Gas Chromatography and Thermal Cycling Absorption Techniques for Hydrogen Isotopes Separation in Water Detritiation Systems
Abstract
1. Introduction
2. Water Detritiation Technologies
3. Materials for Hydrogen Isotopic Separation
3.1. Materials for GC
3.2. Materials for TCAP
4. Gas Chromatography
Gas Chromatography Configurations
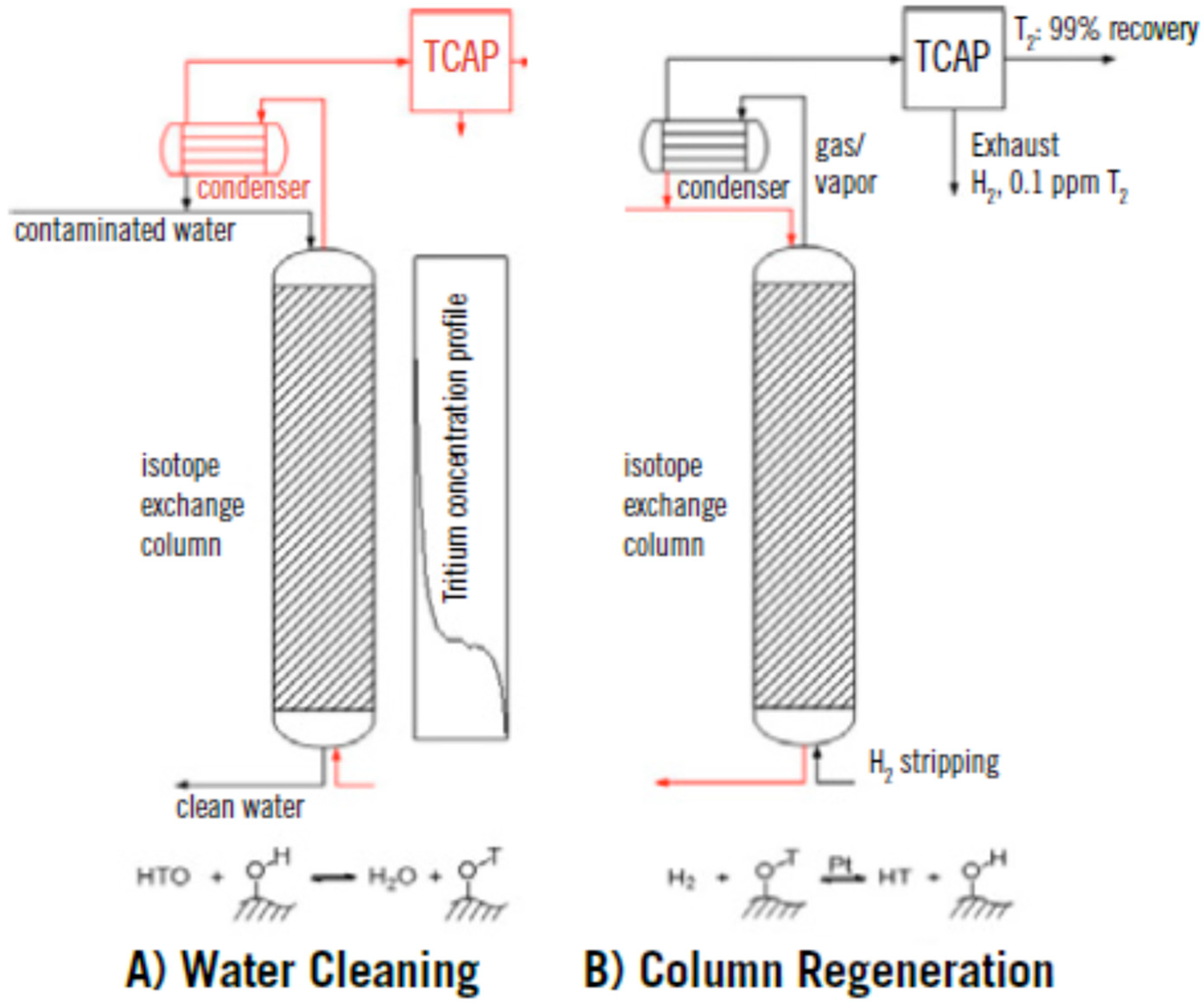
5. Thermal Cycling Absorption Process
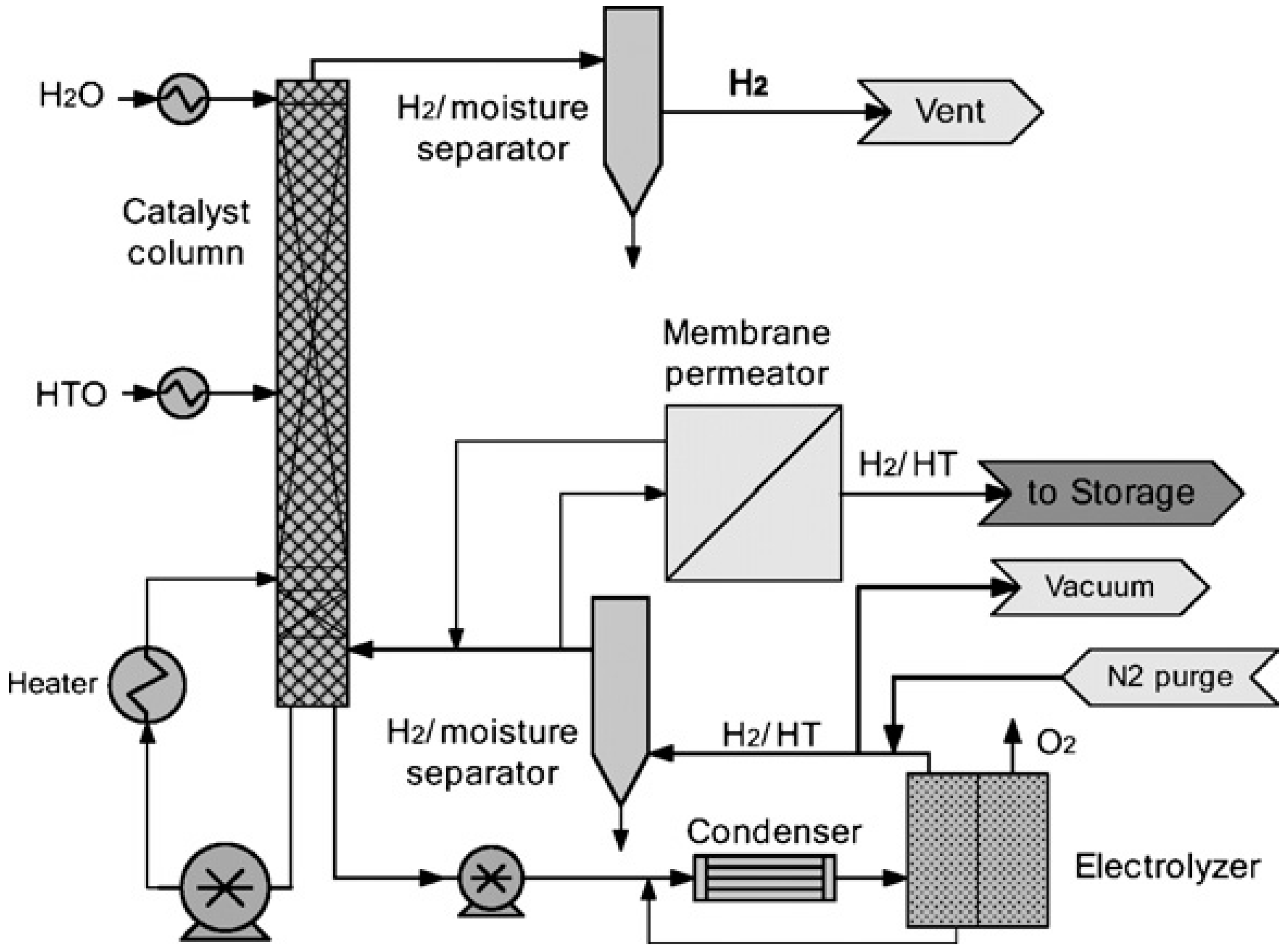
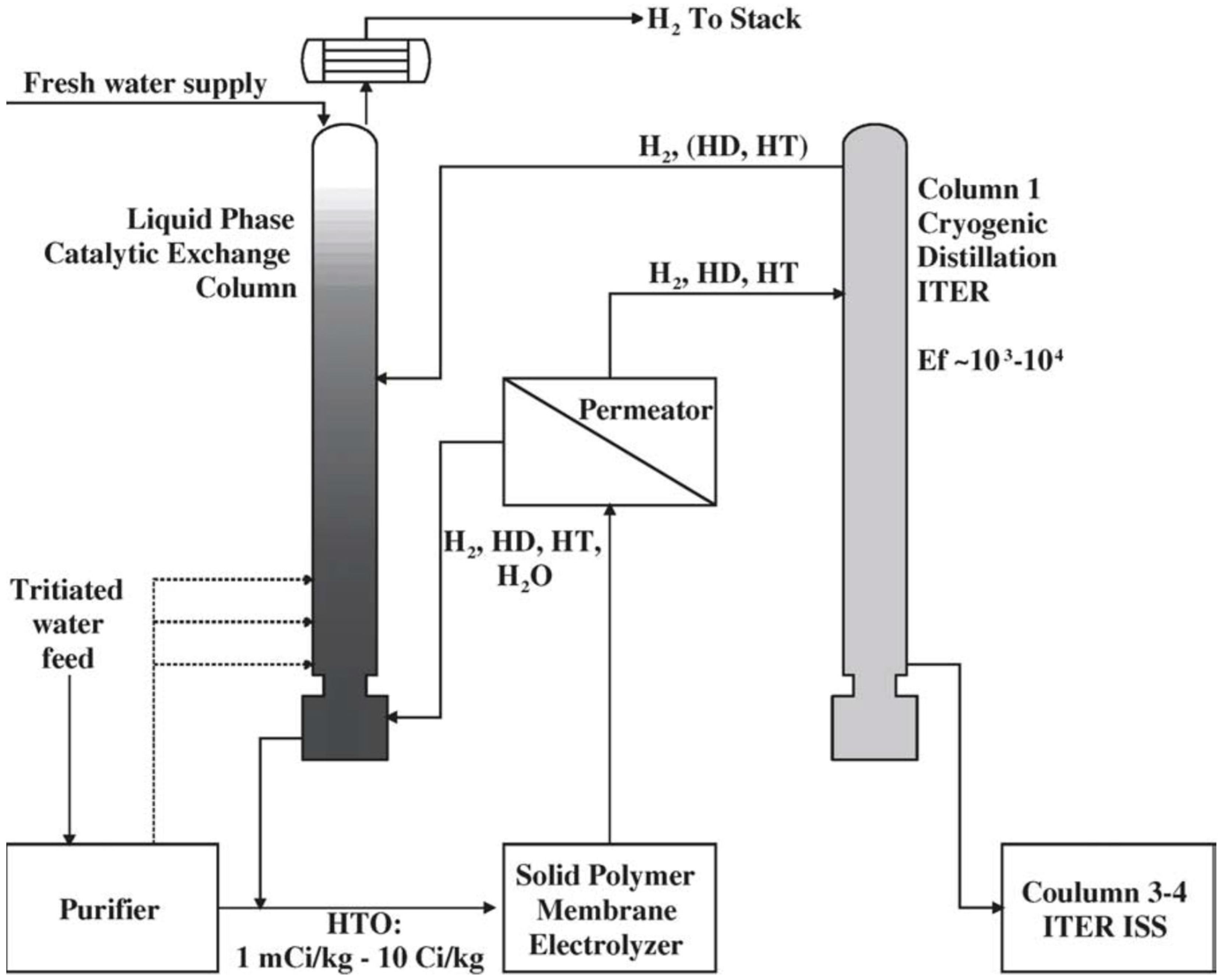
6. Application of GC and TCAP in Water Detritiation Processes
- -
- A feed flow rate of a few L/h,
- -
- A D inlet conc. of 50–99%,
- -
- A T outlet conc. of >98%.
6.1. Gas Chromatography
- -
- deuterium-rich stream: H = 0.17%, D = 99.70%, T = 0.13%,
- -
- -ritium-rich stream: H = 0.03%, D = 0.01%, T = 99.96%.
- -
- the system was capable of separating hydrogen isotope mixtures of six molecules into tritium, deuterium, and protium,
- -
- the best tritium and deuterium qualities achieved were in the range of 99.9 and 99.7%, respectively,
- -
- the size of the system, designed for a feed flow rate of up to 600 NL/h (10 NL/min), when down-scaled to the feed flow rate of the present application (5 NL/h), could be compatible for use in small-size detritiation units,
- -
- the operation at temperatures close to the ambient one makes GC almost immediately ready for separation (unlike the CD system, which needs multiple days to cool down to 19 K),
- -
- the operation showed good safety (no tritium leaks were observed in the various secondary containments of the GC system, with a final global test requirement of no leak indication in the 10−10 Pa m3/s range).
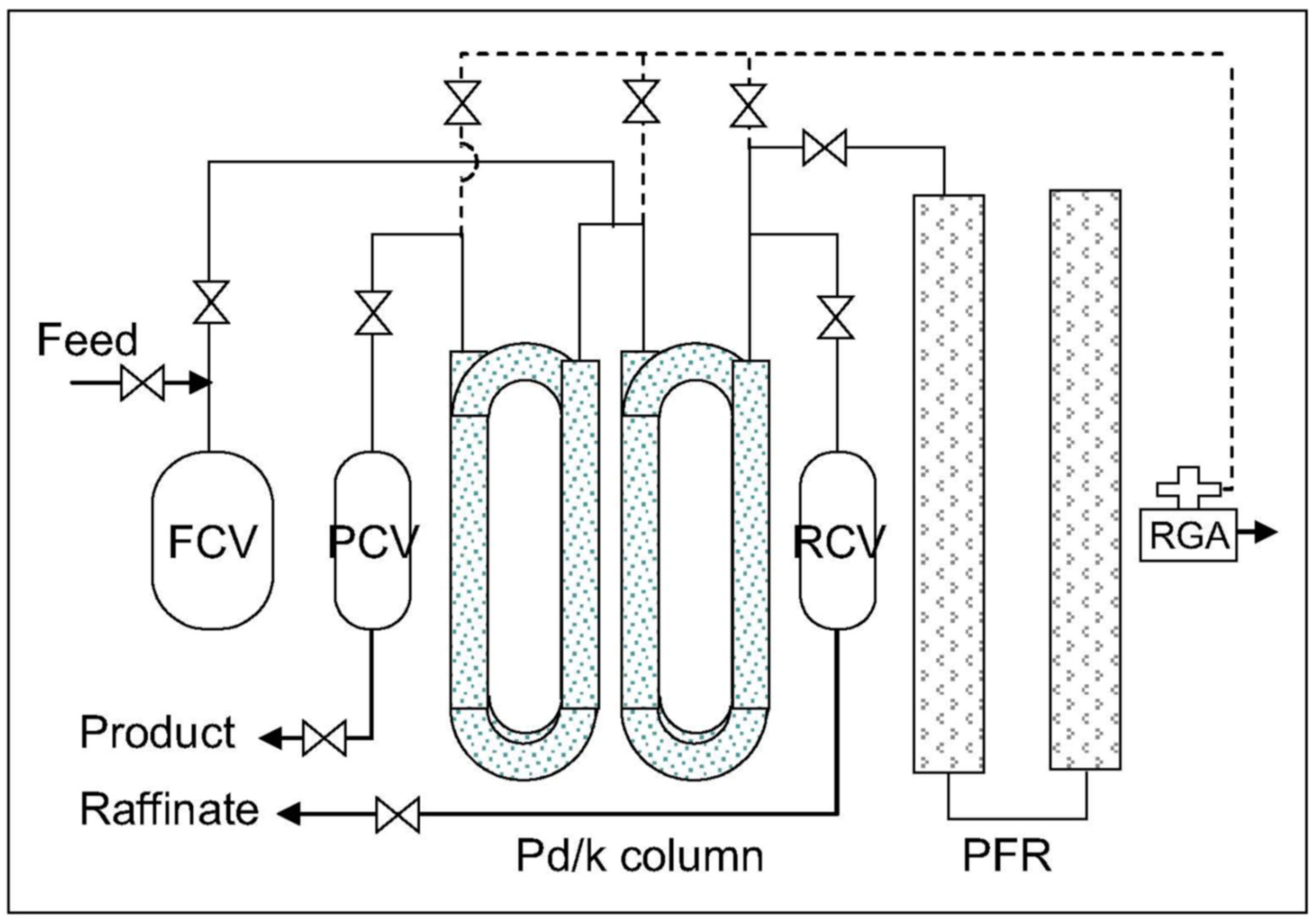
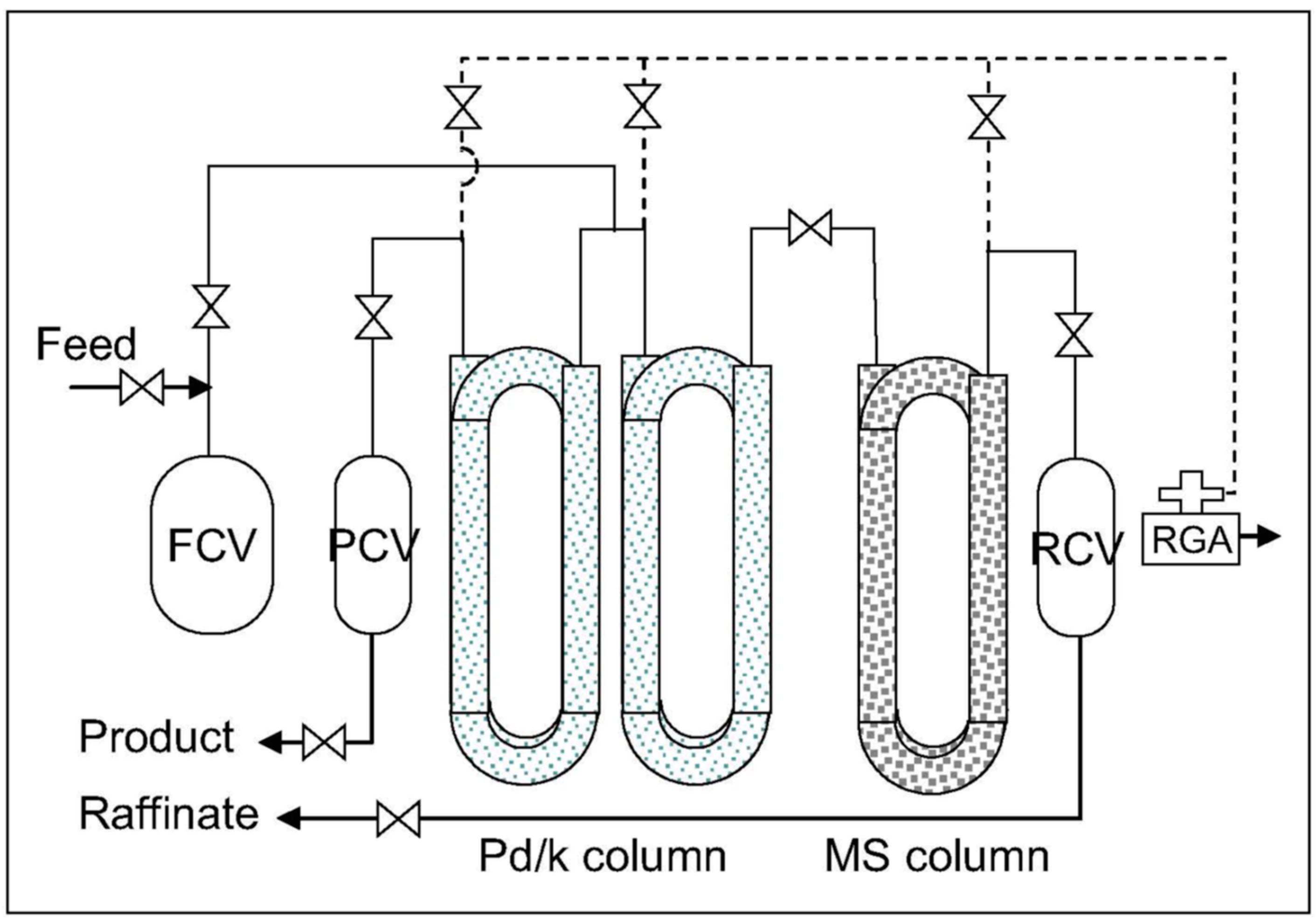
6.2. Thermal Cycling Absorption Process
- -
- the Pd/k column packed in copper tubing (0.95 cm diameter and 6.35 m long) operates from −70 to +145 °C at pressures from 1 to 5500 torr,
- -
- the inverse column (filled with 99 g of MS4A) in stainless steel tubing (0.95 cm diameter and 3.33 m long) operates at temperatures ranging from −190 to −90 °C and pressures ranging from 1 to 9000 torr,
- -
- heating via electrical resistance and cooling via liquid nitrogen.
6.3. Comparison GC/TCAP
7. Recent and Future Applications
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
List of Acronyms
| AGHS | Active Gas Handling System at JET |
| CD | Cryogenic Distillation |
| CEA | Commissariat a l’Energie Atomique et aux Energies Alternatives |
| CECE | Combined Electrolysis and Catalytic Exchange |
| CFFTP | Canadian Fusion Fuel Technology Project |
| FC | Frontal chromatography |
| FDC | Frontal displacement chromatography |
| DC | Displacement Chromatography |
| GC | Gas Chromatography |
| ICSI | Institute for Cryogenic and Isotopic Technologies at Rm. Valcea, Romania |
| JET | Joint European Torus, Culham, UK |
| k | Kieselguhr, a diatomite chiefly composed of amorphous silica |
| LPCE | Liquid Phase Catalytic Exchange |
| MS | Molecular Sieves |
| MS4A | Type 4A Molecular Sieves |
| PFR | Plug Flow Reverser |
| SRNL | Savannah River National Laboratory, USA |
| STP | Standard Temperature and Pressure conditions (273.15 K and 100 kPa) |
| TCAP | Thermal Cycling Absorption Process |
| TLK | Tritium Laboratory of Karlsruhe, DE |
| TRL | Technological Readiness Level |
| VPCE | Vapour Phase Catalytic Exchange |
References
- Chen, C.; Hou, J.; Li, J.; Chen, X.; Xiao, C.; Wang, Q.; Gong, Y.; Yue, L.; Zhao, L.; Ran, G.; et al. A water distillation detritiation facility and its performance test. Fusion Eng. Des. 2020, 153, 111460. [Google Scholar] [CrossRef]
- Iwai, Y.; Kubo, H.; Ohshima, Y.; Noguchi, H.; Taniuchi, J. Recent activities on water detritiation technology in JAEA. Fusion Eng. Des. 2016, 109-111, 1447–1451. [Google Scholar] [CrossRef]
- Kim, K.; Lee, M.; Paek, S.; Yim, S.; Ahn, D.; Chung, H. Operational analysis of a liquid phase catalytic exchange column for a detritiation of heavy water. Sep. Purif. Technol. 2007, 54, 410–414. [Google Scholar] [CrossRef]
- Zamfirache, M.; Bornea, A.; Balteanu, O.; Bucur, C.; Sofilca, N.; Stefanescu, I. Final status of water detritiation system (WDS) for Cernavoda Tritium removal facility (CTRF). Fusion Eng. Des. 2018, 136, 1038–1040. [Google Scholar] [CrossRef]
- Tamm, U.; Cristescu, I.-R.; Glugla, M.; Caldwell-Nichols, C. Investigation of simultaneous tritium and deuterium transfer in a catalytic isotope exchange column for water detritiation. Fusion Eng. Des. 2002, 61-62, 537–542. [Google Scholar] [CrossRef]
- Perevezentsev, A.N.; Bell, A.C. Development of a water detritiation facility for JET. Fusion Eng. Des. 2002, 61–62, 585–589. [Google Scholar] [CrossRef]
- Cristescu, I.R.; Dörr, L.; Glugla, M.; Hellriegel, G.; Michling, R.; Murdoch, D.; Schäfer, P.; Welte, S.; Wurster, W. Commissioning of water detritiation and cryogenic distillation systems at TLK in view of ITER design. Fusion Eng. Des. 2007, 82, 2126–2132. [Google Scholar] [CrossRef]
- Welte, S.; Cristescu, I.; Dittrich, H.; Lohr, N.; Melzer, C.; Michling, R.; Plusczyk, C.; Schaefer, P. Setup and commissioning of a combined water detritiation and isotope separation experiment at the Tritium Laboratory Karlsruhe. Fusion Eng. Des. 2013, 88, 2251–2254. [Google Scholar] [CrossRef]
- Miller, J.M.; Graham, W.; Celovsky, S.L.; Tremblay, J.; Everatt, A.E. Design and Operational Experience with a Pilot-Scale CECE Detritiation Process. Fusion Sci. Technol. 2002, 41, 1077–1081. [Google Scholar] [CrossRef]
- Hammerli, M.; Stevens, W.H.; Butler, J.P. Combined Electrolysis Catalytic Exchange (CECE) Process for Hydrogen Isotope Separation. ACS Symp. Ser. 1978, 68, 110–125. [Google Scholar] [CrossRef]
- Sicking, G. Isotope effects in metal-hydrogen systems. J. Less Common Met. 1984, 101, 169–190. [Google Scholar] [CrossRef]
- Wiswall, R.H.; Reilly, J.J. Inverse hydrogen isotope effects in some metal hydride systems. Inorg. Chem. 1972, 11, 1691–1696. [Google Scholar] [CrossRef]
- Pozio, A.; Tosti, S. Pd-Ag Electrical Resistivity in Hydrogen and Deuterium: Temperature Effect. Materials 2019, 12, 3551. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wu, W.; Zhang, G.; Fang, M.; Jing, W.; Xiong, Y.; Xiong, R.; Tang, T. Study of separation factor and product extraction ratio in hydrogen isotope separation with displacement chromatography. Fusion Eng. Des. 2021, 165, 112246. [Google Scholar] [CrossRef]
- Deng, X.; Luo, D.; Qian, X. Development of separation materials containing palladium for hydrogen isotopes separation. J. Isot. 2010, 23, 53–58. [Google Scholar]
- Sazonov, A.B.; Magomedbekov, E.P. Estimate of the Efficiency of Hydrogen–Metal Hydride (Intermetallic Compound) Systems in the Separation of the Three-Isotope Mixtures H-D-T. At. Energy 2000, 89, 736–744. [Google Scholar] [CrossRef]
- Fukada, S.; Fuchinoue, K.; Nishikawa, M. Hydrogen isotope separation by displacement chromatography with palladium. J. Nucl. Sci. Technol. 1995, 32, 556–564. [Google Scholar] [CrossRef]
- Deng, X.; Luo, D.; Qin, C.; Qian, X.; Yang, W. Hydrogen isotopes separation using frontal displacement chromatography with Pd–Al2O3 packed column. Int. J. Hydrogen Energy 2012, 37, 10774–10778. [Google Scholar] [CrossRef]
- Ducret, D.; Ballanger, A.; Steimetz, J.; Laquerbe, C.; Baudouin, O.; Peyrigain, P.S. Hydrogen isotopes separation by thermal cycling absorption process. Fusion Eng. Des. 2001, 58-59, 417–421. [Google Scholar] [CrossRef]
- Heung, L.K.; Sessions, H.T.; Poore, A.S.; Jacobs, W.D.; Williams, C.S. Next-generation TCAP hydrogen isotope separation process. Fusion Sci. Technol. 2008, 54, 399–402. [Google Scholar] [CrossRef]
- Available online: https://titans-project.eu/ (accessed on 12 July 2023).
- Tosti, S.; Pozio, A. Membrane Processes for the Nuclear Fusion Fuel Cycle. Membranes 2018, 8, 96. [Google Scholar] [CrossRef] [PubMed]
- Brooks, K.; Sevigny, G. (Eds.) Love, Review and Evaluation of Water Detritiation Technologies for Watts Bar Primary Cooling Water, Pacific Northwest National Laboratory, 10 May 2017, EMSL Auditorium, PNNL, PNNL-SA-125901. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwij0M__74v_AhUS7aQKHaRSB4QQFnoECBAQAQ&url=https%3A%2F%2Fwww.energy.gov%2Fsites%2Fprod%2Ffiles%2F2017%2F06%2Ff34%2FMay%252010%2520-%2520Brooks-Review%2520of%2520Water%2520Detritiation%2520Technologies.pdf (accessed on 12 July 2023).
- Kalyanam, K.; Sood, S. A Comparison of Process Characteristics for the Recovery of Tritium from Heavy Water and Light Water Systems. Fusion Technol. 1988, 14, 524–528. [Google Scholar] [CrossRef]
- Libby, W.F. Vibrational Frequencies of the Isotopic Water Molecules; Equilibria with the Isotopic Hydrogens. J. Chem. Phys. 1943, 11, 101–109. [Google Scholar] [CrossRef]
- Paek, S.; Ahn, D.-H.; Choi, H.-J.; Kim, K.-R.; Lee, M.; Yim, S.-P.; Chung, H.; Song, K.-M.; Sohn, S.H. The performance of a trickle-bed reactor packed with a Pt/SDBC catalyst mixture for the CECE process. Fusion Eng. Des. 2007, 82, 2252–2258. [Google Scholar] [CrossRef]
- Park, D.; Urm, J.J.; Lee, J.-U.; Chang, M.H.; Lee, J.M. Dynamic optimization of cryogenic distillation operation for hydrogen isotope separation in fusion power plant. Int. J. Hydrogen Energy 2021, 46, 24135–24148. [Google Scholar] [CrossRef]
- Glugla, M.; Murdoch, D.; Antipenkov, A.; Beloglazov, S.; Cristescu, I.-R.; Day, C.; Laesser, R.; Mack, A. ITER fuel cycle R&D: Consequences for the design. Fusion Eng. Des. 2006, 81, 733–744. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, W.; Xiong, Y.; Zhang, G.; Wei, Y.; Fang, M.; Tang, T. Protium removal from deuterium-tritium mixture with displacement chromatography method: Experiments and simulations. Int. J. Hydrogen Energy 2019, 44, 3824–3833. [Google Scholar] [CrossRef]
- Shugard, A.D.; Buffleben, G.M.; Johnson, T.A.; Robinson, D.B. Isotope exchange between gaseous hydrogen and uranium hydride powder. J. Nucl. Mater. 2014, 447, 304–313. [Google Scholar] [CrossRef]
- Yawny, A.; Friedlmeier, G.; Bolcich, J. Hydrides for hydrogen-deuterium separation. Int. J. Hydrogen Energy 1989, 14, 587–597. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsuyama, M.; Kobayashi, T.; Taguchi, S. Gas chromatographic separation of H2-D2 mixtures by Pd-Pt alloy near room temperature. J. Alloys Compd. 1997, 257, 278–284. [Google Scholar] [CrossRef]
- Watanabe, K.; Matsuyama, M.; Kobayashi, T.; Shu, W.M. Hydrogen isotope separation by gas chromatography using Pd–Pt alloy. Fusion Eng. Des. 1998, 39-40, 1001–1008. [Google Scholar] [CrossRef]
- Maeland, A.; Flanagan, T.B. Lattice Constants and Thermodynamic Parameters of the Hydrogen-Platinum-Palladium and Deuterium-Platinum-Palladium Systems. J. Phys. Chem. 1964, 68, 1419–1426. [Google Scholar] [CrossRef]
- Heung, L.K.; Sessions, H.T.; Xiao, X. TCAP Hydrogen Isotope Separation Using Palladium and Inverse Columns. Fusion Sci. Technol. 2011, 60, 1331–1334. [Google Scholar] [CrossRef]
- Qian, X.J.; Luo, D.L.; Qin, C.; Huang, G.Q.; Yang, W. FTP/P 106 Gas Chromatography Separation of H 2-D 2-Ar Using Pd/K. 2010. Available online: https://www-pub.iaea.org/mtcd/meetings/PDFplus/2010/cn180/cn180_papers/ftp_p1-06.pdf (accessed on 12 July 2023).
- Neffe, G.; Besserer, U.; Dehne, J.; Hutter, E.; Kissel, H.; Penzhorn, R.; Wendel, J.; Brunnader, H. Routine operation of the gas chromatographic isotope separation system of the Tritium Laboratory Karlsruhe. Fusion Eng. Des. 1998, 39-40, 987–993. [Google Scholar] [CrossRef]
- Cheh, C.H. Large Scale Gas Chromatographic Demonstration System for Hydrogen Isotope Separation. Fusion Technol. 1988, 14, 567–573. [Google Scholar] [CrossRef]
- Lässer, R.; Bell, A.C.; Bainbridge, N.; Brennan, D.; Dörr, L.; Hemmerich, J.L.; Knipe, S.; Stagg, R. The preparative gas chromatographic system for the JET Active Gas Handling System—Tritium commissioning and use during and after DTE1. Fusion Eng. Des. 1999, 47, 301–319. [Google Scholar] [CrossRef]
- Shmayda, W.T.; Wittman, M.D.; Earley, R.F.; Reid, J.L.; Redden, N.P. The Laboratory for Laser Energetics’ Hydrogen Isotope Separation System. Fusion Eng. Des. 2016, 109-111, 128–134. [Google Scholar] [CrossRef]
- Klein, J.E. (Ed.) Heung, L.K.; Howard, D.W.; Lee, M.W.; Motyka, T.; Poore, A.S.; Scogin, J.H. Conceptual Design for Consolidation TCAP, Report WSRC.TR-97-00340, 24 October 1997, Savannah River Technology Center. Available online: https://www.osti.gov/servlets/purl/4820 (accessed on 12 July 2023).
- Xiao, X.S.; Heung, L.K.; Sessions, H.T.; Redd, S. Advances in Hydrogen Isotope Separation Using Thermal Cycling Absorption Process (TCAP). Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&ved=2ahUKEwiC-rfq0eT5AhVQXfEDHajvAp4QFnoECAUQAQ&url=https%3A%2F%2Fenergy.gov%2Fsites%2Fprod%2Ffiles%2F2015%2F08%2Ff26%2FSRNL-STI-2013-00230%2520Rev1_Advances%2520in%2520TCAP%2520(Xiao).pdf&usg=AOvVaw1C3s5IDVNlQoqv5FITED4a (accessed on 12 July 2023).
- Clark, E.A. Materials Performance in Prototype Thermal Cycling Absorption Process (TCAP) Columns (U); Report WSRC.TR-92-552-552; Savannah River Technology Center: Aiken, SC, USA. [CrossRef][Green Version]
- Zhou, J.; Zhang, X.; Hao, S.; Huang, W. Dynamic simulation of Thermal Cycling Absorption Process with twin columns for hydrogen isotopes separation. Int. J. Hydrogen Energy 2014, 39, 13873–13879. [Google Scholar] [CrossRef]
- Ana, G.; Niculescu, A.; Bornea, A.; Zamfirache, M.; Draghia, M. TCAP Hydrogen Isotope Separation Process Under Development at ICSI Rm. Valcea. IEEE Trans. Plasma Sci. 2018, 46, 2668–2671. [Google Scholar] [CrossRef]
- Xiao, X.; Randall, B.; Sessions, H.T.; Allgood, R.; Babineau, D. Thermal Cycling Absorption Process Overview, Presentation of Savannah River National Laboratory. Available online: https://www.energy.gov/sites/prod/files/2019/06/f63/Thermal-Cycling-Absorption-Process-Overview.pdf (accessed on 12 July 2023).
- Decontamination of Tritiated Water, TechBriefs SRNL 14.09.2020, SRNL-MS-2020-00101. Available online: https://srnl.doe.gov/tech_transfer/tech_briefs/SRNL_TechBriefs_decontamination-of-tritiated-water.pdf (accessed on 12 July 2023).
- Xiao, X. Decontamination of Tritiated Water. U.S. Patent 10,381,121 B2, 13 August 2019. Available online: https://patents.google.com/patent/US10381121B2/en?oq=48.Xiao%2c+X.+Decontamination+of+Tritiated+Water%2c+Patent+No.+US+10%2c381%2c121+B2+(13+August+2019)file:///D:/ (accessed on 28 August 2023).
- Deng, L.; Chen, C.; Huang, G.; Shi, Y.; Yao, Y.; Hu, J.; Chen, K.; An, Y.; Song, J. The oil bath thermal cycling absorption process design and separation process research. Int. J. Hydrogen Energy 2023, 48, 22132–22140. [Google Scholar] [CrossRef]



| VPCE (473 K) | LPCE (298 K) | |
|---|---|---|
| D/T | 1.22 | 1.67 |
| H/T | 2.13 | 7.14 |
| Streams’ Purity | Processing T | TRL | Safety | |
|---|---|---|---|---|
| GC | >99% | Tamb to 67 °C | 6–7 | inherent safety validated in experiments |
| TCAP * | ≈99% | −70 to +145 °C −190 to −90 °C | 6–7 | inherent safety validated in experiments |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tosti, S. Gas Chromatography and Thermal Cycling Absorption Techniques for Hydrogen Isotopes Separation in Water Detritiation Systems. Hydrogen 2023, 4, 694-708. https://doi.org/10.3390/hydrogen4030044
Tosti S. Gas Chromatography and Thermal Cycling Absorption Techniques for Hydrogen Isotopes Separation in Water Detritiation Systems. Hydrogen. 2023; 4(3):694-708. https://doi.org/10.3390/hydrogen4030044
Chicago/Turabian StyleTosti, Silvano. 2023. "Gas Chromatography and Thermal Cycling Absorption Techniques for Hydrogen Isotopes Separation in Water Detritiation Systems" Hydrogen 4, no. 3: 694-708. https://doi.org/10.3390/hydrogen4030044
APA StyleTosti, S. (2023). Gas Chromatography and Thermal Cycling Absorption Techniques for Hydrogen Isotopes Separation in Water Detritiation Systems. Hydrogen, 4(3), 694-708. https://doi.org/10.3390/hydrogen4030044






