Influence of Rhizosphere Dynamics and Soil Chemical Properties in Arid Environments on the Distribution, Abundance, and Diversity of Arbuscular Mycorrhizal Fungi (AMF)
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling Collection Strategies
2.2. Extraction of AMF Spores
2.3. Measurement AMF Colonization Ratio
2.4. Soil Physicochemical Characteristics
2.5. Statistical Analyses
3. Results and Discussion
3.1. Rhizosphere Soil Nutrients
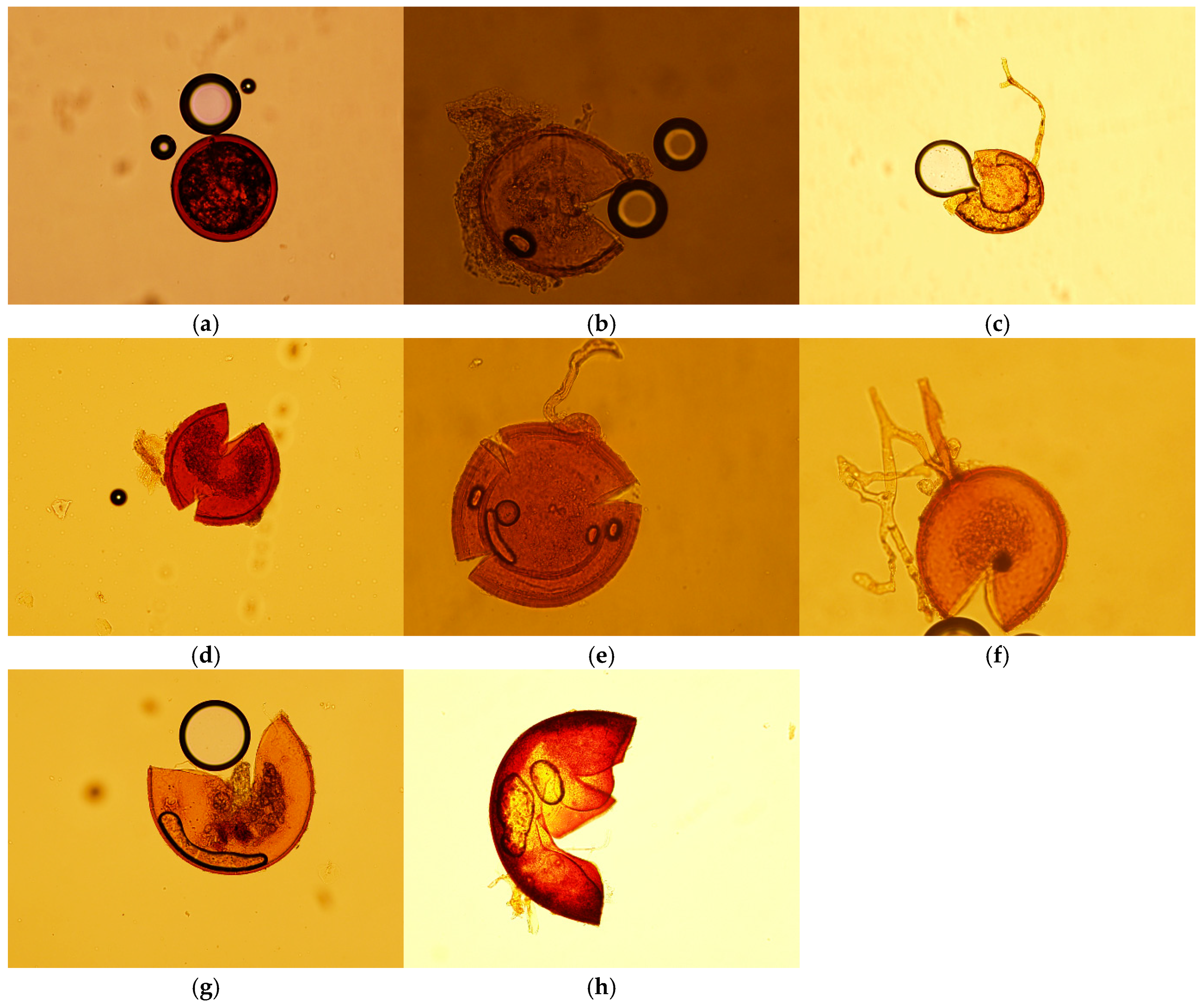
3.2. AMF Spore Count and Community Diversity
3.3. AMF Spore Diversity
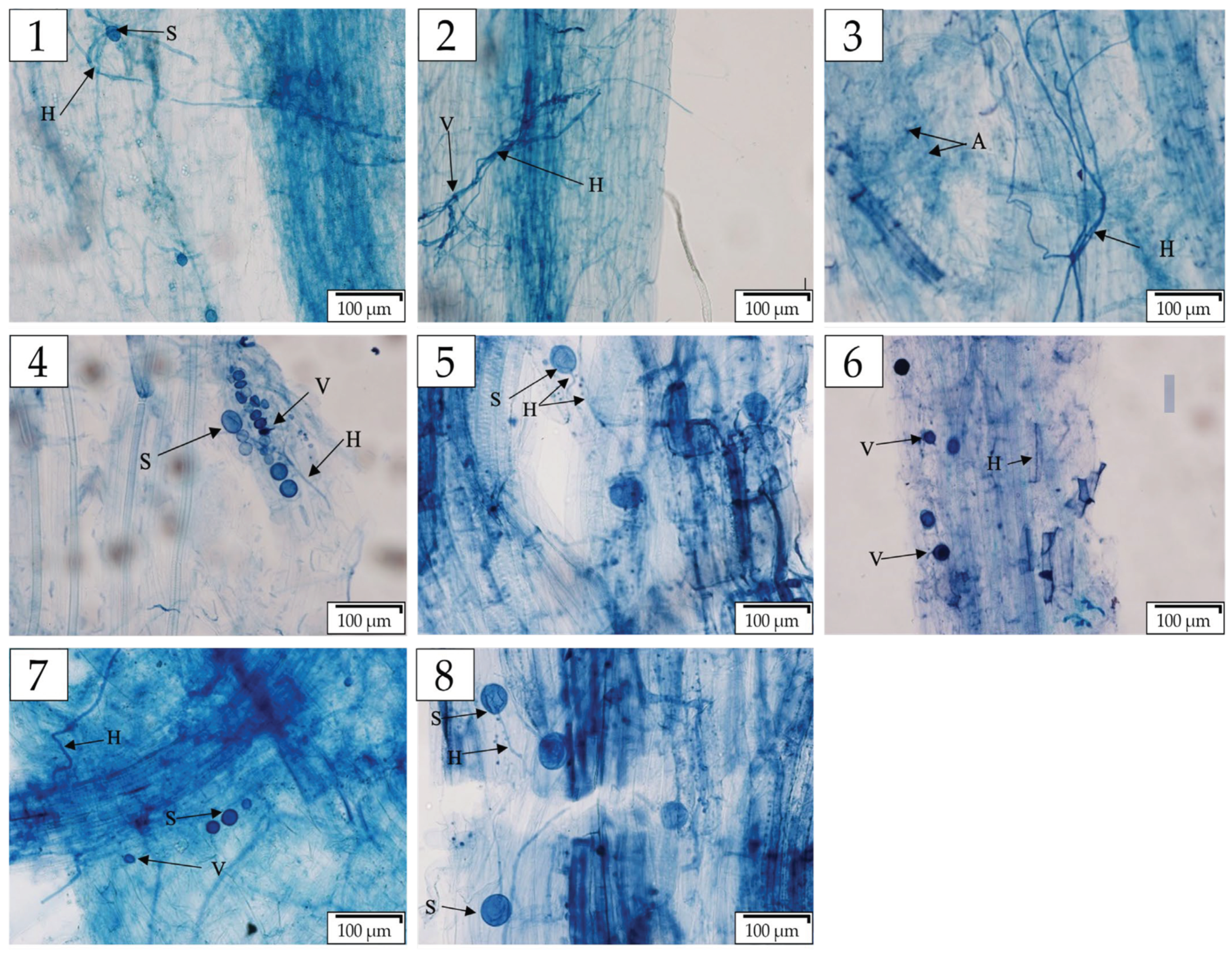
3.4. AMF Structure Colonization Percentage % (Mycelium, Vesicles, and Arbuscules)
3.5. Vesicles and Arbuscules
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, S.E.; Read, D. Mycorrhizal Symbiosis, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2008; ISBN 9780123705266. [Google Scholar]
- Brundrett, M.C.; Tedersoo, L. Evolutionary History of Mycorrhizal Symbioses and Global Host Plant Diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C. Arbuscular Mycorrhizae and Terrestrial Ecosystem Processes. Ecol. Lett. 2004, 7, 740–754. [Google Scholar] [CrossRef]
- Powell, J.R.; Rillig, M.C. Biodiversity of Arbuscular Mycorrhizal Fungi and Ecosystem Function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef]
- Smith, S.E.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Nutrition and Growth: New Paradigms from Cellular to Ecosystem Scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Bohrer, K.E.; Friese, C.F.; Amon, J.P. Seasonal Dynamics of Arbuscular Mycorrhizal Fungi in Differing Wetland Habitats. Mycorrhiza 2004, 14, 329–337. [Google Scholar] [CrossRef]
- Cuenca, G.; Lovera, M. Seasonal Variation and Distribution at Different Soil Depths of Arbuscular Mycorrhizal Fungi Spores in a Tropical Sclerophyllous Shrubland. Botany 2010, 88, 54–64. [Google Scholar] [CrossRef]
- Anderson, E.L.; Millner, P.D.; Kunishi, H.M. Maize Root Length Density and Mycorrhizal Infection as Influenced by Tillage and Soil Phosphorus. J. Plant Nutr. 1987, 10, 1349–1356. [Google Scholar] [CrossRef]
- Brundrett, M.C. Mycorrhizal Associations and Other Means of Nutrition of Vascular Plants: Understanding the Global Diversity of Host Plants by Resolving Conflicting Information and Developing Reliable Means of Diagnosis. Plant Soil 2009, 320, 37–77. [Google Scholar] [CrossRef]
- Yang, F.Y.; Li, G.Z.; Zhang, D.E.; Christie, P.; Li, X.L.; Gai, J.P. Geographical and Plant Genotype Effects on the Formation of Arbuscular Mycorrhiza in Avena Sativa and Avena Nuda at Different Soil Depths. Biol. Fertil. Soils 2010, 46, 435–443. [Google Scholar] [CrossRef]
- Stürmer, S.L.; Siqueira, J.O. Species Richness and Spore Abundance of Arbuscular Mycorrhizal Fungi across Distinct Land Uses in Western Brazilian Amazon. Mycorrhiza 2011, 21, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Friese, C.F.; Koske, R.E. The spatial Dispersion of Spores of Vesicular-Arbuscular Mycorrhizal Fungi in a Sand Dune: Microscale Patterns Associated with the Root Architecture of American Beachgrass. Mycol. Res. 1991, 95, 952–957. [Google Scholar] [CrossRef]
- Bagyaraj, D. Ecology of Vesicular Arbuscular Mycorrhiza. In Handbook on Applied Mycology; Arora, D.K., Rai, B., Mukerji, K.G., Knudsen, G.R., Eds.; Marcel Dekker, Inc.: New York, NY, USA, 1991; Volume 1, pp. 3–34. ISBN 0849356946. [Google Scholar]
- Shukla, A.; Vyas, D.; Jha, A. Soil Depth: An Overriding Factor for Distribution of Arbuscular Mycorrhizal Fungi. J. Soil Sci. Plant Nutr. 2013, 13, 23–33. [Google Scholar] [CrossRef]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Ris, E.-A.; Boller, T.; Wiemken, A. Community Structure of Arbuscular Mycorrhizal Fungi at Different Soil Depths in Extensively and Intensively Managed Agroecosystems. New Phytol. 2005, 165, 273–283. [Google Scholar] [CrossRef]
- Land, S.; Schönbeck, F. Influence of Different Soil Types on Abundance and Seasonal Dynamics of Vesicular Arbuscular Mycorrhizal Fungi in Arable Soils of North Germany. Mycorrhiza 1991, 1, 39–44. [Google Scholar] [CrossRef]
- Karaarslan, E.; Uyanöz, R. Occurrence of Arbuscular Mycorrhizal Fungi in Some Native Plants Grown on Saline Soils Around the Lake Tuz in Turkey and Its Relations with Some Physical and Chemical Properties of Soil. Sci. Res. Essays 2011, 6, 4238–4245. [Google Scholar]
- Titus, J.H.; Titus, P.J.; Nowak, R.S.; Smith, S.D. Arbuscular Mycorrhizae of Mojave Desert Plants. West. N. Am. Nat. 2002, 62, 327–334. [Google Scholar]
- Khan, A.G. The Occurrence of Mycorrhizas in Halophytes, Hydrophytes and Xerophytes, and of Endogone Spores in Adjacent Soils. Microbiology 2000, 81, 7–14. [Google Scholar] [CrossRef]
- Guadarrama, P.; Álvarez-Sánchez, F.J. Abundance of Arbuscular Mycorrhizal Fungi Spores in Different Environments in a Tropical Rain Forest, Veracruz, Mexico. Mycorrhiza 1999, 8, 267–270. [Google Scholar] [CrossRef]
- Adil, S.; Muneer, A.; Imran, M.; Munir, M.Z.; Nisa, Z.; Elahi, H.; Gillani, S.M.N.; Wang, P.; Saifullah, N.-A.; Chaudhry, M.S. Seasonality of Arbuscular Mycorrhiza and Dark Septate Endophytes in Some Grasses under Arid Climatic Conditions. J. Agric. Res. 2017, 55, 601–610. [Google Scholar]
- Lingfei, L.; Anna, Y.; Zhiwei, Z. Seasonality of Arbuscular Mycorrhizal Symbiosis and Dark Septate Endophytes in a Grassland Site in Southwest China. FEMS Microbiol. Ecol. 2005, 54, 367–373. [Google Scholar] [CrossRef]
- Lomolino, M.V.; Riddle, B.R.; Whittaker, R.J. Biogeography: Biological Diversity Across Space and Time; Oxford University Press: Oxford, UK, 2017; ISBN 9781605354729. [Google Scholar]
- Janowski, D.; Leski, T. Factors in the Distribution of Mycorrhizal and Soil Fungi. Diversity 2022, 14, 1122. [Google Scholar] [CrossRef]
- Xu, D.; Yu, X.; Chen, J.; Liu, H.; Zheng, Y.; Qu, H.; Bao, Y. Arbuscular Mycorrhizae Fungi Diversity in the Root–Rhizosphere–Soil of Tetraena Mongolica, Sarcozygium Xanthoxylon, and Nitraria Tangutorum Bobr in Western Ordos, China. Agronomy 2023, 13, 1485. [Google Scholar] [CrossRef]
- Luo, Y.; Wang, Z.; He, Y.; Li, G.; Lv, X.; Zhuang, L. High-Throughput Sequencing Analysis of the Rhizosphere Arbuscular Mycorrhizal Fungi (AMF) Community Composition Associated with Ferula Sinkiangensis. BMC Microbiol. 2020, 20, 335. [Google Scholar] [CrossRef] [PubMed]
- Quilliam, R.S.; Jones, D.L. Fungal Root Endophytes of the Carnivorous Plant Drosera Rotundifolia. Mycorrhiza 2010, 20, 341–348. [Google Scholar] [CrossRef]
- Schenck, N.C.; Perez-Collins, Y. Manual for the Identification of Va Mycorrhizal Fungi; Synergistic Publications: London, UK, 1990. [Google Scholar]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Al-Qarawi, A.A.; Mridha, M.A.U.; Alghamdi, O.M. Diversity of Structural Colonization and Spore Population of Arbuscular Mycorrhizal Fungi in Some Plants from Riyadh, Saudi Arabia. J. Pure Appl. Microbiol. 2012, 6, 1119–1125. [Google Scholar]
- Okalebo, J.R.; Gathua, K.W.; Woomer, P.L. Laboratory Methods of Soil and Plant Analysis: A Working Manual, 2nd ed.; Sustainable Agriculture Centre for Research and Development in East Africa: Nairobi, Kenya, 2002. [Google Scholar]
- Klute, A.; Page, A.L. Methods of Soil Analysis: Chemical and Microbiological Properties; ASA/SSSA. Agronomy; American Society of Agronomy: Madison, WI, USA, 1982; ISBN 9780891180722. [Google Scholar]
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co.: New York, NY, USA, 1997; ISBN 978-0-07-061028-6. [Google Scholar]
- Matekwor Ahulu, E.; Nakata, M.; Nonaka, M. Arum—And Paris—Type Arbuscular Mycorrhizas in a Mixed Pine Forest on Sand Dune Soil in Niigata Prefecture, Central Honshu, Japan. Mycorrhiza 2005, 15, 129–136. [Google Scholar] [CrossRef]
- Tshwene-Mauchaza, B.; Aguirre-Gutiérrez, J. Climatic Drivers of Plant Species Distributions Across Spatial Grains in Southern Africa Tropical Forests. Front. For. Glob. Chang. 2019, 2, 69. [Google Scholar] [CrossRef]
- Jakobsen, T.; Erik Nielsen, N. Vesicular—Arbuscular Mycorrhiza in Field—Grown Crops: I. Mycorrhizal Infection in Cereals and Peas At Various Times and Soil Depths. New Phytol. 1983, 93, 401–413. [Google Scholar] [CrossRef]
- Smith, T. A Note on the Effect of Soil Tillage on the Frequency and Vertical Distribution of Spores of Vesicular-Arbuscular Endophytes. Soil Res. 1978, 16, 359. [Google Scholar] [CrossRef]
- Sutton, J.C. Development of Vesicular-Arbuscular Mycorrhizae in Crop Plants. Can. J. Bot. 1973, 51, 2487–2493. [Google Scholar] [CrossRef]
- Sutton, J.C.; Barron, G.L. Population Dynamics of Endogone Spores in Soil. Can. J. Bot. 1972, 50, 1909–1914. [Google Scholar] [CrossRef]
- Koide, R.T.; Mooney, H.A. Spatial Variation in Inoculum Potential of Vesicular—Arbuscular Mycorrhizal Fungi Caused By Formation of Gopher Mounds. New Phytol. 1987, 107, 173–182. [Google Scholar] [CrossRef]
- Zajicek, J.M.; Hetrick, B.A.D.; Owensby, C.E. The Influence of Soil Depth on Mycorrhizal Colonization of Forbs in the Tallgrass Prairie. Mycologia 1986, 78, 316. [Google Scholar] [CrossRef]
- Higo, M.; Isobe, K.; Yamaguchi, M.; Drijber, R.A.; Jeske, E.S.; Ishii, R. Diversity and Vertical Distribution of Indigenous Arbuscular Mycorrhizal Fungi under Two Soybean Rotational Systems. Biol. Fertil. Soils 2013, 49, 1085–1096. [Google Scholar] [CrossRef]
- Muleta, D.; Assefa, F.; Nemomissa, S.; Granhall, U. Distribution of Arbuscular Mycorrhizal Fungi Spores in Soils of Smallholder Agroforestry and Monocultural Coffee Systems in Southwestern Ethiopia. Biol. Fertil. Soils 2008, 44, 653–659. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; van der Heijden, M.G.A.; Oehl, F. Impact of Conservation Tillage and Organic Farming on the Diversity of Arbuscular Mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
| Parameter | Mean |
|---|---|
| pH (1:1) | 8.1 |
| EC dSm−1 | 2.8 |
| OM % | 1.03 |
| CaCO3 % | 11.91 |
| Sand % | 58 |
| Silt % | 23 |
| Clay % | 19 |
| CEC cmol·kg−1 | 5.81 |
| Plant Species | N | P | K | Na | Ca | Mg | Total Organic Carbon (TOC) | Organic Matter (OM) |
|---|---|---|---|---|---|---|---|---|
| Solanum nigrum | 122 ± 2.9 e | 0.9 ± 0.02 bc | 5.6 ± 0.26 ef | 127 ± 3 e | 2.7 ± 0.2 d | 0.6 ± 0.03 de | 1.2 ± 0.2 cd | 2.2 ± 0.12 bc |
| Zygophyllum coccineum | 79.9 ± 0.9 f | 0.6 ± 0.09 de | 2.4 ± 0.09 gh | 9.4 ± 0.36 fgh | 1.5 ± 0.14 d | 0.5 ± 0.04 de | 0.79 ± 0.06 efg | 1.4 ± 0.27 cd |
| Phragmites australis | 437.8 ± 8.7 a | 1.3 ± 0.1 a | 92.7 ± 2.7 a | 312.6 ± 7 a | 54.9 ± 2.8 a | 20.6 ± 0.04 a | 2.1 ± 0.1 a | 3.4 ± 0.35 a |
| Zygophyllum simplex | 119.8 ± 0.9 e | 0.7 ± 0.02 de | 2.9 ± 0.4 fgh | 16.8 ± 0.4 fg | 2.5 ± 0.19 d | 0.5 ± 0.02 de | 1 ± 0.03 def | 1.4 ± 0.02 cd |
| Malva parviflora | 221.2 ± 1.4 c | 1.2 ± 0.02 a | 14.5 ± 0.35 c | 251.4 ± 2.5 c | 9.4 ± 0.4 c | 4.4 ± 0.6 c | 1.5 ± 0.1 b | 2.6 ± 0.29 ab |
| Cyperus rotundus L. | 233.6 ± 7.4 b | 1.2 ± 0.05 a | 67.3 ± 2 b | 276.9 ± 7 b | 31.4 ± 0.98 b | 17 ± 0.06 b | 1.7 ± 0.3 b | 2.7 ± 0.22 ab |
| Calotropis procera | 70.7 ± 1.1 f | 0.1 ± 0.006 f | 1.3 ± 0.1 h | 0.6 ± 0.03 h | 1.2 ± 0.09 d | 0.2 ± 0.02 e | 0.53 ± 0.04 g | 0.5 ± 0.2 d |
| Pelargonium peltatum | 208.8 ± 0.97 d | 1.1 ± 0.06 ab | 8.6 ± 0.28 d | 164.6 ± 4.6 d | 3.1 ± 0.09 d | 0.9 ± 0.07 d | 1.5 ± 0.1 bc | 2.6 ± 0.17 ab |
| Salsola imbricata | 74.6 ± 1.8 f | 0.5 ± 0.1 de | 2.2 ± 0.08 gh | 5.1 ± 0.1 gh | 1.3 ± 0.03 d | 0.3 ± 0.02 de | 0.7 ± 0.04 fg | 1.3 ± 0.5 cd |
| Citrullus colocynthis | 121.5 ± 2.8 e | 0.8 ± 0.04 cd | 4.8 ± 0.35 efg | 19.7 ± 1.3 f | 2.5 ± 0.09 d | 0.6 ± 0.04 de | 1.1 ± 0.1 de | 1.8 ± 0.26 bc |
| Senna italica | 208.2 ± 3.3 d | 0.9 ± 0.1 c | 5.8 ± 0.37 de | 153.6 ± 9.9 d | 2.9 ± 0.16 d | 0.6 ± 0.04 de | 1.2 ± 0.1 cd | 2.4 ± 0.8 ab |
| Plant Species | N | P | K | Na | Ca | Mg | Total Organic Carbon (TOC) | Organic Matter (OM) |
|---|---|---|---|---|---|---|---|---|
| Solanum nigrum | 130.2 ± 1.2 e | 1.1 ± 0.02 c | 6. ± 0.4 e | 139.8 ± 1.4 f | 2.7 ± 0.07 d | 0.6 ± 0.03 d | 1.4 ± 0.1 cd | 2.7 ± 0.1 ab |
| Zygophyllum coccineum | 82.7 ± 1.2 g | 0.7 ± 0.02 de | 2.6 ± 0.1 fg | 11.5 ± 0.5 h | 1.6 ± 0.14 d | 0.6 ± 0.03 d | 0.9 ± 0.02 def | 1.5 ± 0.7 cd |
| Phragmites australis | 482.5 ± 4.8 a | 1.5 ± 0.2 a | 98. ± 2.6 a | 323.8 ± 2 a | 58.6 ± 3.7 a | 22.2 ± 0.7 a | 2.2 ± 0.03 a | 3.6 ± 0.2 a |
| Zygophyllum simplex | 123.5 ± 1.43 | 0.8 ± 0.1 d | 3.04 ± 0.1 fg | 17.6 ± 0.8 h | 2.7 ± 0.02 d | 0.6 ± 0.1 d | 1.24 ± 0.03 cde | 2.2 ± 0.1 cd |
| Malva parviflora | 225.9 ± 0.98 c | 1.3 ± 0.03 b | 15.8 ± 0.17 c | 259.9 ± 1 c | 14.3 ± 0.24 c | 5.6 ± 0.2 c | 1.7 ± 0.1 abc | 3.01 ± 0.8 ab |
| Cyperus rotundus L. | 254.8 ± 1.6 b | 1.3 ± 0.1 b | 70.7 ± 1.5 b | 285.8 ± 2.9 b | 35.61 ± 0.9 b | 18.6 ± 0.8 d | 1.9 ± 0.1 ab | 3.1 ± 0.2 ab |
| Calotropis procera | 72 ± 1 h | 0.13 ± 0.02 f | 1.4 ± 0.2 g | 0.7 ± 0.1 j | 1.3 ± 0.03 d | 0.3 ± 0.01 d | 0.6 ± 0.3 f | 1.41 ± 0.1 d |
| Pelargonium peltatum | 211.1 ± 1 d | 1.24 ± 0.02 bc | 8.9 ± 0.3 d | 174.5 ± 1.3 d | 3.3 ± 0.1 d | 1.1 ± 0.04 d | 1.7 ± 0.2 bc | 2.9 ± 0.2 ab |
| Salsola imbricata | 78.7 ± 1 g | 0.6 ± 0.02 e | 2.31 ± 0.1 g | 5.4 ± 0.2 i | 1.3 ± 0.02 d | 0.4 ± 0.01 d | 0.84 ± 0.4 ef | 1.48 ± 0.7 cd |
| Citrullus colocynthis | 124.4 ± 1.1 f | 0.8 ± 0.1 d | 5. ± 0.2 ef | 20.4 ± 0.4 g | 2.7 ± 0.02 d | 0.6 ± 0.04 d | 1.3 ± 0.1 cde | 2.6 ± 0.14 abc |
| Senna italica | 211. ± 1 d | 1.23 ± 0.1 bc | 6.21 ± 0.2 de | 166.3 ± 2 e | 3.14 ± 0.13 d | 0.7 ± 0.02 d | 1.7 ± 0.03 bc | 2.9 ± 0.02 ab |
| Plant Species | Spore Count | Myciilum % | Vesicles % | Arbuscules % |
|---|---|---|---|---|
| Solanum nigrum | 125.7 ± 5.7 d | 82.7 ± 2.6 abc | 29 ± 9.8 a | 62.3 ± 4.6 abc |
| Zygophyllum coccineum | 117.3 ± 2.3 d | 75.3 ± 4.6 c | 22.7 ± 4.3 a | 55.6 ± 5.9 abc |
| Phragmites australis | 175 ± 5.3 a | 90 ± 1.7 a | 36 ± 5.9 a | 76 ± 4.7 a |
| Zygophyllum simplex | 120 ± 4.7 d | 77.3 ± 2.9 bc | 24.3 ± 6.9 a | 56.7 ± 11.3 abc |
| Malva parviflora | 157.7 ± 2.9 b | 86 ± 4.3 abc | 34.3 ± 6.9 a | 69.3 ± 4.5 abc |
| Cyperus rotundus L. | 162 ± 3.2 ab | 87.7 ± 2.9 ab | 34.7 ± 4.6 a | 70 ± 8.9 ab |
| Calotropis procera | 101.3 ± 7.9 e | 57.7 ± 4.7 d | 22.3 ± 4.7 a | 49 ± 9.8 c |
| Pelargonium peltatum | 155.3 ± 3.8 bc | 85.3 ± 4.9 abc | 33.3 ± 3.8 a | 65.3 ± 5.6 abc |
| Salsola imbricata | 114.3 ± 4 de | 74.3 ± 4.7 c | 22.3 ± 4.7 a | 52.3 ± 2.9 bc |
| Citrullus colocynthis | 122 ± 2.9 d | 82.7 ± 2.6 abc | 24.7 ± 6.2 a | 57 ± 5.7 abc |
| Senna italica | 142.3 ± 4.4 c | 83.3 ± 5 abc | 32.3 ± 9.8 a | 64.6 ± 6.2 abc |
| Plants Species | Spore Count | Myciilum % | Vesicles % | Arbuscules % |
|---|---|---|---|---|
| Solanum nigrum | 108.7 ± 13.7 abc | 81 ± 2.8 a | 35.7 ± 5.9 ab | 62.7 ± 8 ab |
| Zygophyllum coccineum | 67 ± 7.8 bc | 75 ± 4.6 a | 42.3 ± 7.9 a | 55.7 ± 5.9 ab |
| Phragmites australis | 124 ± 5 a | 84.7 ± 4.4 a | 24.3 ± 4.7 b | 69.3 ± 4.5 a |
| Zygophyllum simplex | 107.3 ± 3.2 abc | 75.7 ± 5.9 a | 37.7 ± 2.3 ab | 55.7 ± 5.9 ab |
| Malva parviflora | 113 ± 6 abc | 84 ± 3 a | 29.3 ± 2.3 ab | 65.3 ± 6.7 ab |
| Cyperus rotundus L. | 116.7 ± 10.8 ab | 84.3 ± 5.9 a | 24.3 ± 5.9 b | 65.7 ± 5.5 ab |
| Calotropis procera | 63 ± 2.8 c | 55 ± 1.2 b | 42.7 ± 4.3 a | 52.7 ± 8.7 b |
| Pelargonium peltatum | 111.7 ± 8.4 abc | 82. ± 8.7 a | 31.3 ± 4.3 ab | 64.7 ± 5.6 ab |
| Salsola imbricata | 64. ± 4 c | 74 ± 5.8 a | 42.7 ± 2.9 a | 54.3 ± 4.6 ab |
| Citrullus colocynthis | 107.7 ± 5.2 abc | 75.7 ± 5.9 a | 35.7 ± 5.9 ab | 59 ± 6.1 ab |
| Senna italica | 109.7 ± 4 abc | 82 ± 3.2 a | 34.7 ± 6.9 ab | 64.7 ± 8 ab |
| Plant Species | Spore Count/100 gm Dry Soil | Shannon (H’) | Simpson (D) | AMF Spore Diversity | |
|---|---|---|---|---|---|
| Summer | Winter | ||||
| Solanum nigrum | 125 | 108 | 0.690 | 0.497 | Glomus ambisporum, Rhizophagus intraradices, Claroideoglomus etunicatum |
| Zygophyllum coccineum | 117 | 67 | 0.656 | 0.463 | Diversispora globifera, Funneliformis geosporum, Rhizophagus intraradices, Claroideoglomus etunicatum, Glomus ambisporum |
| Phragmites australis | 175 | 124 | 0.679 | 0.485 | Rhizophagus intraradices, Funneliformis mosseae, Glomus ambisporum, Claroideoglomus etunicatum, Diversispora globifera, Gigaspora spp. |
| Zygophyllum simplex | 120 | 107 | 0.692 | 0.498 | Diversispora globifera, Funneliformis mosseae, Rhizophagus fasciculatus, Glomus ambisporum, Claroideoglomus etunicatum |
| Malva parviflora | 157 | 113 | 0.680 | 0.487 | Funneliformis mosseae, Glomus ambisporum, Diversispora globifera, Claroideoglomus etunicatum, Rhizophagus fasciculatus |
| Cyperus rotundus L. | 162 | 116 | 0.679 | 0.486 | Diversispora globifera, Funneliformis geosporum, Glomus ambisporum, Rhizophagus intraradices, Claroideoglomus etunicatum |
| Calotropis procera | 101 | 63 | 0.666 | 0.473 | Rhizophagus intraradices, Diversispora globifera, Glomus ambisporum, Claroideoglomus etunicatum, Rhizophagus fasciculatus |
| Pelargonium peltatum | 155 | 111 | 0.679 | 0.486 | Claroideoglomus etunicatum, Diversispora globifera |
| Salsola imbricata | 114 | 64 | 0.653 | 0.461 | Gigaspora spp., Glomus ambisporum, Claroideoglomus etunicatum |
| Citrullus colocynthis | 122 | 107 | 0.691 | 0.498 | Rhizophagus intraradices, Claroideoglomus etunicatum, Diversispora globifera, Glomus ambisporum |
| Senna italica | 142 | 109 | 0.684 | 0.491 | Funneliformis mosseae, Claroideoglomus etunicatum |
| Variables | Summer Seasons | Winter Seasons |
|---|---|---|
| AMF Spore Count | ||
| N (mg·kg−1) | 0.91 ** | 0.71 ** |
| P (mg·kg−1) | 0.94 ** | 0.87 ** |
| K (mg·kg−1) | 0.76 ** | 0.53 * |
| Na (mg·kg−1) | 0.96 ** | 0.76 ** |
| Ca (mg·kg−1) | 0.75 ** | 0.53 * |
| Mg (mg·kg−1) | 0.76 ** | 0.52 * |
| Total Organic Carbon (TOC) (mg·kg−1) | 0.96 ** | 0.92 ** |
| Organic Matter (OM) % | 0.96 ** | 0.96 ** |
| pH | 0.05 | 0.02 |
| Sand % | −0.31 | 0.16 |
| Silt % | 0.17 | 0.07 |
| Caly % | −0.05 | −0.36 |
| EC (dSm−1) | −0.03 | −0.39 |
| CEC (cmol·kg−1) | 0.41 | 0.32 |
| CaCO3 % | 0.07 | −0.11 |
| Spore Count | 1.00 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aloud, S.S.; Alotaibi, F.; Sorrori, S.N.; Alshebe, B. Influence of Rhizosphere Dynamics and Soil Chemical Properties in Arid Environments on the Distribution, Abundance, and Diversity of Arbuscular Mycorrhizal Fungi (AMF). Ecologies 2025, 6, 80. https://doi.org/10.3390/ecologies6040080
Aloud SS, Alotaibi F, Sorrori SN, Alshebe B. Influence of Rhizosphere Dynamics and Soil Chemical Properties in Arid Environments on the Distribution, Abundance, and Diversity of Arbuscular Mycorrhizal Fungi (AMF). Ecologies. 2025; 6(4):80. https://doi.org/10.3390/ecologies6040080
Chicago/Turabian StyleAloud, Saud S., Fahad Alotaibi, Salah N. Sorrori, and Basil Alshebe. 2025. "Influence of Rhizosphere Dynamics and Soil Chemical Properties in Arid Environments on the Distribution, Abundance, and Diversity of Arbuscular Mycorrhizal Fungi (AMF)" Ecologies 6, no. 4: 80. https://doi.org/10.3390/ecologies6040080
APA StyleAloud, S. S., Alotaibi, F., Sorrori, S. N., & Alshebe, B. (2025). Influence of Rhizosphere Dynamics and Soil Chemical Properties in Arid Environments on the Distribution, Abundance, and Diversity of Arbuscular Mycorrhizal Fungi (AMF). Ecologies, 6(4), 80. https://doi.org/10.3390/ecologies6040080






