Functional Morphology and Early Growth of Seedlings of Tropical Species
Abstract
1. Introduction
2. Materials and Methods
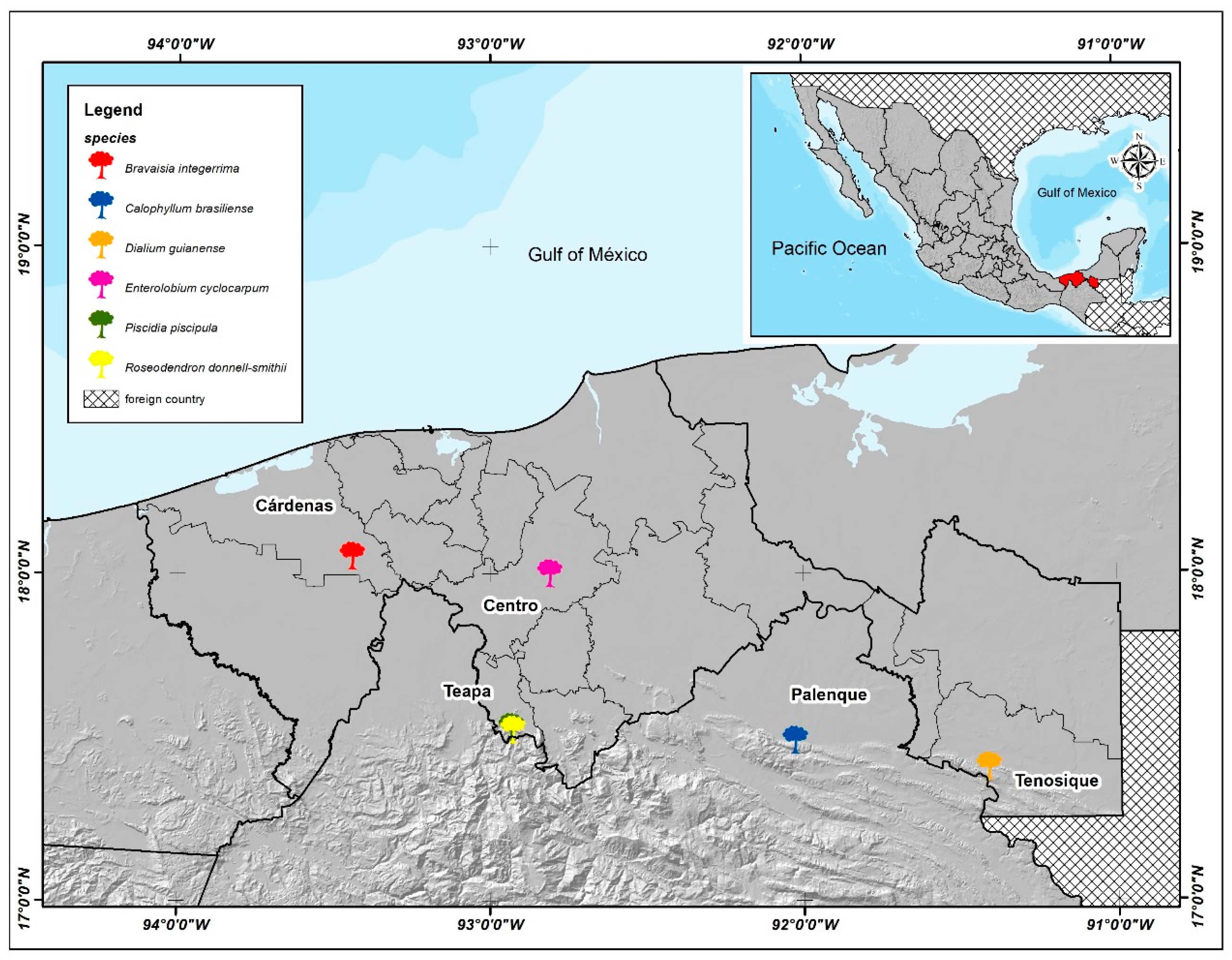
2.1. Survey Site
2.2. Species Selection and Nursery Work
2.3. Morphological Description of Seedlings
2.4. Growth Variables
2.5. Survivorship
2.6. Data Analysis
3. Results
3.1. Plant Germination Documentation
3.1.1. Calophyllum brasiliense
3.1.2. Bravaisia integerrima
3.1.3. Dialium guianense
3.1.4. Piscidia piscipula
3.1.5. Roseodendron donnell-smithii
3.1.6. Enterolobium cyclocarpum
3.2. Growth Variables
3.2.1. Stem Height and Stem Basal Diameter
3.2.2. Number of Juvenile Leaves
3.2.3. Relative Growth Rates
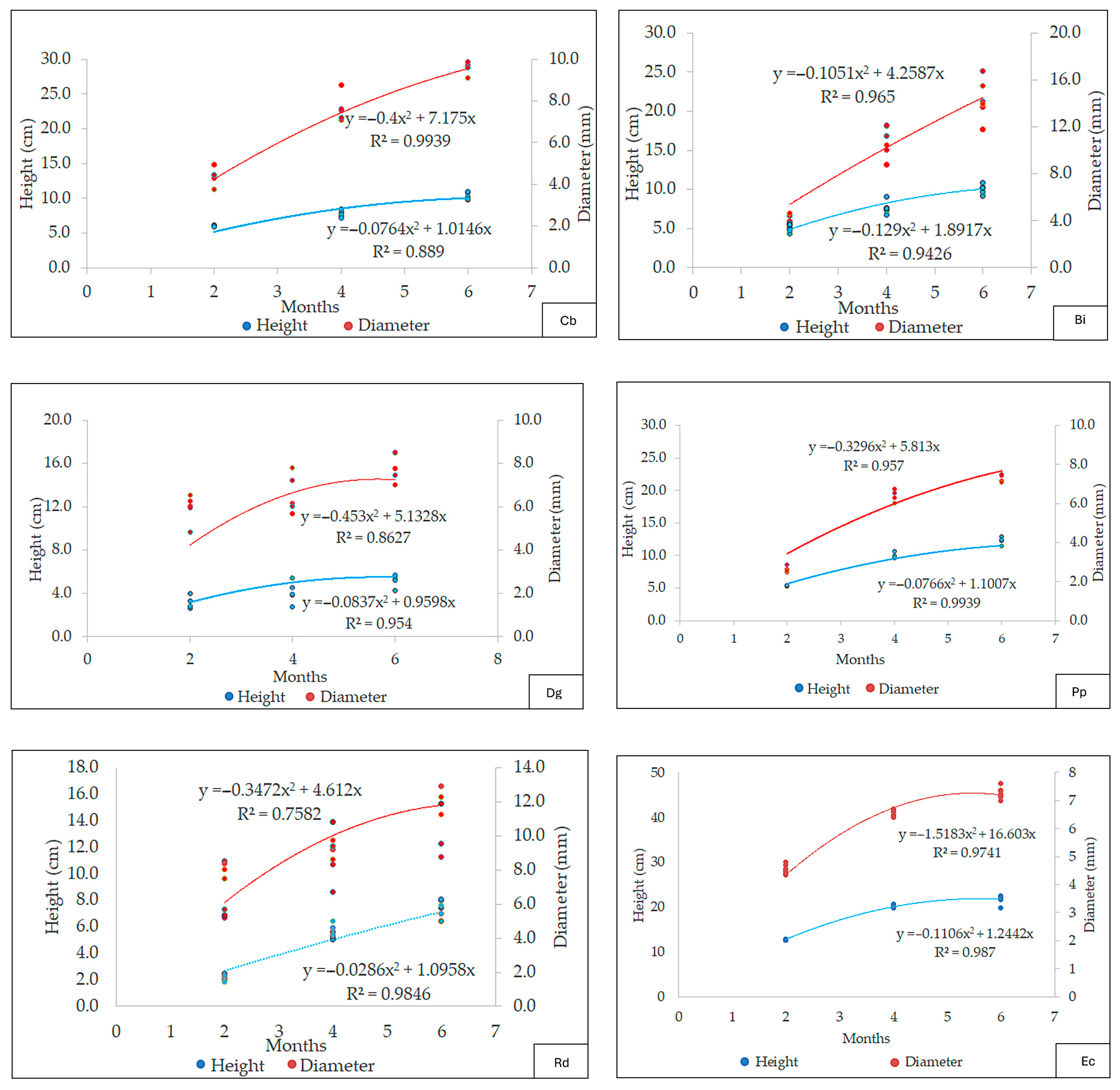
3.2.4. Regression Curves
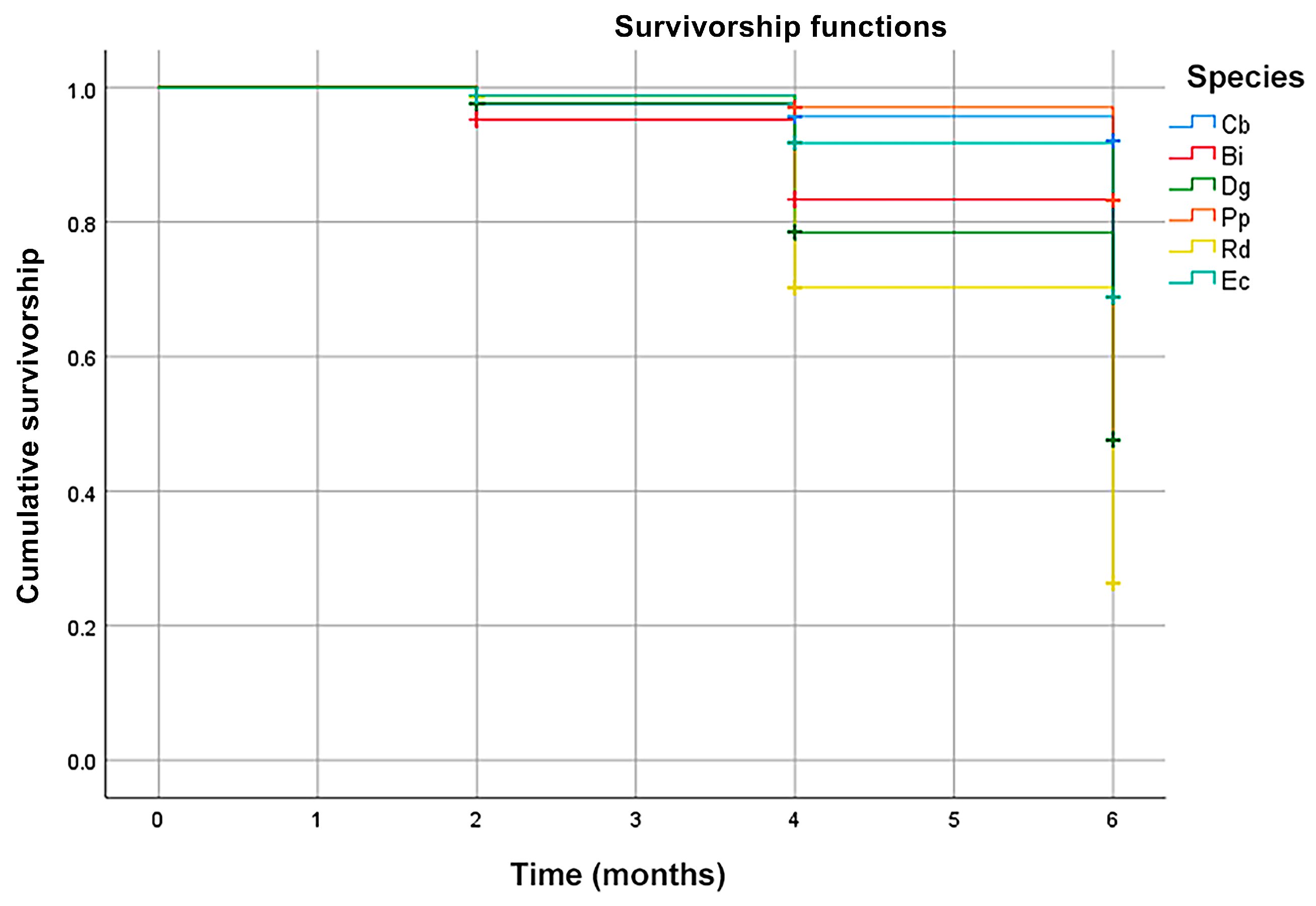
3.2.5. Seedling Survivorship
4. Discussion
4.1. Germination and Morphological Description of Seedlings
4.2. Growth Variables
4.3. Number of Juvenile Leaves
4.4. Relative Growth Rate
4.5. Seedling Survivorship
4.6. Regression Curves
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lopez-Feldman, A. Deforestación en Mexico: Un Análisis Preliminar; Centro de Investigación y Docencia Económicas A.C.: Mexico City, Mexico, 2012. [Google Scholar]
- Castillo Ramiro, J.J.; Gama, L.; Zequeira Larios, C. Análisis de regresión lineal en un sistema de información geográfico para determinar la tasa de deforestación en el estado de Tabasco. Kuxulkab 2008, 15, 15–18. [Google Scholar] [CrossRef]
- Ramos Reyes, R.; Palomeque de la Cruz, M.Á. Cambio de uso del suelo y escenarios prospectivos en el estado de Tabasco (Mexico). An. Geogr. Univ. Complut. 2023, 43, 185–209. [Google Scholar] [CrossRef]
- Bartholomew, D.C.; Shaw, K.; Rivers, M.C.; Baraka, P.; Kigathi, R.N.; Wanja, W.; Wanjiku, C.; Williams, H.F. Overcoming the Challenges of Incorporating Rare and Threatened Flora into Ecosystem Restoration. Restor. Ecol. 2023, 31, e13849. [Google Scholar] [CrossRef]
- Moya-Roque, R.; Tenorio-Monge, C. Características de combustibilidad de diez especies de plantaciones de rápido crecimiento en Costa Rica. Rev. For. Mesoam. Kurú 2013, 10, 26–33. [Google Scholar] [CrossRef]
- de Souza, D.C.; Engel, V.L. Seed Functional Traits as Predictors of Seedling Establishment Success in Brazilian Tropical Forest Restoration. Biotropica 2024, 56, e13355. [Google Scholar] [CrossRef]
- Grossnickle, S.C.; MacDonald, J.E. Seedling Quality: History, Application, and Plant Attributes. Forests 2018, 9, 283. [Google Scholar] [CrossRef]
- González Izquierdo, E.; Cobas López, M.; Bonilla Vichot, M.; Sotolongo Sospedra, R.; Castillo Martínez, I.d.L.C.; García Corona, I.M.; Medina Malagón, M.L. Experiencias en la producción de plantas cultivadas en los viveros forestales en contenedores. Rev. Cuba. Cienc. For. 2014, 2, 118–127. [Google Scholar]
- Adegoke, F.; Akinyele, A.; Ogundwande, O. Effect of Seed Size and Source on Early Seedling Growth of Terminalia ivorensis (Chev.). Agric. For. 2014, 60, 157–166. [Google Scholar]
- Pérez-Hernández, I.; Ochoa-Gaona, S.; Vargas-Simón, G.; Mendoza-Carranza, M.; González-Valdivia, N.A. Germinación y supervivencia de seis especies nativas de un bosque tropical de Tabasco, Mexico. Madera Bosques 2011, 17, 71–91. [Google Scholar] [CrossRef]
- Pérez-Harguindeguy, N.; Díaz, S.; Garnier, E.; Lavorel, S.; Poorter, H.; Jaureguiberry, P.; Bret-Harte, M.S.; Cornwell, W.K.; Craine, J.M.; Gurvich, D.E.; et al. New Handbook for Standardised Measurement of Plant Functional Traits Worldwide. Aust. J. Bot. 2013, 61, 167–234. [Google Scholar] [CrossRef]
- Pennington, T.D.; Sarukhán, J. Manual Para La Identificación de Campo de Los Principales Árboles Tropicales de México; Universidad Nacional Autónoma de Mexico, Fondo de Cultura Económica: Mexico City, Mexico, 1968; ISBN 9683664288. [Google Scholar]
- Royal Botanic Garden Kew Plants of the World Online|Kew Science. Available online: https://powo.science.kew.org/ (accessed on 25 July 2024).
- Tellez, O.; Mattana, E.; Diazgranados, M.; Kühn, N.; Castillo-Lorenzo, E.; Lira, R.; Montes-Leyva, L.; Rodriguez, I.; Flores Ortiz, C.M.; Way, M.; et al. Native Trees of Mexico: Diversity, Distribution, Uses and Conservation. PeerJ 2020, 8, e9898. [Google Scholar] [CrossRef] [PubMed]
- Sudrajat, D.J.; Rustam, E.; Nurhasybi; Widyani, N.; Yulianti; Isnaini, Y.; Aprilianti, P.; Primananda, E.; Zanzibar, M.; Suhartati, S.; et al. Improving the Success of Direct Seeding through the Application of Seed Briquettes, Aquasorb, and Sowing Time: Case Studies on Ceiba pentandra, Enterolobium cyclocarpum, and Calophyllum inophyllum. For. Sci. Technol. 2023, 19, 130–137. [Google Scholar] [CrossRef]
- SEMARNAT. NORMA Oficial Mexicana NOM-059-SEMARNAT-2010, Protección Ambiental-Especies Nativas de Mexico de Flora y Fauna Silvestres-Categorías de Riesgo y Especificaciones Para su Inclusión, Exclusión o Cambio-Lista de Especies en riesgo; Diario Oficial de la Federación: Mexico City, Mexico, 2010.
- CONAFOR. Reglas de Operación 2024 del Programa Desarrollo Forestal Sustentable para el Bienestar; DOF—Diario Oficial de la Federación: Mexico City, Mexico, 2023. [Google Scholar]
- SEMARNAT. Certificación de La Operación de Viveros Forestales-Norma Mexicana NMX-AA-170-SCFI-2016; Diario Oficial de la Federación (DOF): Mexico City, Mexico, 2016.
- Vozzo, J.A. Tropical Tree Seed Manual; FAO—SFM Tool Detail; Department of Agriculture, Forest Service: Washington, DC, USA, 2001.
- Quispe-Mamani, G.; Lelis Duarte, M.; de Almeida, L.S.; Filho, S.M. Non-parametric survivorship analysis in seed germination of forest species. J. Seed Sci. 2024, 46, e202446036. [Google Scholar] [CrossRef]
- Soriano, D.; Huante, P.; Gamboa-de Buen, A.; Orozco-Segovia, A. Seed Reserve Translocation and Early Seedling Growth of Eight Tree Species in a Tropical Deciduous Forest in Mexico. Plant Ecol. 2013, 214, 1361–1375. [Google Scholar] [CrossRef]
- Šerá, B.; Hnilička, F. Genetic and Environmental Factors Affecting Seed Germination. Plants 2023, 12, 4106. [Google Scholar] [CrossRef]
- Nery, F.C.; de Alvarenga, A.A.; Justo, C.F.; Dousseau, S.; Vieira, C.V. Effect of Temperature and Coat in the Germination of Calophyllum brasiliense Seeds. Ciênc. Agrotec. 2007, 31, 1872–1877. [Google Scholar] [CrossRef]
- Maldonado-Sánchez, E.A.; Ochoa-Gaona, S.; Ramos-Reyes, R.; Guadarrama-Olivera, M.d.L.Á.; González-Valdivia, N.; de Jong, B.H.J. La selva inundable de canacoite en Tabasco, Mexico, una comunidad vegetal amenazada. Acta Bot. Mex. 2016, 2016, 75–101. [Google Scholar] [CrossRef]
- Niembro-Rocas, A.; Vázquez-Torres, M.; Sánchez-Sánchez, O. Árboles de Veracruz. 100 Species for Strategical Reforestation; Gobierno del estado de Veracruz, Centro de Investigaciones Tropicales: Veracruz, Mexico, 2010; Volume 1, ISBN 978-607-33-0000-1. [Google Scholar]
- Viveros Viveros, H.; Hernández Palmeros, J.D.; Velasco García, M.V.; Robles Silva, R.; Ruiz Montiel, C.; Aparicio Rentería, A.; Martínez Hernández, M.D.J.; Hernández Villa, J.; Hernández Hernández, M.L. Análisis de semilla, tratamientos pregerminativos de Enterolobium cyclocarpum (Jacq.) Griseb. y su crecimiento inicial. Rev. Mex. Cienc. For. 2018, 6, 52–65. [Google Scholar] [CrossRef]
- Vargas Simón, G.; Pire, R.; Lázaro Dzul, M.O. Crecimiento plantular en condiciones de invernadero de la especie forestal Dialium guianense (Aubl.) Sandwith. Colomb. For. 2017, 21, 58–68. [Google Scholar] [CrossRef]
- Arceo-Gómez, T.M.; Robles-Díaz, E.; Manrique-Ortega, M.D.; Martínez-Campos, Á.R.; Aragón-Gastélum, J.L.; Aguirre-Crespo, F.J.; Ramírez-Albores, J.E.; Pérez-Suárez, M.; Robles, R.; Reyes-Trujeque, J.; et al. Pre-Germinative Treatments and Morphophysiological Traits in Enterolobium cyclocarpum and Piscidia piscipula (Fabaceae) from the Yucatan Peninsula, Mexico. Plants 2022, 11, 2844. [Google Scholar] [CrossRef]
- González-Di Pierro, A.M.; Benítez-Malvido, J.; Lombera, R. Germination Success of Large-Seeded Plant Species Ingested by Howler Monkeys in Tropical Rain Forest Fragments. Am. J. Bot. 2021, 108, 1625–1634. [Google Scholar] [CrossRef] [PubMed]
- SEMARNAT. Manual Para el Manejo de Germoplasma Forestal; Secretaría de Medio Ambiente y Recursos Naturales: Mexico City, Mexico, 2015.
- Dzib-Castillo, B.B.; Van Der Wal, H.; Chanatásig-Vaca, C.I.; Macario Mendoza, A.P.; Pat Fernández, M.J. Emergencia de plántulas de especies maderables nativas de la Península de Yucatán. Rev. Mex. Cienc. For. 2012, 3, 77–87. [Google Scholar] [CrossRef]
- Gurgel, E.S.C.; Dos Santos, J.U.M.; Lucas, F.C.A.; Bastos, M.D.N.D.C. Leguminosae Seedlings Morphology and the Systematic Potential. Rodriguésia 2012, 63, 065–073. [Google Scholar] [CrossRef]
- Silva, L.G.F.d.; Puntieri, J.G.; Melo, N.M.J.; Souza, J.P. Could the Presence of Preformed Leaves inside the Seed Be More Important than Seed Size for the Establishment and Growth of Cerrado Seedlings? Acta Bot. Brasilica 2023, 37, e20230119. [Google Scholar] [CrossRef]
- de Jesus, V.A.M.; Braccini, A.L.; de Souza, L.A.; Moscheta, I.S.; Mariucci, G.E.G.; Santos, F.L. Morphology and Anatomy of the Seedling and the Tirodendro of Calophyllum brasiliense Cambess. (Clusiaceae). Acta Sci. Biol. Sci. 2014, 36, 443–449. [Google Scholar] [CrossRef]
- Domínguez-Liévano, A.; Aguilera-Rodríguez, M.; Espinosa-Zaragoza, S.; Aldrete, A.; Wong-Villarreal, A.; Pérez- de la O, N.B. Sustratos y fertilización para producir planta de Swietenia macrophylla King y Tabebuia donnell-smithii Rose en charolas. Rev. Mex. Cienc. For. 2023, 14, 56–75. [Google Scholar] [CrossRef]
- da Silva, R.C.; Belniaki, A.C.; Vieira, E.S.N.; Cuquel, F.L.; Panobianco, M. Subsidies for Propagation of Native Species in Brazil with Medicinal Potential: Calophyllum brasiliense Cambess. J. Seed Sci. 2019, 41, 318–327. [Google Scholar] [CrossRef]
- Maulidya, A.; Suwignyo, R.A.; Priadi, D.P.; Baral, H.; Choi, E.; Adriansyah, F.; Yang, H. Survivorship and Growth Performance of Calophyllum inophyllum L. Seedlings in Peat Soil and at Different Levels of Groundwater. Land 2024, 13, 879. [Google Scholar] [CrossRef]
- Osaigbovo, A.U.; Nwaoguala, C.N.C. Growth Response of Black Velvet Tamarind (Dialium guineense Willd) Seedling to Different Potting Media. J. Appl. Nat. Sci. 2011, 3, 166–170. [Google Scholar] [CrossRef]
- Le Bec, J.; Courbaud, B.; Le Moguédec, G.; Pélissier, R. Characterizing Tropical Tree Species Growth Strategies: Learning from Inter-Individual Variability and Scale Invariance. PLoS ONE 2015, 10, e0117028. [Google Scholar] [CrossRef] [PubMed]
- Boege, K.; Dirzo, R. Intraspecific Variation in Growth, Defense and Herbivory in Dialium guianense (Caesalpiniaceae) Mediated by Edaphic Heterogeneity. Plant Ecol. 2004, 175, 59–69. [Google Scholar] [CrossRef]
- Aguirre-Medina, J.F.; Culebro-Cifuentes, F.; Cadena-Iñiguez, J.; Aguirre-Cadena, J.F. Crecimiento de Tabebuia donnell-smithii Rose inoculada con hongos micorrízicos y Azospirillum brasilense. Agrociencia 2014, 48, 331–345. [Google Scholar]
- Saraiva, G.F.R.; Souza, G.M.; Rodrigues, J.D. Aclimatação e Fisiologia de Mudas de Guanandi Cultivadas Em Telas de Sombreamento Foto-Protetoras. Colloq. Agrar. 2014, 10, 01–10. [Google Scholar] [CrossRef]
- Budiadi, B.; Widiyatno, W.; Nurjanto, H.H.; Hasani, H.; Jihad, A.N. Seedling Growth and Quality of Avicennia marina (Forssk.) Vierh. under Growth Media Composition and Controlled Salinity in an Ex Situ Nursery. Forests 2022, 13, 684. [Google Scholar] [CrossRef]
- Tamayo-Chim, M.; Reyes-García, C.; Orellana, R. A Combination of Forage Species with Different Responses to Drought Can Increase Year-Round Productivity in Seasonally Dry Silvopastoral Systems. Agrofor. Syst. 2012, 84, 287–297. [Google Scholar] [CrossRef]
- Lisboa, A.C.; dos Santos, P.S.; Neto, S.N.d.O.; De Castro, D.N.; De Abreu, A.H.M. Effect of Volume of Tubes on the Production of Seedlings of Calophyllum brasiliense and Toona ciliata. Rev. Árvore 2012, 36, 603–609. [Google Scholar] [CrossRef]
- Turchetto, F.; Araujo, M.M.; Tabaldi, L.A.; Griebeler, A.M.; Rorato, D.G.; Aimi, S.C.; Berghetti, Á.L.P.; Gomes, D.R. Can Transplantation of Forest Seedlings Be a Strategy to Enrich Seedling Production in Plant Nurseries? For. Ecol. Manage 2016, 375, 96–104. [Google Scholar] [CrossRef]
- Ramos, M.B.P.; Ferraz, I.D.K. Estudos Morfológicos de Frutos, Sementes e Plântulas de Enterolobium schomburgkii Benth. (Leguminosae-Mimosoideae). Rev. Bras. Botânica 2008, 31, 227–235. [Google Scholar] [CrossRef]
- Corby, H.D.L.; Smith, D.L.; Sprent, J.I. Size, Structure and Nitrogen Content of Seeds of Fabaceae in Relation to Nodulation. Bot. J. Linn. Soc. 2011, 167, 251–280. [Google Scholar] [CrossRef]
- Sprent, J.I. Legume Nodulation: A Global Perspective; Wiley-Blackwell: Singapore, 2009; ISBN 9781405181754. [Google Scholar]
- Jardim, I.N.; Matos, M.L.; Rosário, M.O.; Hamada, M.O.S. Osmocote® Proporciona Melhores Mudas de Calophyllum brasiliense Cambess. Sci. Plena 2023, 19, 1–14. [Google Scholar] [CrossRef]
- Luna-Flores, W. Effect of Water Stress on Growth and Water Use Efficiency of Tree Seedlings of Three Deciduous Species. Rev. Terra Latinoam. 2012, 30, 343–353. [Google Scholar]
- Chaturvedi, R.K.; Raghubanshi, A.S.; Singh, J.S. Growth of Tree Seedlings in a Tropical Dry Forest in Relation to Soil Moisture and Leaf Traits. J. Plant Ecol. 2013, 6, 158–170. [Google Scholar] [CrossRef]
- Pommerening, A.; Muszta, A. Relative Plant Growth Revisited: Towards a Mathematical Standardisation of Separate Approaches. Ecol. Modell. 2016, 320, 383–392. [Google Scholar] [CrossRef]
- Griscom, H.P.; Ashton, P.M.S.; Berlyn, G.P. Seedling Survivorship and Growth of Native Tree Species in Pastures: Implications for Dry Tropical Forest Rehabilitation in Central Panama. For. Ecol. Manage 2005, 218, 306–318. [Google Scholar] [CrossRef]
- da Trindade-Lessa, B.F.; Nobre-de Almeida, P.J.P.; Lobo-Pinheiro, C.; Melo-Gomes, F.; Medeiros-Filho, S. Germination and Seedling Growth of Enterolobium contortisiliquum as a Function of Seed Weight and Temperature and Light Conditions. Agrociencia 2015, 49, 315–327. [Google Scholar]
- Rafdinal, R.; Mukhtar, E.; Syamsuardi, S.; Hermansah, H. Survivorship and Growth Rate of Several Climax Species of Tree in Tropical Rains Forest Ulu Gadut West Sumatra Indonesia. Pak. J. Biol. Sci. 2014, 17, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Philipson, C.D.; Dent, D.H.; O’Brien, M.J.; Chamagne, J.; Dzulkifli, D.; Nilus, R.; Philips, S.; Reynolds, G.; Saner, P.; Hector, A. A Trait-Based Trade-off between Growth and Mortality: Evidence from 15 Tropical Tree Species Using Size-Specific Relative Growth Rates. Ecol. Evol. 2014, 4, 3675–3688. [Google Scholar] [CrossRef]
- Viani, R.A.G.; Rodrigues, R.R. Survivorship in Nursery of Native Species Saplings Obtained from Natural Regeneration of Forest Fragments. Pesqui. Agropecu. Bras. 2007, 42, 1067–1075. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Dalling, J.W.; Gallery, R.E.; Maddison, D.R.; Davis, E.C.; Gibson, C.M.; Arnold, A.E. Diversity and Evolutionary Origins of Fungi Associated with Seeds of a Neotropical Pioneer Tree: A Case Study for Analysing Fungal Environmental Samples. Mycol. Res. 2009, 113, 432–449. [Google Scholar] [CrossRef]






| Species | Stem Height (cm) | Stem Basal Diameter (mm) | Number of Eophylls |
|---|---|---|---|
| Calophyllum brasiliensis | 22.0 b | 2.6 d | 8.8 a |
| Bravaisia integerrima | 14.5 cd | 5.0 a | 7.1 b |
| Dialium guianense | 13.1 de | 1.9 e | 7.2 b |
| Piscidia piscipula | 16.3 c | 3.0 cd | 3.9 d |
| Roseodendron donnell-smithii | 11.6 e | 4.2 b | 7.7 ab |
| Enterolobium cyclocarpum | 38.0 a | 3.1 c | 6.0 c |
| Species | Stem Height SRGR (cm cm−1 day−1) | Stem Basal Diameter DRGR (cm cm−1 day−1) |
|---|---|---|
| Calophyllum brasiliensis | 0.0203 ab | 0.0076 a |
| Bravaisia integerrima | 0.0179 b | 0.0108 a |
| Dialium guianense | 0.0154 c | 0.0057 a |
| Piscidia piscipula | 0.0091 d | 0.0080 a |
| Roseodendron donnell-smithii | 0.0147 c | 0.0098 a |
| Enterolobium cyclocarpum | 0.0210 a | 0.0101 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas-Simón, G.; Domínguez-Domínguez, M.; Pire, R.; Martínez-Zurimendi, P. Functional Morphology and Early Growth of Seedlings of Tropical Species. Ecologies 2025, 6, 69. https://doi.org/10.3390/ecologies6040069
Vargas-Simón G, Domínguez-Domínguez M, Pire R, Martínez-Zurimendi P. Functional Morphology and Early Growth of Seedlings of Tropical Species. Ecologies. 2025; 6(4):69. https://doi.org/10.3390/ecologies6040069
Chicago/Turabian StyleVargas-Simón, Georgina, Marivel Domínguez-Domínguez, Reinaldo Pire, and Pablo Martínez-Zurimendi. 2025. "Functional Morphology and Early Growth of Seedlings of Tropical Species" Ecologies 6, no. 4: 69. https://doi.org/10.3390/ecologies6040069
APA StyleVargas-Simón, G., Domínguez-Domínguez, M., Pire, R., & Martínez-Zurimendi, P. (2025). Functional Morphology and Early Growth of Seedlings of Tropical Species. Ecologies, 6(4), 69. https://doi.org/10.3390/ecologies6040069








