1. Introduction
The Hungarian ground beetle
Carabus (
Pachystus)
hungaricus Fabricius, 1792 (Coleoptera: Carabidae), is a zoophagous, mesoxerophilic beetle, which is an endangered stenotopic species, protected or with high conservation status throughout its range from Central Europe to Eastern Siberia [
1]. As a typical steppe species, it represents Pannonian steppe invertebrates in European legislation [
2].
Despite
C. hungaricus being a Natura 2000 species of community importance, there are not many scientific papers about its ecology and biology. Elek et al. [
2] studied the dispersal and mobility of the adults of
C. hungaricus, finding that they are active and mobile beetles frequently moving for tens to hundreds of metres, and although short-distance movements are the most common, individuals are able to cross larger distances, even kilometres. Nevertheless, they were proved to be obstructed from colonising new or uninhabited habitat patches by the high fragmentation of their habitats. Furthermore, being a large-bodied short-winged open habitat specialist beetle,
C. hungaricus is considered to be more prone to significantly decrease, at least across Europe [
3].
Most of the research on
C. hungaricus concern its habitats, conservation status, taxonomy or given populations, e.g., [
2,
4,
5,
6,
7,
8,
9,
10], and even its movement and dispersal patterns [
2,
11] or road mortality [
12], but papers on its ecological relationships and parameters are very scarce, e.g., [
4,
5,
13,
14].
There are still many gaps in the knowledge regarding its populations’ real status, at least in Bulgaria. Current knowledge from the country encompasses only data about just a few localities where the species occurs and some speculative calculations about the population size of
C. hungaricus included in the documentation of the SCI BG0000322 “Dragoman” (see the first part of our study [
15]).
This study represents an additional part of the work on the development of an Action Plan for the conservation of this endangered ground beetle species in Bulgaria [
15,
16]. It includes the results obtained during a three-year study in different xerophytic landscapes in the vicinity of the Sofia Basin in central–western Bulgaria, but here, we give just the analysis of the results from the sites with
C. hungaricus occurrence. With this study, we sought to answer some questions about the taxonomy and the ecological structures of carabid communities where
C. hungaricus lives, using some standard parameters, e.g., dominance, occurrence, life forms, wing morphology, spatial distribution and population characteristics. We also wanted to analyse some population characteristics, such as the seasonal activity, sex ratios, quantitative parameters and catchability of individuals by traps and by periods of collection. The aim was to provide further details on the life history and community structure of this Natura 2000 target species, which would be potentially useful for its further conservation.
2. Materials and Methods
In the period between 24 May 2021 and 10 December 2023, during a survey on the distribution and status of
Carabus hungaricus in Bulgaria, we chose 42 sampling sites in central–western Bulgaria (for a map of the sites, see Teofilova and Kodzhabashev [
15]), where the species is known to occur. We used a total of 252 pitfall traps (6 traps in each site) which operated during 69,903 effective trap-days. Pitfall traps were made out of cut 2 L. plastic bottles filled with 8% formaldehyde.
Carabus hungaricus was found in seven sampling sites, and they are given in
Appendix A, along with the sampling periods and the number of specimens collected.
The analysis of the life forms of the species collected in the carabid complex where
C. hungaricus occurred followed the classification proposed by Sharova [
17].
Species were also classified into three groups according to their hind wing development: macropterous (always possessing wings), wing dimorphic/polymorphic (only part of the population being fully winged), and brachypterous (short-winged/wingless), according to the classification of Den Boer et al. [
18].
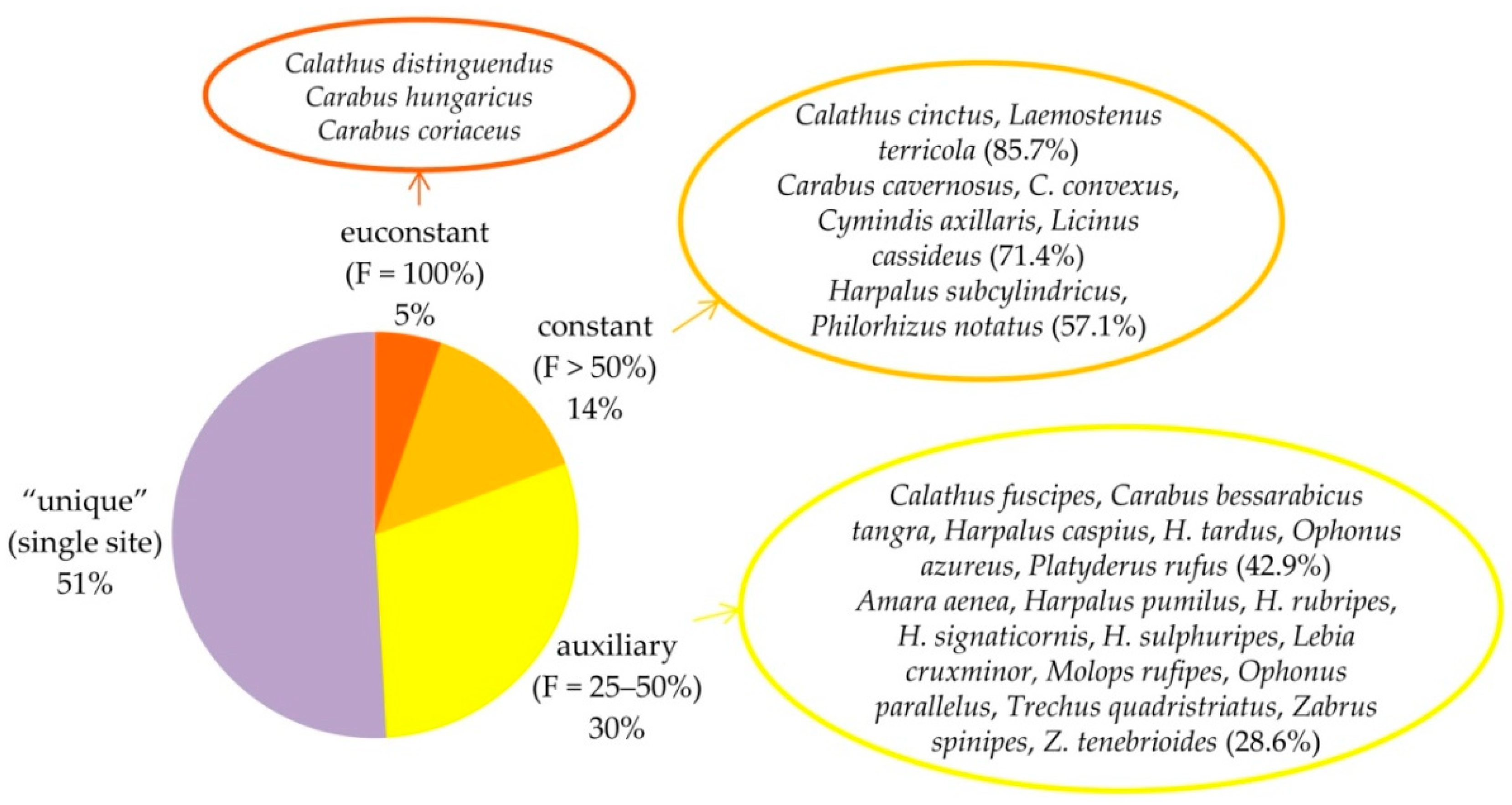
According to their ecological requirements in terms of humidity, the recorded carabid species were divided into six categories: hygrophilous, mesohygrophilous, mesophilous, mesoxerophilous, xerobionts and eurybionts. According to their frequency of occurrence (F), the species were divided into euconstant (F = 100%), constant (F > 50%), auxiliary (F = 25–50%) and accompanying (F < 25%), as the species found in only one sampling site were classified as “unique”.
The dominance structure was calculated on the basis of the classification of Tischler [
19] for soil invertebrates, modified by Sharova [
17] with the initiation of a 5th category “eudominant”: eudominants (with degree of dominance over 10%), dominants (5 to 10%), subdominants (2 to 5%), recedents (1 to 2%) and subrecedents (<1%). When studying carabid coenoses, sometimes, the additional category of “superdominant” (having a degree of dominance over 50%) becomes very useful. For the purpose of our study, we considered it as quantitatively indicative for the specific conditions of the steppe biome in the researched territory, and
Calathus distinguendus (the single superdominant) can be considered diagnostic for the steppe nature of the studied habitats.
Indices for α-diversity reflect the biological diversity within the community or the habitat. Here, the following indices were used: species richness of Margalef (d), Pielou’s index of evenness (J’), Shannon’s index of total species diversity (H’
(loge)) and Simpson’s index of dominance (C
(λ)), and the index of Hill (N1) was calculated for comparison [
20].
To standardise the catches (trapping effort), given the different duration of the samplings and the destruction of some of the traps, we used the term dynamic density (DD), which we calculated as the number of individuals of a given species caught, divided by the effective (realised) number of trap-days (t.d.) multiplied by 100. The resulting values represent the number of individuals of a given species captured per 100 realised trap-days. The dynamic density replaces the abundance, which is unrealistic and incommensurable when traps are non-functional or destroyed. It reflects the actual time and amount of individuals trapped, thereby standardising the data and making it comparable. It is also useful in unifying the results when most species had low numerical values. We have included all days in the calculations of actual trap-days, regardless of the season and weather conditions. During the study period, the winter months had large periods of prolonged warming and little or no snow cover, and therefore, carabids were likely active much of the time.
For the mathematical processing of the data, MS Excel and the software products PRIMER6 v. 6.1.6 [
21] and TWINSPAN v. 2.3 (TWo-way INdicator SPecies ANalysis) [
22] were used. Cluster analysis was performed with the data on species presence/absence (the results based on abundance were the same and are not presented in the paper), and the TWINSPAN calculations were based on the log-transformed values of the dynamic density of the species.
3. Results
During the whole study period, 69,903 effective trap-days and an average dynamic density of about 22 individuals per 100 trap-days were realised. A total of 15,333 ground beetle individuals were identified, belonging to 184 species, of which about one-third were typical steppe specialists.
Carabus hungaricus was found in only 7 of the 42 sampling sites (frequency of occurrence = 16.7%) (see [
15]). These sites are all located in the Chepan Planina and Tri Ushi Mountains, both included in the range of the Western Stara Planina Mts. A total of 198
C. hungaricus specimens were collected, representing just about 1.3% of the total numbers of the carabids. The full list of the species, along with their authors and years of description, is presented in
Appendix B, arranged according to their TWINSPAN classification.
3.1. Taxonomy and Communities
In the seven sites where Carabus hungaricus was collected, we found a total of 57 species belonging to 23 genera and 12 tribes. The average dynamic density (DD) was about 32 ind. per 100 trap-days.
The most species-rich genera were Harpalus (13 sp., 23% of all), Amara (8 sp., 14%) and Carabus (6 sp., 10%). The tribe Harpalini had 19 of the species (33%), and the tribe Amarini was represented by 10 species (17%). Carabini and Sphodrini had six species each, Lebiini had five, Pterostichini had three, Licinini and Brachinini had two species each, and Nebriini, Trechini, Bembidiini and Oodini included only one species each. The taxonomic structure of the carabid fauna from the studied habitats was built mostly from open habitat dwellers. Few exceptions were present, such as the ecotone species Amara communis, Carabus gigas and Harpalus laevipes or the forest inhabitants Molops rufipes, Myas chalybaeus, Platyderus rufus and Pterostichus incommodus, but they were all represented by a single or small number of individuals.
We studied possible relationships of
C. hungaricus within its coenoses, and we found that five other
Carabus species occurred in the same habitats (see
Appendix B). The most numerous was
C. coriaceus (504 ex.), which was also euconstant—collected in all seven sampling sites (frequency of occurrence, F = 100%), and dominant or subdominant in most habitats. Among the constant species, found in five of the sites (F = 71.4%), were
C. cavernosus (with 362 ex.) and
C. convexus (263 ex.).
Carabus bessarabicus tangra was found in three of the sites with a total of 25 ex. The last species,
C. gigas, was recorded with a single specimen from the top of the Chepan Planina Mt. The most abundant species in the carabid complex was
Calathus distinguendus (5976 ex., more than 71% of all carabid specimens), which was euconstant as well (
Figure 1). In all sampling sites, it was superdominant or eudominant. In fact, the abundant presence of
Calatus distingusndus is very striking, and this species (and its percentages) can be successfully used as an indicator of conditions suitable for the presence of the Hungarian ground beetle in the territory of Bulgaria.
Other common and abundant members of the
C. hungaricus communities in Bulgaria were
Calathus cinctus,
Laemostenus terricola,
Cymindis axillaris and
Licinus cassideus (
Figure 1,
Appendix B). The most represented species of the whole carabid complex were the superdominant
Calathus distinguendus with 67% of the total dynamic density, the dominants
Carabus coriaceus (5.66%) and
Laemostenus terricola (5.12%), and the subdominants
Calathus cinctus (4.69%),
Carabus cavernosus (4.63%),
C. hungaricus (3.94%) and
C. convexus (3.36%).
In terms of the ecological relationships of
C. hungaricus, possible competition might be expected from the closely related species
C. (Pachystus) morio Mannerheim, 1830, insofar as they have very similar ecological requirements, and to some extent from
C. (Lamprostus) torosus I. Frivaldszky von Frivald, 1835, which is, however, much more xerophilous. In reality (at least at this stage of the knowledge about the species distribution), such relationships are excluded, as
C. morio and
C. torosus do not occur in the area where
C. hungaricus lives in Bulgaria.
Carabus (Trachycarabus) scabriusculus Olivier, 1795 is another xerophilous inhabitant of open steppe terrains, but it seems to occupy a different ecological niche, and has a much wider tolerance in its requirements for the habitat it prefers; additionally, in the last few decades, this species seems to be declining more and more in Bulgaria (based on own observations). During our study, we found five other representatives of the large beetles of the genus
Carabus—
C. coriaceus,
C. convexus,
C. cavernosus,
C. bessarabicus tangra and
C. gigas. According to the literature, the coexistence of the two rare and protected steppe species,
C. hungaricus and
C. bessarabicus, inhabiting the same localities has also not been recorded, at least not in the last 50 years (for details, see Teofilova and Kodzhabashev [
23]). Other large-sized beetles found were
Zabrus spinipes (Fabricius, 1798) of the carabids and some large Tenebrionidae species (most probably
Gnaptor Brullé, 1832 or
Blaps Fabricius 1775). During our study, we also found some of the potential predators of
C. hungaricus—the banded centipede
Scolopendra cingulata Latreille, 1829 (Chilopoda: Scolopendridae), some wolf spiders (Arachnida: Lycosidae), three species of lizards (Reptilia: Lacertidae) and three species of insectivorous mammals (Mammalia: Eulipotyphla).
The ecological parameters and indices of the seven sampling sites varied widely due to factors of different natures and strengths (
Table 1). In terms of the number of species recorded, the highest site in the Chepan Planina Mt. (site 16, at about 1180 m a.s.l.) has the maximum number of species. It lies on a southern slope and has a mosaic landscape, tightly sodded between the stones and boulders, with spots of dwarf almond, lilac and smoke tree. The soil is structured and rich in organics and the exposed limestone rocks comprise approximately 25%. Despite the strong drainage of the soil, humidity is relatively high throughout the year. The habitat is undisturbed by human activities. It has been traditionally used as a pasture in the past, but with very low grazing intensity due to the presence of a number of adjacent good-quality pastures in the low, flat areas. In addition to the study record of 39 collected species, comprising 68% of the whole species composition, 58% of the individuals in the total catch were caught in this sampling site, as well. Respectively, the dynamic density also had high values of 78.33 ind./100 t.d., which is almost two and a half times higher than the average of all the studied sampling sites. The α-diversity indices of this site showed very high species richness values, but low evenness and high degree of dominance due to a single species,
Calathus distinguendus, with a contribution of 78% of the total catch.
In the other six sampling sites with records of C. hungaricus, the number of associated species found ranged from 11 to 19 species. In the two sites with the most abundant catches of C. hungaricus (sites 33 and 40), the number of species was 15 and 12, with dynamic densities of 23 and 24 ind./100 t.d., and other biodiversity values were similar. In the two sites where only one C. hungaricus individual was recorded for the whole study period (sites 35 and 39), the low dynamic density (15.6 and 16.3), which was twice as low as the average for all sites, and the relatively low degree of evenness were remarkable.
3.2. Life Forms
The ground beetles from the studied habitats related to two classes of life forms proposed by Sharova [
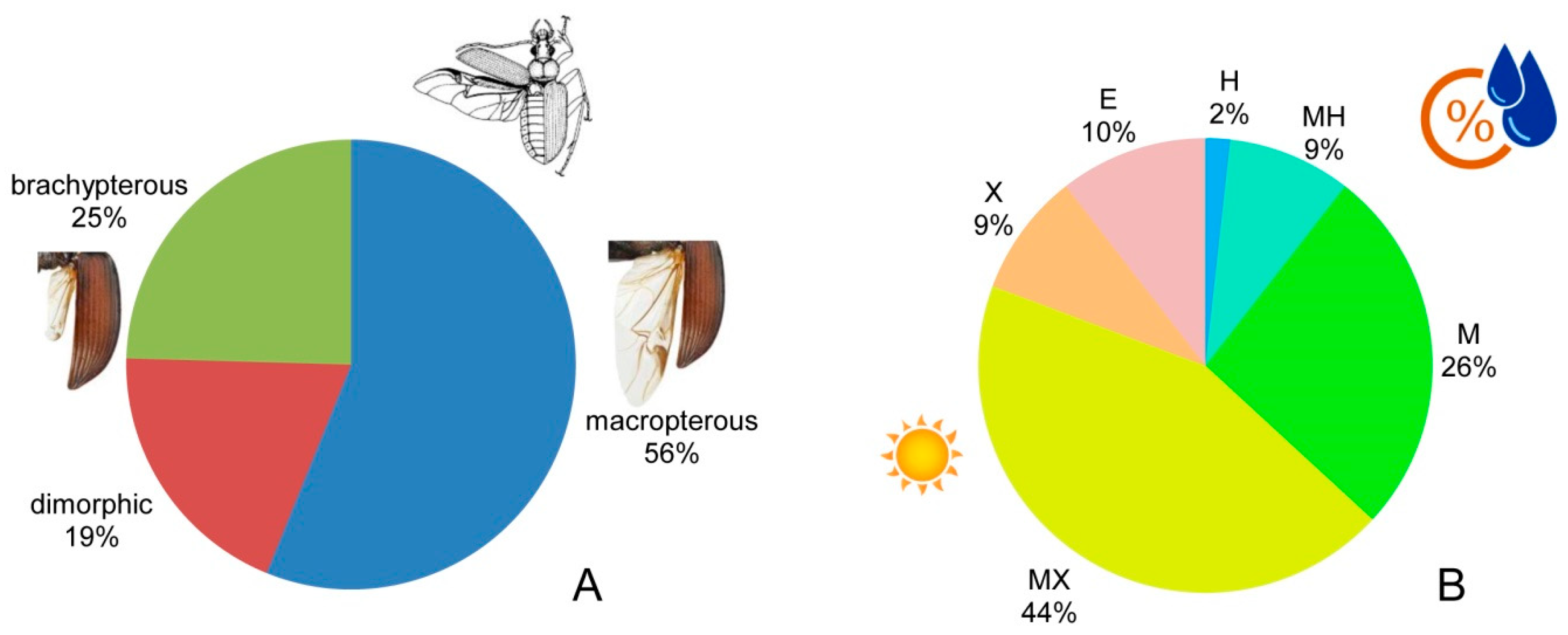
17], with almost equal proportions between the class Zoophagous (28 species, 49.1%) and the class Mixophytophagous (29 species, 50.9%) (
Figure 2). This ratio between the classes is quite remarkable and unique for Bulgaria.
3.3. Wing Morphology
The analysis of the degree of hind wing development of carabids from the study area showed the presence of all three groups: macropterous (winged)—32 species (56%); brachypterous (hind wings shorter than elytra, or missing)—14 species (25% of all); and di(poly)morphic (some individuals have fully developed wings, others only vestigial ones)—11 species (19%) (
Figure 3A).
3.4. Humidity Preferences
The analysis of the humidity preferences (
Figure 3B) of carabids showed that in the studied sites, the mesoxerophilous species had the largest share (25 species, 44% of all). Mesophilous carabids comprised 15 species (26%), and 6 species (10%) were eurybiontic in relation to the humidity. The groups of the xerophilous and mesohygrophilous beetles included five species (9%) each. Hygrophilous was only one species, the flying
Badister bullatus, collected with a single specimen in the site at the highest part of the Chepan Planina. Among the mesohygrophiles found in this sampling plot was also the rare marshland species
Oodes gracilis. This site is situated above the Dragomansko Blato Marsh, which undoubtedly supports the penetration of hygrophilous and mesohygrophilous or some ecotone species.
3.5. Analysis of β-Diversity and Spatial Distribution
The results from the clustering and TWINSPAN classification were remarkably similar, both detaching the sites 14 and 40 in a separate group (
Figure 4,
Appendix B).
The splitting of the dendrogram at the first level combined into one branch two of the sampling sites having abundant catches of Carabus hungaricus and a landscape with a strong predominance of open rock passages (sites 14 and 40), a likely reason for the similar species composition and its proportional distribution. Both habitats are very insolated and there are large water basins in the vicinity of both sites, at about one or two kilometres away. The second division branched into two clusters. One grouped the two sites from the Chepan Planina Mountain, which are in relatively close proximity to each other and have similar microhabitat and phytocoenotic complexes. The three sites in the second cluster have a similar type of vegetation distribution and landscape mosaic. In addition to the open, sodded habitats, the three sites contain tufts of native woody and shrub vegetation dominated by the typical steppe indicators. This cluster includes the sites with the highest level of similarity (35 and 39), where a single specimen of C. hungaricus was established in each site.
The TWINSPAN analysis generated a separation in only one level, indicating the great resemblance between the habitats where the target species C. hungaricus lives. The indicator for the separation of the negative group of habitats (all sites except 14 and 40) was Carabus convexus. This group unites the two sites from the Chepan Planina Mt., the site near Beledie Han and the two sites above Bezden. The positive group included only the site above Opitsvet and the site near the dam of Bezden (sites 14 and 40, with a high abundance of C. hungaricus).
In relation to the species classification, the negative group of species included 41 species. Among them were the 21 species characteristic only for the Chepan Planina and the negative preferentials (according to the TWINSPAN classification) Carabus bessarabicus tangra, Harpalus tardus, Philorhizus notatus and Platyderus rufus. The positive group of species included only 16 species, but among them were 4 of the dominant species, including the superdominant Calathus distinguendus, as well as all 3 euconstant species, and almost all constant species with a degree of occurrence > 70%, with the exception of Carabus cavernosus, Carabus convexus and Laemostenus terricola. Positive preferentials (according to the TWINSPAN classification) were Harpalus pumilus and Harpalus flavicornis.
3.6. Analysis of Carabus hungaricus Population
3.6.1. Seasonal Activity and Sex Ratio
Data for the quantitative analysis were collected in the period 25 May 2021–10 December 2023 and are presented in
Appendix A. In total, for the entire study period, 198 individuals were collected, and 60% of them (119 individuals) were females and 40% (79 individuals) were males.
The seven sampling sites with individuals of
C. hungaricus were set up successively during the three years. In sites 14 and 16, surveys were carried out for a three-year period, and in sites 33 and 35, for a two-year period. Simultaneous surveys in all seven localities were carried out only during the last year, in the period 10 March–10 December 2023, with four seasonal samplings. The catch in 2023 accounted for 70% of the total beetles collected (139 specimens out of 198), which allowed us to establish several significant facts about the activity, sex structure and spatial distribution of the population (
Table 2).
During the first collection period of 2023 (March–May), only one female was captured, which, for a third consecutive year, confirmed the relatively late activation of the species. Thus, our first important finding, over the three-year study period with permanently set traps, was the spring activation of the species, which we recorded to be after mid-May. Only in the lowest site (40), at about 650 m a.s.l., was a single individual trapped before that (between 10 March and 15 May 2023).
During the second period (May–July), a total of 50 individuals were captured, accounting for 36% of the catch for the year. This catch is strongly dominated by females (represented by 36 individuals and 72% of the catch), repeating the 2022 results for both sites 14 and 33. In the third catch (July–September), 29 individuals were caught (21% of the total catch for the year). Here, males predominated (19 individuals, 66%). In the last period (September–December), 59 individuals were recorded (43% of the total catch for the year). In this catch, the distribution of individuals was 47% females and 53% males, indicating a relative evenness of the two sexes during the period of preparation for the upcoming hibernation (
Table 2).
Since the sampling method we used is not very reliable for phenological surveys, in order to try to further clarify the phenology of the species, in April 2024, we conducted soil excavations in two different habitats with proven presence of the species. The soil profiles were 1 m2 in size and 0.5 m in depth, but despite the vast quantities of soil excavated and accurately turned over (according to a successful methodology), no larvae or imagoes of the species were found. This unsuccess might be due to inopportune time and climatic conditions.
Sampling site 40 has the highest number of individuals caught, although the trapping occurred for one year only. In this area, the sex structure is very impressive, with a female–male ratio of approximately 2:1. The most pronounced here was the significant predominance of females in the spring–summer season (14 f: 3 m), of males in the summer–autumn season (3 f: 13 m) and the equalisation of the two sexes in late autumn (17 f: 14 m). It is likely that the high spring–summer (15 May–15 July) activity and density of females may be due to an active search for food to nourish the eggs in their bodies, but also due to a search for a suitable place and substrate for oviposition, assuming that copulation occurred in autumn. If the females breed in the autumn and emerge in the spring, it is logical that there is also activity of males with which to copulate. The appearance of high activity of males during the summer–autumn period (15 July–15 September) is probably due to the search for mates for copulation. The equilibration of the two sexes during the late autumn period (15 September–10 December) shows the relatively equal activity and density, when both sexes are probably preparing for hibernation.
3.6.2. Trends and Recurrence of Catches in the Surveyed Sampling Sites in the Different Years
Only sampling sites 14 and 16 were operated throughout the whole three-year period. In these sampling sites, catches in the three years had very different values (
Table 3), despite the constant locations and conditions of the traps. Similar results were obtained from simultaneous surveys in four sites in 2022, when we had two additional plots with
C. hungaricus present. In 2022, in two of the sites, we observed increased activity and presence of females during the spring–summer period, and in the other two sites, we found increased activity and presence of males during the summer–autumn period. During the 2021 and 2022 biennium, the established female/male sex ratio was 4:1 (
Table 3). This result has no analogue in the literature, and therefore, interpretation is impossible at this stage of knowledge. The results in 2023 showed no significant differences in the sex structure of the species, but confirmed the different activity of the two sexes during the spring–summer and summer–autumn periods. This fact requires confirmation with future investigations and establishing its regular recurrence or randomness.
3.6.3. Quantitative Characterisation—Dynamic Density (DD) and Percentage Presence
During the field studies, significant differences in the distribution of the species in different parts of the studied territory were registered. Various methodologies and approaches are been used to determine the quantitative characteristics in the distribution of C. hungaricus and in the assessment of numbers. In the present work, we made an attempt to analyse the various standard methodologies for quantitative reporting. We accounted for the quantitative characteristics of the Hungarian ground beetle with the help of the dynamic density (DD), given as the number of individuals captured per 100 trap-days and the percentage of the species to the total number of individuals. We believe that practices to calculate an absolute abundance of small invertebrates should be excluded from the legislative regulations as a criterion and indicator of the status of any species. We consider its accounting impossible, unnecessary and erroneous for the following reasons:
- -
Extremely large fluctuations across years of research for the same sampling sites with the same exploitation and number of traps;
- -
Varying density and activity of individuals in different seasons and inability to determine when the actual peak of imagoes’ moulting is;
- -
Different natural density and activity of the species in different parts of the relatively large occupied area;
- -
Inability to use the method of live capture, mark and recapture in a sufficiently large number of sampling sites (in order to overcome differences in density in different parts of the habitat) and to use a subsequent extrapolation for the whole area where the species is recorded;
- -
Data on the abundance are only accurate for a particular habitat when obtained by the recapture method within a defined area during the established peak of appearing of the adults;
- -
Such studies are very expensive and financially unjustified, given the need for a large number of experts engaged over a long period of time for several years. In the same area, it is necessary to have several teams in the field three times a year for 10 days each, manning several hundred traps daily (considering that in an area with established individuals, a minimum of 12 traps are needed for every 1 decare; this would provide for obtaining of objective, correct and suitable for biological extrapolation in the following years information).
For assessment of the quantitative parameters taking into account the relative abundance in small territories, we could use the sampling sites in which the catches were carried out at the same time, in the same way and with the same effort, i.e., as the catches were carried out in 2023, when we simultaneously collected biological information from all seven sampling sites (with six traps set and collected at the same time).
The dynamic density, quantitative representativeness (percentage, significance) and absolute abundance of individuals from each sampling site could be used to account for, evaluate and analyse quantitative characteristics. For the purposes of practice, when taking into account the results of this study, it is appropriate to use digital scales according to which the parameters are divided into measurement groups. The scales are consistent with the obtained minimum and maximum values and their distribution in three main measurement groups.
Calculated dynamic densities showed significant differences in the maximum and minimum values—from about 0.1 to 6.0 ind./100 t.d. For practical purposes, according to the results, we have divided the densities into three groups: low (DD = 0.1–1 ind./100 t.d.), medium (DD = 1–4), and high (DD > 4). In two of the sampling sites, just one individual was recorded for the study period (two years for site 35 and one year for site 39). For these sites, the dynamic density was the minimum for the study (0.1 ind./100 t.d.). Low dynamic density (0.53 ind./100 t.d.) also occurred in site 16, which was studied during the whole three-year period, but it occupied the highest part of the Chepan Planina Mountain. Sites 14 and 41 had an average DD of 1.1 and 1.35 ind./100 t.d. They were very similar in relation to landscape, exposure and biotic conditions, despite the relatively long distance between them. High DD values were recorded in site 33 (4.1 ind./100 t.d.) and site 40 (6.0 ind./100 t.d.). These habitats were predominantly covered with open karst and very sparse vegetation, and the species composition of carabid fauna was poor, with 12 and 15 species, respectively. The analysis and the findings can be considered preliminary, given the absence of other similar research and the possibility of comparability.
When using the percentage of C. hungaricus individuals from the total number of individuals caught, three of the sites (16, 33 and 35) had percentages below 1%, two had percentages between 1 and 5%, and two had percentages above 5%. Like the previous one, this quantitative criterion might also be applied for benchmarking purposes when it is necessary to analyse and typify the monitored areas.
When comparing the seven sampling sites by the results of the total number of individuals caught in 2023, the sites were divided into three groups, as well—with catches up to 9 individuals, 10 to 30 individuals, and over 30 individuals, respectively. Thus, sites 35 and 39 fall in the first group (with only one individual each), three of the sites (14, 16 and 41) fall in the middle range, and sites 33 and 40 fall in the third group. The difference between the sites with one individual versus the single site with a maximum catch of 64 individuals is very pronounced. The reasons for these significant differences are difficult to interpret with our knowledge at this stage, but according to the specific conditions in the given sampling site, they give a real idea of the unevenness of the distribution and can facilitate the search for possible root causes.
Overall, all three quantitative characteristics used to detect differences in density and abundance are reasonably accurate and, most of all, comparable, especially if used comparatively for the same collecting period and under the same operational conditions.
3.7. Distribution of the Catches by Traps
Microhabitats are extremely important for the distribution of individuals, although they visually look very similar or akin to each other. In our study, the results of the catchability by trap in the seven sampling sites indicated that the distribution of individuals, when present, was not uniform (
Table 4).
Variation was particularly strong where densities were high. Conversely, at relatively low densities, the distribution across the sampling site was relatively uniform and the variation was lower. Of particular interest are the traps with catches of more than 15 individuals, where further studies on the microhabitat features could be carried out. In sampling site 33, with a two-year period of trapping, the catches in the two years were almost identical (32 and 35 ind.), and the proportional distribution of individuals per trap was maintained, indicating the great importance of microhabitat specificity for the distribution and density of individuals. The results in
Table 4 show the fortuitous distribution of the species across the traps and demonstrate that the importance of the specific microhabitat takes priority over the duration of the sampling. The practical contribution of such analysis is that, in order to ensure comparability of results, it is appropriate to conduct monitoring in the same sampling sites using the same methodology and to monitor possible changes in the parameters of previous surveys.
4. Discussion
Bearing in mind that
Carabus hungaricus is a species with high conservation significance, we tried to study as much as possible of its biology and ecology. We set our research in various habitats in a wider territory than the species was known to occur in Bulgaria, since we wanted to better explore not only its distributional limits but also its environmental requirements (see [
15]). We also studied the ecological relationships of
C. hungaricus and its co-inhabitants. As noted by Bérces et al. [
6],
C. hungaricus lives mostly in habitats where mainly no other
Carabus species occur; however, there are exceptions [
5]. We found five other
Carabus species in the sites where
C. hungaricus was present. The most interesting finding, undoubtedly, was
C. bessarabicus; it has been recently reported for the first time in Bulgaria [
23] and is represented by the newly described subspecies
tangra. While in Hungary
Carabus scabriusculus is reported as an often-found typical steppe species [
6], we did not succeed in collecting it in our steppe habitats. Another difference with communities of
C. hungaricus in Hungary was the great presence of
Calathus distinguendus, which was found in great abundance in all sites where
C. hungaricus occurred in Bulgaria. In terms of the ecological relationships of
C. hungaricus, possible competitors, except the other
Carabus species, could be large tenebrionids and
Zabrus spinipes of the carabids, as also found by Bérces et al. [
5]. Besides other
Carabus spp. and
Blaps spp., Pokluda et al. [
13] pointed out the representatives of the genera
Calosoma Weber, 1801 (Coleoptera: Carabidae),
Meloe Linnaeus, 1758 (Coleoptera: Meloidae) and
Dorcadion Dalman, 1817 (Coleoptera: Cerambycidae), as potential competitors of
C. hungaricus, but the authors also suggested that these beetles occupy different ecological niches. As for the potential predators of
C. hungaricus, some further details have been presented by Teofilova and Kodzhabashev [
23]. When studying
C. hungaricus, it is appropriate to study all potential groups of animals, such as possible food resources and those of the potential predators, and the method we used allows for the collection of such biological information on the potential prey or natural enemies of
C. hungaricus. At this stage, we have not been able to identify all the prey and predators, which we set as a task for the future.
We also used the analysis of the ecological parameters and indices in an attempt to explain differences in the distribution of individuals between the habitats which are seemingly identical or very similar, but at the same time have large differences in the number of individuals and their density. The results showed no direct correlation with the quantitative presence of the Hungarian ground beetle in the studied sites, which may be due to the relatively short period of research and the different exposure period of the traps, despite the use of the dynamic density as an objective quantitative characteristic aimed at equalising the actual trapping period of the individuals.
The analysis of the life forms of carabids from the
C. hungaricus coenose showed equal proportions between the zoophages and mixophytophages, which is most similar to and in complete accordance with the results obtained by Sharova [
17] for the steppe zone of Eurasia—50% for each of the classes or a little above 50% for the mixophytophages. Such a ratio, with a predominance of the mixophytophages (even though it is very weak), has never been found in all previously analysed regions in Bulgaria (based on our own investigations). The closest were the results from: unvegetated or sparsely vegetated inland cliffs, rock pavements and outcrops; screes; different habitats in the Eastern Rhodope Mts.; the urbanised region of the city of Plovdiv. The established ratios between the zoophages and mixophytophages were, respectively, 54%: 46%; 56%: 44%; 56.5%: 43.5% and 57%: 43% [
24,
25,
26]. A relatively lower share of the zoophages was found in pseudomaquises in SW Bulgaria, where it revealed the effect of the xerothermic Transitional–Mediterranean environmental factors [
27].
Another important characteristic in ecological and morphological research, especially in regard to important indicators such as carabid beetles, is their wing morphology. The wings of insects, which enable them to fly, are one of the main reasons for the incredible biological progress of this group of animals. Thanks to their highly chitinised elytra, Coleopterans have specialised for a terrestrial lifestyle and are able to use narrow spaces for shelter. The ability to fly in carabids is a specific adaptation dependent on the need to migrate when adverse conditions arise. Steppe-dwelling, open-ranged beetles evolved two different types of adaptive strategies to survive prolonged summer droughts—falling into summer diapause or migration. The first one is characteristic primarily of large carnivorous species, while the latter is a tool of phytophilous phyto- and myxophytophagous species [
17,
28,
29,
30,
31,
32].
The here-established ratio between the macropterous, wing dimorphic/polymorphic and brachypterous carabids has not been reported so far in the other regions in Bulgaria studied in this discipline, where the share of the brachypterous species has always been the lowest. Furthermore, here, we found a relatively low number of winged species, comparable only with typical montane habitats. The ratios between the winged, pteridimorphic and wingless species were as follows: 73%, 17% and 10% in Bulgarian rapeseed (
Brassica napus L.) fields [
33]; 69%, 22% and 8% in Zlatiya Plateau [
34]; 67%, 21% and 6% in the region of the city of Plovdiv [
26]; 57%, 22% and 16% in the Sarnena Gora Mts. [
35]; 45%, 32% and 23% in a house yard in the Western Rhodope Mts. [
36]. While wingless carabid assemblages are characteristic of ecologically homogeneous and stable environments, where resources are sufficient for beetles’ entire life cycle, the proportion of flight-capable pioneer species increases with increasing disturbance (see Teofilova and Kodzhabashev [
37]). The established structure of the wing forms in the studied carabid coenosis is consistent with steppe communities from other parts of the biome, which in our opinion is due to the naturalness of the karst steppes in the study area. Therefore, we could conclude that the studied complex of steppe habitats appears to be stable in relation to the wing morphology of its ground beetle fauna. Given the results of the analysis of the wing forms, we can suggest that the steppe complexes of carabids had been established a long time ago in the surrounding areas of the Sofia Basin and have been preserved in their natural state, probably due to the unsuitability of the lands for agricultural and other anthropogenic purposes.
Another interesting aspect of our research was the analysis of the humidity preferences of the beetles from the studied carabid complex. The majority of the group of the xerophilous and mesoxerophilous species, more than half (53%) of all species, is quite remarkable among the other regions in Bulgaria studied in this discipline. Larger shares (more than 65%) have been noted only for the specific xerothermic pseudomaquis habitats in the extreme south-west of the country [
27]. These values were 47%, 46% and 45% in the carabid complexes from the relatively dry regions of the Zlatiya loess plateau (NW Bulgaria), the city and surroundings of Plovdiv, and the Eastern Rhodopes, respectively [
25,
26,
34].
In order to better illustrate our findings, we conducted mathematical processing of the data. The dendrogram, based on the species composition, divided the sampling sites according to the number of carabids captured, but at this stage of our knowledge, we cannot explain the reasons for this pattern, but only express hypotheses. According to the TWINSPAN classification, the separation of the two main groups was the same as in the cluster analysis. Willner et al. [
38] point out that the lower mountain ranges, high topographic heterogeneity and mostly calcareous substrates provide potentially suitable habitats and refugia for steppe species, and our sampling sites seem to follow this rule.
The three-year study allowed us to make some conclusions and/or suggestions about the seasonal activity and sex ratio of
C. hungaricus. The established trend of increased activity during the spring–summer and autumn periods, with a maximum at the second peak, has been found in other parts of the range as well [
8,
14,
39]. In our study, no summer diapause was detected, although such has been recorded in the eastern part of the species range [
8]. For the study area inhabited by the species in Bulgaria, activity was observed during all warm seasons of the year, although the activity of the species varies considerably during different phases of its life cycle, given the seasonal distribution of catches and their sex structure.
The trend of different activities and densities of males and females during the second and third periods of the study has not been recorded or discussed in other parts of the species’ range and requires further studies. It is likely that the increased activity of females after the winter diapause, soon after their spring moulting or overwintering, may be due to their preparation for nourishing the eggs in their body and the respective searching for prey. The increased activity of the males during the summer–autumn period (July–September) is probably due to the search for mates for copulation. Bérces et al. [
5] and Bérces and Elek [
7] also revealed that males were more active during the breeding season than females. The equilibration of the two sexes during the last (autumn) period is probably after the breeding is complete, when individuals are preparing for the upcoming hibernation.
It is very likely that the life cycle has more than one opportunity for the species to reproduce—an adaptation that increases the chance of its successful survival. The steppes are characterised by extreme climatic amplitudes in summer and winter temperature values and by prolonged summer drought [
38,
40,
41,
42]. These are concrete factors to which xerothermophilic stenobiont species (such as
C. hungaricus) have been able to adapt.
Possible life cycle variations have not been investigated in this particular population and territory, so for now, we can only make hypotheses based on studies in other areas. The life history traits found for
C. hungaricus in different parts of its range vary considerably, with suggestions of polycyclicality [
5], both in terms of life span (two years instead of one) and the number of ovipositions (one or two).
Given the results we obtained, we attempted to analyse the life history traits of
C. hungaricus. Assuming that females have a monocyclic life cycle, the following question remains: What happens to the males during their hibernation, so they emerge in the spring with far fewer individuals than females? Is it possible that females have two breeding cycles, one in spring and one in autumn, or is the doubling of the percentage of females not a defence mechanism of the species against the severely limited space suitable for the realisation of the life cycle and the resulting need for compensatory doubling of the reproductive capacity? At the extremes of its range, each species is subjected to intense competition, and for the less abundant and spatially restricted stenobiont, in the face of constantly changing environmental factors, the lack of guarantees for its future may underlie the modification of its mechanisms of reproduction and adaptation to particular environmental factors. Is it possible that the life cycle has two different scenarios with an overwintering last larval instar and subsequent spring moulting and then overwintering eggs, the larvae of which will moult the following summer? These questions are raised by the different cycles of the species found at the two edges of its range, west and east [
6,
9,
10,
14,
43], where moulting occurs at different times and there are established summer diapauses, as well as life cycles involving parts of two calendar years [
9,
14] and moulted forms with life spans of more than one year [
8].
In order to clarify the life cycle of the population in Bulgaria, a long-term multi-year study of phenological features is needed, with marking of individuals at different times of the year and their subsequent tracking for possible recapture. To help clarify the life cycle, larvae captured during excavations can be used to identify the period of hatching and to estimate the time of pupation. It is likely that the phenology of C. hungaricus in this extreme refugium differs from that currently known in other parts of the species range.
The dynamic densities found in our study were calculated for the entire period of the year when the traps were operated, regardless of the season and the presence of snow cover, given the inability to correctly monitor and account for this climatic feature. During our visit in site 33 on 19 November 2022, an active female was found in one of the traps, indicating that the species may be active during late autumn. Surprisingly or not, our data were supported by Bérces and Růžičková [
11], who found active
C. hungaricus beetles even around temperatures near 0 °C. It is also known that species as
C. hungaricus are oligothermic and successfully survive low temperatures [
43]. The situation was similar during the dry and hot summer period, as evidenced by the finding of an active individual at site 16 on 18 July 2023, which rules out the possibility of summer diapause. Our results differ from the known activity of
C. hungaricus in Hungary, where it is aestivating during the hottest summer days in July [
7]. As an inhabitant of severe habitats such as the steppes,
C. hungaricus is probably a temperature-tolerant species, which was also pointed out in the work of Bérces and Růžičková [
11].
Due to climate changes, the period of activity of carabids has been extended, i.e., if possible, it is best if the surveys are performed year-round or cover the entire period with positive temperatures. The extended period of trap setting in our study can be considered optimal, given the variation in annual activity arising from factors of different natures and strengths, strongly influencing the timing and specificity of the life cycle in a particular year. The period from the end of March to the beginning of December with monthly collections should be considered the optimum period for research, and for establishing population and phenological characteristics through capture, marking and recapture, the periods from 15 May to 30 June and from 15 September to 30 October are purposive. The proposed timelines are based on the results established to date and cannot be considered final, but they should be envisaged in the monitoring scheme for the species in Bulgaria.
5. Conclusions
In this paper, we provided details on the life history and community structure of the Natura 2000 target species Carabus hungaricus, which are potentially useful for its further monitoring and conservation. For the first time in Bulgaria, some characteristics related to the life history, associated taxa, community structure and potential competitors, prey and predators of this endangered and protected species have been studied. Ecological data on the abiotic and biotic factors of its specific habitats were also obtained. Assessments based on the absolute abundance of small invertebrates are often impossible, unnecessary or erroneous, and our study proves that the wrong approach was applied in the past. We suggest that it would be better for such quantitative characteristics to be calculated with the help of the dynamic density, given as the number of individuals captured per 100 trap-days and the percentage of the species to the total number of individuals.
Based on the results, we could claim that in relation to its ground beetle fauna, the studied complex of habitats is quite remarkable for Bulgaria and appears to be stable and characteristic for the steppe biome. The ratio between the Zoophagous and Mixophytophagous species is extraordinary for Bulgaria, but typical for the steppe zone of Eurasia. The relatively low number of winged species is comparable only to typical montane habitats. In relation to their humidity preferences, mesoxerophilous carabids had the largest share, while hygrophilous comprised only one species. The classification analysis indicated the great resemblance between the habitats where the target species C. hungaricus lives. The applied ecological analysis of the studied traps in habitats with a presence of individuals of C. hungaricus showed a complex set of patterns in the distribution of individuals and their accompanying assemblages of ground beetle species.
Here, for the first time, we present data on the peculiarities of the phenology of C. hungaricus, its sexual and seasonal activity, as well as its distribution depending on the nature of its habitats in the southernmost limit of its steppe range, which the karst steppes around the Sofia Basin seem to represent. In Bulgaria, at present, C. hungaricus is found in a relatively restricted area, where its distribution is patchy and with large density fluctuations, the reasons for which remain to be elucidated. The life history of the species and its duration also require continued study to establish its specificity and likely adaptive variability. We established a trend of increased activity during the spring–summer and autumn periods, with a maximum at the second peak. No summer diapause was detected. However, this requires future investigation and establishing its regular recurrence or randomness.
Analyses of the species-important environmental gradients can be considered momentary and primary, given the lack of previous similar studies. The results obtained here are transitory and not final, given the relatively short 3-year study period and the need to investigate the patterns found in the context of significant global environmental changes.