Chemotypic Plasticity of Potentilla erecta (L.) Raeusch. Across Elevational Gradients in the Ukrainian Carpathians
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Plant Collection
2.2. Determination of Tannin Content
2.3. Determination of Flavonoid Content
2.4. Statistical Analysis
3. Results
3.1. Tanin Content
3.2. Total Flavonoid Content
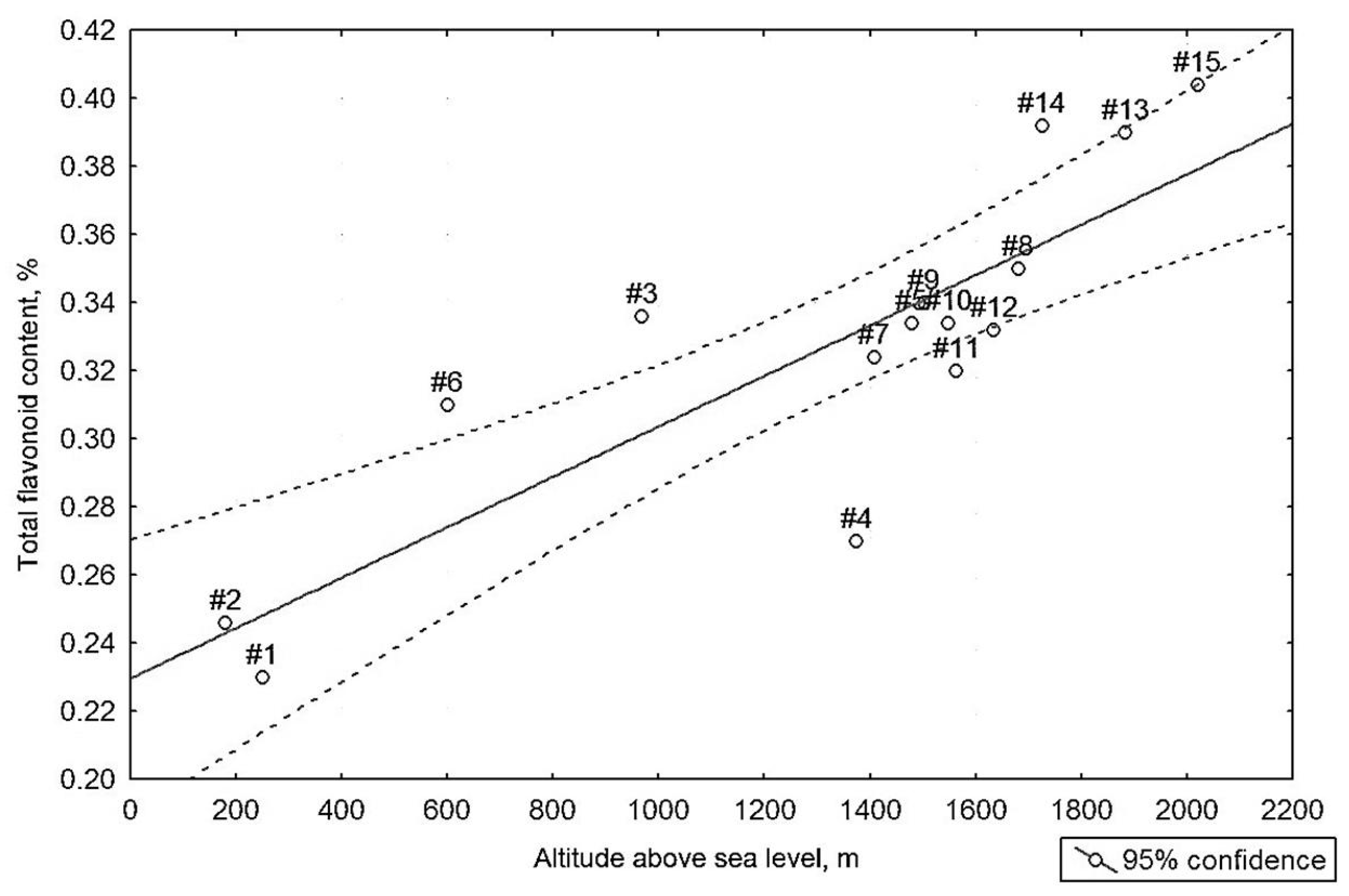
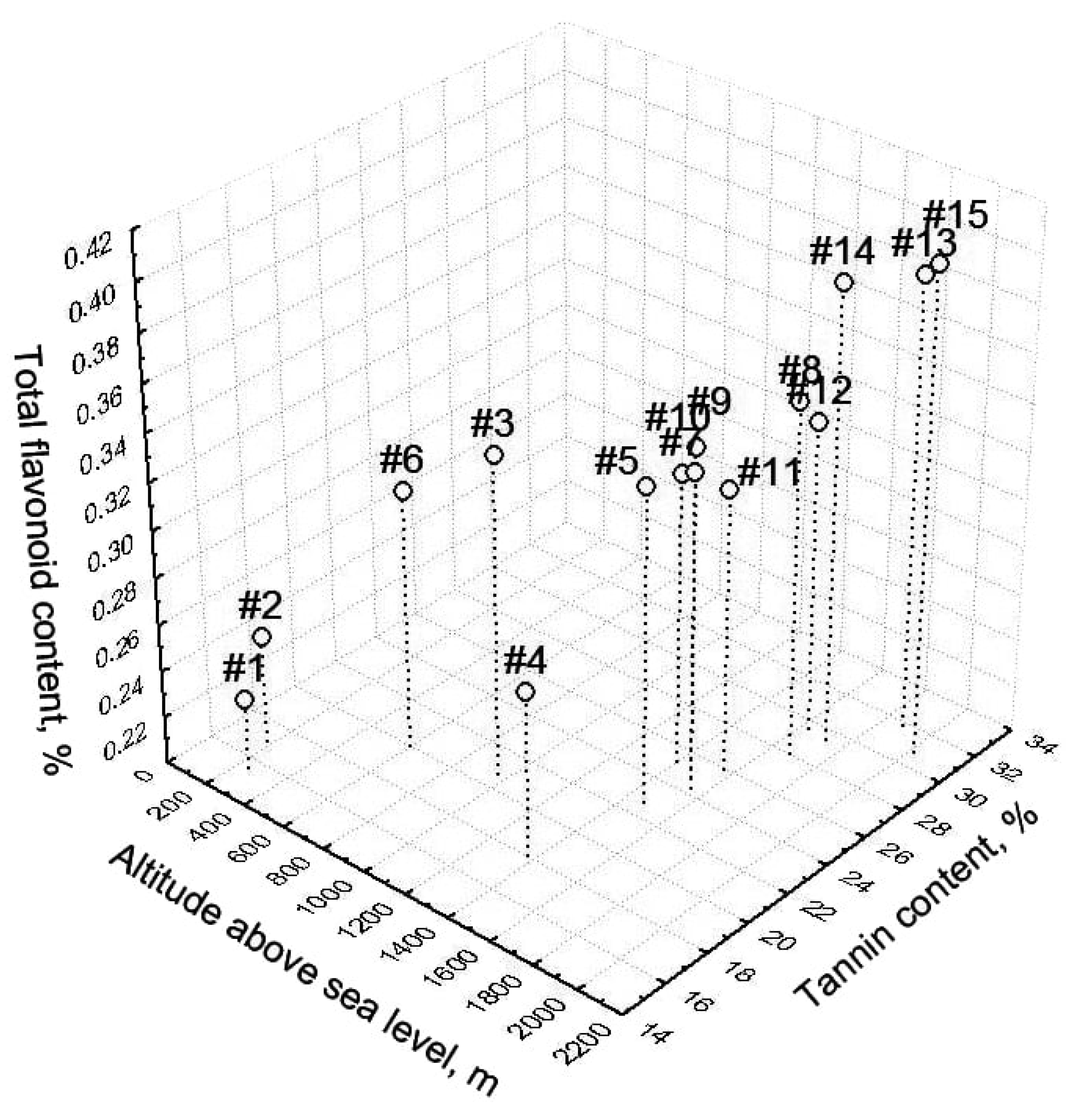
3.3. Relationship with Altitude
3.4. Statistical Interpretation
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of Variance |
| DW | Dry Weight |
| HSD | Honestly Significant Difference (Tukey’s test) |
| Rf | Retention Factor (in thin-layer chromatography) |
| SD | Standard Deviation |
| TLC | Thin-Layer Chromatography |
| UV | Ultraviolet |
References
- Dai, J.; Mumper, R.J. Plant phenolics: Extraction, analysis and their antioxidant and anticancer properties. Molecules 2010, 15, 7313–7352. [Google Scholar] [CrossRef]
- Li, Y.; Kong, D.; Fu, Y.; Sussman, M.R.; Wu, H. The effect of developmental and environmental factors on secondary metabolites in medicinal plants. Plant Physiol. Biochem. 2020, 148, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Körner, C. Mountain biodiversity, its causes and function. AMBIO J. Hum. Environ. 2004, 33, 11–17. [Google Scholar] [CrossRef]
- Vellend, M. Conceptual synthesis in community ecology. Q. Rev. Biol. 2010, 85, 183–206. [Google Scholar] [CrossRef]
- Tutin, T.G.; Heywood, V.H.; Burges, N.A.; Moore, D.M.; Valentine, D.H.; Walters, S.M.; Webb, D.A. Flora Europaea; Cambridge University Press: Cambridge, UK, 1969; Volume 2. [Google Scholar]
- Kurtto, A. Rosaceae (pro parte majore). In The Information Resource for Euro-Mediterranean Plant Diversity; Euro+Med PlantBase: Berlin, Germany, 2009; Available online: http://www.europlusmed.org (accessed on 20 March 2024).
- Council of Europe. European Pharmacopoeia, 9th ed.; Council of Europe: Strasbourg, France, 2017. [Google Scholar]
- State Pharmacopoeia of Ukraine. State Pharmacopoeia of Ukraine, 2nd ed.; State Enterprise Ukrainian Scientific Pharmacopoeial Quality Centre for Medicines: Kharkiv, Ukraine, 2014; Volume 3, pp. 416–418. [Google Scholar]
- Tomczyk, M.; Leszczyńska, K.; Jakoniuk, P.H. Antimicrobial activity of Potentilla species. Fitoterapia 2008, 79, 592–594. [Google Scholar] [CrossRef]
- Tomczyk, M.; Latté, K.P. Potentilla—A review of its phytochemical and pharmacological profile. J. Ethnopharmacol. 2009, 122, 184–204. [Google Scholar] [CrossRef]
- Augustynowicz, D.; Latté, K.P.; Tomczyk, M. Recent phytochemical and pharmacological advances in the genus Potentilla L. sensu lato—An update covering the period from 2009 to 2020. J. Ethnopharmacol. 2021, 266, 113412. [Google Scholar] [CrossRef]
- Șöhretoğlu, D.; Sari, S.; Özel, A.; Barut, B. α-Glucosidase inhibitory effect of Potentilla astracanica and some isoflavones: Inhibition kinetics and mechanistic insights through in vitro and in silico studies. Int. J. Biol. Macromol. 2017, 105, 1062–1070. [Google Scholar] [CrossRef]
- Wölfle, U.; Hoffmann, J.; Haarhaus, B.; Rao, M.V.; Schempp, C.M. Anti-inflammatory and vasoconstrictive properties of Potentilla erecta—A traditional medicinal plant from the northern hemisphere. J. Ethnopharmacol. 2017, 204, 86–94. [Google Scholar] [CrossRef]
- Melzig, M.F.; Böttger, S. Tormentillae rhizoma—Review for an underestimated European herbal drug. Planta Med. 2020, 86, 1050–1057. [Google Scholar] [CrossRef]
- Dróżdż, P.; Sentkowska, A.; Pyrzynska, K. Potentilla erecta (L.) rhizomes as a source of phenolic acids. Nat. Prod. Res. 2019, 33, 2128–2131. [Google Scholar] [CrossRef]
- Camas, N.; Radusiene, J.; Ivanauskas, L.; Jakstas, V.; Cirak, C. Altitudinal changes in the content of bioactive substances in Hypericum orientale and Hypericum pallens. Acta Physiol. Plant. 2014, 36, 675–686. [Google Scholar] [CrossRef]
- Cirak, C.; Radusiene, J.; Jakstas, V.; Ivanauskas, L.; Seyis, F.; Yayla, F. Altitudinal changes in secondary metabolite contents of Hypericum androsaemum and Hypericum polyphyllum. Biochem. Syst. Ecol. 2017, 70, 108–115. [Google Scholar] [CrossRef]
- Monschein, M.; Jaindl, K.; Buzimkić, S.; Bucar, F. Content of phenolic compounds in wild populations of Epilobium angustifolium growing at different altitudes. Pharm. Biol. 2015, 53, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, L.S. Conserving mountain biodiversity in protected areas. In Mountain Biodiversity: A Global Assessment; Körner, C., Spehn, E.M., Eds.; Routledge: London, UK, 2002; pp. 295–306. [Google Scholar] [CrossRef]
- Kumari, S.; Seth, A.; Sharma, S.; Attri, C. A holistic overview of different species of Potentilla, a medicinally important plant, along with their pharmaceutical significance: A review. J. Herb. Med. 2021, 29, 100460. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef]
- Pietta, P.G. Flavonoids as antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A review of its structure, synthesis, pharmacology, pharmacokinetics and toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Ren, J.; Barton, C.D.; Zhan, J. Engineered production of bioactive polyphenolic O-glycosides. Biotechnol. Adv. 2023, 65, 108146. [Google Scholar] [CrossRef]
- Seyis, F.; Yurteri, E.; Özcan, A.; Cirak, C. Altitudinal impacts on chemical content and composition of Hypericum perforatum, a prominent medicinal herb. S. Afr. J. Bot. 2020, 135, 391–403. [Google Scholar] [CrossRef]
- Boyarskikh, I.G.; Artemov, I.A.; Kuznetsov, A.A.; Kostikova, V.A. Changes in profiles of classes and of individual polyphenols in leaves of Spiraea chamaedryfolia and Spiraea media along an altitudinal gradient. Plants 2023, 12, 2977. [Google Scholar] [CrossRef]
- Morais, N.D.A.; Martins, D.H.N.; Fagg, C.W.; Simeoni, L.A.; Magalhães, P.O.; Silveira, D.; Fonseca-Bazzo, Y.M. Seasonal monitoring of the antioxidant activity of Erythroxylum suberosum A. St.-Hil. leaves: Correlation with hyperoside and isoquercitrin contents. Indian J. Tradit. Knowl. 2022, 21, 373–382. [Google Scholar] [CrossRef]
- Synowiec, A.; Gniewosz, M.; Bączek, K.; Przybył, J.L. Antimicrobial effect of an aqueous extract of Potentilla erecta rhizome. Herba Pol. 2014, 60, 18–28. [Google Scholar] [CrossRef]
- Benomari, F.Z.; Sarazin, M.; Chaib, D.; Pichette, A.; Boumghar, H.; Boumghar, Y.; Djabou, N. Chemical variability and chemotype concept of essential oils from Algerian wild plants. Molecules 2023, 28, 4439. [Google Scholar] [CrossRef] [PubMed]
- Clancy, M.V.; Mamin, M.; Flückiger, G.; Quijano-Medina, T.; Pérez-Niño, B.; Abdala-Roberts, L.; Turlings, T.C.J.; Bustos-Segura, C. Terpene chemotypes in Gossypium hirsutum (wild cotton) from the Yucatan Peninsula, Mexico. Phytochemistry 2023, 205, 113454. [Google Scholar] [CrossRef] [PubMed]
- Dussarrat, T.; Schweiger, R.; Ziaja, D.; Nguyen, T.T.; Krause, L.; Jakobs, R.; Eilers, E.J.; Müller, C. Influences of chemotype and parental genotype on metabolic fingerprints of tansy plants uncovered by predictive metabolomics. Sci. Rep. 2023, 13, 11645. [Google Scholar] [CrossRef]
- Pluhár, Z.; Kun, R.; Cservenka, J.; Neumayer, É.; Tavaszi-Sárosi, S.; Radácsi, P.; Gosztola, B. Variations in essential oil composition and chemotype patterns of wild thyme (Thymus) species in the natural habitats of Hungary. Horticulturae 2024, 10, 150. [Google Scholar] [CrossRef]
- Leht, M.; Paal, J. Variation of Potentilla sect. Potentilla (Rosaceae) in Estonia and neighbouring countries. Ann. Bot. Fenn. 2004, 41, 53–61. [Google Scholar]
- Paule, J. Evolutionary Patterns and Processes in the Genus Potentilla L. (Rosaceae). Ph.D. Thesis, Ruperto-Carola University of Heidelberg, Heidelberg, Germany, 2010. [Google Scholar]
- Schaal, B.A.; Hayworth, D.A.; Olsen, K.M.; Rauscher, J.T.; Smith, W.A. Phylogeographic studies in plants: Problems and prospects. Mol. Ecol. 1998, 7, 465–474. [Google Scholar] [CrossRef]

| No. | Sampling Site (Locality) | Altitude (m) | Latitude (°) | Longitude (°) |
|---|---|---|---|---|
| 1. | Ostra wildlife sanctuary, Kamianytsia, Uzhhorod Rayon | 250 | 49.1479 | 22.6582 |
| 2. | Offshoot slopes of Synatoriya Range between Perechyn and Simer | 180 | 49.2210 | 22.8547 |
| 3. | Mt Antalovetska Poliana (Synatoriya Range) | 968 | 49.1256 | 22.8235 |
| 4. | Mt Lutianska Holytsia (western part of Polonyna Range) | 1375 | 49.4219 | 23.2694 |
| 5. | Mt Polonyna Runa (western part of Polonyna Range) | 1479 | 49.3410 | 23.3519 |
| 6. | Environs of Uzhok Village, Uzhhorod Rayon | 600 | 49.6392 | 23.4367 |
| 7. | Mt Pikui (West Beskydy Range) | 1408 | 49.3834 | 23.0024 |
| 8. | Mt Stoi (Borzhava Polonynas Range) | 1681 | 49.0358 | 23.3192 |
| 9. | Mt Darvaika (Pishkonia Range) | 1502 | 48.8074 | 24.2876 |
| 10. | Mt Topas (Krasna Range) | 1548 | 48.6383 | 24.2481 |
| 11. | Mt Syhliansky (Krasna Range) | 1563 | 48.6085 | 24.3273 |
| 12. | Mt Tempa (western part of Svydovets Range) | 1634 | 48.4581 | 24.0776 |
| 13. | Mt Blyznytsi (eastern part of Svydovets Range) | 1883 | 48.3733 | 24.385 |
| 14. | Mt Sheshul (western part of Chornohora Range) | 1726 | 48.2593 | 24.6174 |
| 15. | Mt Petros (Chornohora Range) | 2020 | 48.2883 | 24.7043 |
| Population * | Mean ± SD (%) | CV (%) |
|---|---|---|
| 1 | 15.57 ± 1.16 | 7.45 |
| 2 | 17.05 ± 1.79 | 10.50 |
| 3 | 20.71 ± 1.54 | 7.44 |
| 4 | 18.05 ± 1.22 | 6.76 |
| 5 | 22.70 ± 1.75 | 7.71 |
| 6 | 19.98 ± 0.87 | 4.35 |
| 7 | 25.16 ± 1.18 | 4.69 |
| 8 | 28.00 ± 1.07 | 3.82 |
| 9 | 24.82 ± 1.51 | 6.08 |
| 10 | 24.31 ± 1.62 | 6.66 |
| 11 | 25.86 ± 1.19 | 4.60 |
| 12 | 29.55 ± 1.26 | 4.26 |
| 13 | 31.82 ± 1.13 | 3.55 |
| 14 | 29.48 ± 1.79 | 6.07 |
| 15 | 30.79 ± 1.77 | 5.75 |
| Population * | Mean ± SD (%) | CV (%) |
|---|---|---|
| 1 | 0.23 ± 0.016 | 6.96 |
| 2 | 0.25 ± 0.023 | 9.20 |
| 3 | 0.34 ± 0.024 | 7.06 |
| 4 | 0.27 ± 0.016 | 5.93 |
| 5 | 0.33 ± 0.021 | 6.36 |
| 6 | 0.31 ± 0.016 | 5.16 |
| 7 | 0.32 ± 0.021 | 6.56 |
| 8 | 0.35 ± 0.016 | 4.57 |
| 9 | 0.34 ± 0.016 | 4.71 |
| 10 | 0.33 ± 0.021 | 6.36 |
| 11 | 0.32 ± 0.016 | 5.00 |
| 12 | 0.33 ± 0.024 | 7.27 |
| 13 | 0.39 ± 0.016 | 4.10 |
| 14 | 0.39 ± 0.024 | 6.15 |
| 15 | 0.40 ± 0.021 | 5.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kolesnyk, A.; Kolesnyk, O.; Leno, Y.; Kosztyuné Krajnyák, E.; Szabó, B.; Hörcsik, Z.T.; Cziáky, Z.; Dobránszki, J.; Németh, A.; Csabai, J. Chemotypic Plasticity of Potentilla erecta (L.) Raeusch. Across Elevational Gradients in the Ukrainian Carpathians. Ecologies 2025, 6, 73. https://doi.org/10.3390/ecologies6040073
Kolesnyk A, Kolesnyk O, Leno Y, Kosztyuné Krajnyák E, Szabó B, Hörcsik ZT, Cziáky Z, Dobránszki J, Németh A, Csabai J. Chemotypic Plasticity of Potentilla erecta (L.) Raeusch. Across Elevational Gradients in the Ukrainian Carpathians. Ecologies. 2025; 6(4):73. https://doi.org/10.3390/ecologies6040073
Chicago/Turabian StyleKolesnyk, Anzhela, Oleksandra Kolesnyk, Yurij Leno, Edit Kosztyuné Krajnyák, Béla Szabó, Zsolt Tibor Hörcsik, Zoltán Cziáky, Judit Dobránszki, Anikó Németh, and Judit Csabai. 2025. "Chemotypic Plasticity of Potentilla erecta (L.) Raeusch. Across Elevational Gradients in the Ukrainian Carpathians" Ecologies 6, no. 4: 73. https://doi.org/10.3390/ecologies6040073
APA StyleKolesnyk, A., Kolesnyk, O., Leno, Y., Kosztyuné Krajnyák, E., Szabó, B., Hörcsik, Z. T., Cziáky, Z., Dobránszki, J., Németh, A., & Csabai, J. (2025). Chemotypic Plasticity of Potentilla erecta (L.) Raeusch. Across Elevational Gradients in the Ukrainian Carpathians. Ecologies, 6(4), 73. https://doi.org/10.3390/ecologies6040073






