The Diversity Indices of Culturable Bacteria from the Rhizosphere of Pennisetum clandestinum and Pseudelephantopus spicatus in Urban Soil
Abstract
1. Introduction
2. Materials and Methods
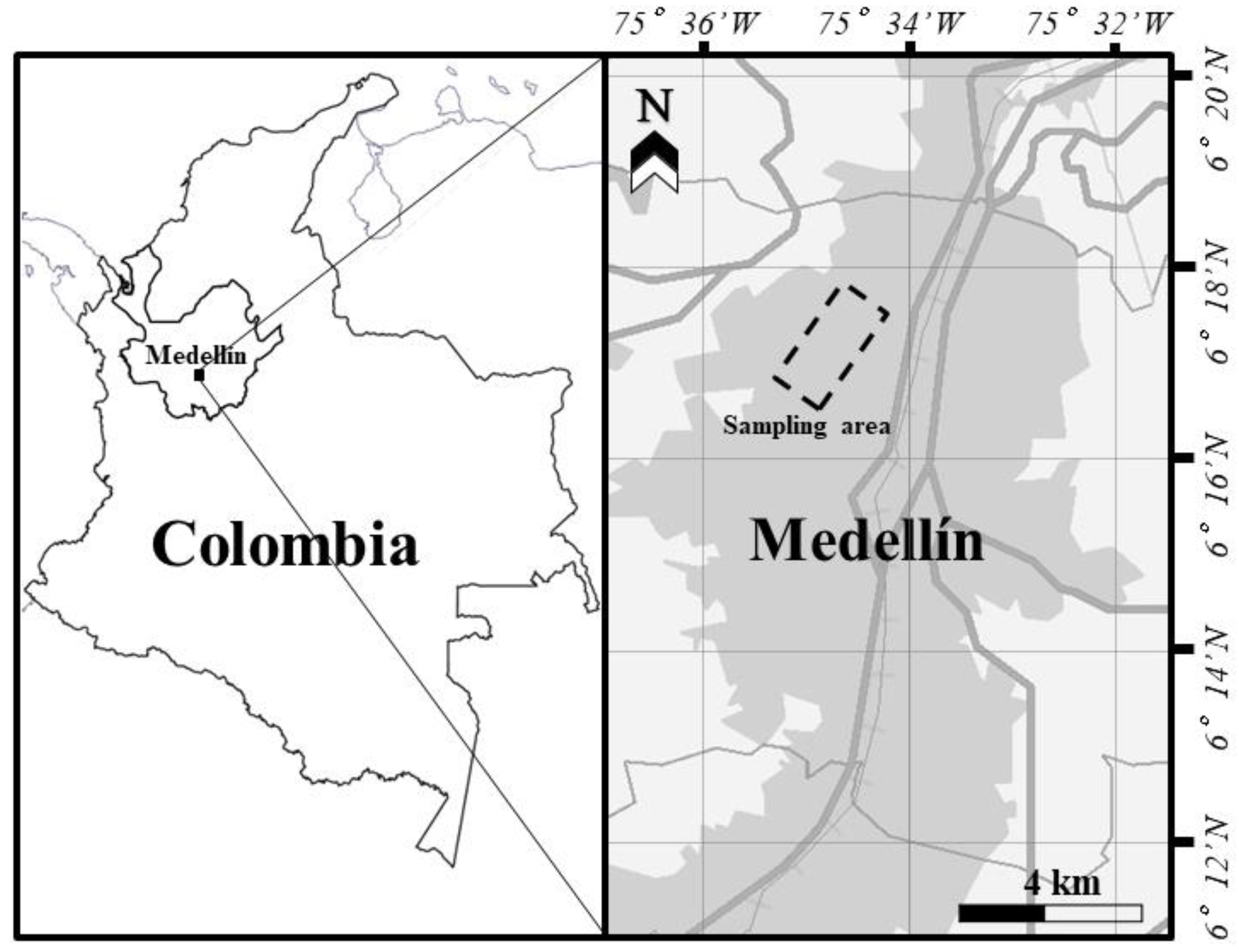
2.1. Study Area and Sampling
2.2. Soil Analyses and Microorganisms Culture
2.3. Statistics and Microbial Diversity Analyses
3. Results and Discussion
3.1. Soil Characteristics
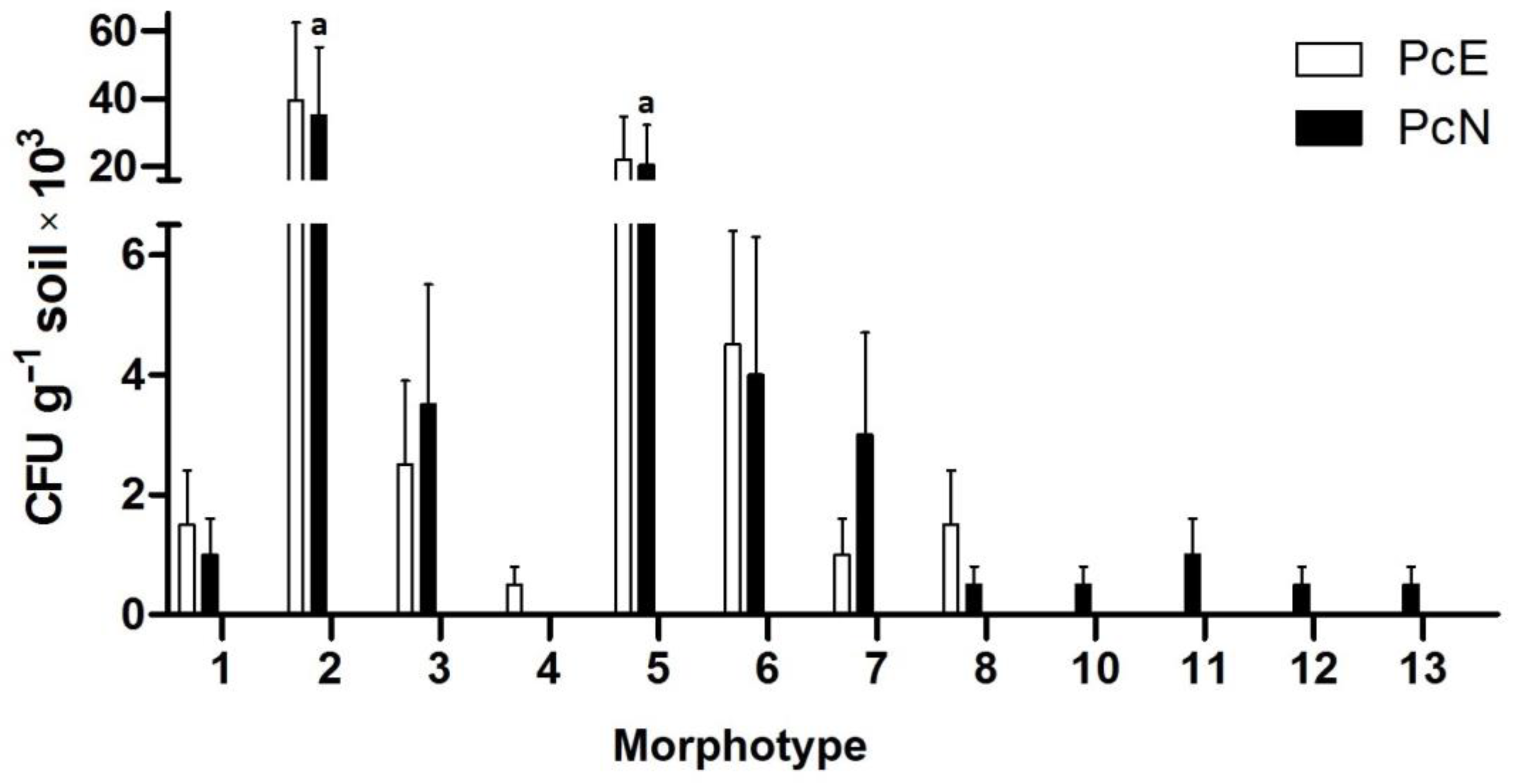
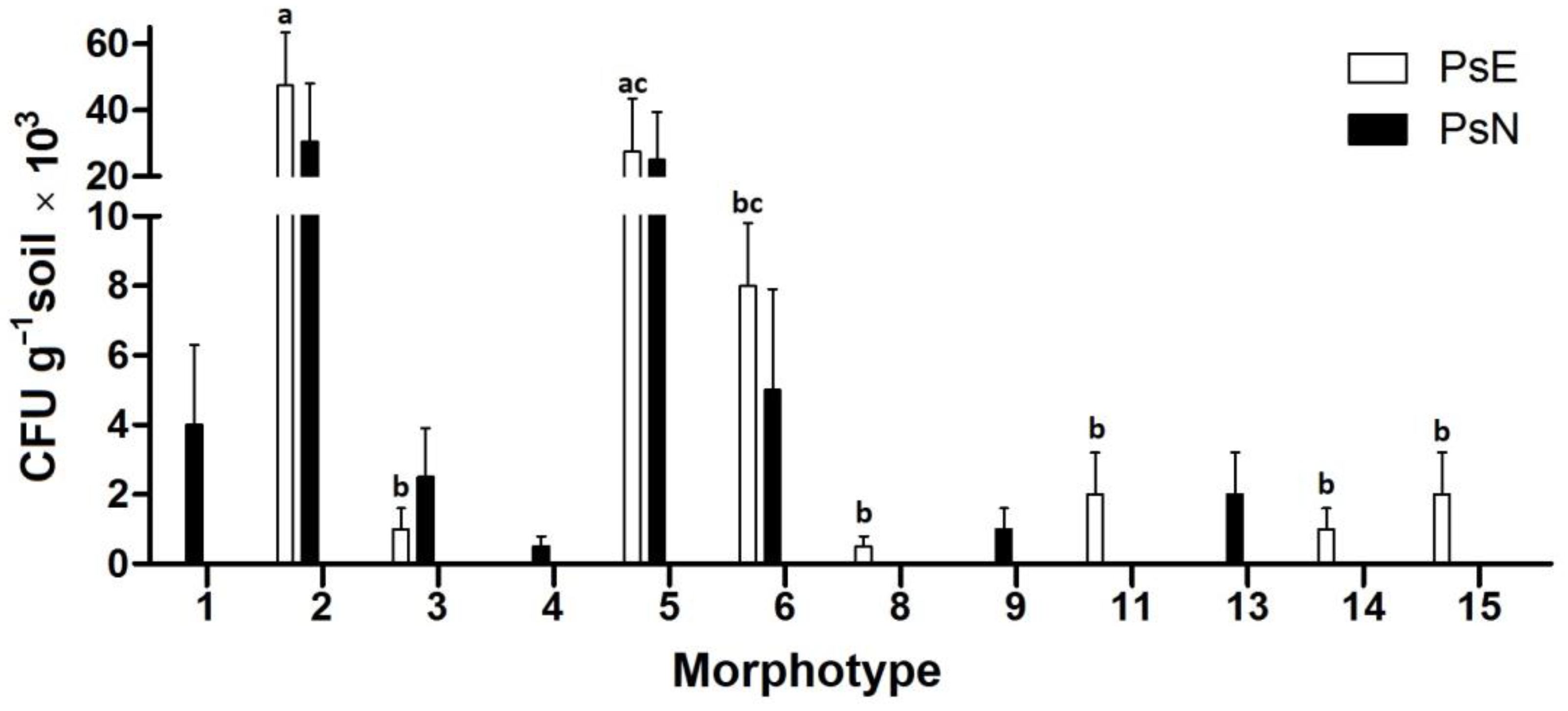
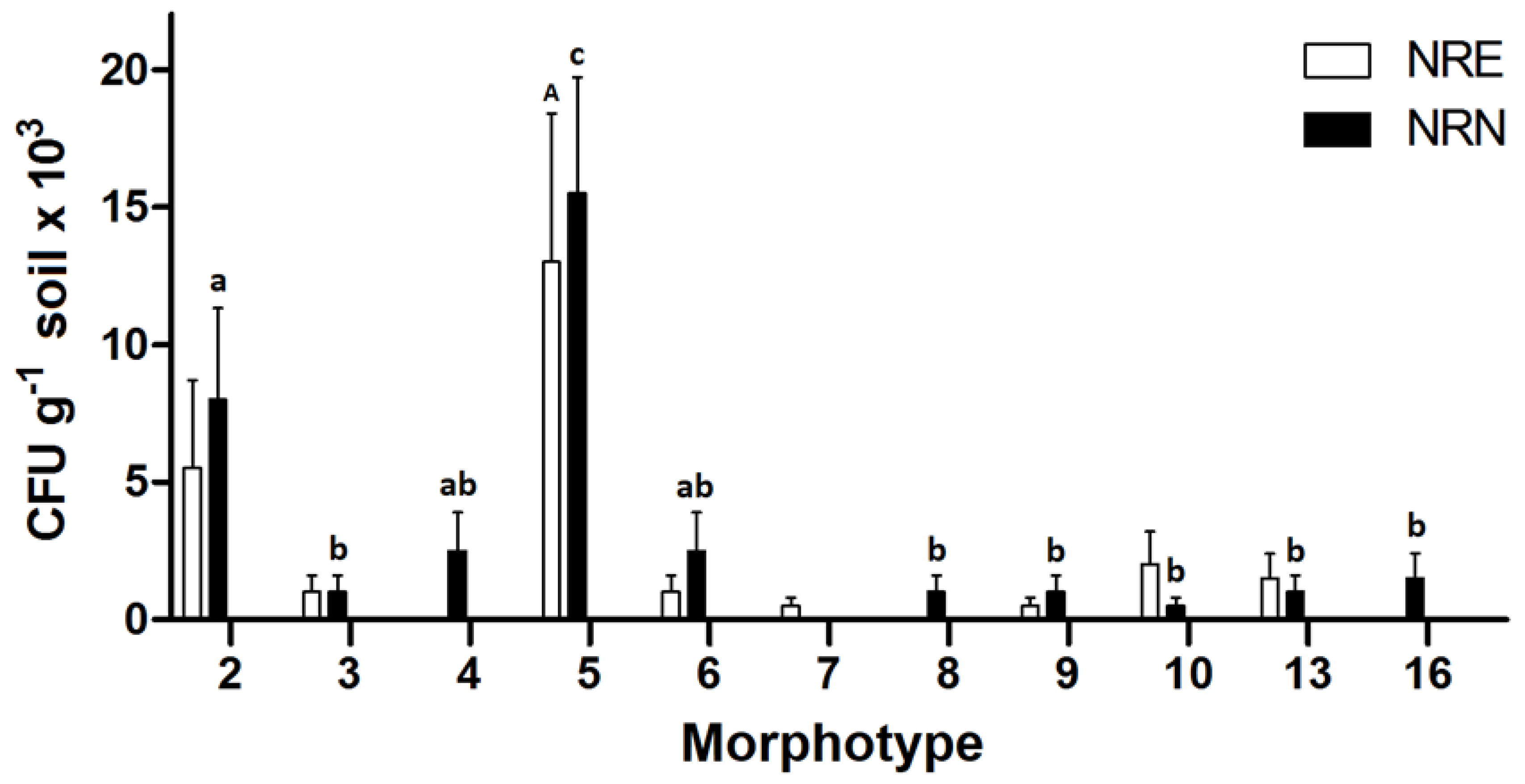
3.2. Description of Culturable Microorganisms
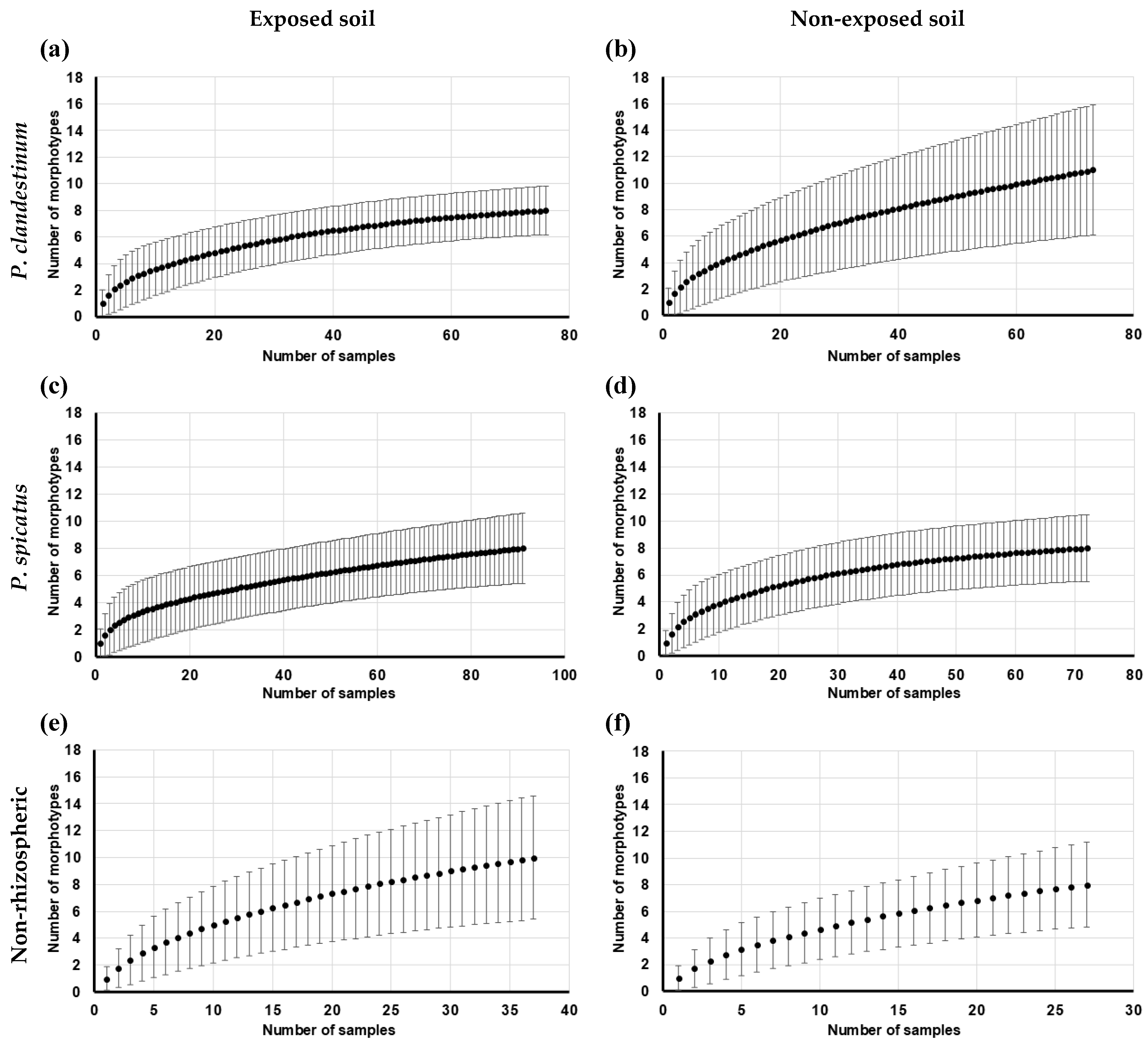
3.3. Rarefaction Assessment
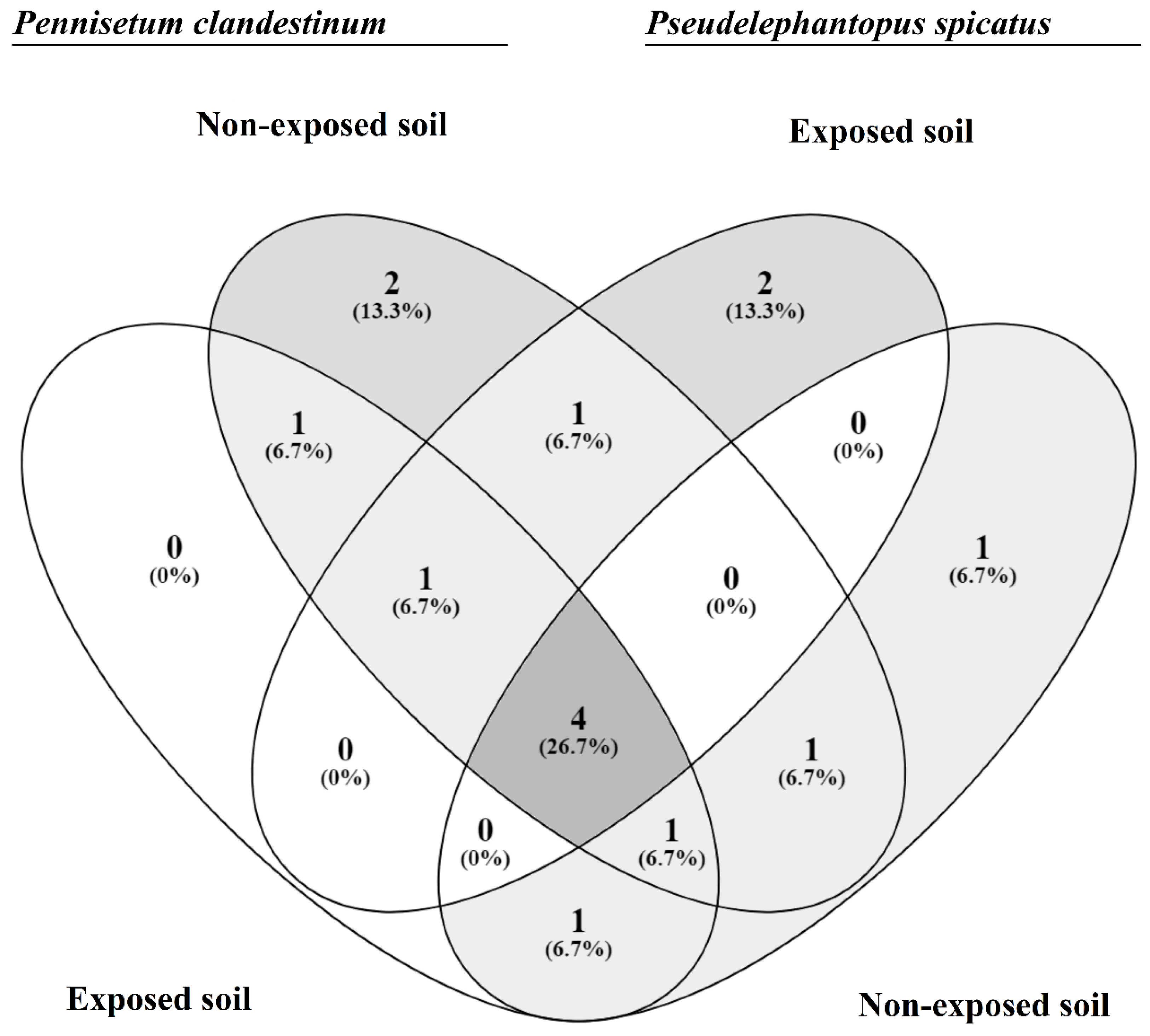
3.4. Microbial Diversity Indices and Community Comparisons
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- FAO; ITPS. Status of the World’s Soil Resources (SWSR)—Main Report; FAO: Rome, Italy, 2015; ISBN 9789251090046. [Google Scholar]
- FAO; UNEP Global Assessment of Soil Pollution; FAO; UNEP: Rome, Italy, 2021; ISBN 978-92-5-134469-9.
- FAO; ITPS; GSBI; SCBD; EC. State of Knowledge of Soil Biodiversity—Status, Challenges and Potentialities; FAO: Rome, Italy, 2020; ISBN 978-92-5-133582-6. [Google Scholar]
- Maron, P.A.; Sarr, A.; Kaisermann, A.; Lévêque, J.; Mathieu, O.; Guigue, J.; Karimi, B.; Bernard, L.; Dequiedt, S.; Terrat, S.; et al. High Microbial Diversity Promotes Soil Ecosystem Functioning. Appl. Environ. Microbiol. 2018, 84, e02738-17. [Google Scholar] [CrossRef] [PubMed]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Eida, A.A.; Ziegler, M.; Lafi, F.F.; Michell, C.T.; Voolstra, C.R.; Hirt, H.; Saad, M.M. Desert Plant Bacteria Reveal Host Influence and Beneficial Plant Growth Properties. PLoS ONE 2018, 13, e0208223. [Google Scholar] [CrossRef] [PubMed]
- Dhankher, O.P.; Foyer, C.H. Climate Resilient Crops for Improving Global Food Security and Safety. Plant Cell Environ. 2018, 41, 877–884. [Google Scholar] [CrossRef]
- Yan, B.; Li, J.; Xiao, N.; Qi, Y.; Fu, G.; Liu, G.; Qiao, M. Urban-Development-Induced Changes in the Diversity and Composition of the Soil Bacterial Community in Beijing. Sci. Rep. 2016, 6, 38811. [Google Scholar] [CrossRef]
- Beltrán-Pineda, M.E.; Rocha-Gil, Z.E.; Bernal-Figueroa, A.A.; Pita-Morales, L.A. Functional Microorganisms in Soil with and without Revegetation in the Municipality of Villa de Leyva-Boyaca. Colomb. For. 2017, 20, 158–170. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, J.; Lu, J.; Sun, Y. Study on the Influence of Soil Microbial Community on the Long-Term Heavy Metal Pollution of Different Land Use Types and Depth Layers in Mine. Ecotoxicol. Environ. Saf. 2019, 170, 218–226. [Google Scholar] [CrossRef]
- Yrjälä, K.; Keskinen, A.K.; Åkerman, M.L.; Fortelius, C.; Sipilä, T.P. The Rhizosphere and PAH Amendment Mediate Impacts on Functional and Structural Bacterial Diversity in Sandy Peat Soil. Environ. Pollut. 2010, 158, 1680–1688. [Google Scholar] [CrossRef]
- Jacobsen, C.S.; Hjelmsø, M.H. Agricultural Soils, Pesticides and Microbial Diversity. Curr. Opin. Biotechnol. 2014, 27, 15–20. [Google Scholar] [CrossRef]
- Guo, M.; Gong, Z.; Miao, R.; Rookes, J.; Cahill, D.; Zhuang, J. Microbial Mechanisms Controlling the Rhizosphere Effect of Ryegrass on Degradation of Polycyclic Aromatic Hydrocarbons in an Aged-Contaminated Agricultural Soil. Soil Biol. Biochem. 2017, 113, 130–142. [Google Scholar] [CrossRef]
- Hill, G.T.; Mitkowski, N.A.; Aldrich-Wolfe, L.; Emele, L.R.; Jurkonie, D.D.; Ficke, A.; Maldonado-Ramirez, S.; Lynch, S.T.; Nelson, E.B. Methods for Assessing the Composition and Diversity of Soil Microbial Communities. Appl. Soil Ecol. 2000, 15, 25–36. [Google Scholar] [CrossRef]
- Kirk, J.L.; Beaudette, L.A.; Hart, M.; Moutoglis, P.; Klironomos, J.N.; Lee, H.; Trevors, J.T. Methods of Studying Soil Microbial Diversity. J. Microbiol. Methods 2004, 58, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Alain, K.; Querellou, J. Cultivating the Uncultured: Limits, Advances and Future Challenges. Extremophiles 2009, 13, 583–594. [Google Scholar] [CrossRef]
- Stefani, F.O.P.; Bell, T.H.; Marchand, C.; De La Providencia, I.E.; El Yassimi, A.; St-Arnaud, M.; Hijri, M. Culture-Dependent and -Independent Methods Capture Different Microbial Community Fractions in Hydrocarbon-Contaminated Soils. PLoS ONE 2015, 10, e0128272. [Google Scholar] [CrossRef]
- Hill, T.C.J.; Walsh, K.A.; Harris, J.A.; Moffett, B.F. Using Ecological Diversity Measures with Bacterial Communities. FEMS Microbiol. Ecol. 2003, 43, 1–11. [Google Scholar] [CrossRef]
- Hughes, J.B.; Hellmann, J.J.; Ricketts, T.H.; Bohannan, B.J.M. Counting the Uncountable: Statistical Approaches to Estimating Microbial Diversity. Appl. Environ. Microbiol. 2001, 67, 4399–4406. [Google Scholar] [CrossRef]
- Orlóci, L.; Anand, M.; Pillar, V.D. Biodiversity Analysis: Issues, Concepts, Techniques. Community Ecol. 2002, 3, 217–236. [Google Scholar] [CrossRef]
- Li, H.; Su, J.Q.; Yang, X.R.; Zhu, Y.G. Distinct Rhizosphere Effect on Active and Total Bacterial Communities in Paddy Soils. Sci. Total Environ. 2019, 649, 422–430. [Google Scholar] [CrossRef]
- Chandra, P.; Dhuli, P.; Verma, P.; Singh, A.; Choudhary, M.; Prajapat, K.; Rai, A.K.; Yadav, R.K. Culturable Microbial Diversity in the Rhizosphere of Different Biotypes under Variable Salinity. Trop. Ecol. 2020, 61, 291–300. [Google Scholar] [CrossRef]
- García-Villaraco Velasco, A.; Probanza, A.; Gutierrez Mañero, F.J.; Ramos, B.; Lucas García, J.A. Functional Diversity of Rhizosphere Microorganisms from Different Genotypes of Arabidopsis Thaliana. Community Ecol. 2009, 10, 111–119. [Google Scholar] [CrossRef]
- Aislabie, J.; Julie, R. Deslippe Soil Microbes and Their Contribution to Ecosystem Services. In Ecosystem Services in New Zealand—Conditions and Trends; Dymond, J., Ed.; Manaaki Whenua Press: Lincoln, New Zealand, 2013; pp. 143–161. ISBN 9780478347364. [Google Scholar]
- López, J.E.; Gallego, J.L.; Vargas-Ruiz, A.; Peña-Mosquera, A.L.; Zapata-Zapata, A.D.; López-Sánchez, I.J.; Botero-Botero, L.R. Aspergillus tubingensis and Talaromyces islandicus Solubilize Rock Phosphate Under Saline and Fungicide Stress and Improve Zea Mays Growth and Phosphorus Nutrition. J. Soil Sci. Plant Nutr. 2020, 20, 2490–2501. [Google Scholar] [CrossRef]
- Wozniak, G.; Markowicz, A.; Borymski, S.; Piotrowska-Seget, Z.; Chmura, D.; Besenyei, L. The Relationship between Successional Vascular Plant Assemblages and Associated Microbial Communities on Coal Mine Spoil Heaps. Community Ecol. 2015, 16, 23–32. [Google Scholar] [CrossRef]
- Arias, M.E.; González-Pérez, J.A.; González-Vila, F.J.; Ball, A.S. Soil Health—A New Challenge for Microbiologists and Chemists. Int. Microbiol. 2005, 8, 13–21. [Google Scholar]
- Sousa, A.M.; Machado, I.; Nicolau, A.; Pereira, M.O. Improvements on Colony Morphology Identification towards Bacterial Profiling. J. Microbiol. Methods 2013, 95, 327–335. [Google Scholar] [CrossRef]
- Overmann, J.; Abt, B.; Sikorski, J. Present and Future of Culturing Bacteria. Annu. Rev. Microbiol. 2017, 71, 711–730. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Mucha, A.P.; Tomasino, M.P.; Almeida, C.M.R.; Carvalho, M.F. Combining Culture-Dependent and Independent Approaches for the Optimization of Epoxiconazole and Fludioxonil-Degrading Bacterial Consortia. Microorganisms 2021, 9, 2109. [Google Scholar] [CrossRef]
- Seyedpour Layalestani, S.S.; Shavandi, M.; Haddadi, A.; Amoozegar, M.A.; Dastgheib, S.M.M. Analysis of Bacterial Diversity during Bioremediation of Gasoil-Contaminated Soils with Different Salinities. Soil Sediment Contam. 2021, 30, 622–638. [Google Scholar] [CrossRef]
- Hinsu, A.; Dumadiya, A.; Joshi, A.; Kotadiya, R.; Andharia, K.; Koringa, P.; Kothari, R. To Culture or Not to Culture: A Snapshot of Culture-Dependent and Culture-Independent Bacterial Diversity from Peanut Rhizosphere. PeerJ 2021, 9, e12035. [Google Scholar] [CrossRef]
- Thrash, J.C. Culturing the Uncultured: Risk versus Reward. mSystems 2019, 4, e00130-19. [Google Scholar] [CrossRef]
- Ajmone-Marsan, F.; Certini, G.; Scalenghe, R. Describing Urban Soils through a Faceted System Ensures More Informed Decision-Making. Land Use Policy 2016, 51, 109–119. [Google Scholar] [CrossRef]
- Birt, H.W.G.; Bonnett, S.A.F. Microbial Mechanisms of Carbon and Nitrogen Acquisition in Contrasting Urban Soils. Eur. J. Soil Biol. 2018, 88, 1–7. [Google Scholar] [CrossRef]
- Thompson, G.L.; Kao-Kniffin, J. Diversity Enhances NPP, N Retention, and Soil Microbial Diversity in Experimental Urban Grassland Assemblages. PLoS ONE 2016, 11, e0155986. [Google Scholar] [CrossRef] [PubMed]
- Christel, A.; Dequiedt, S.; Chemidlin-Prevost-Bouré, N.; Mercier, F.; Tripied, J.; Comment, G.; Djemiel, C.; Bargeot, L.; Matagne, E.; Fougeron, A.; et al. Urban Land Uses Shape Soil Microbial Abundance and Diversity. Sci. Total Environ. 2023, 883, 163455. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Villada, C.; Garizábal-Carmona, J.A.; Martínez-Arias, V.M.; Mancera-Rodríguez, N.J. Built vs. Green Cover: An Unequal Struggle for Urban Space in Medellín (Colombia). Urban Ecosyst. 2024, 27, 1055–1065. [Google Scholar] [CrossRef]
- Montoya-Tangarife, C.; Villamizar Duarte, N.; Jorquera Guajardo, F.; Cardenas, M.F.; Giraldo-Ospina, T. Accessibility to Public Spaces: Boosting Ecosystem Services in Urban Areas in Four Latin American Cities. Front. Sustain. Cities 2022, 4, 796122. [Google Scholar] [CrossRef]
- Garcia Ferrari, S.; Smith, H.; Coupe, F.; Rivera, H. City Profile: Medellin. Cities 2018, 74, 354–364. [Google Scholar] [CrossRef]
- Mondragón, V.; Hurtado, F.M.; Jaramillo Jaramillo, D.F. Soil Organic Carbon Stocks and Properties Are Affected by Plant Cover Types in an Urban Ecosystem in Colombia. S. Afr. J. Plant Soil 2022, 39, 322–330. [Google Scholar] [CrossRef]
- Zhang, F.; Qian, H. A Comprehensive Review of the Environmental Benefits of Urban Green Spaces. Environ. Res. 2024, 252, 118837. [Google Scholar] [CrossRef]
- Canizales, S.A.; Celemín, J.S.; Mora-Delgado, J. Diversity and Uses of Weeds on Pastures of Livestock Farms in the Department of Tolima (Colombia). Zootec. Trop. 2010, 28, 427–437. [Google Scholar]
- Jimenez Usuga, N.D.S.; Malafronte, N.; Durango, E.J.O.; Braca, A.; De Tommasi, N. Phytochemical Investigation of Pseudelephantopus spiralis (Less.) Cronquist. Phytochem. Lett. 2016, 15, 256–259. [Google Scholar] [CrossRef]
- Sánchez, D.B.; Gómez, R.M.; García, A.M.; Bonilla, R.R. Phosphate Solubilizing Bacteria Isolated from Pennisetum clandestinum Associate to Livestock Systems in the Andean Area. Rev. U.D.C.A Actual. Divulg. Científica Divulg. Científica 2014, 17, 423–431. [Google Scholar]
- Schmidt, D.J.E.; Pouyat, R.; Szlavecz, K.; Setälä, H.; Kotze, D.J.; Yesilonis, I.; Cilliers, S.; Hornung, E.; Dombos, M.; Yarwood, S.A. Urbanization Erodes Ectomycorrhizal Fungal Diversity and May Cause Microbial Communities to Converge. Nat. Ecol. Evol. 2017, 1, 123. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.S.; Lee, A.; McGuire, K.L. Phylogenetic and Functional Diversity of Total (DNA) and Expressed (RNA) Bacterial Communities in Urban Green Infrastructure Bioswale Soils. Appl. Environ. Microbiol. 2017, 83, e00287-17. [Google Scholar] [CrossRef]
- Charlop-Powers, Z.; Pregitzer, C.C.; Lemetre, C.; Ternei, M.A.; Maniko, J.; Hover, B.M.; Calle, P.Y.; McGuire, K.L.; Garbarino, J.; Forgione, H.M.; et al. Urban Park Soil Microbiomes Are a Rich Reservoir of Natural Product Biosynthetic Diversity. Proc. Natl. Acad. Sci. USA 2016, 113, 14811–14816. [Google Scholar] [CrossRef]
- Wang, H.; Cheng, M.; Dsouza, M.; Weisenhorn, P.; Zheng, T.; Gilbert, J.A. Soil Bacterial Diversity Is Associated with Human Population Density in Urban Greenspaces. Environ. Sci. Technol. 2018, 52, 5115–5124. [Google Scholar] [CrossRef]
- Xie, F.; Pathom-aree, W. Actinobacteria from Desert: Diversity and Biotechnological Applications. Front. Microbiol. 2021, 12, 765531. [Google Scholar] [CrossRef]
- Díaz-Rodríguez, A.M.; Chávez-Luzanía, R.A.; Rojas Anaya, E.; Villalobos, S.; de los, S. Bioprospection of Beneficial Bacteria for Bioproducts Innovation in Agriculture. In New Insights, Trends, and Challenges in the Development and Applications of Microbial Inoculants in Agriculture; Academic Press: Cambridge, MA, USA, 2024; pp. 53–58. [Google Scholar] [CrossRef]
- Sprocati, A.R.; Alisi, C.; Tasso, F.; Fiore, A.; Marconi, P.; Langella, F.; Haferburg, G.; Nicoara, A.; Neagoe, A.; Kothe, E. Bioprospecting at Former Mining Sites across Europe: Microbial and Functional Diversity in Soils. Environ. Sci. Pollut. Res. 2014, 21, 6824–6835. [Google Scholar] [CrossRef]
- Baltz, R.H. Natural Product Drug Discovery in the Genomic Era: Realities, Conjectures, Misconceptions, and Opportunities. J. Ind. Microbiol. Biotechnol. 2019, 46, 281–299. [Google Scholar] [CrossRef]
- Hurtado Moreno, G.; González, O.C.; Cadena, M.; Benavides, H.; Rúiz, F.; Montealegre, E.; Ortiz Royero, J.C.; Montoya Ramírez, R.D. Atlas Climatológico de Colombia, 2nd ed.; Instituto de Hidrología Meteorología y Estudios Ambientales IDEAM: Bogotá, Colombia, 2017; ISBN 978-958-8067-95-7. [Google Scholar]
- IGAC. Suelos y Tierras de Colombia, 1st ed.; Instituto Geográfico Agustín Codazzi: Bogotá, Colombia, 2016; ISBN 9789588323831. [Google Scholar]
- García Castellanos, L.E.; Díaz Ávila, J.H.; Burgos Revelo, L.A.; Ortíz; Peña, L.E.; González Nivia, J.; Vera Raigosa, D.F.; Martínez Burgos, R.; Peña Hernández, G.; Fabían, R.; et al. Estudio General de Suelos y Zonificacion de Tierras: Departamento de Antioquia, 1st ed.; Codazzi., I.G.A., Ed.; Imprenta Nacional de Colombia: Bogota, Colombia, 2007; ISBN 9789588323077. [Google Scholar]
- Barillot, C.D.C.; Sarde, C.O.; Bert, V.; Tarnaud, E.; Cochet, N. A Standardized Method for the Sampling of Rhizosphere and Rhizoplan Soil Bacteria Associated to a Herbaceous Root System. Ann. Microbiol. 2013, 63, 471–476. [Google Scholar] [CrossRef]
- Gallego, J.L.; Olivero-Verbel, J. Cytogenetic Toxicity from Pesticide and Trace Element Mixtures in Soils Used for Conventional and Organic Crops of Allium cepa L. Environ. Pollut. 2021, 276, 116558. [Google Scholar] [CrossRef]
- Walkley, A.; Black, I.A. An Examination of the Degtjareff Method for Determining Soil Organic Matter, and a Proposed Modification of the Chromic Acid Titration Method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Margalef, R. Information Theory in Ecology. Gen. Syst. 1958, 3, 36–71. [Google Scholar]
- Berger, W.H.; Parker, F.L. Diversity of Planktonic foraminifera in Deep-Sea Sediments. Science 1970, 168, 1345–1347. [Google Scholar] [CrossRef]
- Pielou, E.C. The Measurement of Diversity in Different Types of Biological Collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Menhinick, E.F. A Comparison of Some Species-Individuals Diversity Indices Applied to Samples of Field Insects. Ecology 1964, 45, 859–861. [Google Scholar] [CrossRef]
- Colwell, R. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples; Version 8.2.0; University of Connecticut: Stors, CT, USA, 2009. [Google Scholar]
- Sanjerehei, M.M.; Rundel, P.W. A Comparison of Methods for Detecting Association between Plant Species. Ecol. Inform. 2020, 55, 101034. [Google Scholar] [CrossRef]
- Oliveros, J.C. VENNY. An Interactive Tool for Comparing Lists with Venn Diagrams. 2007. Available online: https://bioinfogp.cnb.csic.es/tools/venny/ (accessed on 17 January 2025).
- Håkansson, I.; Lipiec, J. A Review of the Usefulness of Relative Bulk Density Values in Studies of Soil Structure and Compaction. Soil Tillage Res. 2000, 53, 71–85. [Google Scholar] [CrossRef]
- Reynolds, W.D.; Bowman, B.T.; Drury, C.F.; Tan, C.S.; Lu, X. Indicators of Good Soil Physical Quality: Density and Storage Parameters. Geoderma 2002, 110, 131–146. [Google Scholar] [CrossRef]
- Osorio, N.W. Como Interpretar Los Resultados Del Análisis de Fertilidad Del Suelo. Boletín Manejo Integr. Suelo Nutr. Veg. 2012, 1, 1–3. [Google Scholar]
- Marais, J.P. Factors Affecting the Nutritive Value of Kikuyu Grass (Pennisetum clandestinum)—A Review. Trop. Grassl. 2001, 35, 65–84. [Google Scholar]
- Herrera Ramírez, D.A.; León Peláez, J.D.; Ruiz Rendón, M.; Osorio Vega, N.W.; Correa Londoño, G.; Ricardo, R.E.; Uribe Bravo, A. Evaluation of Nutritional Requirements in Nurseries of Tropical Species Used in Urban Greening. Rev. EIA/Engl. Version 2014, 11, 41–54. [Google Scholar]
- Stoma, G.V.; Manucharova, N.A.; Belokopytova, N.A. Biological Activity of Microbial Communities in Soils of Some Russian Cities. Eurasian Soil Sci. 2020, 53, 760–771. [Google Scholar] [CrossRef]
- Curd, E.E.; Martiny, J.B.H.; Li, H.; Smith, T.B. Bacterial Diversity Is Positively Correlated with Soil Heterogeneity. Ecosphere 2018, 9, e02079. [Google Scholar] [CrossRef]
- Bach, E.M.; Williams, R.J.; Hargreaves, S.K.; Yang, F.; Hofmockel, K.S. Greatest Soil Microbial Diversity Found in Micro-Habitats. Soil Biol. Biochem. 2018, 118, 217–226. [Google Scholar] [CrossRef]
- Hamood Altowayti, W.A.; Almoalemi, H.; Shahir, S.; Othman, N. Comparison of Culture-Independent and Dependent Approaches for Identification of Native Arsenic-Resistant Bacteria and Their Potential Use for Arsenic Bioremediation. Ecotoxicol. Environ. Saf. 2020, 205, 111267. [Google Scholar] [CrossRef]
- Ukaegbu-Obi, K.M.; Mbakwem-Aniebo, C.C. Bioremediation Potentials of Bacteria Isolated from Rhizosphere of Some Plants of Oil Contaminated Soil of Niger Delta. J. Appl. Environ. Microbiol. 2014, 2, 194–197. [Google Scholar] [CrossRef]
- Hughes, J.B.; Hellmann, J.J. The Application of Rarefaction Techniques to Molecular Inventories of Microbial Diversity. Methods Enzymol. 2005, 397, 292–308. [Google Scholar] [CrossRef]
- Kim, B.R.; Shin, J.; Guevarra, R.B.; Lee, J.H.; Kim, D.W.; Seol, K.H.; Lee, J.H.; Kim, H.B.; Isaacson, R.E. Deciphering Diversity Indices for a Better Understanding of Microbial Communities. J. Microbiol. Biotechnol. 2017, 27, 2089–2093. [Google Scholar] [CrossRef]
- Morales, G.E.; Gallego, J.L. Determinación de Los Efectos Tóxicos Del Mercurio En La Especie Brachiaria dictyoneura (Fig. & De Not.) Stapf. Av. Cienc. Ing. 2013, 4, 1–17. [Google Scholar]
- Cruz, Y.; Carmago, G.; Gallego, J.L.; Saldarriaga, J.F. A Kinetic Modelling of the Growth Rate of Lolium perenne for Phytotoxicity Bioassays. Chem. Eng. Trans. 2019, 74, 1441–1446. [Google Scholar] [CrossRef]
- Cruz, Y.; Villar, S.; Gutiérrez, K.; Ruiz, C.M.; Gallego, J.L.; Delgado, P.; Saldarriaga, J.F. Gene Expression and Morphological Responses of Lolium perenne L. Exposed to Cadmium (Cd2+) and Mercury (Hg2+). Sci. Rep. 2021, 11, 11257. [Google Scholar] [CrossRef] [PubMed]
- Chang Kee, J.; Gonzales, M.J.; Ponce, O.; Ramírez, L.; León, V.; Torres, A.; Corpus, M.; Loayza-Muro, R. Accumulation of Heavy Metals in Native Andean Plants: Potential Tools for Soil Phytoremediation in Ancash (Peru). Environ. Sci. Pollut. Res. 2018, 25, 33957–33966. [Google Scholar] [CrossRef]
- Yetgin, A. The Dynamic Interplay of Root Exudates and Rhizosphere Microbiome. Soil Stud. 2023, 12, 111–120. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root Exudates: From Plant to Rhizosphere and Beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Muscolo, A.; Panuccio, M.R.; Eshel, A. Ecophysiology of Pennisetum clandestinum: A Valuable Salt Tolerant Grass. Environ. Exp. Bot. 2013, 92, 55–63. [Google Scholar] [CrossRef]
- Cheung, P.K.; Nice, K.A.; Livesley, S.J. Impacts of Irrigation Scheduling on Urban Green Space Cooling. Landsc. Urban Plan. 2024, 248, 105103. [Google Scholar] [CrossRef]
- Zhou, Y.; Lambrides, C.J.; Li, J.; Xu, Q.; Toh, R.; Tian, S.; Yang, P.; Yang, H.; Ryder, M.; Denton, M.D. Nitrifying Microbes in the Rhizosphere of Perennial Grasses Are Modified by Biological Nitrification Inhibition. Microorganisms 2020, 8, 1687. [Google Scholar] [CrossRef]
- Singh, N.; Megharaj, M.; Kookana, R.S.; Naidu, R.; Sethunathan, N. Atrazine and Simazine Degradation in Pennisetum Rhizosphere. Chemosphere 2004, 56, 257–263. [Google Scholar] [CrossRef]
- Lu, A.J.D.T.; Navarro, F.J.B.; Pascual, R.Z.; Clemen-Pascual, L. Phytoremediation Potential of Dilang-Aso (Pseudelephantopus spicatus (Juss.) Rohr) in Lead-Contaminated Soil. J. Agric. Res. Dev. Ext. Technol. 2020, 2, 40–52. [Google Scholar]
- Ali, H.; Shariful, I.; Mohammad, M.H.; Rahman, H.; Asheka, R.; Mohammad, A.T.; Tanvir, M. Studies of Phytochemical Analysis, In Vitro and In Vivo Evaluation of the Local Pseudoelephantopus spicatus Plant. Afr. J. Pharm. Pharmacol. 2024, 18, 114–126. [Google Scholar] [CrossRef]
- Odonne, G.; Herbette, G.; Eparvier, V.; Bourdy, G.; Rojas, R.; Sauvain, M.; Stien, D. Antileishmanial Sesquiterpene Lactones from Pseudelephantopus spicatus, a Traditional Remedy from the Chayahuita Amerindians (Peru). Part III. J. Ethnopharmacol. 2011, 137, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Acevedo-Barrios, R.; Tirado-Ballestas, I.; Bertel-Sevilla, A.; Cervantes-Ceballos, L.; Gallego, J.L.; Leal, M.A.; Tovar, D.; Olivero-Verbel, J. Bioprospecting of Extremophilic Perchlorate-Reducing Bacteria: Report of Promising Bacillus spp. Isolated from Sediments of the Bay of Cartagena, Colombia. Biodegradation 2024, 5, 601–620. [Google Scholar] [CrossRef]
- Das, R.; Kazy, S.K. Microbial Diversity, Community Composition and Metabolic Potential in Hydrocarbon Contaminated Oily Sludge: Prospects for In Situ Bioremediation. Environ. Sci. Pollut. Res. 2014, 21, 7369–7389. [Google Scholar] [CrossRef]
- Das, S.; Nurunnabi, T.R.; Parveen, R.; Nur Mou, A.; Emdadul Islam, M.; Didarul Islam, K.M.; Rahman, S.M.M. Isolation and Characterization of Indole Acetic Acid Producing Bacteria from Rhizosphere Soil and Their Effect on Seed Germination. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1237–1245. [Google Scholar] [CrossRef]
- Alexandrino, D.A.M.; Ribeiro, I.; Pinto, L.M.; Cambra, R.; Oliveira, R.S.; Pereira, F.; Carvalho, M.F. Biodegradation of Mono-, Di- and Trifluoroacetate by Microbial Cultures with Different Origins. New Biotechnol. 2018, 43, 23–29. [Google Scholar] [CrossRef]
- Saldarriaga, J.F.; Gallego, J.L.; López, J.E. Determination of Kinetics Parameters for Composting Process of the Organic Fraction of Municipal Solid Waste Separated at Source. Chem. Eng. Trans. 2018, 70, 217–222. [Google Scholar] [CrossRef]
- Romero-Murillo, P.; Gallego, J.L.; Leignel, V. Marine Pollution and Advances in Biomonitoring in Cartagena Bay in the Colombian Caribbean. Toxics 2023, 11, 631. [Google Scholar] [CrossRef]
- Narváez-Valderrama, J.F.; Alzate-B, S.V.; Correa-Gil, V.; García-L, J.J.; Bedoya-Soto, J.M.; Molina-P, F.J.; Pauta-Calle, G.G.; Vázquez-Guillén, G.B.; Ramos-Contreras, C.D. Traffic and Industrial Contributions of Particle-Bound PAHs during an Air Pollution Event in the Metropolitan Area of Medellin-Colombia: Inhalation Intake Risk during Pregnancy. Atmosphere 2024, 15, 173. [Google Scholar] [CrossRef]
- Calzolari, C.; Tarocco, P.; Lombardo, N.; Marchi, N.; Ungaro, F. Assessing Soil Ecosystem Services in Urban and Peri-Urban Areas: From Urban Soils Survey to Providing Support Tool for Urban Planning. Land Use policy 2020, 99, 105037. [Google Scholar] [CrossRef]
- González-Méndez, B.; Chávez-García, E. Re-Thinking the Technosol Design for Greenery Systems: Challenges for the Provision of Ecosystem Services in Semiarid and Arid Cities. J. Arid Environ. 2020, 179, 104191. [Google Scholar] [CrossRef]
- Anne, B.; Geoffroy, S.; Cherel, J.; Warot, G.; Marie, S.; Noël, C.J.; Louis, M.J.; Christophe, S. Towards an Operational Methodology to Optimize Ecosystem Services Provided by Urban Soils. Landsc. Urban Plan. 2018, 176, 1–9. [Google Scholar] [CrossRef]
- O’Riordan, R.; Davies, J.; Stevens, C.; Quinton, J.N.; Boyko, C. The Ecosystem Services of Urban Soils: A Review. Geoderma 2021, 395, 115076. [Google Scholar] [CrossRef]
| Class | Color | Shape | Surface | Border |
|---|---|---|---|---|
| Subclass | Beige | Circular | Rought | Whole |
| Yellow | Irregular | Smooth | Curly | |
| Light yellow | Filamentous | Opaque | Lobed | |
| White | Rhizoid | Bright | Filiform |
| Index | Calculation | Description |
|---|---|---|
| Richness | : Total number of morphotypes identified in a sample | |
| Margalef | : Sum of the frequencies of the morphotypes observed in a sample | |
| Menhinick | ||
| Shannon | Morphotype frequency ; Proportional abundance of the i morphotype | |
| Simpson | ||
| Berger-Parker | : Frequency of the most abundant morphotype. | |
| Pielou’s equity | ||
| Jaccard similarity coefficient | : 1 On-site morphotypes. : 2 On-site morphotypes. : Common morphotypes at sites 1 and 2. | |
| Ochiai coefficient |
| Property | Units | Non-Exposed Soil | Exposed Soil |
|---|---|---|---|
| Sand | % | 73 | 76 |
| Clay | % | 19 | 8 |
| Lime | % | 8 | 16 |
| Texture | Sandy loam | Sandy loam | |
| Db | Mg/m3 | 1.4 | 1.6 |
| pH H2O | 6.3 | 5.9 | |
| pH KCl | 5.5 | 5.6 | |
| OC | % | 1.4 | 1.3 |
| Ca | cmol/kg | 11.9 | 12.7 |
| Mg | cmol/kg | 7.6 | 6.1 |
| K | cmol/kg | 0.3 | 0.4 |
| P | mg/kg | 8.7 | 5.8 |
| Morphotype | Color | Shape | Border | Surface |
|---|---|---|---|---|
| 1 | White | Rhizoid | Filiform | Flat |
| 2 | Light yellow | Irregular | Curly | Bright curly |
| 3 | White | Filamentous | Filiform | Flat |
| 4 | White | Irregular | Lobed | Wrinkled |
| 5 | White | Circular | Complete | Flat |
| 6 | Yellow | Irregular | Curly | Bright flat |
| 7 | White | Filamentous | Filiform | Wrinkled opaque |
| 8 | White | Irregular | Lobed | Flat |
| 9 | Beige | Filamentous | Filiform | Wrinkled |
| 10 | White | Irregular | Curly | Wrinkled opaque |
| 11 | Light yellow | Filamentous | Filiform | Flat |
| 12 | Beige | Filamentous | Filiform | Wrinkled |
| 13 | White | Filamentous | Filiform | Bright flat |
| 14 | White | Rhizoid | Lobed | Flat |
| 15 | Beige | Rhizoid | Filiform | Wrinkled |
| 16 | White | Irregular | Curly | Flat |
| Index | Rhizospheric Soil | Non Rhizospheric Soil | p-Value * | ||||
|---|---|---|---|---|---|---|---|
| P. clandestinum | P. spicatus | E | NE | ||||
| E | NE | E | NE | ||||
| S | 8 | 11 | 8 | 8 | 10 | 8 | |
| Margalef | 1.64 ab | 2.45 c | 1.62 ab | 1.75 ab | 1.46 a | 1.80 b | 0.0004 |
| Menhinick | 0.95 ab | 1.42 d | 0.92 a | 1.08 abc | 1.21 bcd | 1.32 cd | 0.0117 |
| Shannon | 1.77 | 1.86 | 1.71 | 1.77 | 1.83 | 2.05 | 0.5313 |
| Simpson | 0.63 | 0.63 | 0.62 | 0.61 | 0.64 | 0.66 | 0.9534 |
| Berger–Parker | 0.49 | 0.51 | 0.53 | 0.53 | 0.52 | 0.51 | 0.9967 |
| Pielou | 0.59 a | 0.54 a | 0.57 a | 0.59 a | 0.76 b | 0.73 b | 0.0101 |
| Jaccard | P.c.-E | P.c.-NE | P.s.-E | P.s.-NE | NR-E | NR-NE | |
|---|---|---|---|---|---|---|---|
| Ochai | |||||||
| P.c-E | 0.58 | 0.45 | 0.60 | 0.50 | 0.45 | ||
| P.c.-NE | 0.43 | 0.46 | 0.46 | 0.50 | 0.58 | ||
| P.s.-E | 0.38 | 0.39 | 0.33 | 0.38 | 0.33 | ||
| P.s.-NE | 0.43 | 0.39 | 0.33 | 0.64 | 0.60 | ||
| NR-E | 0.40 | 0.40 | 0.36 | 0.44 | 0.64 | ||
| NR-NE | 0.38 | 0.43 | 0.33 | 0.43 | 0.44 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gallego, J.L.; Agudelo, A.M.; Morales, C.M.; Tamayo-Londoño, A.; Soler-Arango, J.; Tirado-Ballestas, I.P.; Arango-Correa, A. The Diversity Indices of Culturable Bacteria from the Rhizosphere of Pennisetum clandestinum and Pseudelephantopus spicatus in Urban Soil. Ecologies 2025, 6, 49. https://doi.org/10.3390/ecologies6030049
Gallego JL, Agudelo AM, Morales CM, Tamayo-Londoño A, Soler-Arango J, Tirado-Ballestas IP, Arango-Correa A. The Diversity Indices of Culturable Bacteria from the Rhizosphere of Pennisetum clandestinum and Pseudelephantopus spicatus in Urban Soil. Ecologies. 2025; 6(3):49. https://doi.org/10.3390/ecologies6030049
Chicago/Turabian StyleGallego, Jorge L., Ana M. Agudelo, Clara M. Morales, Andrea Tamayo-Londoño, Juliana Soler-Arango, Irina P. Tirado-Ballestas, and Alejandro Arango-Correa. 2025. "The Diversity Indices of Culturable Bacteria from the Rhizosphere of Pennisetum clandestinum and Pseudelephantopus spicatus in Urban Soil" Ecologies 6, no. 3: 49. https://doi.org/10.3390/ecologies6030049
APA StyleGallego, J. L., Agudelo, A. M., Morales, C. M., Tamayo-Londoño, A., Soler-Arango, J., Tirado-Ballestas, I. P., & Arango-Correa, A. (2025). The Diversity Indices of Culturable Bacteria from the Rhizosphere of Pennisetum clandestinum and Pseudelephantopus spicatus in Urban Soil. Ecologies, 6(3), 49. https://doi.org/10.3390/ecologies6030049







