Abstract
Hydrogen gas inhalation has not yet been elucidated to improve blood behaviors or antioxidant activity in blood. In the present study, the PEM (proton-exchange-membrane)-/platinum-plated electrode-equipped electrolyzer was examined as a hydrogen gas inhaler, which was estimated to supply 3% hydrogen as rapidly as post-operating 10–15 min, together with continuous 30 min retention of 20.8% oxygen being nearly equal to atmospheric oxygen contents. The 40 min inhalation of 3% hydrogen gas and thereafter 60 min rest were shown to enhance the antioxidant ability in human serum, as evaluated by ORAC (oxygen radical absorbing capacity)-based fluorometry, although scarcely enhanced for air dummy inhalation. Unexpectedly, antioxidant ability was 2.50-fold more enhanced for post-inhalational 0–60 min rest than during 40 min inhalation. Oxidative stress-suffering erythrocytes formed a rosary-chain-like aggregation composed of 3–6 cells, together with loss of a single hollow/biconcave-discoid structure in the cell central-part being necessary for erythrocyte passing through capillary vessels, both of which were prevented by 3% hydrogen gas inhalation. Hydrogen gas inhalation increased the intracellular foreign bodies, being distinguished from vacuole/cyst, in leucocytes, suggesting the hydrogen-activated leukocyte phagocytosis-associated events. Thus, 3%-hydrogen gas inhalation is suggested to potentially improve both the erythrocyte rheological/morphologic behaviors and the leucocyte phagocytosis-associated activity, concurrently with the enhanced antioxidant ability in blood.
1. Introduction
Aging is accompanied by increases in oxidative stress, which can lead to the formation of blood clots and immune disorders in the human body [1,2,3]. In the circulation system, erythrocytes are exposed to oxygen, and they are rich in iron contents, which promotes, in combination with diverse iron-containing proteins including transferrin and ferritin etc., the formation of reactive oxygen species (ROS) through Haber–Weiss/Fenton reactions etc. [4]. Moreover, leukocyte functions are influenced by the antioxidant/pro-oxidant balance. Because immune cells produce ROS to execute their immune action, meanwhile adequate amounts of antioxidants are required to prevent oxidative damage to immune cells themselves [3].
Recently, molecular hydrogen has been suggested to contribute to improvement of pressure ulcer care, neurological disorders, pulmonary hypertension, blood fluidity and heat retention in bathing, etc., owing to its antioxidant ability and the marked permeability into organ depth, leading to systemic distribution through the whole body [5,6,7,8,9]. In our previous studies, hydrogen-rich water was found to exert beneficial effects such as suppression of melanin production, cell-death prevention against ultraviolet ray irradiation, and inhibition of cancerous cell growth [10,11,12]. In terms of medical application, it is reported that hydrogen gas inhalation improved the delayed brain injury after subarachnoid hemorrhage, the function of lung organ donated after cardiac injuries, and the disordered immune system of senescent cancer patients [13,14,15]. Hydrogen-gas inhalation enhances alveolar macrophage phagocytosis in ovalbumin-induced asthmatic mice [16]. Here, when hydrogen is inhaled as gaseous matter, we can utilize hydrogen effectively, overcoming drawbacks of hydrogen water such as low water solubility and easy evaporation from water. This is an interesting concept, whereby we may be able to exert effects of hydrogen gas on both the rheological behavior of erythrocytes and the leukocyte phagocytotic functions, because hydrogen gas can approach blood cells through lung capillaries, which has not yet been elucidated substantially.
Thus, the objective of the present study is to assess the effects of hydrogen gas inhalation on antioxidant capacity in serum, and the associated rheological/morphological status of erythrocytes and phagocytotic activity of leukocytes. The present study was designed as a randomized double-blind test to compare the effects of hydrogen gas inhalation versus air dummy inhalation on middle-aged healthy subjects.
2. Materials and Methods
2.1. The Device for Hydrogen Gas Inhalation
The proton exchange membrane (PEM)-/platinum (Pt)-plated electrode-equipped electrolysis-generated hydrogen gas inhaler LitaAir (H2-inhaler LA, WCJ Co. Ltd., Osaka, Japan) was used. The performance of H2-inhaler LA at a flow rate of 100 mL/minute was evaluated in comparison with another commercially widespread ordinary device (400 mL/minute), according to manufacturers’ protocols, being set to ruled electrode amperage and air-pump dilution, with the semiconductor-sensing hydrogen gas monitor HYDlyzer mBA-31 (TAIYO Instruments Inc., Osaka, Japan) and the dissolved oxygen gas polarographic monitor DO-5519E (FUSO Co., Ltd. Tokyo, Japan). Hydrogen or oxygen gas concentrations were quantified on the basis of authentic gas calibrations and gas collection with a gas-impermeable aluminum bag. Each gas sample was delivered as the code-name sample from the sample collector to another sample estimator with randomized sequence. The experiments were independently conducted three times.
2.2. The Participants in the Trial of Hydrogen gas Inhalation with Informed Consents
In this trial, after objective screening-out of fifteen candidate parsons in view of healthy conditions without chronic diseases, no medical treatment and more than 60-day-dificiency of hydrogen treatments, four healthy subjects, a male (46 years old) and three females (34, 46 and 52 years old, respectively) participated after their proposal of informed consent, and the official certification as Research Number #19G01 from Research Ethic Committee of Non-Profitable Organization Corporate Japanese Center for AntiAging MedSciences that was officially authenticated by Hiroshima Prefectural Government. Our experiments were designed as a randomized double-blind test to compare the effects of hydrogen gas inhalation versus air dummy inhalation.
2.3. Antioxidant Capacity in Serum Determined by the ORAC Assay
H2-inhaler LA was operated to generate hydrogen gas with adjustments at concentrations of approximately 3%. Out of four chosen subjects (Section 2.2), three subjects (46-year-old male, and 34- and 52-year-old females) were selected because of the unified inhaling protocol, inhaled the hydrogen gas or dummy air for 40 min, and, after an interval of 14 days, inhaled dummy air or hydrogen gas, respectively, for six subjects in the total, with the randomized-sequential hydrogen/dummy-air cross-over. Then the device was shut down. The serum samples were obtained at each time point during 40 min inhalation and for 60 min after termination of inhalation by cooled centrifugation of collected blood. The antioxidant activity in serum of the subjects was evaluated by oxygen radical absorption capacity (ORAC) assay with the OxiSelect ORAC Activity Assay Kit (Cell Biolabs, Inc., San Diego, CA, USA) followed by a fluorescence microplate reader SH-900Lab (Ex/Em = 480 nm/520 nm, Hitachi High-Tech Science Corp., Tokyo, Japan). The integral values of antioxidant capacity in every serum sample of subjects were also estimated and expressed without leakage.
2.4. Effects of Hydrogen gas Inhalation on Rheological/Morphological Statuses of Erythrocytes and Phagocytotic Activity of Leukocytes
Out of four chosen subjects (Section 2.2), two subjects (46-year male and 34-year female) were excluded because of maximum or minimum values for changes of integral values for antioxidant capacity. The whole blood was collected from two resultant subjects (46-year and 52-year females) inhaling 3% hydrogen gas for 20 min, immediately before or post-inhaling 20–60 min, which was executed at an interval of 14 days after serum antioxidant activity experiment (Section 2.3). The inhalation duration time was set to be shorter than for experiments on serum antioxidant capacity, because morphologic changes of erythrocyte/leucocyte may demand the quick response of cytoskeleton to microenvironments in vigorously flowing blood-stream. The whole blood was video-graphed under a microscope (type ECL-Ci-L, Nicon Co. Ltd., Tokyo, Japan), immediately after blood collection. Out of two subjects, one subject (18/10 specimens of video-extracted still images before/after hydrogen inhalation, respectively) was substantially examined for erythrocyte morphology, in contrast to another subject being scarcely observed for erythrocyte aggregates or central-pallor disappearance, even before hydrogen inhalation. For leucocyte phagocytotic activity, meanwhile, two subjects (16/17 specimens before/after hydrogen inhalation, respectively) were in detail examined. The rouleaux-chain-shaped erythrocyte-aggregates were counted in the images of microscopic visual fields, and the aggregation rates were evaluated. The biconcave-discoid shaped part called “central pallor” was assessed as the gray value on the line-selected 3–6 erythrocytes with a software Image J. Then, the phagocytotic activity of leukocytes were observed for two selected subjects, indicating foreign endogenous bodies, which were distinguished from vacuoles or cysts. The results were compared between values of immediately before/after 20 min inhalation, and were compared to air dummy inhalation, which was conducted under the same conditions, after intervals as long as 14 days, and shown scarcely to significantly affect erythrocyte aggregation/morphology and leucocyte phagocytotic activity. All the still-image specimens were transferred, from the image preparer to another image evaluator, with code assignment at randomized image-sequences.
2.5. Statistical Analysis
Results are expressed as means ± standard deviation (SD). Differences between groups were determined by Student’s t-test. p-values < 0.05 were considered to be statistically different.
3. Results
3.1. Concentrations of Hydrogen/Oxygen Gas being Emitted from Cannula Terminal of the Hydrogen Inhaler
Two types of hydrogen gas inhalers, the H2-inhaler LA and another ordinary electrolysis-based device model, were operated for 30 min, and then concentrations of hydrogen and oxygen in the cannula terminal gas were quantified. Hydrogen concentrations for the H2-inhaler LA reached 3.0% at times as short as post-operating 10–15 min, when hydrogen gas was observed as dilute as 1.11–1.42% for another device, demonstrating that the H2-inhaler LA produced hydrogen gas more than 2.1-fold faster than another device model. Meanwhile, H2-inhaler LA stably maintained oxygen concentrations at 20.72–20.88%, being nearly equal to the atmospheric concentration of 21%, for continuous 30 min operation from the start, whereas oxygen concentrations gradually lowered, and reduced to 20.49% for another device (Figure 1).
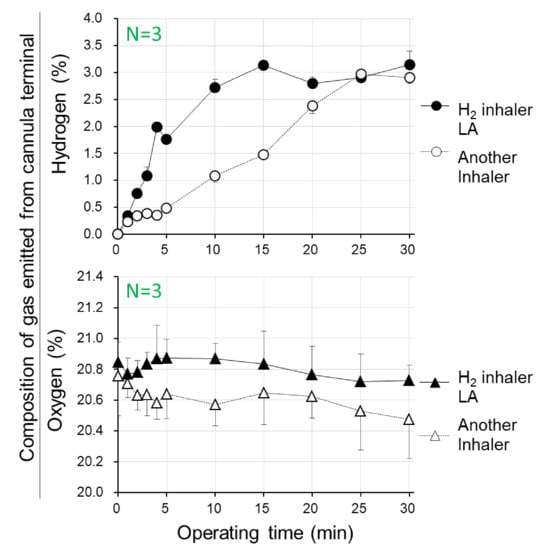
Figure 1.
Time courses of concentrations of hydrogen and oxygen in gas which were emitted from the cannula terminal during operation of hydrogen gas inhalation apparatus. Two types of hydrogen gas inhalers, the proton exchange membrane (PEM)-/Pt-plated electrode-based, electrolysis-generated hydrogen gas inhaler LitaAir (H2 inhaler LA) and another device model, were operated for 30 min, and the concentrations of hydrogen and oxygen in the cannula terminal for gas inspiratory usage were measured. Mean ± SD, n = 3.
As concerned with H2-inhaler LA, practically beneficial values were certified for prompt achievement to 3% hydrogen, which is a concentration safe for the avoidance of hydrogen burning/exploding, in contrast to more than 4% of hydrogen gas beginning to burn. Furthermore, appropriate concentrations of oxygen gas should be retained within concentration ranges being strictly necessary for preventions against either dilute-oxygen-induced hypoxemia or dense-oxygen-induced oxygen toxicity. From both viewpoints, the H2-inhaler LA is recommended as a device suitable for both device-function performances and healthy-body preservation.
3.2. Antioxidant Capacity in Serum Determined by ORAC Assay
The ORAC-fluorometry-based antioxidant ability in serum was demonstrated to be appreciably increased for 3% hydrogen gas inhalation LA for 40 min in three healthy subjects (Figure 2). In contrast, no marked increase in serum antioxidant ability was brought by air sham inhalation under similar conditions, which was conducted as the dummy treatment. Time-course bent-line graphs were calculated as “± values” versus the initial antioxidant capacity for the areas under the bent line, and integrated for times (Figure 2). The integral values for each subject were expressed both “during inhalation” and “after inhalation arrest”, respectively. The tendencies were observed typically for two subjects of approximately 40 and 50 years (Figure 2 upper graph A,B) more obviously, than a hydrogen-responsive subject of 30 s (ibid C).
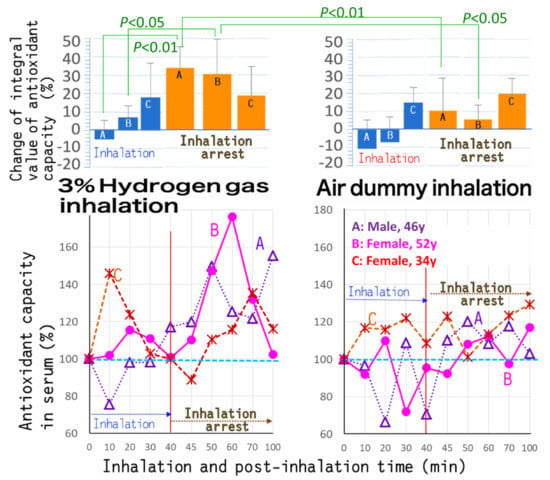
Figure 2.
Antioxidant capacity in human serum, before/during/after inhalation of hydrogen gas or air. The hydrogen gas inhaler H2 inhaler LA was operated to generate hydrogen gas, and adjusted at a concentration of 3%. Three healthy subjects (a 46-year-old male, and 52- and 34-year-old females, respectively) inhaled for 40 min, and then the device was shut down. The antioxidant capacity in serum of the subjects during 40 min inhalation and for 60 min after termination of hydrogen gas inhalation was estimated for the oxygen radical absorption capacity (ORAC)-based antioxidant activity. The results were compared to those of air dummy inhalation which was conducted under the same conditions.
At post-inhalational 0–60 min in particular, as it was an inhalation-arrest state, the antioxidant capacity in serum was shown to be larger than during 40 min inhalation. The findings indicated that the increases in antioxidant capacity were heightened by 28.0% versus the initial value for hydrogen gas inhalation, in contrast to increases as low as 11.2% (Figure 2). Thus, antioxidant capacity increments at the post-inhalation state were estimated to be as large as approximately 2.51-fold versus those at the inhalation state. These delayed antioxidant-ability-heightened effects at post-inhalational times might be exerted by 40 to 70 min time lag being necessary for translocation of inhaled hydrogen via the respiratory organ into blood.
3.3. Preventive Effects of Hydrogen Inhalation against Erythrocyte Aggregation
Aggregation or isolation of erythrocytes was examined for whole blood from healthy subjects that inhaled 3% hydrogen using the hydrogen gas inhaler LA for 20 min and thereafter rested for 20 min. The video-graphic images and micro-photographs for the collected whole blood were analyzed and expressed as typical data for a female at the age of 40 (Figure 3B). The states of isolated erythrocytes were observed for hydrogen gas inhalation, in contrast to the state of rosary-chain-shaped aggregated erythrocytes (3–6 cells) for pre-inhalation time. Degrees of erythrocyte aggregation were quantified by opticometry and the resultant line histograms of intracellular distances versus opticometric brightness, which express degrees of cell–cell mutual overlaying or single-cell isolation (Figure 3C). The results showed that rosary-chain-shaped aggregation was prevented by hydrogen gas inhalation, which made erythrocytes to the cell-isolated states.
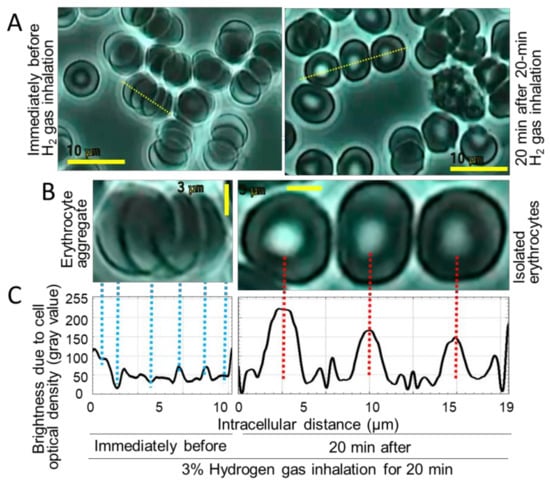
Figure 3.
Suppressive effects of hydrogen gas inhalation on erythrocyte rosary-chain-like aggregation, together with retention of biconcave-discoid-shaped morphology in the erythrocyte center-part, called “central pallor”. (A) The micro-photograms immediately before inhalation and at 20 minutes after 20-min 3%-hydrogen-gas inhalation for a 46-year female subject. Bar: 10 μm. (B) Enlarged micro-photograms of yellow dotted line portions of (A). Bar: 3 μm. (C) Line histograms of intracellular optimetrical distribution for (B).
A subject (female, 46 years old) inhaled 3% hydrogen gas for 20 min, while whole blood was collected before/after the inhalation. (A) The erythrocytes were observed to be aggregated or individually separated under a microscope, either of which is indicated with the yellow line-selected erythrocytes (3–5 cells); (B) the microscopic photographs were enlarged for both the concerned cellular states; (C) erythrocytes at states of aggregation/separation were scanned along the yellow lines, and expressed as opticometric line histograms, with a software Image J, indicating that the peak heights become larger as cell-regional optical densities (cell-regional brightness; gray values) are more marked.
3.4. Preservative Effects of Hydrogen Inhalation on the Cell-Center Hollow-Shaped Morphology of Erythrocytes
Biconcave-discoid-shaped morphology in the erythrocyte center-part called “central pallor” [17] was found to disappear concurrently with erythrocyte rosary-chain-like aggregation, under the pre-inhalational conditions (Figure 3A,B). The central pallor, however, was shown to be preserved by hydrogen gas inhalation. The intracellular opticometric line-histograms interior to erythrocytes were obtained (Figure 3C), resulting in numerical expression for the degrees of both the erythrocyte aggregation and erythrocyte central pallor retention (Figure 4A,B). The Y-axis in Figure 4A represents the rate of the number of aggregate-consisting erythrocytes (not the number of erythrocyte aggregates) versus the total erythrocyte number. The results showed that erythrocyte aggregation was repressed to 33.0% by 20 min 3% hydrogen inhalation simultaneously to the retention of erythrocyte center-part hollow shapes to an increase of 25.9%.
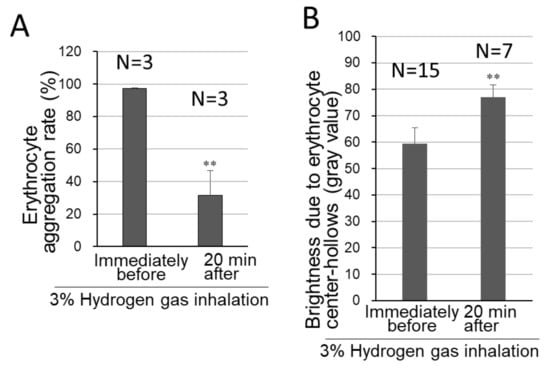
Figure 4.
Degrees of erythrocyte-aggregation suppression and biconcave-discoid shape-retention by hydrogen gas inhalation. Before/after inhalation of 3% hydrogen gas for 20 min, whole blood was collected and analyzed for erythrocyte morphology, as in Figure 3. N represents the number of the examined video-extracted still images before/after hydrogen inhalation. (A) The erythrocyte-aggregation rates were quantified for numbers of aggregated erythrocytes (Figure 3A,B). (B) The opticometry in the cell-central parts containing “central pallor”, was conducted for the biconcave discoid morphology necessary for either erythrocyte cell-flexibility or passing through the capillary vessels, as shown in the cell-brightness-based line-histogram (Figure 3C). The still images and microscopic photographs were analyzed, and shown as mean ± SD, erythrocyte aggregation rate: n = 3; average of gray values: n = 15, immediately before inhalation and n = 7 for 20 min after the inhalation ended. ** p < 0.01 (versus immediately before 3% hydrogen gas inhalation).
3.5. Activation to Phagocytosis of Leukocytes by Hydrogen Gas Inhalation
Leukocytes, existing at ratios as few as 1:800–1:1200 versus erythrocyte numbers, were observed in whole blood of hydrogen-gas-inhaling subjects, and then microscopically filmed. According to analysis for the numerous still images extracted from the video-graphs, the intracellular foreign bodies being distinguished from merely vacuoles/cysts were scarcely observed in blood from pre-inhalational subjects, but abundantly in blood from subjects that inhaled 3% hydrogen gas for 20 min using H2-gas inhaler LA (Figure 5).
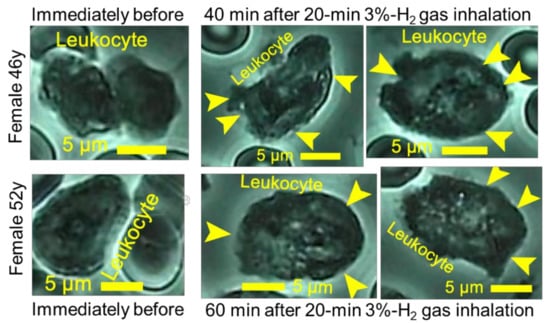
Figure 5.
Promotive effects of hydrogen gas inhalation on leukocyte phagocytosis. The subjects of healthy females 46 and 52 years old inhaled 3% hydrogen gas for 20 min. Whole blood was collected immediately before the inhalation and at 40 or 60 min after the inhalation, and then filmed for 5 min under a microscope. The still images of huge numbers were prepared out of the video-graphs, and were analyzed for leukocytes existing at ratios as few as 1:800 to 1:1200 versus erythrocyte numbers. The microscopic photographs of leukocytes were shown together with yellow arrowheads indicating the foreign endogenous bodies which were distinguished from merely vacuoles or cysts.
The analysis for still images of leukocytes demonstrated that numbers of the foreign bodies per one leukocyte were as few as 4.1–5.9 immediately before hydrogen application, in contrast to numbers as great as 22.1–27.7 after 20 min hydrogen gas inhalation (Figure 6).
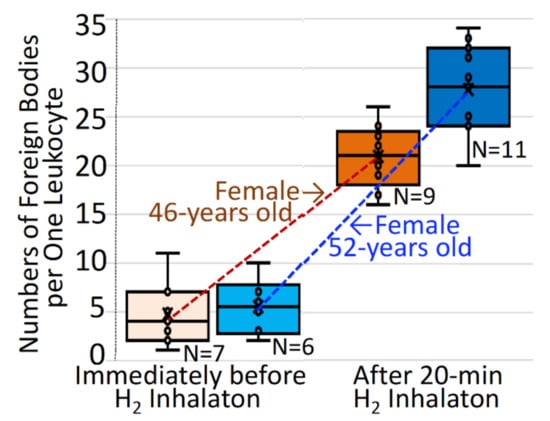
Figure 6.
Increases in numbers of intracellular foreign bodies in leukocytes after hydrogen gas inhalation. The subjects inhaled 3% hydrogen gas for 20 min, as in Figure 5, and were evaluated for numbers of foreign endogenous bodies with no count of vacuoles or cysts, per single leukocyte. The intracellular foreign bodies per one leucocyte were quantitatively analyzed for specified number (n) of microscopic still-image specimens, each of which contained mostly one, in part two, leucocytes being coexistent among abundant erythrocytes, before/after hydrogen inhalation in two subjects of 46-year-old and 52-year-old females. The individual values for the intracellular foreign bodies per single cell are indicated by open circles, and expressed by a quartile method-based box–whisker plot.
4. Discussion
In the present study, hydrogen gas inhalation into healthy subjects by the PEM-/Pt-plated electrode-equipped electrolyzer was demonstrated to promptly achieve 3% hydrogen, together with retention of oxygen nearly equal to concentrations in atmosphere, for H2-inhaler LA, in contrast to both slow attainment to 3% hydrogen and dilute oxygen for another H2-inhaler device (Figure 1), both types of which were manipulated according to manufacturers’ protocols with ruled settings for electrode amperages and gas-diluting pump powers. Diluted oxygen being not in proportion to hydrogen concentrations for another H2-inhaler device might be attributed to improper IC-sensor-based adjustment, together with an oxygen-detecting sensitivity defect and/or mechanical disorder for gas mixing.
The hydrogen gas inhalation for 20–40 min appreciably promoted the ORAC fluorometry-based antioxidant capacity in serum, in contrast to no promotion for air dummy inhalation (Figure 2), and the delayed antioxidant response may be because it takes some time for serum H2 concentration to bring about the intracellular H2 concentration necessary for serum antioxidant-increasing effects. There is a possibility that hydrogen cannot necessarily scavenge most amounts/types of ROS, and is likely to be related indirectly to its modulation for endogenous antioxidant abilities via the Nrf2 (nuclear factor erythroid 2)-signaling pathway, rather than to directly scavenge hydroxyl radicals. The finding for scarce effects of air-dummy inhalation might exclude a possibility for any effects of inhaling actions themselves, including cannula-wearing into the nasal cavity and the possible consciously deep respiration. The results might demonstrate the direct-trigger effect of inhaled hydrogen itself.
The hydrogen gas inhalation exerted beneficial effects on both repression of erythrocyte aggregation and preservation of erythrocyte center-part hollow-shaped morphology designated as the “central pallor”, (Figure 3 and Figure 4) being necessary for erythrocyte passing through capillary vessels [17]. Most of erythrocytes consisting of erythrocyte aggregate lost the central pallor, because of rosary-shaped aggregate-caused cellular morphologic distortion, but not because of erythrocyte-overlapping-derived concealing hindrance of the central pallor, whereas the non-aggregated and isolated erythrocytes were occupied mostly by central-pallor-possessing erythrocytes, suggesting an inverse correlation between numerical values (Figure 4A,B). Regarding some causes for age-related erythrocyte aggregation, plasma fibrinogen was reported to promote erythrocyte aggregate formation, and erythrocyte aggregations in plasma are higher in the age group of 50–59 years than that of 20–29 years [18]. The negative charge of the erythrocyte surface is attributed to the sialic acid moiety in the cell membrane, but the sialic acid content of erythrocytes decreases along with human aging, which enhances rouleaux-shaped formation of erythrocytes [19]. Hydrogen gas that was dissolved in blood might cause erythrocyte surfaces to be electrostatically negative charged and lower the zeta potentials, which is suggested to promote the intercellular mutual repulsive power between erythrocytes, whereas oxidative stress-suffered erythrocytes are implied to be increased for the zeta potentials, resulting in aggregation through loss of the repulsive power [20].
The biconcave-discoid shape due to the lack of nuclei is an important morphological feature of erythrocytes [19,21]. The size of erythrocytes is larger than the capillary diameter, and therefore blood flow requires that the biconcave-discoid erythrocytes deform to squeeze through capillary vessels to convey and deliver oxygen to the tissues [22]. Under low-shear conditions of blood flow, erythrocytes show a biconcave-discoid shape, and at stasis they aggregate into structures resembling a stack of coins, which is a reversible process [19,21]. It is notable that decreased erythrocyte deformability is associated with oxygen free-radical damage to erythrocytes [23,24] which is observed in older humans [19]. Thus, hydrogen-achieved morphological preservation of erythrocyte central-pallor might be reversible [19,21] brought about by promoted antioxidant ability due to hydrogen gas inhalation, as found for Figure 2, together with prevention against ROS-caused cell injuries to erythrocyte [23,24], especially for persons such as the 40–50-year-old subjects examined in the present study. The improvement effects are emphatically anticipated to be more obviously achieved for continuous daily practices than for a single hydrogen gas inhalation such as in the present study.
In contrast to comparatively static aspects of both erythrocyte aggregation and hollow-shape rates, as observed in the present study, dynamic effects were exerted either for the enlargement of capillary-vessel size caused by the hydrogen-warm-water bath-, as compared with a normal warm tap-water bath by CCD-based capillaroscopy [5], or for the hydrogen-intensified prevention against bloodstream clogging in a comb-shaped blood rheological apparatus [6].
Marked increases in the intracellular foreign bodies, which were taken up but not digested in leukocytes, were brought by hydrogen gas inhalation (Figure 5 and Figure 6), suggesting the activation of leukocyte phagocytosis through hydrogen-executed scavenging to excess of ROS being generated upon phagocytosis, rather than through hydrogen-induced surplus generation of the intracellular foreign bodies. This is because the similar events were also shown by enhancing effects of hydrogen gas inhalation on macrophage phagocytosis to bacterial particles [16], and by correlation of phagocytotic activity with antioxidant capacity [25]. The still images for leucocytes suggested that hydrogen gas inhalation might activate leukocyte-based phagocytosis, which is considered to ensue from scavenging by hydrogen gas inhalation to ROS that is generated by diverse phagocytes such as macrophages, neutrophils and dendritic cells through ROS-generating enzymes, including myeloperoxidase and NADPH-oxidase.
Thus, hydrogen gas inhalation might exert potentials to improve blood fluidity through erythrocyte-aggregate repression together with preservation of an erythrocyte center-part hollow-shape-part called “central pallor”, and to intensify leukocyte-executed phagocytosis of intracellular non-self substances through the enhanced antioxidant ability.
Author Contributions
Conceptualization, N.M.; methodology, N.M. and Y.T.; software, N.M. and Y.T.; validation, N.M. and Y.T.; formal analysis, N.M. and Y.T.; investigation, N.M.; resources, N.M.; data curation, N.M. and Y.T.; writing—original draft preparation, N.M.; writing—review and editing, N.M. and Y.T.; visualization, N.M.; supervision, N.M.; project administration, N.M.; funding acquisition, Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
The present study was supported in part by a Grant-in-Aid No. 2003 to YT for the Scientific Research on Anti-Aging Promotion from the Non-profitable Organization Corporate Japanese Center for Anti-Aging MedSciences, authenticated by Hiroshima Prefectural Government.
Data Availability Statement
The data presented in this study are available within the article. There are no databases associated with this manuscript.
Acknowledgments
The authors would express their gratitude to Li Xiao (The Nippon Dental University) for the technical assistance.
Conflicts of Interest
The author declares no conflict of interest.
References
- Wang, Q.; Zennadi, R. Oxidative Stress and Thrombosis during Aging: The Roles of Oxidative Stress in RBCs in Venous Thrombosis. Int. J. Mol. Sci. 2020, 21, 4259. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, C.; Karlsson, A.; Bylund, J. Measurement of respiratory burst products generated by professional phagocytes. Methods Mol. Biol. 2007, 412, 349–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarado, C.; Alvarez, P.; Puerto, M.; Gausserès, N.; Jiménez, L.; De la Fuente, M. Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition 2006, 22, 767–777. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Temiz, A.; Meiselman, H.J. Effect of superoxide anions on red blood cell rheologic properties. Free Radic. Biol. Med. 1998, 24, 102–110. [Google Scholar] [CrossRef]
- Kato, S.; Takada, Y.; Miwa, N. Heat-retention effects of hydrogen-rich water bath assessed by thermography for humans. J. Therm. Biol. 2021, 95, 102805. [Google Scholar] [CrossRef]
- Kato, S.; Hokama, R.; Okayasu, H.; Saitoh, Y.; Iwai, K.; Miwa, N. Colloidal platinum in hydrogen-rich water exhibits radical-scavenging activity and improves blood fluidity. J. Nanosci. Nanotechnol. 2012, 12, 4019–4027. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kato, S.; Matsuoka, D.; Tanaka, H.; Miwa, N. Hydrogen water intake via tube-feeding for patients with pressure ulcer and its reconstructive effects on normal human skin cells in vitro. Med. Gas Res. 2013, 3, 20. [Google Scholar] [CrossRef] [Green Version]
- Iketani, M.; Ohsawa, I. Molecular Hydrogen as a Neuroprotective Agent. Curr Neuropharmacol. 2017, 15, 324–331. [Google Scholar] [CrossRef] [Green Version]
- Lazar, H.L. Molecular hydrogen: A novel therapy for the treatment of pulmonary hypertension. J. Thorac. Cardiovasc. Surg. 2015, 150, 654–655. [Google Scholar] [CrossRef]
- Kato, S.; Saitoh, Y.; Miwa, N. Inhibitions by hydrogen-occluding silica microcluster to melanogenesis in human pigment cells and tyrosinase reaction. J. Nanosci. Nanotechnol. 2013, 13, 52–59. [Google Scholar] [CrossRef]
- Kato, S.; Saitoh, Y.; Iwai, K.; Miwa, N. Hydrogen-rich electrolyzed warm water represses wrinkle formation against UVA ray together with type-I collagen production and oxidative-stress diminishment in fibroblasts and cell-injury prevention in keratinocytes. J. Photochem. Photobiol. B 2012, 106, 24–33. [Google Scholar] [CrossRef]
- Kato, S.; Saitoh, Y.; Miwa, N. Hydrogen-bubbled platinum-colloid suppresses human esophagus- or tongue-carcinoma cells with intracellular platinum-uptake and the diminished normal-cell mortality. Hum. Cell 2020, 33, 1294–1301. [Google Scholar] [CrossRef]
- Kumagai, K.; Toyooka, T.; Takeuchi, S.; Otani, N.; Wada, K.; Tomiyama, A.; Mori, K. Hydrogen gas inhalation improves delayed brain injury by alleviating early brain injury after experimental subarachnoid hemorrhage. Sci. Rep. 2020, 10, 12319. [Google Scholar] [CrossRef]
- Haam, S.; Lee, J.G.; Paik, H.C.; Park, M.S.; Lim, B.J. Hydrogen gas inhalation during ex vivo lung perfusion of donor lungs recovered after cardiac death. J. Heart Lung Transplant. 2018, 37, 1271–1278. [Google Scholar] [CrossRef]
- Chen, J.B.; Kong, X.F.; Qian, W.; Mu, F.; Lu, T.Y.; Lu, Y.Y.; Xu, K.C. Two weeks of hydrogen inhalation can significantly reverse adaptive and innate immune system senescence patients with advanced non-small cell lung cancer: A self-controlled study. Med. Gas Res. 2020, 10, 149–154. [Google Scholar] [CrossRef]
- Huang, P.; Wei, S.; Huang, W.; Wu, P.; Chen, S.; Tao, A.; Wang, H.; Liang, Z.; Chen, R.; Yan, J.; et al. Hydrogen gas inhalation enhances alveolar macrophage phagocytosis in an ovalbumin-induced asthma model. Int. Immunopharmacol. 2019, 74, 105646. [Google Scholar] [CrossRef]
- Matsumoto, N. Morphology of erythrocytes: Hematological examination for erythrocyte morphological abnormalities. Medicina 1989, 26, 1704–1705. [Google Scholar] [CrossRef]
- Christy, R.M.; Baskurt, O.K.; Gass, G.C.; Gray, A.B.; Marshall-Gradisnik, S.M. Erythrocyte aggregation and neutrophil function in an aging population. Gerontology 2010, 56, 175–180. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Meiselman, H.J.; Baskurt, O.K. Blood rheology and aging. J. Geriatr. Cardiol. 2013, 10, 291–301. [Google Scholar] [CrossRef]
- Izumida, Y.; Seiyama, A.; Maeda, N. Erythrocyte aggregation: Bridging by macromolecules and electrostatic repulsion by sialic acid. Biochim. Biophys. Acta 1991, 1067, 221–226. [Google Scholar] [CrossRef]
- Baskurt, O.K.; Meiselman, H.J. Lessons from comparative hemorheology studies. Clin. Hemorheol. Microcirc. 2010, 45, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Pries, A.R.; Secomb, T.W.; Gaehtgens, P. Biophysical aspects of blood flow in the microvasculature. Cardiovasc. Res. 1996, 32, 654–667. [Google Scholar] [CrossRef]
- Machiedo, G.W.; Powell, R.J.; Rush, B.F., Jr.; Swislocki, N.I.; Dikdan, G. The incidence of decreased red blood cell deformability in sepsis and the association with oxygen free radical damage and multiple-system organ failure. Arch. Surg. 1989, 124, 1386–1389. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, M.J.; Meiselman, H.J.; Marshall-Gradisnik, S.M.; Pyne, M.; Kakanis, M.; Keane, J.; Brenu, E.; Christy, R.; Baskurt, O.K. Assessment of oxidant susceptibility of red blood cells in various species based on cell deformability. Biorheology 2011, 48, 293–304. [Google Scholar] [CrossRef]
- Brajovich, M.I.; Rucci, A.; Acosta, I.L.; Cotorruelo, C.; García Borrás, S.; Racca, L.; Biondi, C.; Racca, A. Effects of aging on antioxidant response and phagocytosis in senescent erythrocytes. Immunol. Investig. 2009, 38, 551–559. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).