A Case Study Approach: Summary of Some Results on the Effects of Hydrogen Exposure on the Mechanical Properties of Palladium and the Alloy Systems Pd1−xMx, M = Ag, Cu, Mn; x = 5 − 0.25
Abstract
1. Introduction
2. Materials and Methods
3. Results
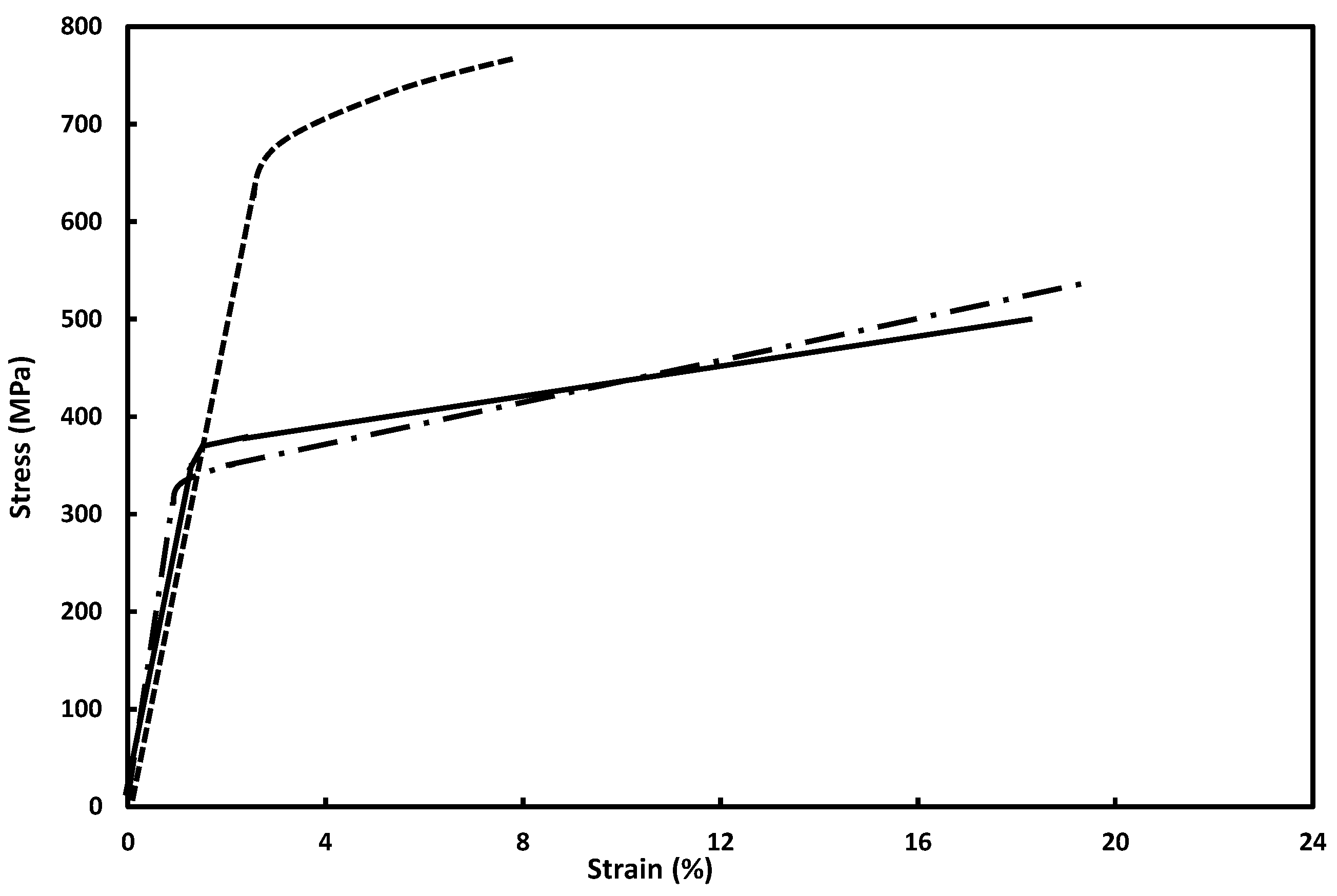
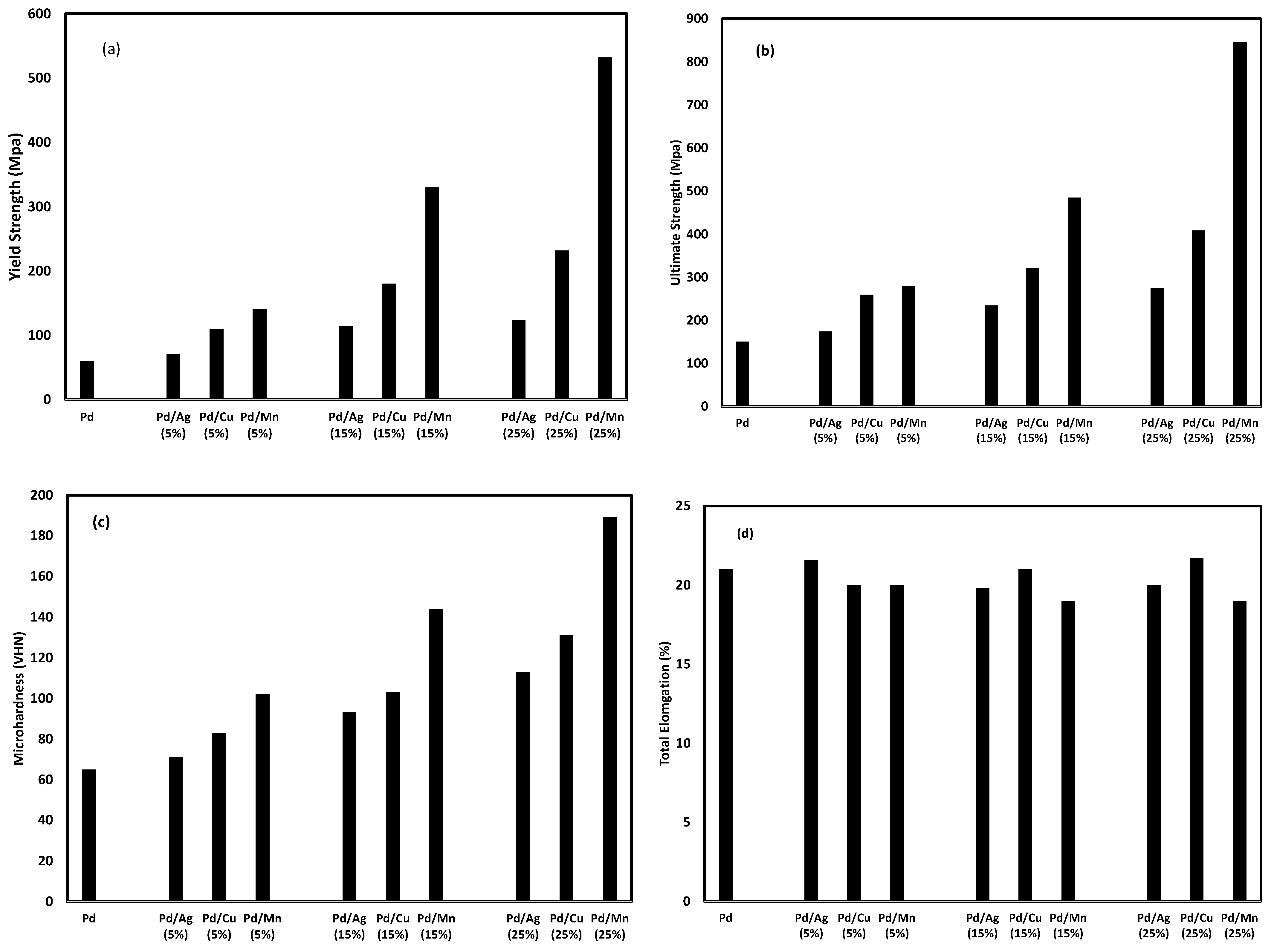
3.1. Mechanical Properties of Vacuum-Annealed Materials
3.2. Case 1: Pure Palladium
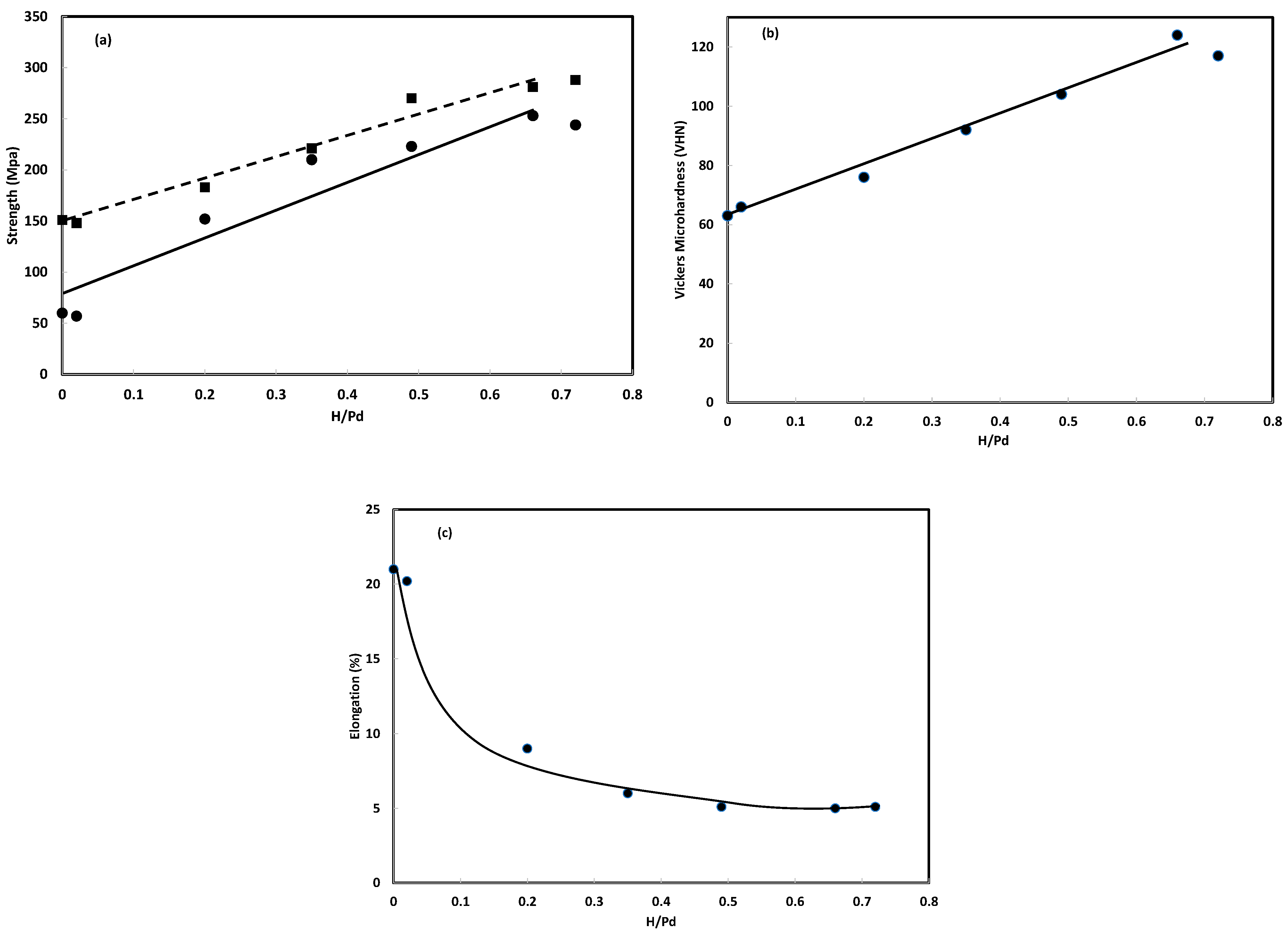
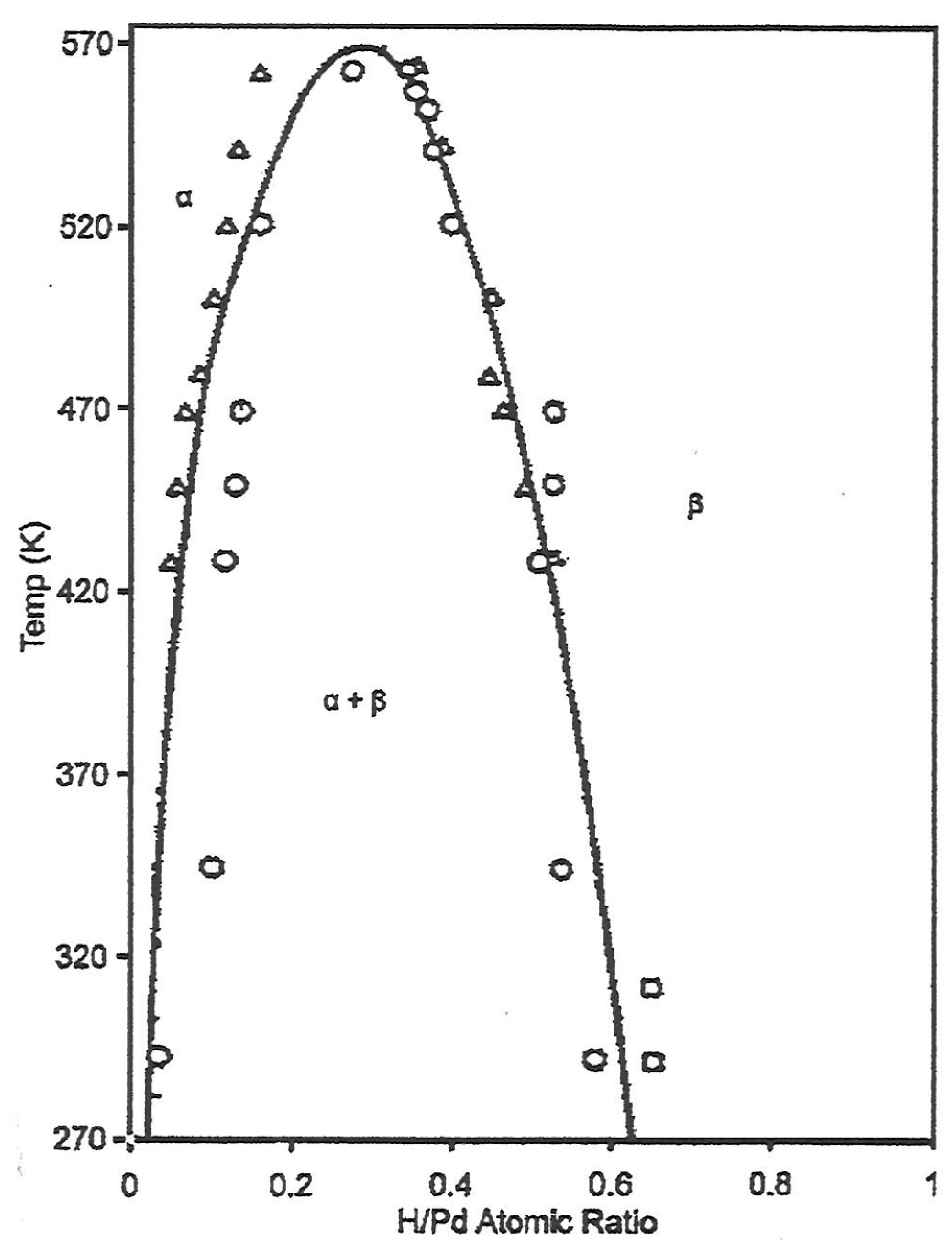
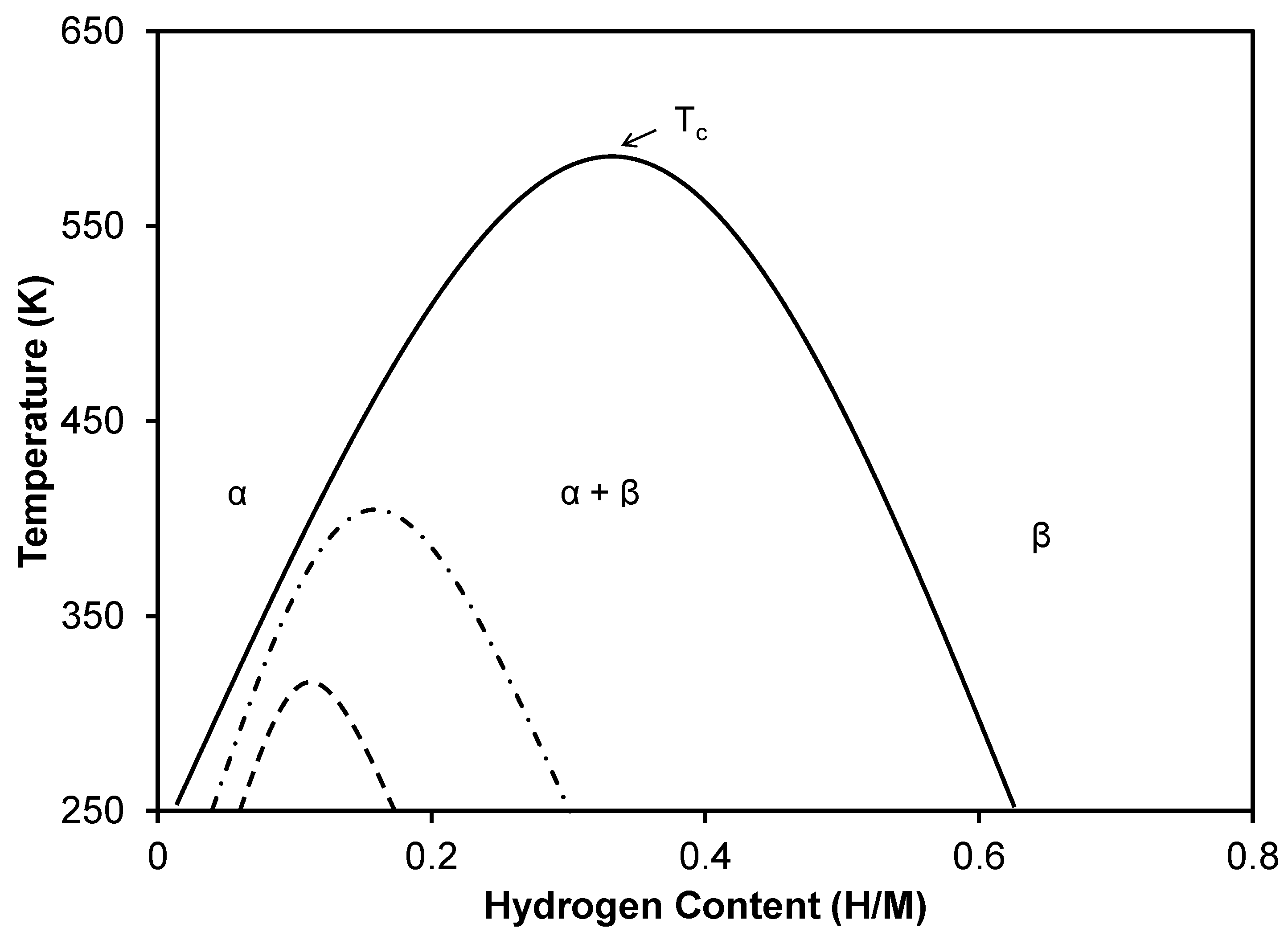
3.2.1. Amount of Hydrogen Absorbed
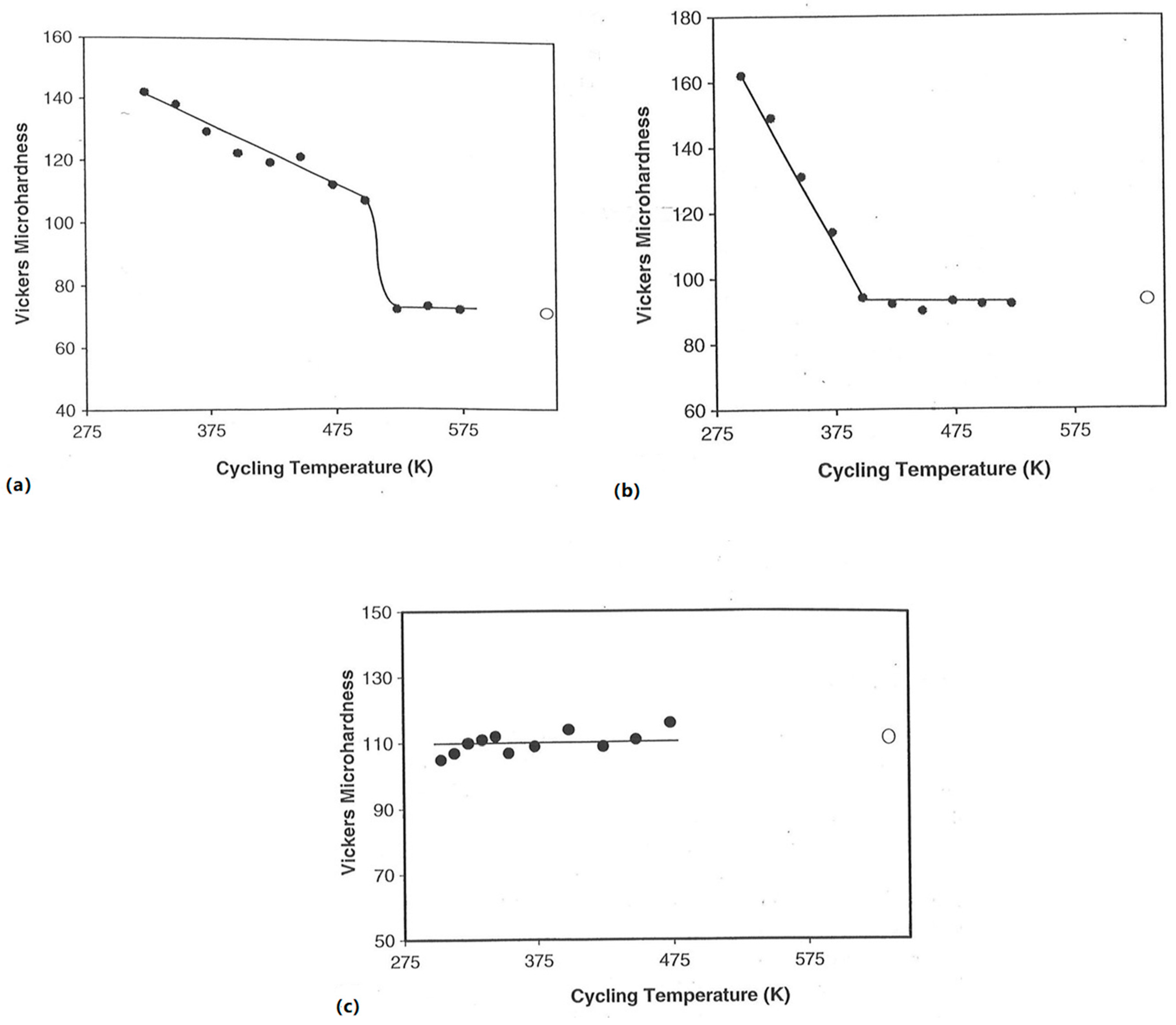
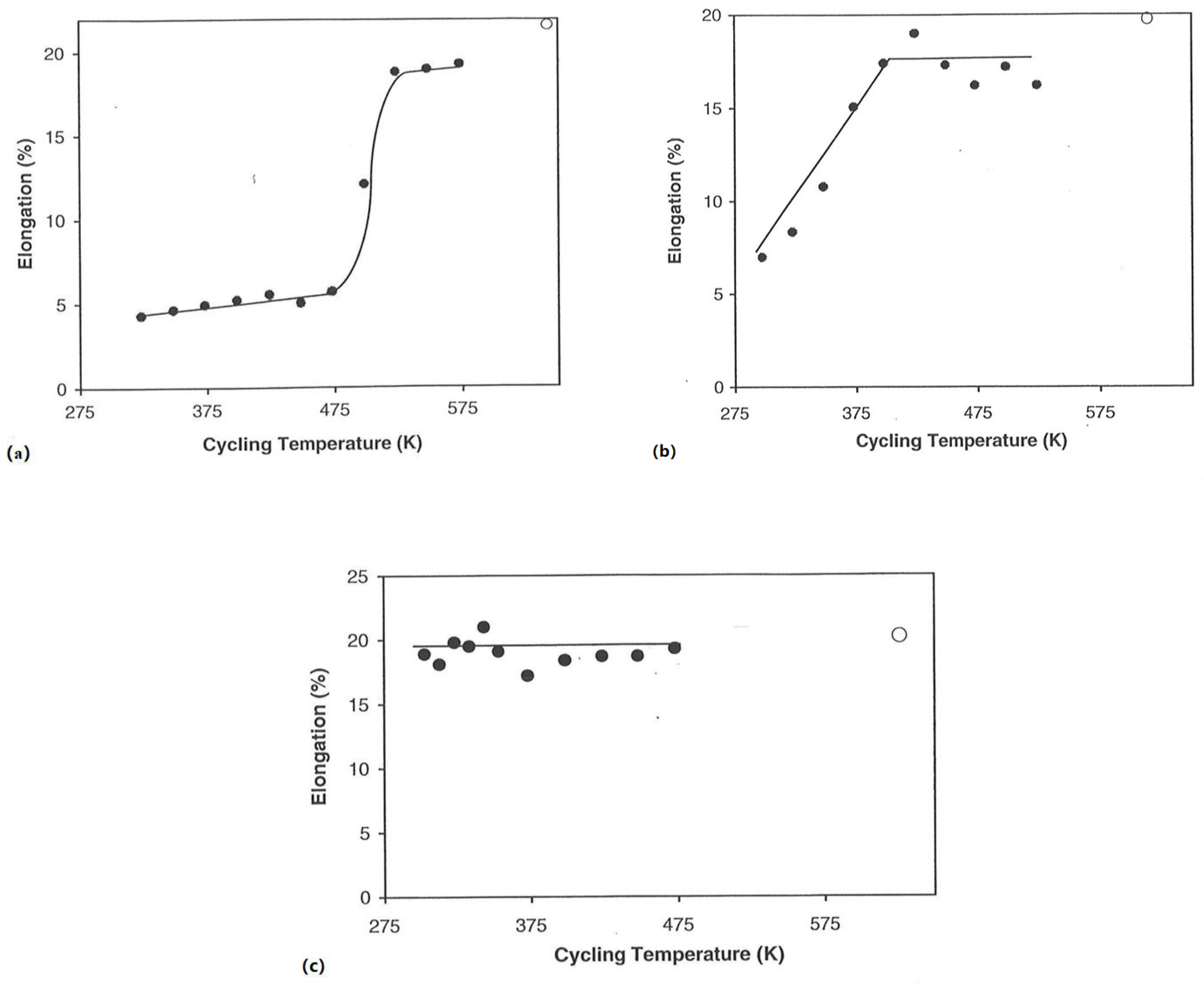
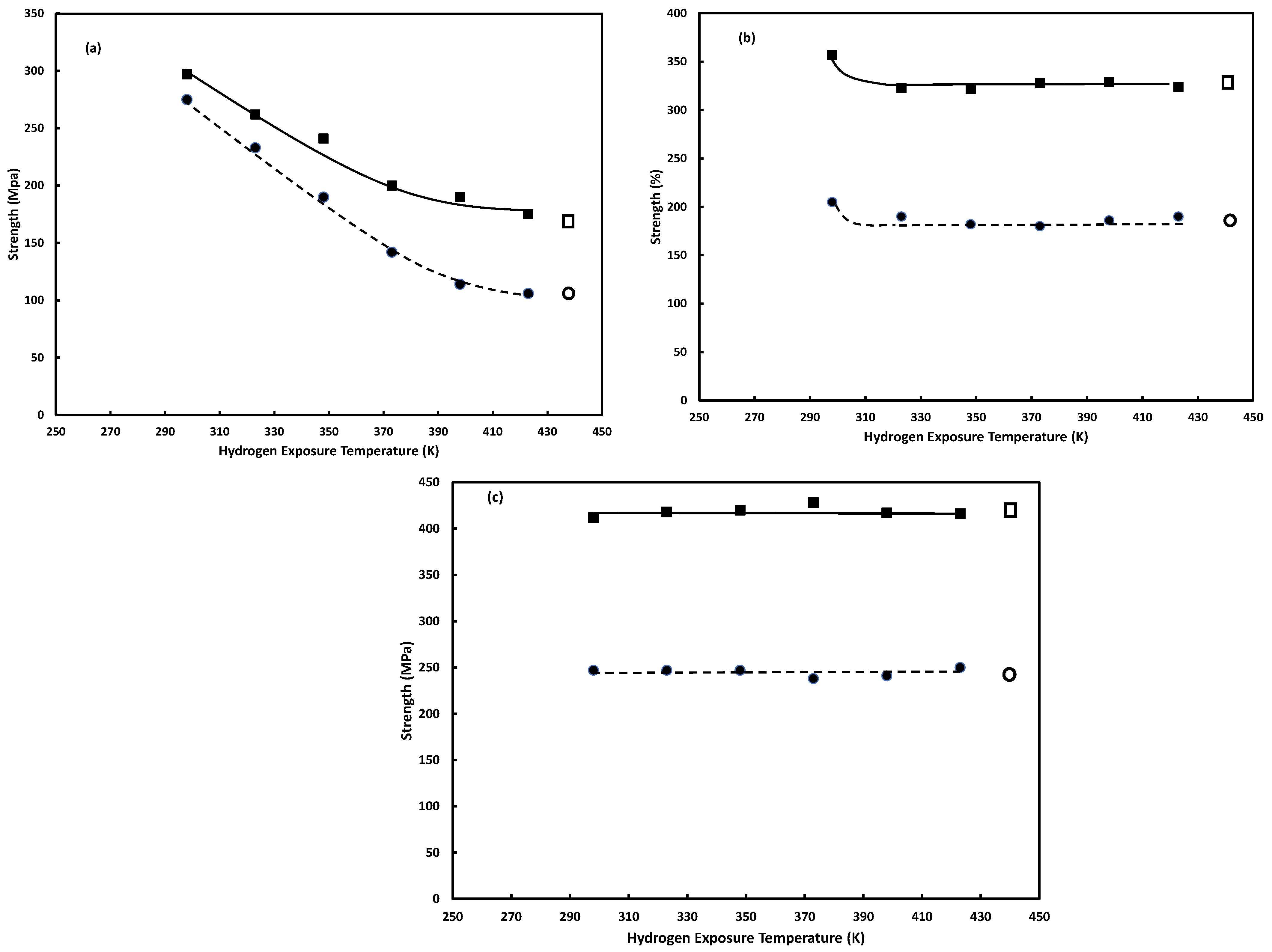
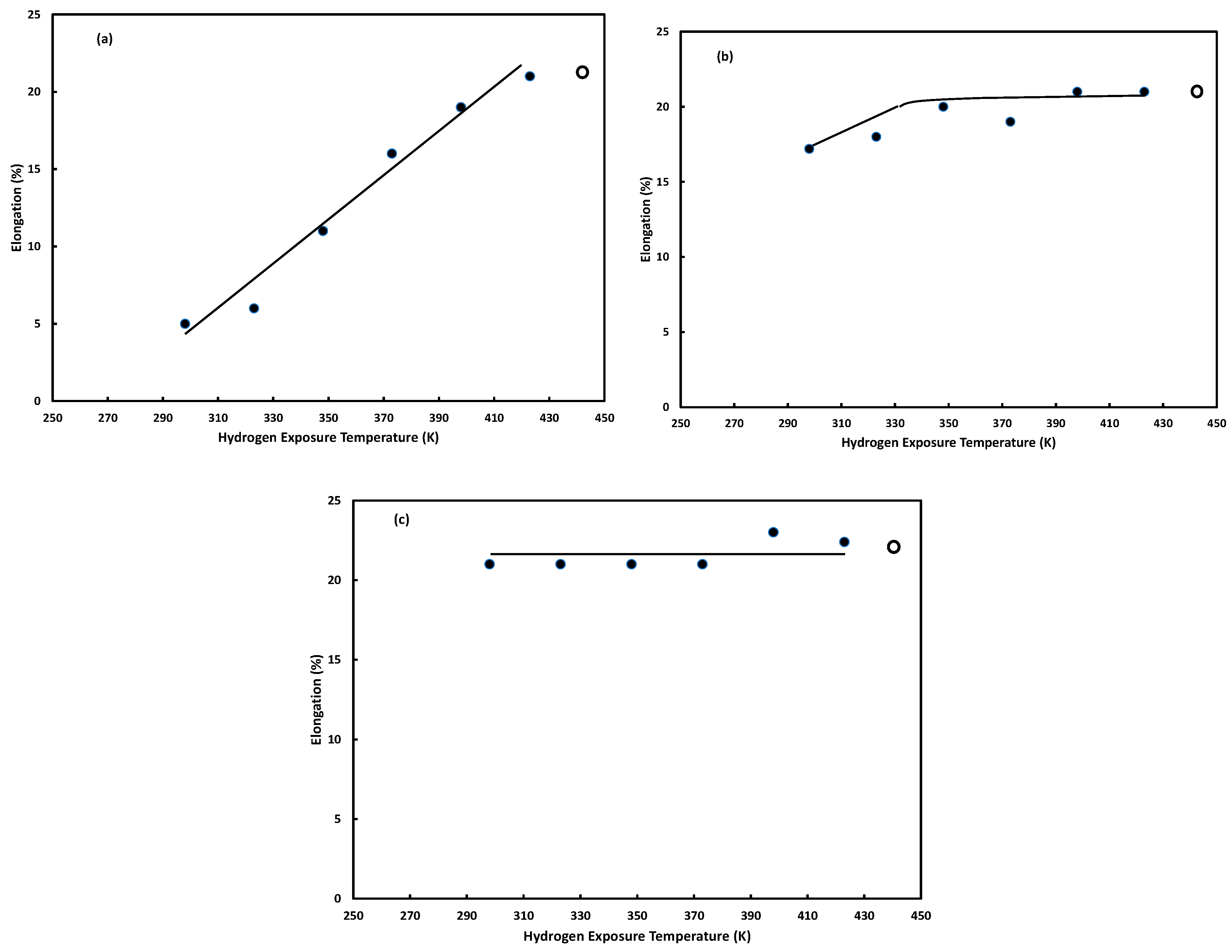
3.2.2. Hydrogen Exposure Temperature
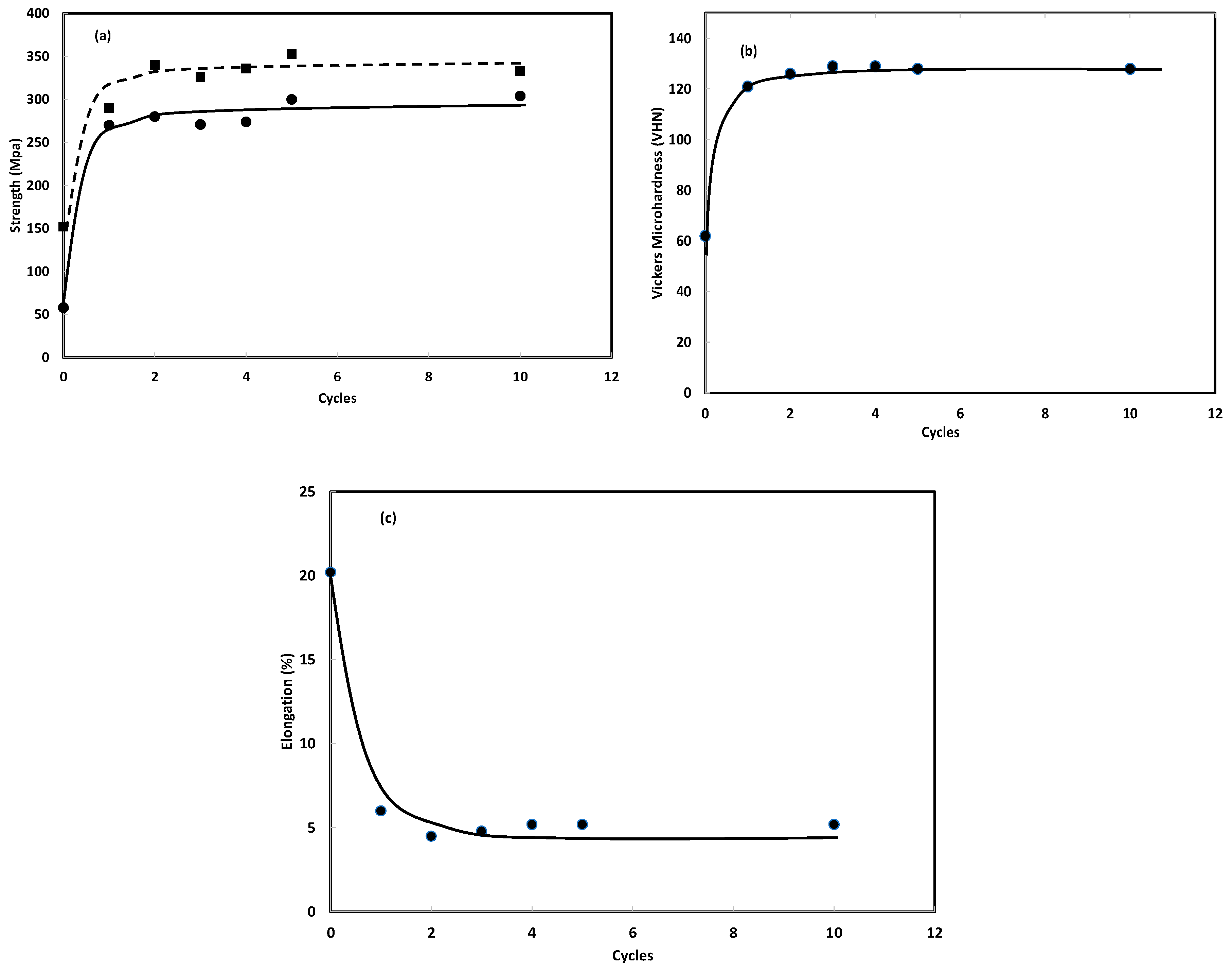
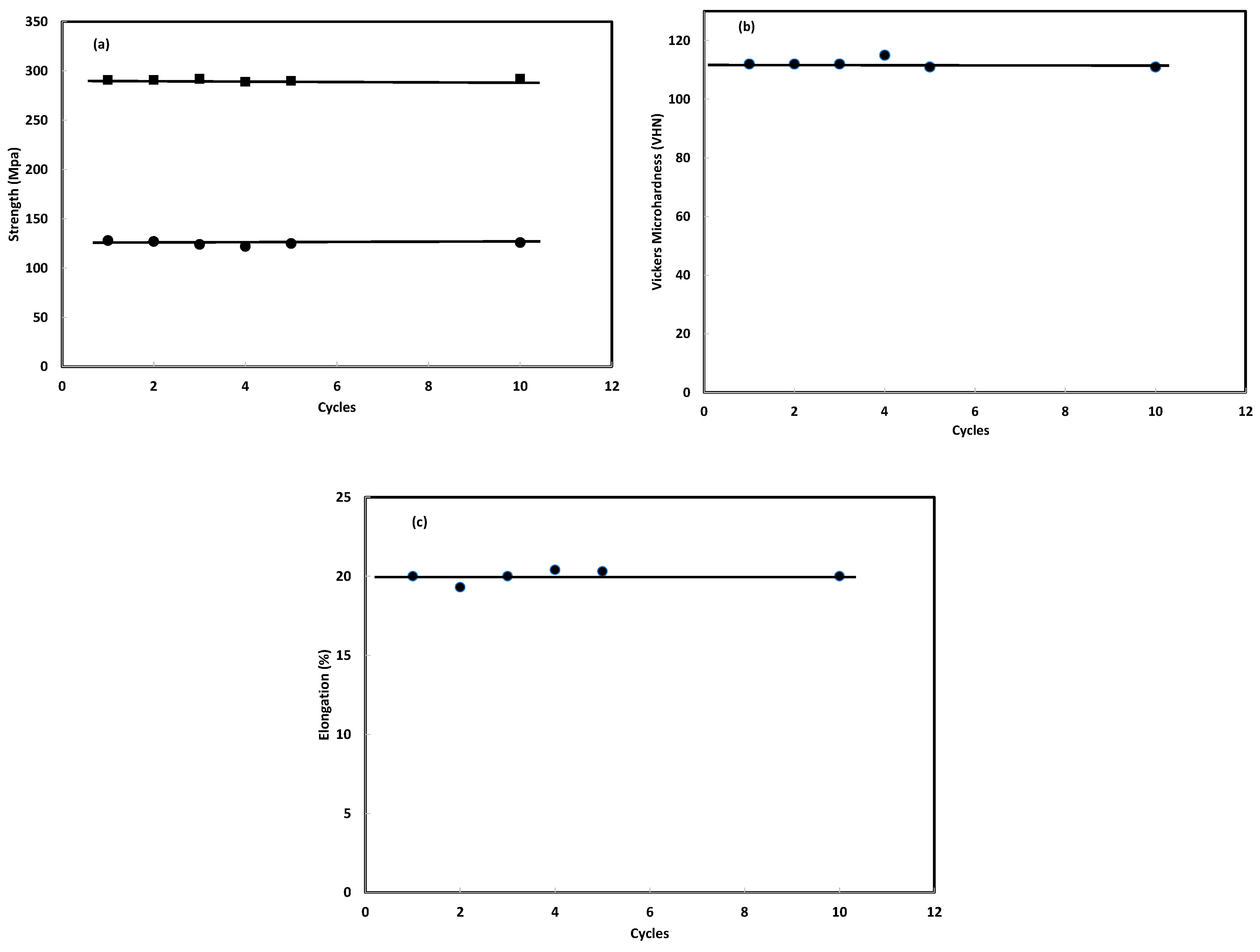
3.2.3. Number of Hydrogen Absorption/Desorption Cycles
3.3. Case 2: Palladium–Silver
3.3.1. Hydrogen Exposure Temperature
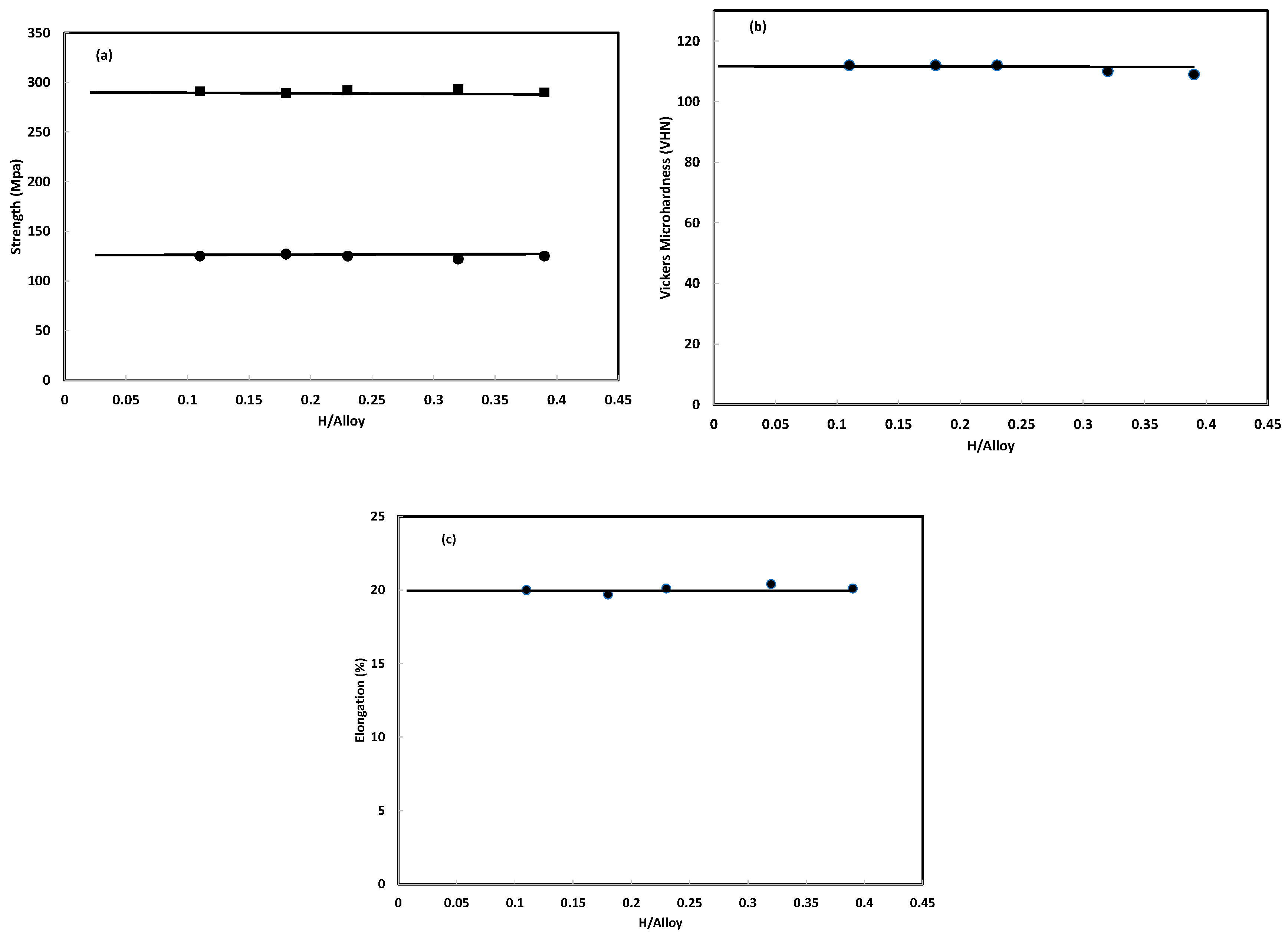
3.3.2. Amount of Hydrogen Absorbed
3.3.3. Number of Hydrogen Absorption/Desorption Cycles
3.4. Case 3: Palladium–Copper
Hydrogen Exposure Temperature
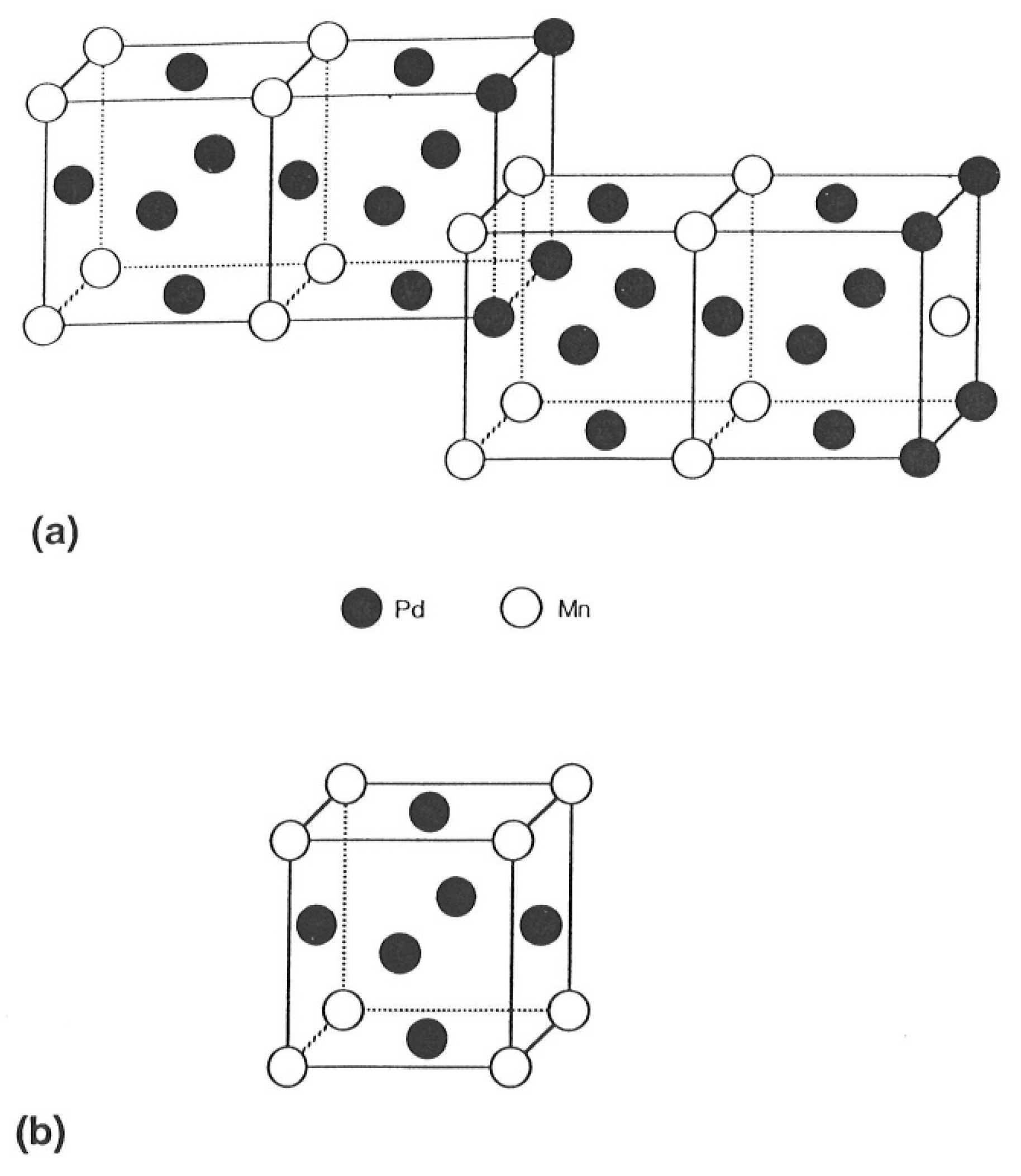
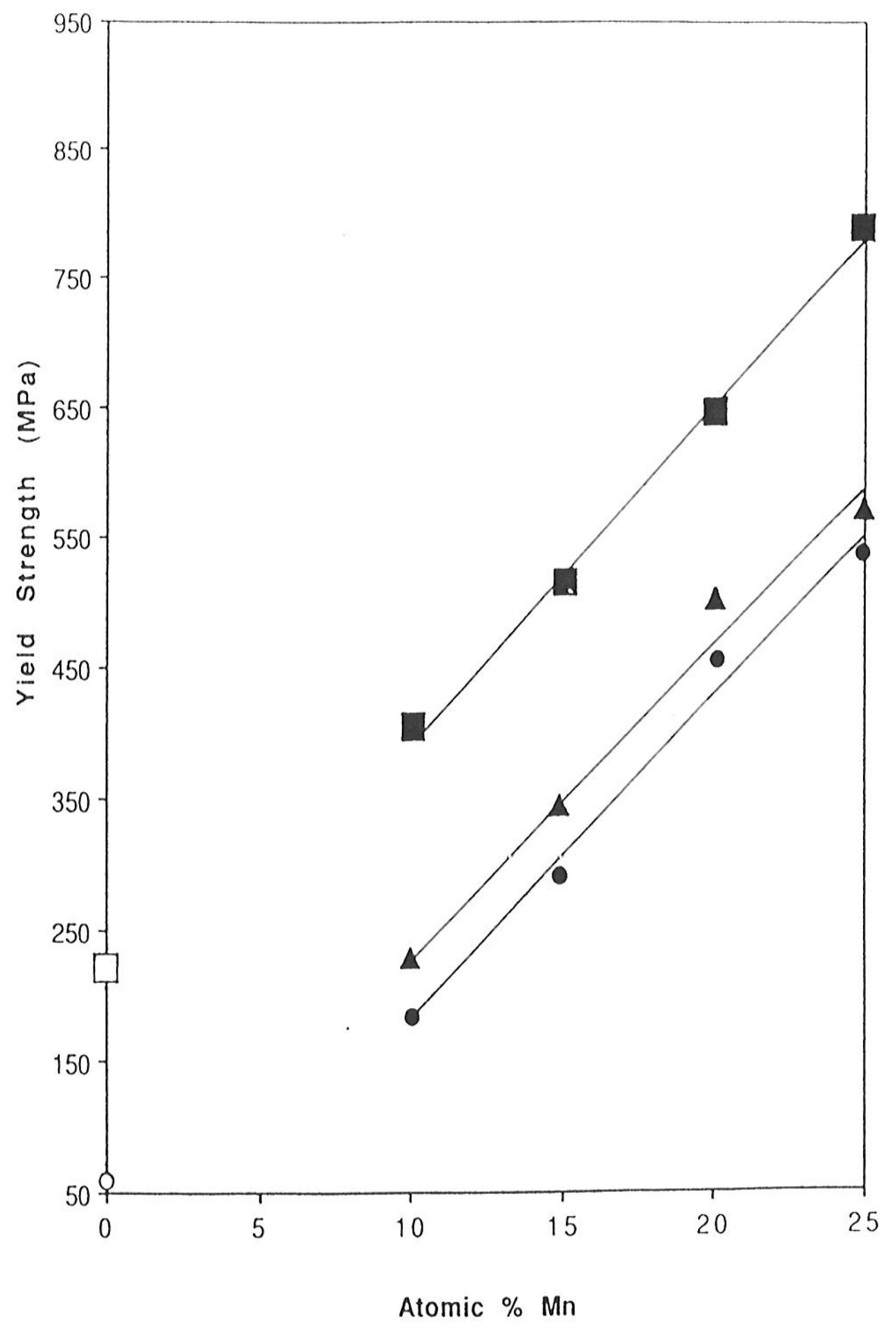
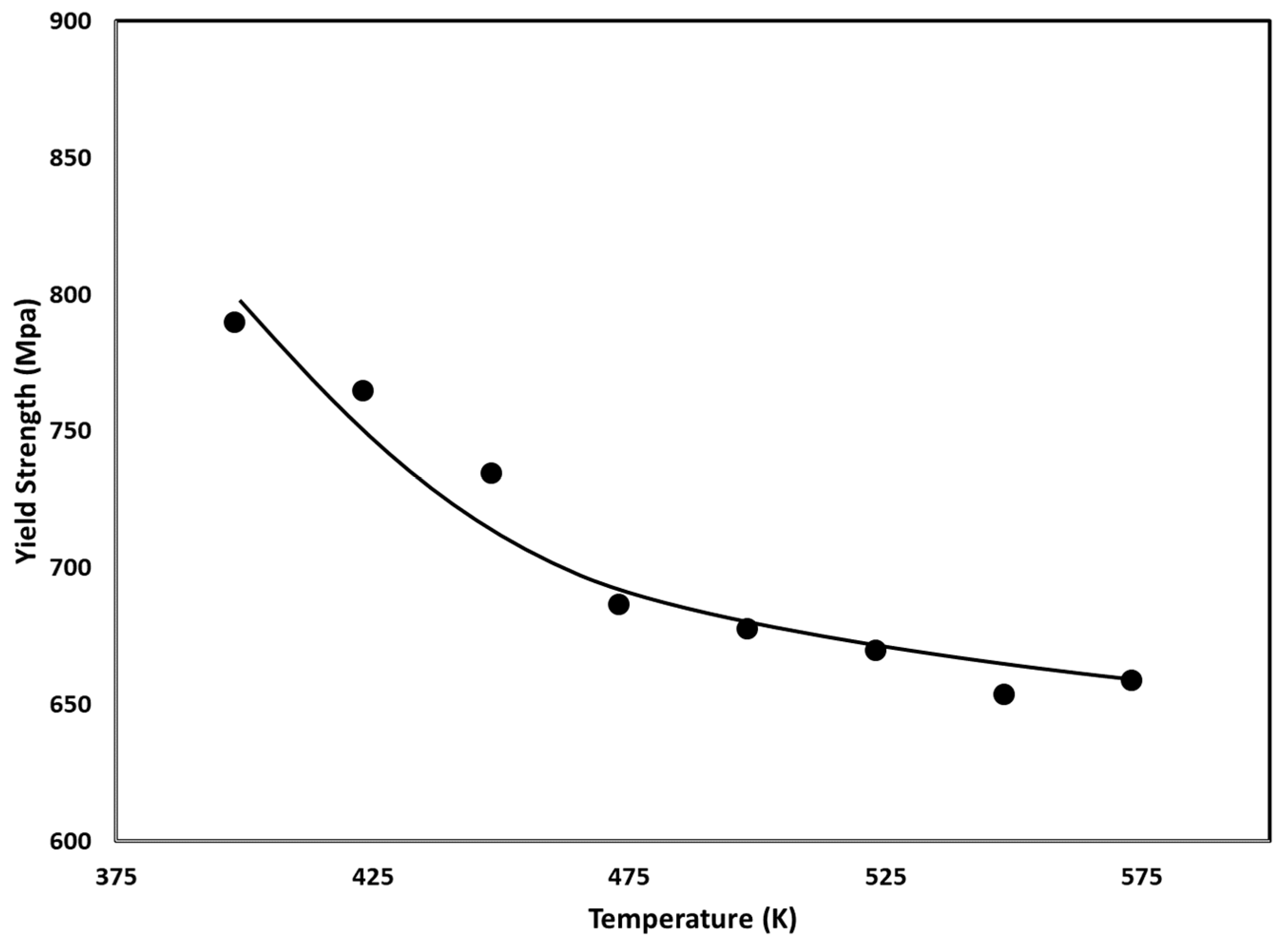
3.5. Case 4: Palladium–Manganese
3.5.1. Stress–Strain Characteristics
3.5.2. Effect of Hydrogen Evacuation Temperature
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mond, L.; Ramsay, W.; Shields, J. On the occlusion of hydrogen and oxygen by palladium. Philos. Trans. R. Soc. Lond. Ser. A 1898, 191, 105–126. [Google Scholar]
- Vargas, W.; Rojas, I.; Azofeifa, D.; Clark, N. Optical and electrical properties of hydrided palladium thin films studied by an inversion approach from transmittance measurements. Thin Solid Film 2006, 496, 189–196. [Google Scholar] [CrossRef]
- Clark, N.; Vargas, W.; Azofeifa, D. Dielectric function of Pd hydride thin films in terms of hydrogen concentration and film’s thickness: A parametric formulation. J. Alloys Compd. 2015, 645, S320–S324. [Google Scholar] [CrossRef]
- Kawae, T.; Inagaki, Y.; Wen, S.; Hirota, S.; Itou, D.; Kimura, T. Superconductivity in palladium hydride systems. J. Phys. Soc. Jpn. 2020, 89, 051004. [Google Scholar] [CrossRef]
- Cattania, M.; Penka, V.; Behm, R.; Christmann, K.; Ertl, G. Interaction of hydrogen with a palladium (110) surface. Surf. Sci. 1983, 126, 382–391. [Google Scholar] [CrossRef]
- Burch, R.; Buss, R.G. Absorption of hydrogen by palladium–copper alloys. Part 1.—Experimental measurements. J. Chem. Soc. Faraday Trans. 1 Phys. Chem. Condens. Phases 1975, 71, 913–921. [Google Scholar] [CrossRef]
- Baba, K.; Sakamoto, Y.; Flanagan, T.; Kuji, T.; Craft, A. Electrical resistance anomalies and hydrogen solubilities in the disorder-order system Pd3Mn. Scr. Metall. 1987, 21, 299–303. [Google Scholar] [CrossRef]
- Richardson, T.; Slack, J.; Farangis, B.; Rubin, M. Mixed metal films with switchable optical properties. App. Phys. Lett. 2002, 80, 1349–1351. [Google Scholar] [CrossRef]
- Wang, M.; Feng, Y. Palladium–silver thin film for hydrogen sensing. Sens. Actuators B Chem. 2007, 123, 101–106. [Google Scholar] [CrossRef]
- Hara, M.; Sakurai, J.; Akamaru, S.; Hashizume, K.; Nishimura, K.; Mori, K.; Okabe, T.; Watanabe, K.; Matsuyama, M. Thermodynamic and magnetic properties of Pd0.93Mn0.07 hydride. Mater. Trans. 2007, 48, 3154–3159. [Google Scholar] [CrossRef]
- Vocaturo, R.; Tresca, C.; Ghiringhelli, G.; Profeta, G. Prediction of ambient-pressure superconductivity in ternary hydride PdCuH x. J. Appl. Phys. 2022, 131, 033903. [Google Scholar] [CrossRef]
- Sivasamy, R.; Venugopal, P.; Kumar, K.V.; Espinoza-González, R. Synthesis and characterizations of Pd/Mn (Mn1.36Pd0.64)O4 nanocomposite: An experimental and theoretical approach. Vacuum 2020, 182, 109683. [Google Scholar] [CrossRef]
- Gryaznov, V.; Serebryannikova, O.; Serov, Y.; Ermilova, M.; Karavanov, A.; Mischenko, A.; Orekhova, N. Preparation and catalysis over palladium composite membranes. Appl. Catal. A Gen. 1993, 96, 15–23. [Google Scholar] [CrossRef]
- Padama, A.; Kasai, H.; Budhi, Y. Hydrogen absorption and hydrogen-induced reverse segregation in palladium–silver surface. Int. J. Hydrogen Energy 2013, 38, 4715–14724. [Google Scholar] [CrossRef]
- Dillon, E.; Jimenez, G.; Davie, A.; Bulak, J.; Nesbit, S.; Craft, A. Factors influencing the tensile strength, hardness and ductility of hydrogen-cycled palladium. Mater. Sci. Eng. A 2009, 524, 89–97. [Google Scholar] [CrossRef]
- Manganese: Crystal Structures. Available online: https://www.webelements.com/manganese/crystal_structure.html (accessed on 22 March 2023).
- Manchester, F.; San-Martin, A.; Pitre, J. The H-Pd (Hydrogen-Palladium) System. J. Phase Equilibria 1994, 15, 62–83. [Google Scholar] [CrossRef]
- Flanagan, T.; Park, C. Hydrogen Storage Materials: Materials Science Forum; Barnes, R., Ed.; Trans Tech Publications: Aedermannsdorf, Switzerland, 1988; pp. 297–305. [Google Scholar]
- Wang, D.; Flanagan, T.; Balasubramaniam, R. Hydrogen solubility as a probe for dislocation formation, rearrangement, and annihilation Pd and Pd/Al2O3 composites. Scr. Mater. 1999, 41, 517–521. [Google Scholar] [CrossRef]
- Polfus, J.; Peters, T.; Bredesen, R.; Lovvik, O. Vacancy diffusion in palladium hydrides. Phys. Chem. Chem. Phys. 2021, 23, 13680–13686. [Google Scholar] [CrossRef] [PubMed]
- Millet, P.; Ngameni, R.; Decaux, C.; Grigoriev, S. Hydrogen sorption by Pd77Ag23 metallic membranes. Role of hydrogen content, temperature and sample microstructure. Int. J. Hydrogen Energy 2011, 36, 4262–4269. [Google Scholar] [CrossRef]
- Okazaki, J.; Tanaka, D.; Tanco, M.; Wakui, Y.; Mizukami, F.; Suzuki, T. Hydrogen permeability study on the thin film Pd-Ag alloy membranes in the temperature range across the α → β transition. J. Membr. Sci. 2006, 282, 370–374. [Google Scholar] [CrossRef]
- Conde, J.; Marono, M.; Sanchez-Hervas, J. Pd-based membranes for hydrogen separation. Review of alloying elements and their influence on membrane properties. Sep. Purif. Rev. 2017, 46, 152–177. [Google Scholar] [CrossRef]
- Zhang, K.; Way, J. Palladium-copper membranes for hydrogen separation. Sep. Purif. Technol. 2017, 186, 39–44. [Google Scholar] [CrossRef]
- Flanagan, T.; Chisdes, D. Solution of Hydrogen in Palladium/Copper Alloys. J. Solid State Chem. 1977, 20, 147–158. [Google Scholar]
- Wang, D.; Flanagan, T.; Shanahan, K. Diffusion of H through Pd-Ag alloys (423–523 K). J. Phys. Chem. B 2008, 112, 1135–1148. [Google Scholar] [CrossRef]
- Ahlzen, P.; Andersson, R.; Tellgren, R.; Rodic, R.; Flanagan, T.; Sakamoto, Y. A neutron powder diffraction study of Pd3MnHx. Z. Phys. Chem. N.F. 1989, 163, 213–218. [Google Scholar] [CrossRef]
- Flanagan, T.; Craft, A.; Kuji, T.; Baba, K.; Sakamoto, Y. Hydrogen induced order-disorder transition in Pd3Mn. Scr. Metall. 1986, 20, 1745–1750. [Google Scholar] [CrossRef]
- Jonsen, D.; Moss, A.; Shenk, J.; Rebeiz, K.; Nesbit, S.; Foley, R.; Craft, A. Influence of hydrogen-induced ordering on the tensile and fatigue characteristics of the alloy system Pd1−xMnx (x = 0.1–0.25). Mater. Sci. Eng. A 1995, 199, 131–138. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Craft, A. A Case Study Approach: Summary of Some Results on the Effects of Hydrogen Exposure on the Mechanical Properties of Palladium and the Alloy Systems Pd1−xMx, M = Ag, Cu, Mn; x = 5 − 0.25. Hydrogen 2023, 4, 237-256. https://doi.org/10.3390/hydrogen4020017
Craft A. A Case Study Approach: Summary of Some Results on the Effects of Hydrogen Exposure on the Mechanical Properties of Palladium and the Alloy Systems Pd1−xMx, M = Ag, Cu, Mn; x = 5 − 0.25. Hydrogen. 2023; 4(2):237-256. https://doi.org/10.3390/hydrogen4020017
Chicago/Turabian StyleCraft, Andrew. 2023. "A Case Study Approach: Summary of Some Results on the Effects of Hydrogen Exposure on the Mechanical Properties of Palladium and the Alloy Systems Pd1−xMx, M = Ag, Cu, Mn; x = 5 − 0.25" Hydrogen 4, no. 2: 237-256. https://doi.org/10.3390/hydrogen4020017
APA StyleCraft, A. (2023). A Case Study Approach: Summary of Some Results on the Effects of Hydrogen Exposure on the Mechanical Properties of Palladium and the Alloy Systems Pd1−xMx, M = Ag, Cu, Mn; x = 5 − 0.25. Hydrogen, 4(2), 237-256. https://doi.org/10.3390/hydrogen4020017




