The Synergistic Effects of Alloying on the Performance and Stability of Co3Mo and Co7Mo6 for the Electrocatalytic Hydrogen Evolution Reaction
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. Materials Characterisation
2.3. Electrochemical Characterisation
2.4. Gas Chromatography Measurements
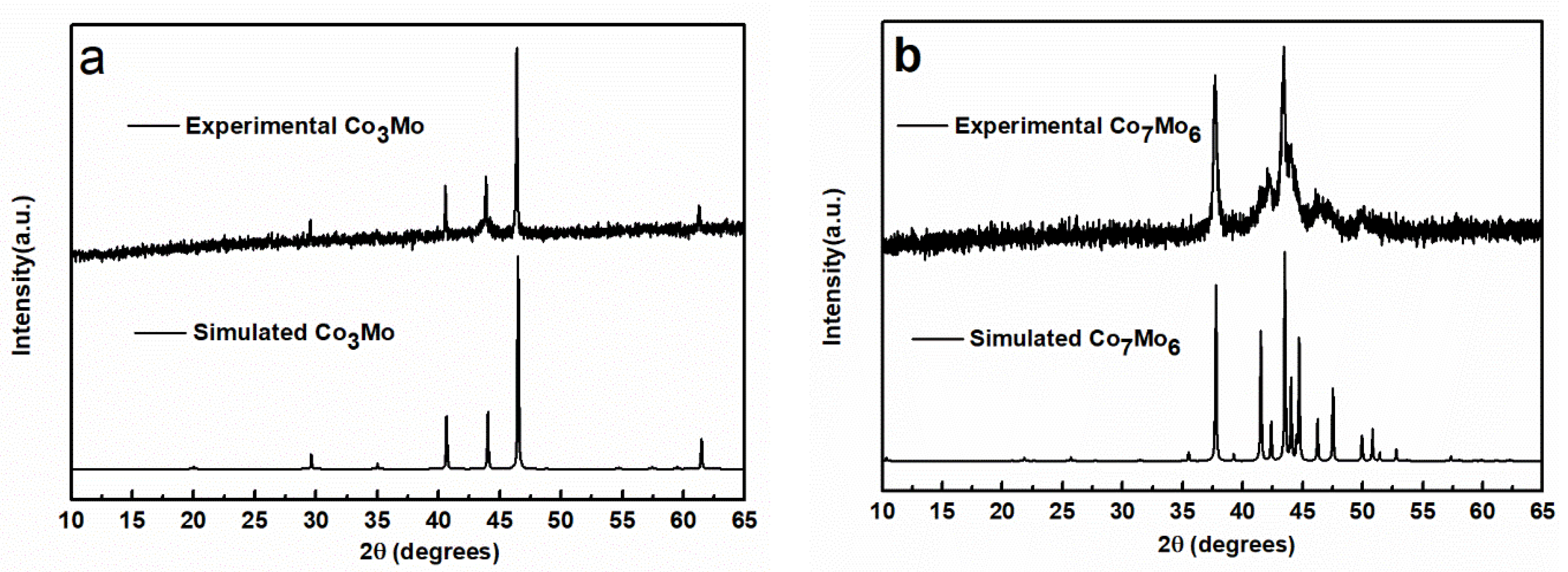
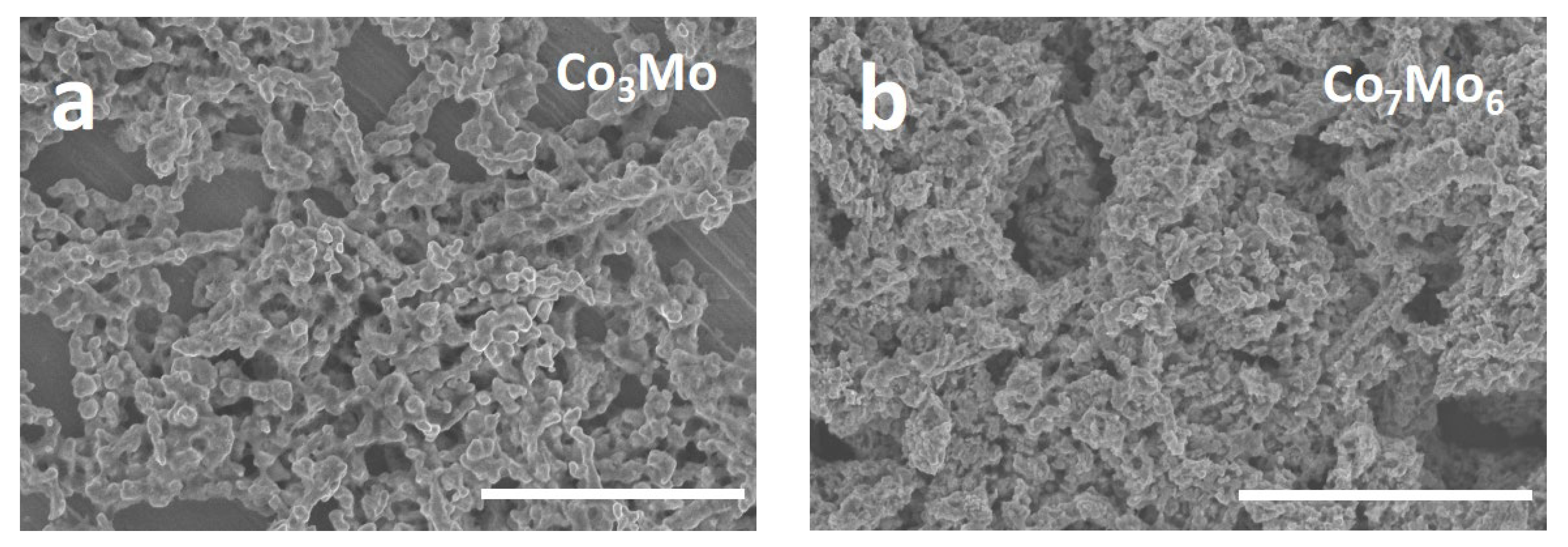
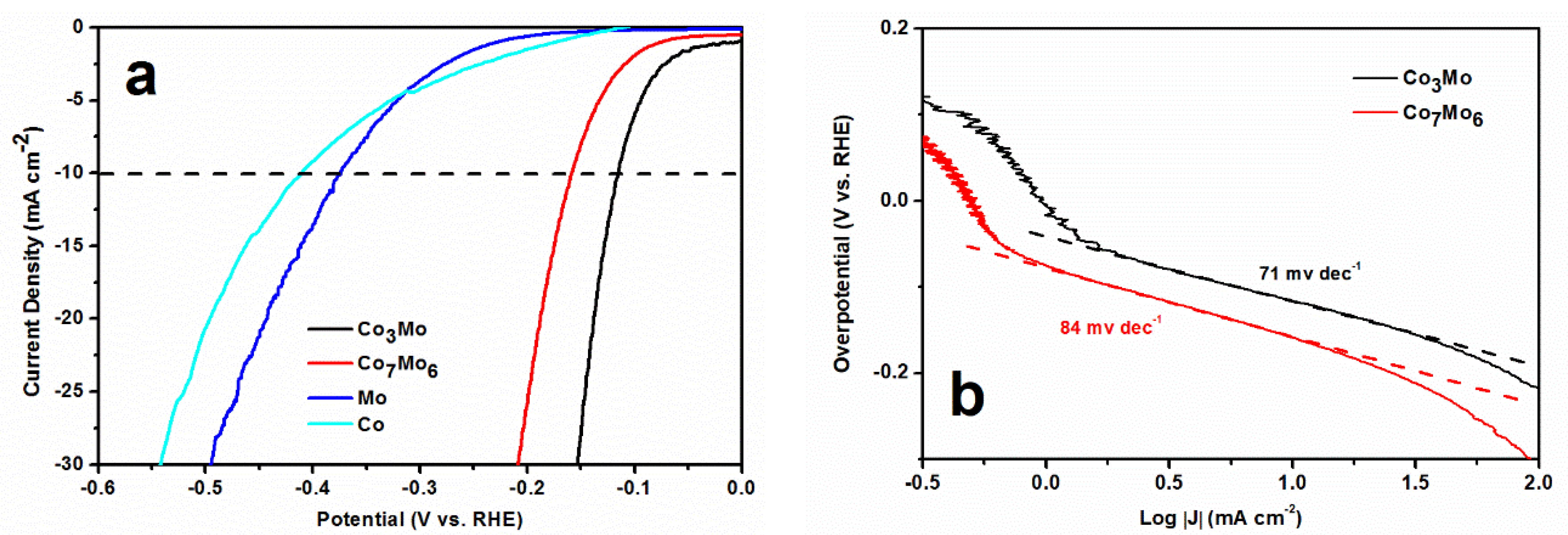
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- IEA Global Energy Review 2020; IEA: Paris, France, 2020; Available online: https://www.iea.org/reports/global-energy-review-2020 (accessed on 26 August 2020).
- IEA Monthly Electricity Statistics; IEA: Paris, France, 2020; Available online: https://www.iea.org/reports/monthly-electricity-statistics (accessed on 25 September 2020).
- Fan, X.; Liu, B.; Liu, J.; Ding, J.; Han, X.; Deng, Y.; Lv, X.; Xie, Y.; Chen, B.; Hu, W.; et al. Battery Technologies for Grid-Level Large-Scale Electrical Energy Storage. Trans. Tianjin Univ. 2020, 26, 92–103. [Google Scholar] [CrossRef] [Green Version]
- IEA The Future of Hydrogen; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 26 August 2020).
- Chehade, Z.; Mansilla, C.; Lucchese, P.; Hilliard, S.; Proost, J. Review and analysis of demonstration projects on power-to-X pathways in the world. Int. J. Hydrogen Energy 2019, 44, 27637–27655. [Google Scholar] [CrossRef]
- Baroutaji, A.; Wilberforce, T.; Ramadan, M.; Olabi, A.G. Comprehensive Investigation on Hydrogen and Fuel Cell Technology in the Aviation and Aerospace Sectors. Renew. Sustain. Energy Rev. 2019, 106, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Buttler, A. Current status of water electrolysis for energy storage, grid balancing and sector coupling via power-to-gas and power-to-liquids: A review. Renew. Sustain. Energy Rev. 2018, 82, 2440–2454. [Google Scholar] [CrossRef]
- Roger, I.; Shipman, M.A.; Symes, M.D. Earth-abundant catalysts for electrochemical and photoelectrochemical water splitting. Nat. Rev. Chem. 2017, 1, 1–13. [Google Scholar] [CrossRef]
- Sequeira, C.A.C.; Cardoso, D.S.P.; Amaral, L.; Šljukić, B.; Santos, D.M.F. On the performance of commercially available corrosion-resistant nickel alloys: A review. Corros. Rev. 2016, 34, 187–200. [Google Scholar] [CrossRef]
- Keçebaş, A.; Kayfeci, M.; Bayat, M. Electrochemical hydrogen generation. In Solar Hydrogen Production: Processes, Systems and Technologies; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–317. [Google Scholar] [CrossRef]
- Kongkanand, A.; Mathias, M.F. The Priority and Challenge of High-Power Performance of Low-Platinum Proton-Exchange Membrane Fuel Cells. J. Phys. Chem. Lett. 2016, 7, 1127–1137. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.G.; Martinez, J.M.P.; Carter, E.A. Why Do We Use the Materials and Operating Conditions We Use for Heterogeneous (Photo)Electrochemical Water Splitting? ACS Catal. 2020, 10, 11177–11234. [Google Scholar] [CrossRef]
- Jaksic, M.M. Hypo-Hyper-d-Electronic Interactive Nature of Interionic Synergism in Catalysis and Electrocatalysis for Hydrogen Reactions. Int. J. Hydrogen Energy 2001, 26, 559. [Google Scholar] [CrossRef]
- Rößner, L.; Armbrüster, M. Electrochemical Energy Conversion on Intermetallic Compounds: A Review. ACS Catal. 2019, 9, 2018–2062. [Google Scholar] [CrossRef]
- Schalenbach, M.; Zeradjanin, A.R.; Kasian, O.; Cherevko, S.; Mayrhofer, K.J.J. A Perspective on Low-Temperature Water Electrolysis –Challenges in Alkaline and Acidic Technology. Int. J. Electrochem. Sci. 2018, 13, 1173–1226. [Google Scholar] [CrossRef]
- Highfield, J.G.; Claude, E.; Oguro, K. Electrocatalytic Synergism in Ni/Mo Cathodes for Hydrogen Evolution in Acid Medium: A New Model. Electrochim. Acta 1999, 44, 2805. [Google Scholar] [CrossRef]
- Jin, D.; Yu, A.; Lee, Y.; Kim, M.H.; Lee, C. NixRh1−x bimetallic alloy nanofibers as a pH-universal electrocatalyst for the hydrogen evolution reaction: The synthetic strategy and fascinating electroactivity. J. Mater. Chem. A 2020, 8, 8629–8637. [Google Scholar] [CrossRef]
- Nsanzimana, J.M.V.; Peng, Y.; Miao, M.; Reddu, V.; Zhang, W.; Wang, H.; Xia, B.Y.; Wang, X. An Earth-Abundant Tungsten–Nickel Alloy Electrocatalyst for Superior Hydrogen Evolution. ACS Appl. Nano Mater. 2018, 1, 1228–1235. [Google Scholar] [CrossRef]
- Schalenbach, M.; Speck, F.D.; Ledendecker, M.; Kasiana, O.; Goehl, D.; Mingers, A.M.; Breitbach, B.; Springer, H.; Cherevko, S.; Mayrhofer, K.J.J. Nickel-molybdenum alloy catalysts for the hydrogen evolution reaction: Activity and stability revised. Electrochimica Acta 2018, 259, 1154–1161. [Google Scholar] [CrossRef]
- Chen, J.; Ge, Y.; Feng, Q.; Zhuang, P.; Chu, H.; Cao, Y.; Smith, W.R.; Dong, P.; Ye, M.; Shen, J. Nesting Co3Mo Binary Alloy Nanoparticles onto Molybdenum Oxide Nanosheet Arrays for Superior Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 9002. [Google Scholar] [CrossRef]
- Shi, H.; Zhou, Y.T.; Yao, R.Q.; Wan, W.B.; Ge, X.; Zhang, W.; Wen, Z.; Lang, X.Y.; Zheng, W.T.; Jiang, Q. Spontaneously Separated Intermetallic Co3Mo from Nanoporous Copper as Versatile Electrocatalysts for Highly Efficient Water Splitting. Nat. Commun. 2020, 11, 1. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, P.A. Wood, Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
- McGlynn, J.C.; Friskey, M.; Ganin, A.Y. Parameter Optimisation for Electrochemically Activated MoTe2. Sustain. Energy Fuels 2020, 4, 4473–4477. [Google Scholar] [CrossRef]
- Raydt, U.; Tammann, G. Alloys of molybdenum and cobalt. Z. Anorg. Allg. Chem. 1914, 83, 246–252. [Google Scholar] [CrossRef]
- Takei, T. Equilibrium diagram of the cobalt-molybdenum system. J. Study Metals 1928, 5, 364–376. [Google Scholar]
- Sykes, W.P.; Graff, H.F. The cobalt-molybdenum system. T. Am. Soc. Metal. 1935, 23, 249–283. [Google Scholar]
- Hashimoto, U. Effect of additions to cobalt on its allotropic transformation. Nippon Kinzoku Gakkaishi 1937, 1, 177–189. [Google Scholar]
- Forsyth, J.B.; D’Alte da Veiga, L.M. Refinement of the structure of the phase Co3Mo. Acta Cryst. 1965, 18, 855–857. [Google Scholar]
- D’Alte da Veiga, L.M. The structure of the μ-Co7Mo6. Acta. Cryst. 1962, 15, 543–546. [Google Scholar]
- Sort, J.; Suriñach, S.; Muñoz, S.; Baró, D.; Wojcik, M.; Jedryka, E.; Nadolski, S.; Sheludko, N.; Nogués, J. Role of Stacking Faults in the Structural and Magnetic Properties of Ball-Milled Cobalt. Phys. Rev. B Condens. Matter Mater. Phys. 2003, 68, 014421. [Google Scholar] [CrossRef]
- Hiraga, K.; Yamamoto, T.; Hirabayashi, M. Intermetallic compounds of the μ- and P-phases of Co7Mo6 studied by 1 MV Electron Microscopy. Trans. Jpn. Inst. Met. 1983, 24, 421–428. [Google Scholar] [CrossRef] [Green Version]
- Vie, D.; Valero, N.; Martínez, E.; Sapiña, F.; Folgado, J.-V.A. Beltrána A new approach to the synthesis of intermetallic compounds: Mild synthesis of submicrometric CoxMy (M = Mo, W; x:Y = 3:1 and 7:6) particles by direct reduction of freeze-dried precursors. J. Mat. Chem. 2002, 12, 1017–1021. [Google Scholar] [CrossRef]
- Lasia, A. Mechanism and kinetics of the hydrogen evolution reaction. Int. J. Hydrogen Energy 2019, 36, 19484–19518. [Google Scholar] [CrossRef]
- Li, D.; Batchelor-McAuley, C.; Compton, R.G. Some thoughts about reporting the electrocatalytic performance of nanomaterials. Appl. Mater. Today 2020, 18, 100404. [Google Scholar] [CrossRef]
- Csernica, P.M.; McKone, J.R.; Mulzer, C.R.; Dichtel, W.R.; Abruña, H.D.; DiSalvo, F.J. Electrochemical Hydrogen Evolution at Ordered Mo7Ni7. ACS Catal. 2017, 7, 3375–3383. [Google Scholar] [CrossRef]
- Voiry, D.; Chhowalla, M.; Gogotsi, Y.; Kotov, N.A.; Li, Y.; Penner, R.M.; Schaak, R.E.; Weiss, P.S. Best Practices for Reporting Electrocatalytic Performance of Nanomaterials. ACS Nano 2018, 12, 9635–9638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGlynn, J.C.; Dankwort, T.; Kienle, L.; Bandeira, N.A.G.; Fraser, J.P.; Gibson, E.K.; Cascallana-Matías, I.; Kamarás, K.; Symes, M.D.; Miras, H.N.; et al. The Rapid Electrochemical Activation of MoTe2 for the Hydrogen Evolution Reaction. Nat. Commun. 2019, 10, 4916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benck, J.D.; Hellstern, T.R.; Kibsgaard, J.; Chakthranont, P.; Jaramillo, T.F. Catalyzing the hydrogen evolution reaction (HER) with molybdenum sulfide nanomaterials. ACS Catal. 2014, 4, 3957–3971. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real surface area measurements in electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Soares, D.M. Hydride Effect on the Kinetics of the Hydrogen Evolution Reaction on Nickel Cathodes in Alkaline Media. J. Electrochem. Soc. 1992, 139, 98–105. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Ge, X.; Wang, L.; Liu, J.; Wang, Y.; Feng, L. A Free Standing Porous Co/Mo Architecture as a Robust Bifunctional Catalyst toward Water Splitting. RSC Adv. 2017, 7, 11568. [Google Scholar] [CrossRef] [Green Version]

| Co3Mo | Co7Mo6 | |||
|---|---|---|---|---|
| Co | Mo | Co | Mo | |
| at. % Exp. | 73.57 ± 1.79 | 28.5 ± 2.1 | 50.55 ± 0.82 | 49.5 ± 0.82 |
| at. % Theory | 75 | 25 | 53.8 | 46.2 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Ganin, A.Y. The Synergistic Effects of Alloying on the Performance and Stability of Co3Mo and Co7Mo6 for the Electrocatalytic Hydrogen Evolution Reaction. Hydrogen 2020, 1, 11-21. https://doi.org/10.3390/hydrogen1010002
Sun Y, Ganin AY. The Synergistic Effects of Alloying on the Performance and Stability of Co3Mo and Co7Mo6 for the Electrocatalytic Hydrogen Evolution Reaction. Hydrogen. 2020; 1(1):11-21. https://doi.org/10.3390/hydrogen1010002
Chicago/Turabian StyleSun, Youyi, and Alexey Y. Ganin. 2020. "The Synergistic Effects of Alloying on the Performance and Stability of Co3Mo and Co7Mo6 for the Electrocatalytic Hydrogen Evolution Reaction" Hydrogen 1, no. 1: 11-21. https://doi.org/10.3390/hydrogen1010002
APA StyleSun, Y., & Ganin, A. Y. (2020). The Synergistic Effects of Alloying on the Performance and Stability of Co3Mo and Co7Mo6 for the Electrocatalytic Hydrogen Evolution Reaction. Hydrogen, 1(1), 11-21. https://doi.org/10.3390/hydrogen1010002






