Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment
Abstract
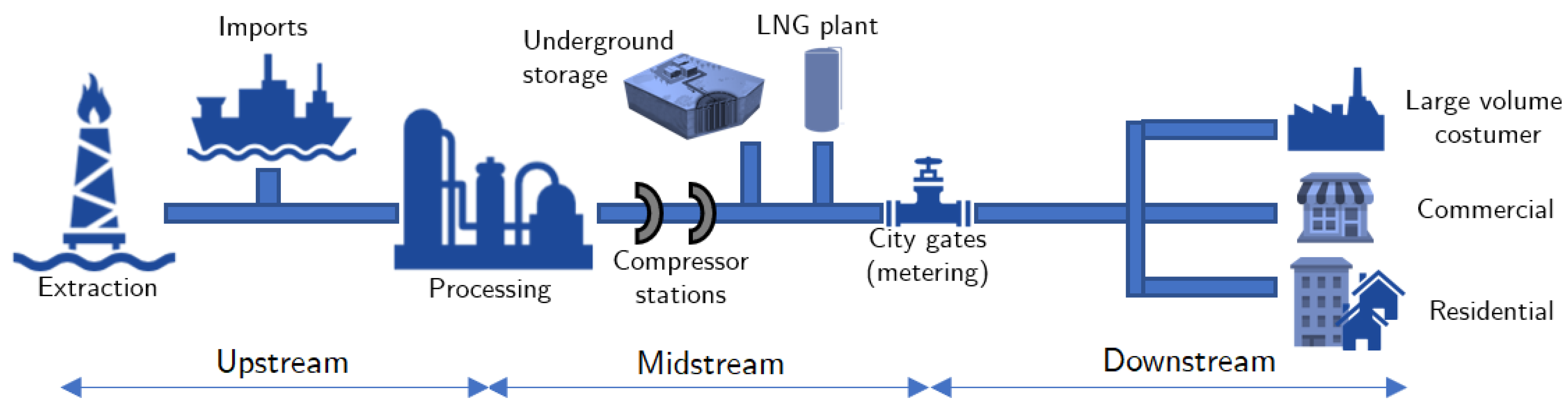
:1. Introduction
2. Means and Methods
Modelling
3. Results and Discussion
3.1. Transmission and Distribution
3.1.1. Material Aspects
3.1.2. Energetic Aspects
3.1.3. Operational Aspects
3.2. Consumption
Interchangeability Analysis
4. Conclusions
- The introduction of renewable gases in gas pipelines, particularly hydrogen, will produce a significant increase in volumetric flow rate due to their lower heating values (). Thus, compression stations and pressure reduction stations will have to be adapted to accommodate the expected flow rate increase. Furthermore, for hydrogen concentrations above 30%, steel pipelines may suffer from hydrogen embrittlement. For distribution networks, sections built in cast iron and steel will likely have to be replaced by polyethylene to reduce H2 leakage when hydrogen admixture is above 30% by volume.
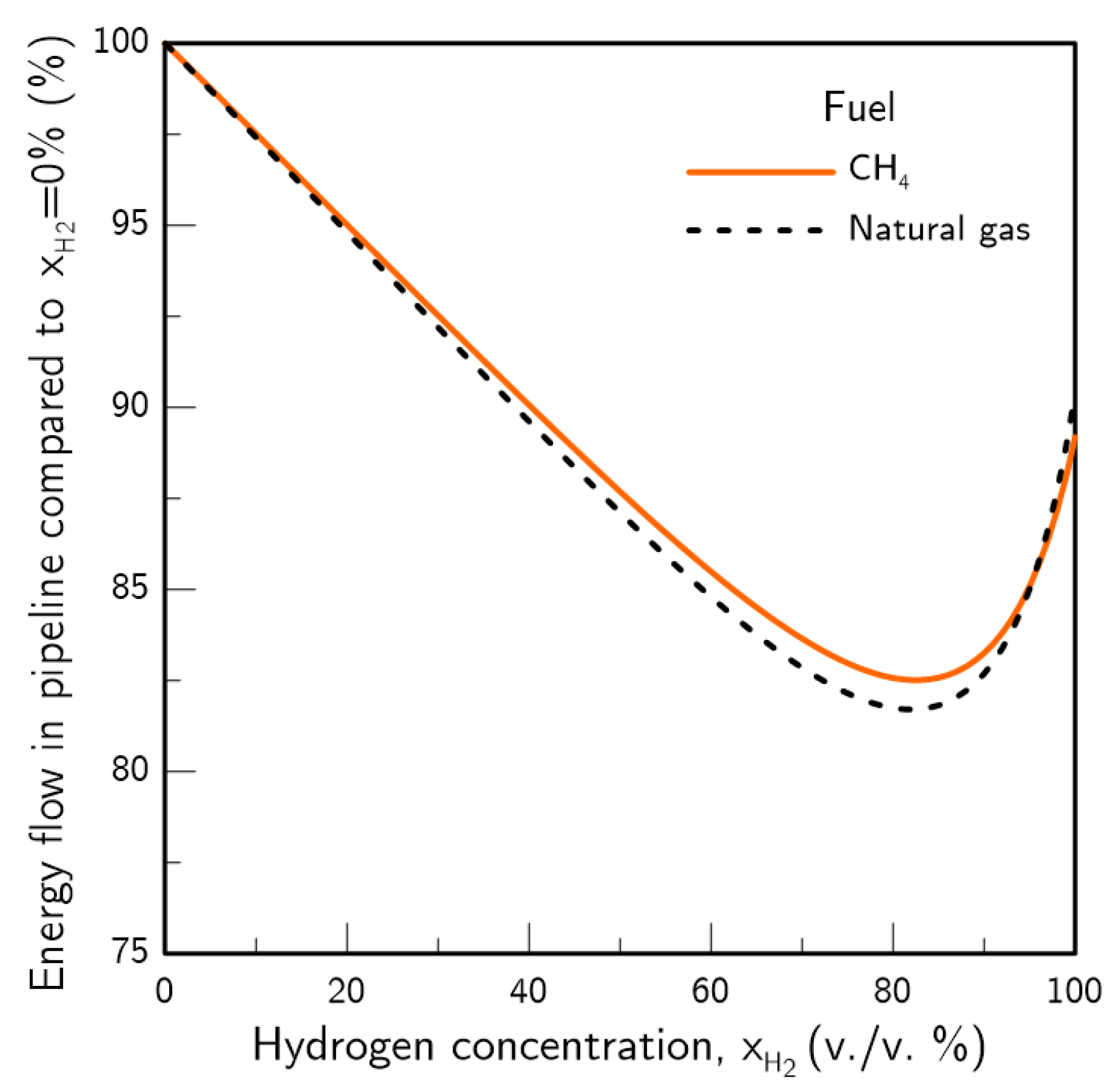
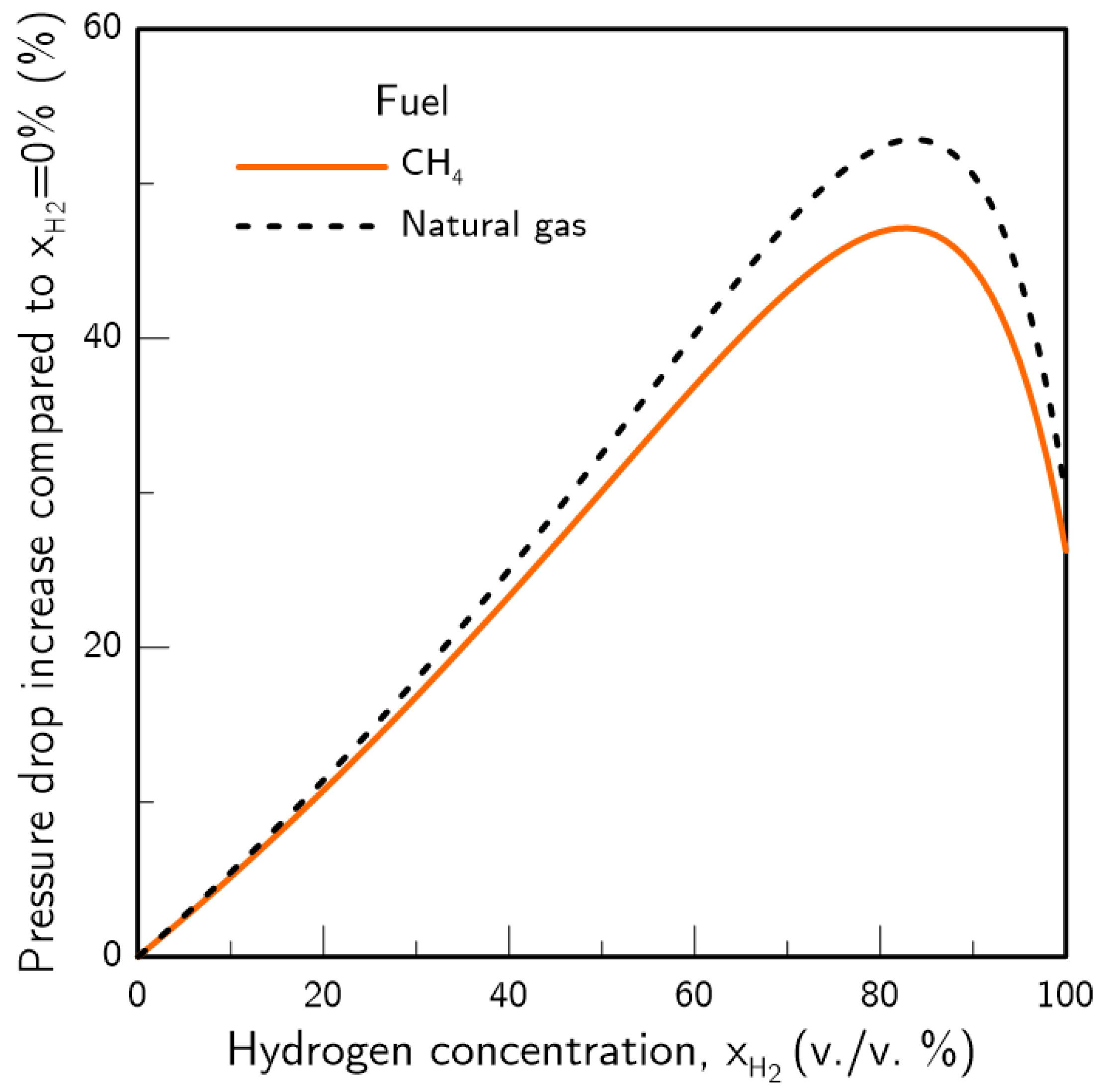
- If pipeline dimensions and pressure drop are maintained, the introduction of hydrogen will reduce the amount of energy transported in the system. A shift towards a grid with 100% hydrogen would produce an estimated loss of 10% in the transported energy. If energy demand and pipeline dimensions are maintained, an increase in pipeline pressure drop will occur, which will be around 11% for a 20% H2 content in the pipeline. For TSOs, this may force an increase in pipeline operating pressure or additional compression stations. For DSOs, the increase in pressure drop may be compensated by increasing pipeline pressure or through network meshing.
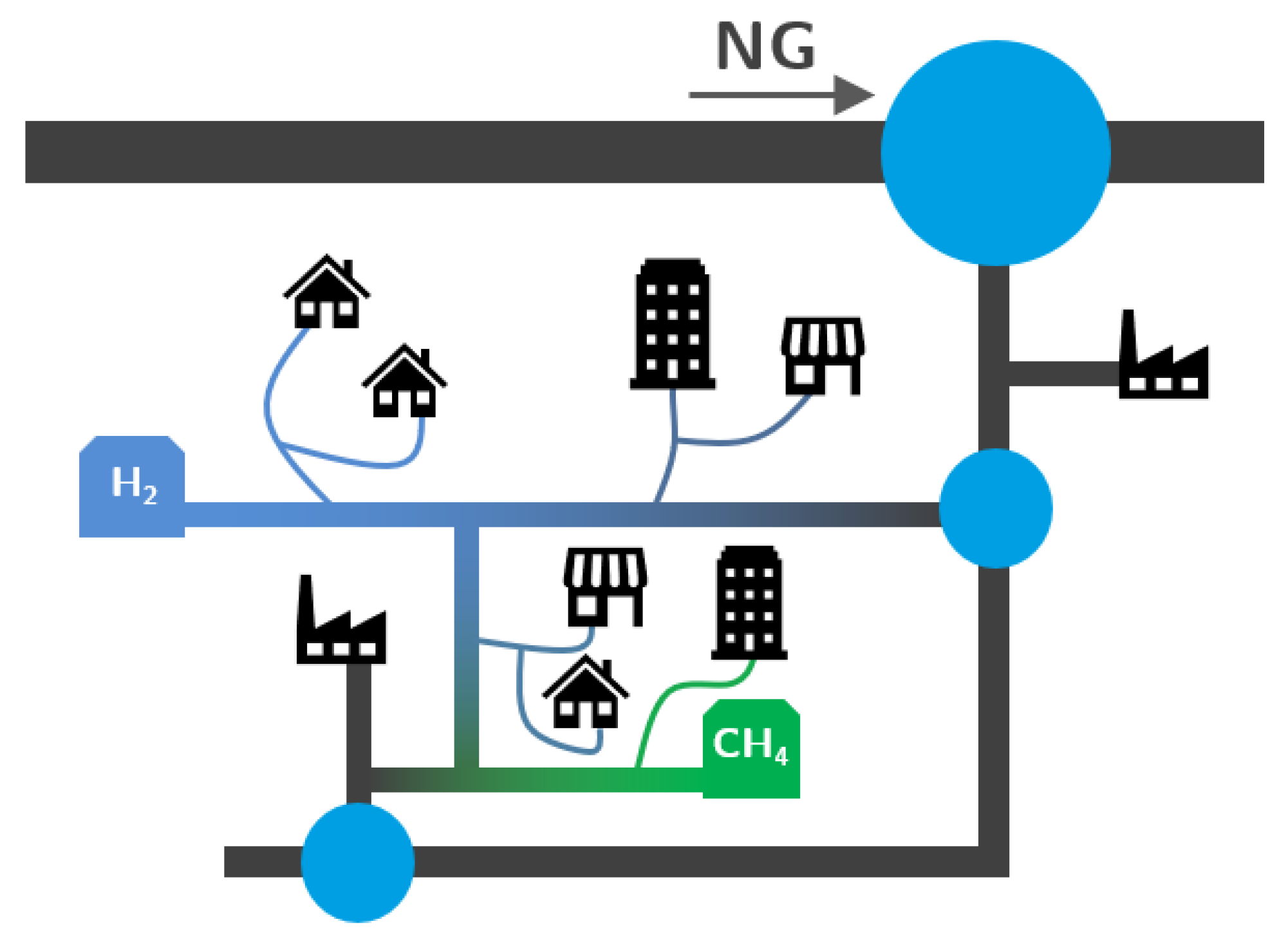
- The decentralized nature of hydrogen and biomethane production will produce a distributed injection system, with several renewable gas injection points across distribution networks. To track gas composition and determine the GCV of the gas consumed by each customer (fair billing), DSOs will require numerical methods to support network management. Such a system has already been tested by Abeysekera et al. [49] with good results.
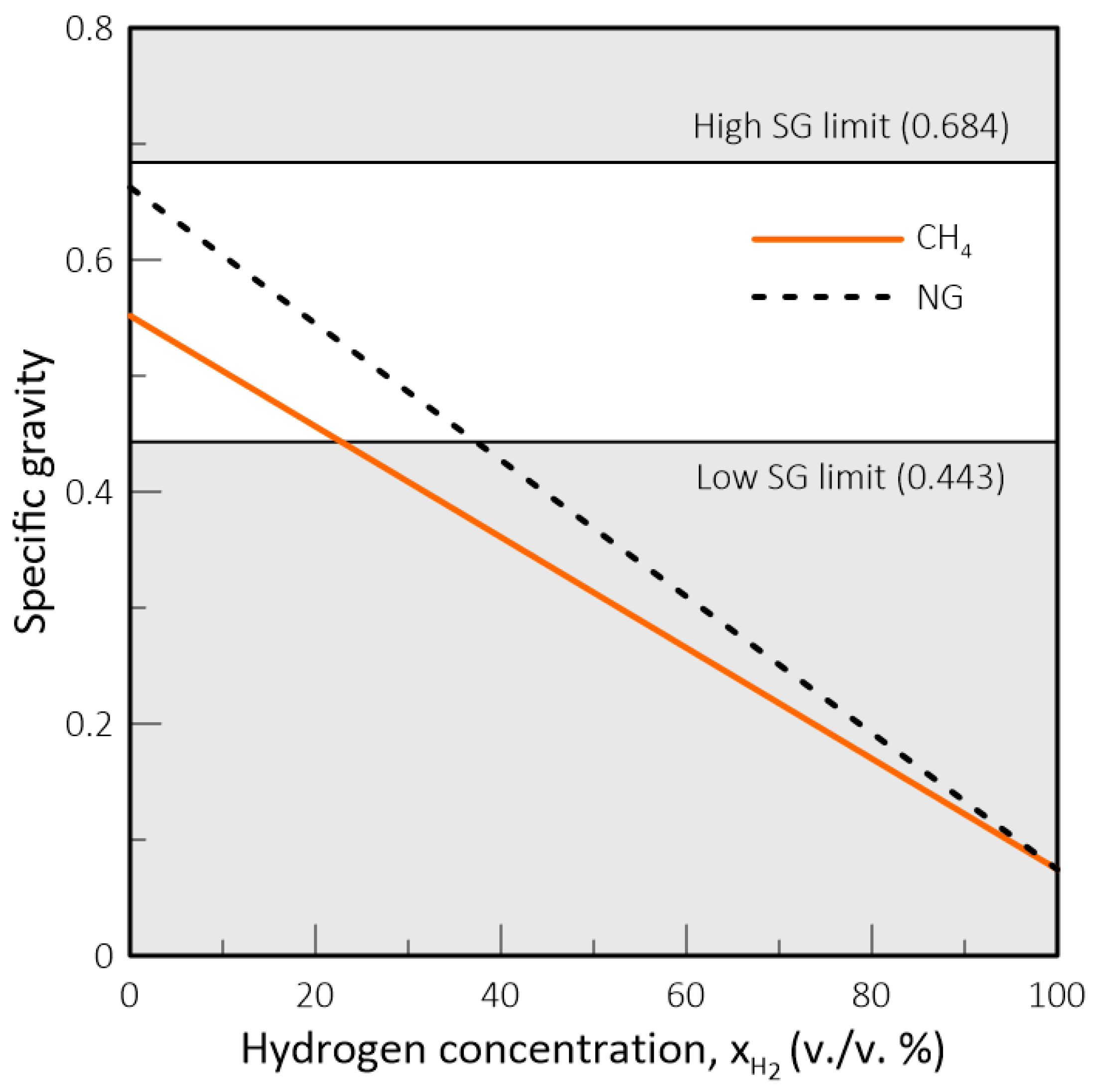
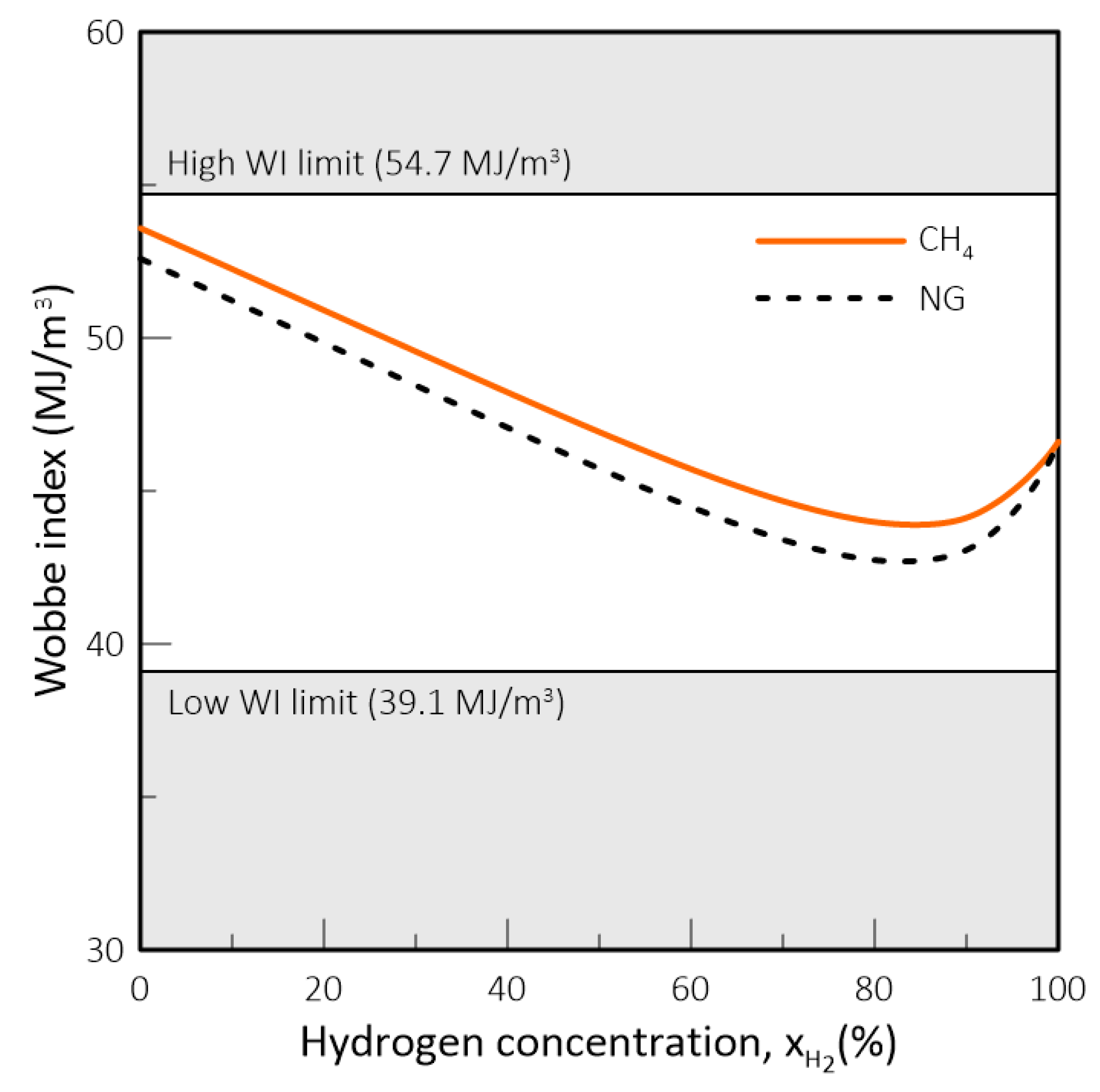
- In natural gas systems, the Wobbe Index (WI) and Specific Gravity (SG) have been widely adopted as interchangeability criteria and gas quality measurements form a regulatory standpoint. However, these criteria fail to capture the impact of hydrogen on parameters such as the methane number, critical for co-generation equipment and engines in general, and the burning velocity, important in gas equipment design and domestic appliances. Thus, in a changing market structure scenario where hydrogen and biomethane are often blended with natural gas, criteria other than WI and SG will have to be used to assess the suitability of a determined gas composition to each application.
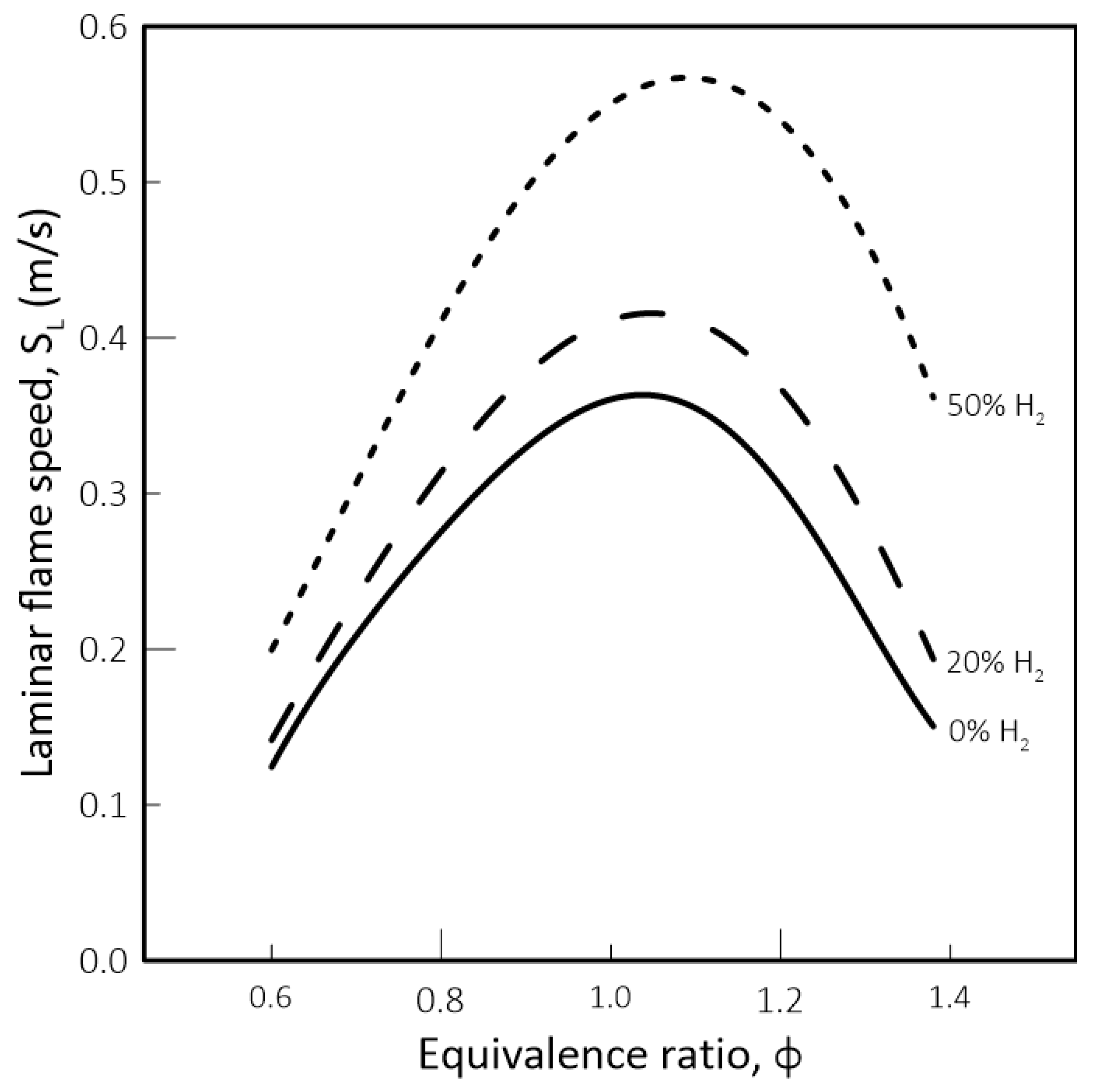
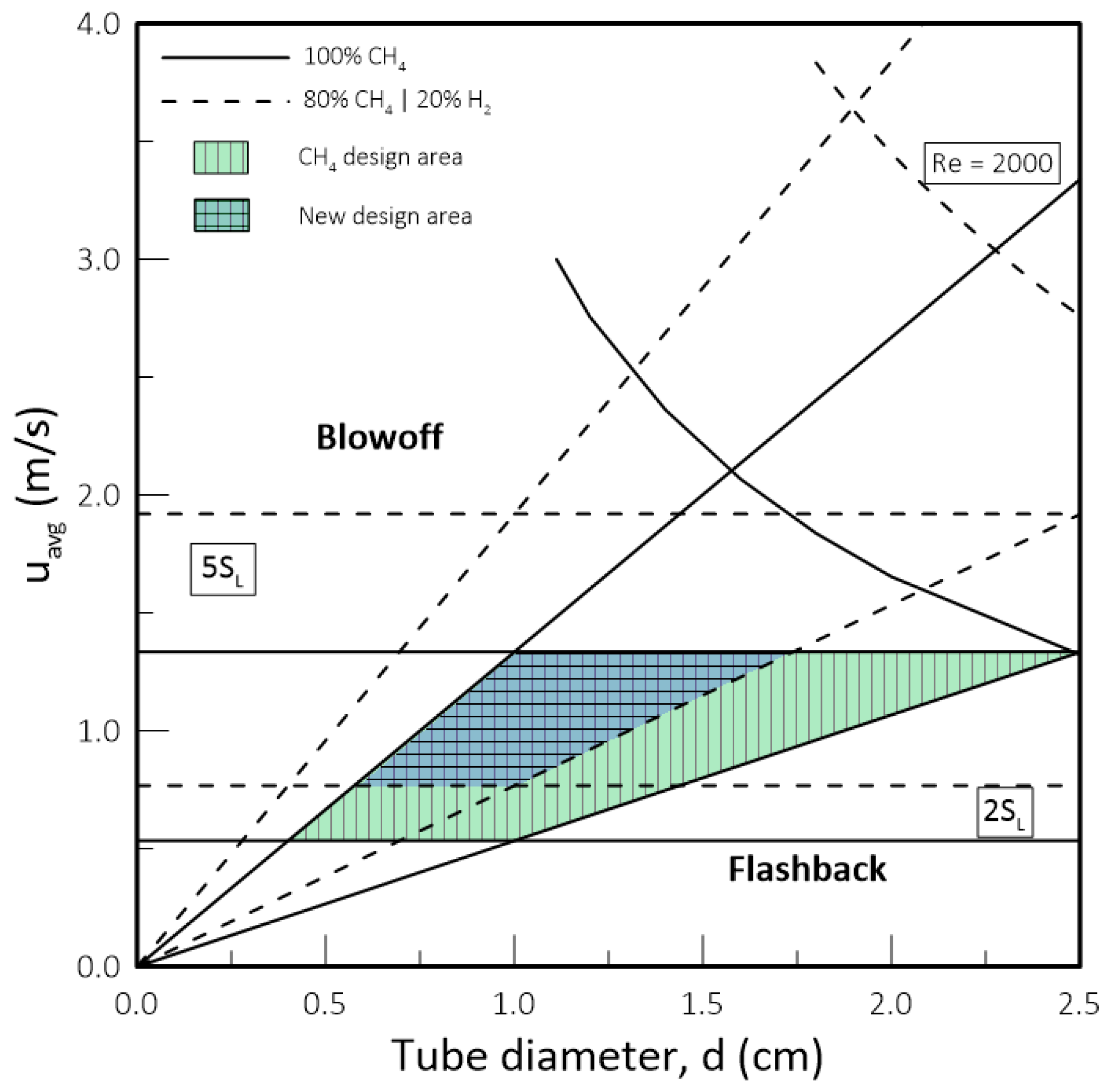
- While biomethane is interchangeable with natural gas, hydrogen is not. The interchangeability analysis performed for domestic appliances showed that the admixture of 20% of H2 by volume, often mentioned as tolerable for most applications, produces a reduction of around 30% in design area for appliances currently in operation. This result indicates that for this level of blending, some appliances may see their turnover reduced for lower power settings. Nevertheless, most domestic equipment is expected to cope well with this level of H2 content.
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1D | One dimensional |
| DSO | Distribution System Operator |
| TSO | Transmission System Operator |
| NG | Natural Gas |
| LNG | Liquefied Natural Gas |
| HHV | Higher Heating Value |
| LHV | Lower Heating Value |
| GCV | Gross Calorific Value |
| UGS | Underground Storage |
| LPG | Liquefied Petroleum Gas |
| SG | Specific Gravity |
| WI | Wobbe Index |
| MN | Methane Number |
| FPF | Freely propagating flame |
| USC | University of Southern California |
References
- European Union. Hydrogen Roadmap Europe: A Sustainable Pathway for the European Energy Transition; Technical Report; Publications Office of the European Union: Brussels, Belgium, 2019. [Google Scholar]
- IEA. World Energy Outlook—China and India Insights; Technical Report; OECD/IEA: Paris, France, 2007. [Google Scholar]
- Scott, M.; Powells, G. Blended Hydrogen: The UK Public’s Perspective; Newcastle University: Newcastle upon Tyne, UK, 2019. [Google Scholar]
- Rotunno, P.; Lanzini, A.; Leone, P. Energy and economic analysis of a water scrubbing based biogas upgrading process for biomethane injection into the gas grid or use as transportation fuel. Renew. Energy 2017, 102, 417–432. [Google Scholar] [CrossRef]
- Urban, W. Biomethane injection into natural gas networks. In The Biogas Handbook; Elsevier: Amsterdam, The Netherlands, 2013; pp. 378–403. [Google Scholar]
- Liemberger, W.; Halmschlager, D.; Miltner, M.; Harasek, M. Efficient extraction of hydrogen transported as co-stream in the natural gas grid—The importance of process design. Appl. Energy 2019, 233, 747–763. [Google Scholar] [CrossRef]
- Isaac, T. HyDeploy: The UK’s first hydrogen blending deployment project. Clean Energy 2019, 3, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Cells, F.; Undertaking, H.J. Hydrogen in Gas GridS: A Systematic Validation Approach at Various Admixture Levels into High-Pressure Grids. Available online: https://www.higgsproject.eu/ (accessed on 10 July 2021).
- Leicher, J.; Schaffert, J.; Carpentier, S.; Albus, R.; Görner, K. Impact of Hydrogen Admixture on Combustion Processes—Part I: Theory; THyGA Project: Brussels, Belgium, 2020. [Google Scholar]
- Schaffert, J.; Fischer, A.; Leicher, J.; Burmeister, F.; Flayyih, M.; Cigarida, H.; Albus, R.; Görner, K.; Milin, P.; Carpentier, S.; et al. Impact of Hydrogen Admixture on Combustion Processes—Part II: Practice; THyGA Project: Brussels, Belgium, 2020. [Google Scholar]
- Speirs, J.; Balcombe, P.; Johnson, E.; Martin, J.; Brandon, N.; Hawkes, A. A greener gas grid: What are the options. Energy Policy 2018, 118, 291–297. [Google Scholar] [CrossRef]
- IRENA. Green Hydrogen Cost Reduction: Scaling up Electrolysers to Meet the 1.5C Climate Goal; Technical Report; International Renewable Energy Agency: Abu Dhabi, United Arab Emirates, 2020. [Google Scholar]
- Gas for Climate. European Hydrogen Backbone: Analysing Future Demand, Supply, and Transport of Hydrogen; Technical Report; Guidehouse: Utrecht, The Netherlands, 2021. [Google Scholar]
- Haeseldonckx, D.; D’haeseleer, W. The use of the natural-gas pipeline infrastructure for hydrogen transport in a changing market structure. Int. J. Hydrogen Energy 2007, 32, 1381–1386. [Google Scholar] [CrossRef]
- Melaina, M.W.; Antonia, O.; Penev, M. Blending Hydrogen into Natural Gas Pipeline Networks: A Review of Key Issues; National Renewable Energy Laboratory: Golden, CO, USA, 2013. [Google Scholar]
- Altfeld, K.; Pinchbeck, D. Admissible hydrogen concentrations in natural gas systems. Gas Energy 2013, 2103, 1–2. [Google Scholar]
- Gondal, I.A. Hydrogen integration in power-to-gas networks. Int. J. Hydrogen Energy 2019, 44, 1803–1815. [Google Scholar] [CrossRef]
- Pellegrini, M.; Guzzini, A.; Saccani, C. A Preliminary Assessment of the Potential of Low Percentage Green Hydrogen Blending in the Italian Natural Gas Network. Energies 2020, 13, 5570. [Google Scholar] [CrossRef]
- Lee, S.; Speight, J.G.; Loyalka, S.K. Handbook of Alternative Fuel Technologies; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Faramawy, S.; Zaki, T.; Sakr, A.E. Natural gas origin, composition, and processing: A review. J. Nat. Gas Sci. Eng. 2016, 34, 34–54. [Google Scholar] [CrossRef]
- Gasodutos, R. Specification M-00000-SPC-MI-0002-Rev6; Technical Report; Gas Properties: Setúbal, Portugal, 2021. [Google Scholar]
- Goodwin, D.G.; Moffat, H.K.; Speth, R.L. Cantera: An Object-Oriented Software Toolkit for Chemical Kinetics, Thermodynamics, and Transport Processes; Caltech: Pasadena, CA, USA, 2009. [Google Scholar]
- Amirante, R.; Distaso, E.; Tamburrano, P.; Reitz, R.D. Laminar flame speed correlations for methane, ethane, propane and their mixtures, and natural gas and gasoline for spark-ignition engine simulations. Int. J. Engine Res. 2017, 18, 951–970. [Google Scholar] [CrossRef]
- Wang, H.; You, X.; Joshi, A.V.; Davis, S.G.; Laskin, A.; Egolfopoulos, F.; Law, C.K.; Version, U.M., II. High-Temperature Combustion Reaction Model of H2; Technical Report; CO/C1-C4 Compounds. 2007. Available online: http://ignis.usc.edu/USC_Mech_II.htm (accessed on 10 July 2021).
- Zhang, Y.; Jiang, X.; Wei, L.; Zhang, J.; Tang, C.; Huang, Z. Experimental and modeling study on auto-ignition characteristics of methane/hydrogen blends under engine relevant pressure. Int. J. Hydrogen Energy 2012, 37, 19168–19176. [Google Scholar] [CrossRef]
- Quintino, F.; Fernandes, E. Analytical correlation to model diluent concentration repercussions on the burning velocity of biogas lean flames: Effect of CO2 and N2. Biomass Bioenergy 2018, 119, 354–363. [Google Scholar] [CrossRef]
- Quintino, F.M.; Fernandes, E.C. Numerical Investigation of the Impact of H2 Enrichment on Lean Biogas/Air Flames: An Analytical Modelling Approach. Energies 2021, 14, 369. [Google Scholar] [CrossRef]
- Mohitpour, M.; Solanky, H.; Vinjamuri, G.K. Materials selection and performance criteria for hydrogen pipeline transmission. In Proceedings of the ASME/JSME 2004 Pressure Vessels and Piping Conference, San Diego, CA, USA, 25–29 July 2004; pp. 241–251. [Google Scholar]
- Moradi, R.; Groth, K.M. Hydrogen storage and delivery: Review of the state of the art technologies and risk and reliability analysis. Int. J. Hydrogen Energy 2019, 44, 12254–12269. [Google Scholar] [CrossRef]
- Vanderoost, T.; Vannoppen, G. Ontwerp van een Roadmap Voor de Omschakeling van een Aardgaseconomie Naar een Waterstofeconomie: Production, Transport, Stockage, Eindconversie en Veiligheidsaspecten van Waterstof. Master’s Thesis, KU Leuven, Department of Applied Thermodynamics and Energy, Leuven, Belgium, 2004. [Google Scholar]
- Kölle, W.; Strebel, O.; Böttcher, J. Reduced sulphur compounds in sandy aquifers and their interactions with groundwater. In Groundwater Monitoring and Management; IAHS-AISH Publication: Wallingford, UK, 1990; pp. 23–30. [Google Scholar]
- Ozarslan, A. Large-scale hydrogen energy storage in salt caverns. Int. J. Hydrogen Energy 2012, 37, 14265–14277. [Google Scholar] [CrossRef]
- Preuster, P.; Alekseev, A.; Wasserscheid, P. Hydrogen storage technologies for future energy systems. Annu. Rev. Chem. Biomol. Eng. 2017, 8, 445–471. [Google Scholar] [CrossRef]
- Gillette, J.; Kolpa, R. Overview of Interstate Hydrogen Pipeline Systems; Technical Report; Argonne National Lab. (ANL): Argonne, IL, USA, 2008. [Google Scholar]
- Lemieux, A.; Sharp, K.; Shkarupin, A. Preliminary assessment of underground hydrogen storage sites in Ontario, Canada. Int. J. Hydrogen Energy 2019, 44, 15193–15204. [Google Scholar] [CrossRef]
- Hemme, C.; Van Berk, W. Hydrogeochemical modeling to identify potential risks of underground hydrogen storage in depleted gas fields. Appl. Sci. 2018, 8, 2282. [Google Scholar] [CrossRef] [Green Version]
- Taamallah, S.; Vogiatzaki, K.; Alzahrani, F.M.; Mokheimer, E.M.; Habib, M.; Ghoniem, A.F. Fuel flexibility, stability and emissions in premixed hydrogen-rich gas turbine combustion: Technology, fundamentals, and numerical simulations. Appl. Energy 2015, 154, 1020–1047. [Google Scholar] [CrossRef]
- Ando, Y.; Oozawa, H.; Mihara, M.; Irie, H.; Urashita, Y.; Ikegami, T. Demonstration of SOFC-micro gas turbine (MGT) hybrid systems for commercialization. Mitsubishi Heavy Ind. Tech. Rev. 2015, 52, 47–52. [Google Scholar]
- Gondal, I. Hydrogen transportation by pipelines. In Compendium of Hydrogen Energy; Elsevier: Amsterdam, The Netherlands, 2016; pp. 301–322. [Google Scholar]
- Alban, T. Blending Hydrogen into Existing Gas Grid: Opportunities and Challenges for Pipeline e-Motor Compressor System Design. In Proceedings of the Pipeline Technology Conference 2020, Berlin, Germany, 30 March–2 April 2020. [Google Scholar]
- Scholten, F.L.; Wolters, M. Hydrogen diffusion through plastic pipes. In Proceedings of the 17th International Plastic Fuel-Gas Pipeline Symposium, San Francisco, CA, USA, 20–23 October 2002; pp. 280–284. [Google Scholar]
- Miyake, H.; Matsuyama, M.; Ashida, K.; Watanabe, K. Permeation, diffusion, and solution of hydrogen isotopes, methane, and inert gases in/through tetrafluoroethylene and polyethylene. J. Vac. Sci. Technol. A Vac. Surf. Film 1983, 1, 1447–1451. [Google Scholar] [CrossRef]
- Sadler, D.; Cargill, A.; Crowther, M.; Rennie, A.; Watt, J.; Burton, S.; Haines, M. H21 Leeds City Gate; Northern Gas Networks: Leeds, UK, 2016. [Google Scholar]
- Jaworski, J.; Kułaga, P.; Blacharski, T. Study of the effect of addition of hydrogen to natural gas on diaphragm gas meters. Energies 2020, 13, 3006. [Google Scholar] [CrossRef]
- Ouyang, L.B.; Aziz, K. Steady-state gas flow in pipes. J. Pet. Sci. Eng. 1996, 14, 137–158. [Google Scholar] [CrossRef]
- Schouten, J.; Michels, J.; Janssen-van Rosmalen, R. Effect of H2-injection on the thermodynamic and transportation properties of natural gas. Int. J. Hydrogen Energy 2004, 29, 1173–1180. [Google Scholar] [CrossRef]
- Abd, A.A.; Naji, S.Z.; Thian, T.C.; Othman, M.R. Evaluation of hydrogen concentration effect on the natural gas properties and flow performance. Int. J. Hydrogen Energy 2021, 46, 974–983. [Google Scholar] [CrossRef]
- Ogden, J.; Jaffe, A.M.; Scheitrum, D.; McDonald, Z.; Miller, M. Natural gas as a bridge to hydrogen transportation fuel: Insights from the literature. Energy Policy 2018, 115, 317–329. [Google Scholar] [CrossRef]
- Abeysekera, M.; Wu, J.; Jenkins, N.; Rees, M. Steady state analysis of gas networks with distributed injection of alternative gas. Appl. Energy 2016, 164, 991–1002. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Gao, W.; Yu, Y.; Wang, M.; Chen, C. Primary air ratio change and gas interchangeability index correct for domestic gas cooker burning multi-source natural gases. J. Nat. Gas Sci. Eng. 2016, 35, 276–282. [Google Scholar] [CrossRef]
- Klimstra, J. Interchangeability of Gaseous Fuels—The Importance of the Wobbe-Index; SAE Transactions: New York, NY, USA, 1986; pp. 962–972. [Google Scholar]
- European Committee for Standardization. EN 437:2003+A1, Test Gases–Test Pressures–Appliance Categories; Technical Report; European Union: Brussels, Belgium, 2009. [Google Scholar]
- Che, D.; Liu, Y.; Gao, C. Evaluation of retrofitting a conventional natural gas fired boiler into a condensing boiler. Energy Convers. Manag. 2004, 45, 3251–3266. [Google Scholar] [CrossRef]
- Durbin, D.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Sofianopoulos, A.; Assanis, D.; Mamalis, S. Effects of hydrogen addition on automotive lean-burn natural gas engines: Critical review. J. Energy Eng. 2016, 142, E4015010. [Google Scholar] [CrossRef]
- Park, C.; Kim, C.; Choi, Y. Power output characteristics of hydrogen-natural gas blend fuel engine at different compression ratios. Int. J. Hydrogen Energy 2012, 37, 8681–8687. [Google Scholar] [CrossRef]
- Gondal, I.A. Infrastructure Analysis for Renewable Hydrogen Production in Pakistan; Technical Report; Department of Mechanical Engineering, Faculty of Mechanical and Aeronautical Engineering, University of Engineering and Technology: Taxila, Pakistan, 2012. [Google Scholar]
- Turns, S.R. Introduction to Combustion; McGraw-Hill Companies: New York, NY, USA, 1996; Volume 287. [Google Scholar]
- Jones, H.R.N. The Application of Combustion Principles to Domestic Gas Burner Design; Taylor & Francis: Abingdon, UK, 1989. [Google Scholar]
- Lewis, B.; Von Elbe, G. Combustion, Flames and Explosions of Gases; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Glassman, I.; Yetter, R.A.; Glumac, N.G. Combustion; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- De Vries, H.; Mokhov, A.V.; Levinsky, H.B. The impact of natural gas/hydrogen mixtures on the performance of end-use equipment: Interchangeability analysis for domestic appliances. Appl. Energy 2017, 208, 1007–1019. [Google Scholar] [CrossRef]
- Hindasageri, V.; Vedula, R.P.; Prabhu, S.V. Blowoff Stability of Methane-Air Premixed Flame on Tube Burners. Int. J. Emerg. Multidiscip. Fluid Sci. 2011, 3, 209–225. [Google Scholar] [CrossRef]
| Property | CH4 | NG | H2 |
|---|---|---|---|
| M (kg/kmol) | 16.043 | 17.919 | 2.016 |
| (kg/m3) | 0.668 | 0.802 | 0.0899 |
| d (adm) | 0.554 | 0.620 | 0.075 |
| LdV (adm) | 9.52 | 10.23 | 2.38 |
| HHV (MJ/m3) | 39.8 | 42.8 | 12.7 |
| LHV (MJ/m3) | 35.8 | 38.8 | 10.8 |
| WI (MJ/m3) | 53.5 | 54.4 | 46.4 |
| Fuel Blend | |
|---|---|
| 100% CH4 | 1.25 |
| 80% CH4/20% H2 | 1.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quintino, F.M.; Nascimento, N.; Fernandes, E.C. Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment. Hydrogen 2021, 2, 301-318. https://doi.org/10.3390/hydrogen2030016
Quintino FM, Nascimento N, Fernandes EC. Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment. Hydrogen. 2021; 2(3):301-318. https://doi.org/10.3390/hydrogen2030016
Chicago/Turabian StyleQuintino, Filipe M., Nuno Nascimento, and Edgar C. Fernandes. 2021. "Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment" Hydrogen 2, no. 3: 301-318. https://doi.org/10.3390/hydrogen2030016
APA StyleQuintino, F. M., Nascimento, N., & Fernandes, E. C. (2021). Aspects of Hydrogen and Biomethane Introduction in Natural Gas Infrastructure and Equipment. Hydrogen, 2(3), 301-318. https://doi.org/10.3390/hydrogen2030016








