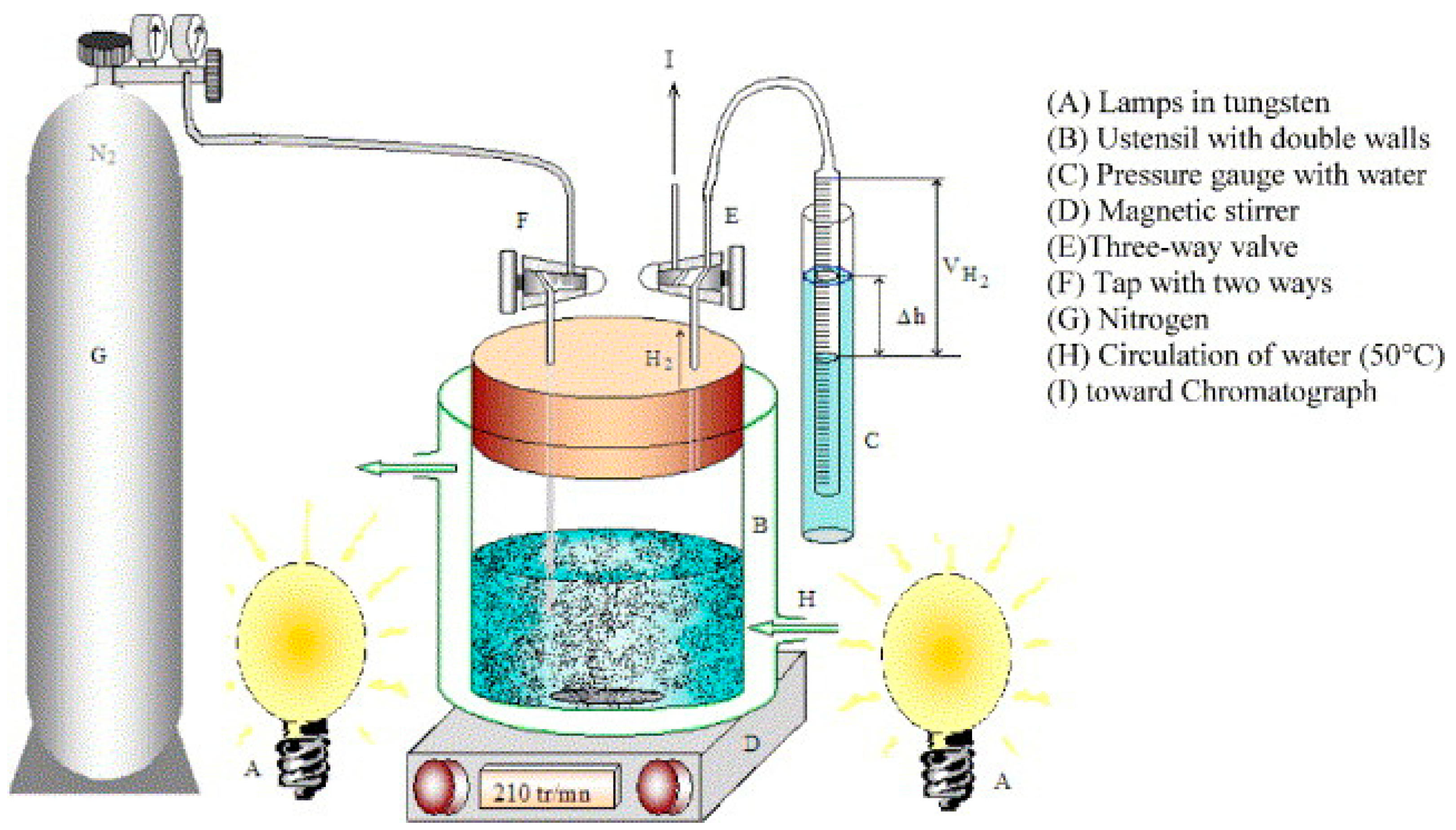




| Catalyst | H2 Production Rate (mL STP min−1 g-cat−1) | ||||
|---|---|---|---|---|---|
| 250 °C | 300 °C | 350 °C | 400 °C | Ref. | |
| CuYO2 | 1107.9 | 1735.65 | 1390.05 | 1375.2 | [95] |
| CuFeO2 | 705.188 | 464.869 | 1452.975 | 2010.600 | [125] |
| CuCrO2 | 279.169 | 753.205 | 1480.365 | 1720.300 | [126] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chfii, H.; Bouich, A.; Soucase, B.M. The Use of Copper-Based Delafossite to Improve Hydrogen Production Performance: A Review. Hydrogen 2024, 5, 39-58. https://doi.org/10.3390/hydrogen5010004
Chfii H, Bouich A, Soucase BM. The Use of Copper-Based Delafossite to Improve Hydrogen Production Performance: A Review. Hydrogen. 2024; 5(1):39-58. https://doi.org/10.3390/hydrogen5010004
Chicago/Turabian StyleChfii, Hasnae, Amal Bouich, and Bernabé Mari Soucase. 2024. "The Use of Copper-Based Delafossite to Improve Hydrogen Production Performance: A Review" Hydrogen 5, no. 1: 39-58. https://doi.org/10.3390/hydrogen5010004
APA StyleChfii, H., Bouich, A., & Soucase, B. M. (2024). The Use of Copper-Based Delafossite to Improve Hydrogen Production Performance: A Review. Hydrogen, 5(1), 39-58. https://doi.org/10.3390/hydrogen5010004







