Abstract
This study analyzes 1980–2020 landings data from three Mediterranean coastal lagoons—the Logarou and Rodia-Tsoukalio Lagoons (NW Greece) and the Mar Menor Lagoon (SE Spain)—to assess ecosystem changes and fishing pressure dynamics. The findings classify these systems as low-yielding, with productivity ranked as follows: Yield Logarou > Yield Rodia-Tsoukalio = Yield Mar Menor. Mean trophic level analysis (mTrL) revealed significant differences driven by the contribution of detritivorous and mid-level carnivorous species (TrL Mar Menor > TrL Rodia-Tsoukalio > TrL Logarou). The fishing pressure indices suggest reduced fishing intensity in the Greek lagoons, while in Mar Menor, a stable Fisheries in Balance (FiB) trend corresponded with stable yields despite eutrophication. Cluster analysis (CA) and principal component analysis (PCA) linked ecosystem differences to sediment characteristics and changes in habitat structure. These results underscore a transition of Mediterranean coastal lagoons toward new ecological states, highlighting the urgent need for habitat conservation and adaptive management strategies to ensure sustainable fisheries under increasing environmental pressures. These findings may be extrapolated to similar transitional coastal ecosystems facing comparable anthropogenic stressors worldwide, providing a broader framework for understanding and managing lagoon systems under changing environmental conditions.
1. Introduction
Coastal lagoons are among the most productive ecosystems globally, offering essential goods and services to humans [1,2]. However, they are particularly vulnerable to the numerous environmental pressures arising from both land-based and marine activities taking place in their surroundings. Such pressures not only influence their ecological and environmental status [1,3] but also lead to alterations in fish species distribution [4,5] and annual fisheries landings [6,7,8,9,10,11]. Despite their importance, scientific knowledge about fisheries in coastal lagoons, their ecological mechanisms, and their functioning remains limited and fragmented [12].
Time series data on landings are valuable tools for assessing the ecological status of coastal lagoons, enabling accurate assessment of fishing grounds and providing reliable fisheries data [13]. Since catch composition partially reflects the fish assemblage in such ecosystems, three indicators are particularly useful for evaluating trophic interactions. The Trophic Level index (TL) reflects the trophic structure of fish communities and its variation [14,15,16]. The cumulative relative Biomass Trophic Level Spectra (BTLS) identifies how different pressures, like habitat loss, influence the balance of fish populations over time [17]. Lastly, the Trophic Category (TrC) index converts species landings into trophic groupings, useful for monitoring ecosystem changes [18]. To evaluate the ecological balance of fisheries, the Fishing in Balance (FiB) index, the Catch per Unit Effort (CPUE), and the maximum sustainable yield are used as representative indicators [8,19,20].
This study analyzes time series landings data from 1980 to 2020 to examine diachronic patterns in fishing yield, trophic dynamics, and fisheries management across three Mediterranean lagoon systems: the Logarou Lagoon and the Rodia-Tsoukalio Lagoon Complex (Northwest Greece), and the Mar Menor Lagoon (Southeast Spain) (Figure 1). The analysis also explores how physical, chemical, and sedimentological characteristics influence these dynamics. Special emphasis is placed on understanding the temporal occurrence and intensity of recorded pressures, considering each lagoon’s geographic location, size, and ecological significance within the Mediterranean region. By investigating these interactions, this research aims to offer insights into how environmental and anthropogenic factors shape fishing activities and contribute to ecosystem resilience.
Figure 1.
The study areas: the Mar Menor Lagoon in southeast Spain and Rodia-Tsoukalio and Logarou Lagoons in northwestern Greece.
2. Materials and Methods
2.1. Target Area
The Greek lagoons are situated in the northern part of the Amvrakikos Gulf (38°58′383″ N, 20°59′500″ Ε), which covers approximately 405 km2 and is one of the largest enclosed fjord-type gulfs in Greece (Figure 1). The area is shaped by the inflow of three rivers—Louros, Arachthos, and Vouvos—creating a complex of river plains, deltas, marshes, and lagoons. This region has been recognized as a critical habitat protected under the RAMSAR International Convention since 1975 [21]. The Logarou Lagoon, covering 25.3 km2 with an average depth of 0.5 m, is a restricted system characterized by a barrier with seven tapered openings functioning as fish traps. The Rodia-Tsoukalio complex consists of Rodia Lagoon, a large brackish swamp (12.87 km2), and Tsoukalio Lagoon (16.41 km2), which is connected to Rodia via a narrow passage. Both lagoons are surrounded by wetlands and are characterized by complex structures with numerous islets. Rodia receives substantial freshwater inflow from the Louros river, while Tsoukalio has limited freshwater input and is separated from the open sea by a thin barrier with nine fish traps [22].
The Mar Menor Lagoon (37°42′943″ N, 0°47′098″ W), in Southeast Spain (Figure 1), is a hypersaline coastal lagoon covering approximately 136 km2 with an average depth of 4.4 m [23]. It is classified as a choked lagoon [24], due to its restricted connection to the Mediterranean Sea. It is isolated from the open sea by a sand bar called La Manga which is crossed by three inlets. Historically, these inlets were used for traditional fishing practices, but modifications such as dredging and silting have altered the system’s hydrodynamics and the fishing activities over time [1]. Like the other lagoons in this study, it has been designated by the RAMSAR Convention as a wetland of international importance and is subject to numerous other national and regional protections that, however, do not effectively prevent the impact of the pressure and consequences of human activities on its environment.
2.2. Datasets
Annual landings during the period 1980–2020 were estimated for January to December each year. The Greek lagoons have been leased to the same fishery cooperatives since 1971; the number of fishermen members has varied per season mainly according to fisheries production [25]. Data based on the logbooks of the corresponding fishery cooperatives were kindly provided by the local fisheries department of Arta Prefecture. For Mar Menor Lagoon, data on the commercial landings and fishing efforts were sourced from the Fishermen’s Association of San Pedro del Pinatar and the Fisheries Service of the Ministry of Agriculture and Water of Murcia Regional Government, complemented by the 13-volume series of the “Anuario de Pesca Marítima” which provides yearly catch data from 1973 to 1986 [26].
For the Greek lagoons, annual fisheries landings data were primarily derived from fish barrier traps, which account for up to 70% of total landings, and from nets, including trammel nets and fyke nets. In the Rodia and Tsoukalio Lagoons, landings data included those using fishing gear in both lagoons, as well as from the Tsoukalio Lagoon’s outlet to the sea. The barrier traps are passive-fixed fishing gears that form part of a fence (which is a construction hammered into the lake bed, sustaining a net of reeds that separates the lagoons from the sea) and are used from July to January. Trammel nets (mainly 80–88 mm of stretch mesh size in the inner part of the net and 360 mm in the outer panel), and 30 mm stretch mesh fyke nets (specifically used for eel and goby fisheries) are used all year round but with lower density. Since the late 1980s, the fence and barrier traps have been gradually replaced by cement frame and plastic net installations, with the aim primarily of conserving the catch of market-sized fish and assuming that the efficiency of the replaced fishing gear remains stable regardless of changes in the construction materials [27].
Fishing in the Mar Menor is primarily artisanal, with boats averaging length of approximately 8 m and engine power ranging from 8 to 389 horsepower (HP). The fishing techniques are based on traditional gear, the use of which is regulated by Decree 91/1984 (2 August) of the former Ministry of Agriculture, Livestock and Fisheries of the Region of Murcia. This decree outlines the technical specifications of the gear, target species, permitted fishing periods and areas, as well as limits to fishing effort, including the number of gears per vessel and the number of fishermen on board. The decree also designates 17 preferential fishing areas, known as Trozos de Compañías, which are assigned weekly through a draw conducted by the fishermen. Vessels use a basic set of predominantly static nets, which are seasonally rotated and modified to optimize catches of target species. The main types of gear employed are paranzas and langostineras (also known as charamitas). These consist of a guide net (travesía) that channels fish into either a snail-shaped net (moruna) or a trap (paranza), which is a rectangular box-like net with a bottom and a roof designed to hold fish alive. In addition to these main types of gear, other types of fixed gear—such as trammel nets and longlines—are used on a smaller scale. Also noteworthy is the traditional use of reed nets (encañizadas) in the lagoon’s inlets. These structures allow the entry of juvenile migratory species into the lagoon but retain adults as they attempt to return to the open sea, directing them into paranza-type receptacles.
2.3. Data Analysis
Time series were examined according to the criterion proposed by [28], to establish whether each of the annual production values was independent of previous values or whether the time series exhibited a positive or negative trend in general (critical statistics were compared with the Anderson table).
Information connecting the fisheries’ production and the ecosystem structure was obtained using the mTL ecological index. Trophic level (TL) expresses the position of organisms within food webs that are largely defined by the aquatic ecosystems [8,14], computed from the following formula:
where Yjk represents the landing in t of species j in a given year k, TLj is the trophic level of species j (ranging from 2 to 5 expressing the position of species j in the trophic chain according to the composition of its diet), Yk represents the total landing in t in the year k, TE is the transfer efficiency, set at 10% in agreement with the average value according to analysis of a suite of different marine ecosystems [29], and mTLk represents the mean trophic level of the landings for the year k.
mean trophic level index (mTLk) = Σ(Yjk × TLj)/Yk × log(1/TE)
The exploited fishery species were categorized into four groups: Trophic Category 1 (TrC1, TL: 2.00–2.50) consisting of herbivores and detritivores species, Trophic Category 2 (TrC2, TL: 2.51–3.00) consisting of omnivores species, Trophic Category 3 (TrC3, TL: 3.01–3.50) consisting of mid-level carnivorοus species, and Trophic Category 4 (TrC4, TL > 3.50) consisting of top predator species. Specific analysis determined the contribution of each category to the total landings, which is considered useful for monitoring ecosystem change [30].
Additionally, to analyze the time series temporal patterns, the cumulative biomass trophic level spectra (BTLS) were applied as a potential indicator of multifactor impacts on the ecosystem [17]. To facilitate the analysis of the relationships between landings and the trophic structure of the food web, a scatter plot of the yield versus mTrL of the lagoons was employed [30].
The families and species that represent the main catches in the studied lagoons analyzed in this work are listed in Table 1. Individual values of TL for each species were taken from [31,32]. The commercial categories of the five species of grey mullet (Mugil cephalus Linnaeus, 1758, Chelon labrosus (Risso, 1827), Chelon saliens (Risso, 1810), Chelon auratus (Risso, 1810) and Chelon ramada (Risso, 1827)) and the two species of Mullidae (Mullus barbatus Linnaeus, 1758 and Mullus surmuletus Linnaeus, 1758) were grouped as functional groups (Mugilidae, and Mullidae respectively). In Logarou and Rodia-Tsoukalio Lagoons, the composition of the gobiid fish assemblages is dominated by the grass goby Zosterisessor ophiocephalus (Pallas, 1814) [27], but due to the lack of analytical data on the recorded landings of gobies, the trophic level of this group was estimated. In Mar Menor lagoon, four Gobiidae species were recorded, dominated by the giant goby Gobius cobitis Pallas, 1814 [33].

Table 1.
Species composition of landings from the studied systems, trophic category (TrC) and trophic level (TL) values. (ALL = All lagoons, MM = Mar Menor lagoon). Source: [32].
To assess whether a certain level of exploitation can be sustained by a given marine ecosystem and to detect bottom-up effects, the FiB index was used [8]. This index represents the ratio between the energy required to sustain the fishery landings and the baseline value from the first year of the time series. The FiB was estimated for each year, considering both the landings and their mTL, combined in the following final expression [8]:
where Yk and mTLk represent the total landing and its mTL for the year k, and Yo and mTLo represent the total landing and its mTL for the first year of the time series.
FiB = log (Yk/Yo) + (mTLk-mTLo)
Therefore, a positive trend in the FiB time series may be caused by an increase in the fishing effort (expanding fisheries) or by an increase in nutrient availability which, in turn, leads to an increase in the productivity of the system and, consequently, in landings [16]. Since catch per unit effort (CPUE in t/fisherman) is considered a reliable indicator of species catches that can determine changes over time [20], the observed trends were interpreted by plotting CPUE against FiB to facilitate analysis of the relationships between landings and fishing activity.
The Gordon–Schaefer equilibrium model [19,34] was used to estimate reasonable figures of the maximum yield and the maximum fishing effort in conjunction with the recorded values throughout the time series period, according to Equation (1):
where f is the annual CPUE (as t/fisherman).
Annual Production = af + af2
A meta-analysis of indicators in the form of principal component analysis (PCA) was used to examine the major variations among the target lagoons based on their time-series-averaged physical, chemical, and sedimentological characteristics. The degree of similarity among the lagoons was calculated using cluster analysis (CA), computed using the Euclidean distances.
The comparison of the fishing yields is presented as tonnes per square kilometer (t/km2). One-way analysis of variance (ANOVA) was applied to test for significant differences among the lagoons in mean annual fishing yields, mTL values, and FiB values over the period 1980–2020, at a 95.0% confidence level. Additionally, to identify trends on the yields, trophic levels, trophic categories, fisheries in balance, and main species landings within the study period, simple linear regression analysis at 95.0% confidence was applied [35]. The PRIMER software package, version 6.0, was used for the multivariate statistics analysis.
3. Results
3.1. Time Series Analysis
The independence test showed that the time series landings data for the studied systems exhibited a positive serial correlation in the population (Von Neumann Ratio method, K < k), indicating that no structural break was present in the data [28].
The species composition data of the landings and the trophic levels in each lagoon, as well as the formed trophic category groups, are summarized in Table 1, where 23 species or sets of species grouped into families are recorded in total. In Logarou and Rodia-Tsoukalio Lagoons, fisheries data are mainly dominated by the Mugilidae family, the gilt head sea bream, and the eel, which constituted more than 85% of the annual mean production (87% and 92% respectively) in the studied period. In contrast, in the Mar Menor Lagoon, a group of 8 species and families (eel, gilt head sea bream, Mugilidae, Mullidae, big-scale sand smelt, annular sea bream, sand steenbras, and European anchovy) comprised 81% of the annual average landings. The contribution of pelagic species (mainly anchovies, sardines, and sand smelt) approached about 37% of the annual average time series production in the studied system, indicating a different trophic structure.
3.2. Logarou Lagoon
The fishing yield of this lagoon was characterized by a continuous decreasing trend (p < 0.05) throughout the study period (Figure 2). Annual values ranged from 0.86 t/km2 in 2020 to 8.94 t/km2 in 1981, with a mean value of 4.39 t/km2 (±1.83), Additionally, the main target species (Mugilidae, sea bream, Gobiidae, and eels) also showed a significant decreasing trend (Figure 3).
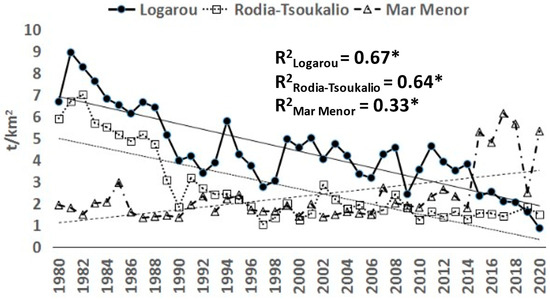
Figure 2.
Annual fluctuations of the fishing yield (t/km2) in the systems studied for the period 1980–2020 * Statistically significant trend at 0.05 level.
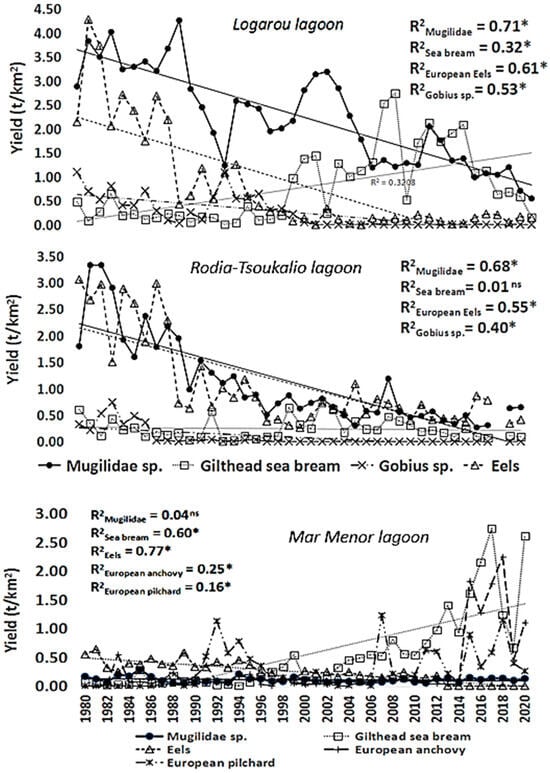
Figure 3.
Fishing yield trends in main target species or families throughout the period 1980–2020 in the studied systems. *: statistically significant correlation at 0.05 level. ns: no statistically significant trend. Trends in Mar Menor Lagoon: Mugilidae, sea bream, eels, European anchovy, and European pilchard species only.
The mTrL index (Figure 4), ranging from 2.39 (in 1989) to 3.08 (2008), with a mean value of 2.81 (±0.18), revealed a stable trend (p > 0.05). However, the pattern of trophic categories (Figure 4) featured an upward trend of the TrC3 contribution (p < 0.05, 32.02 ± 18.57) and a substantially decreased contribution from TrC4 (p < 0.05, 14.43 ± 14.36). In contrast, TrC1 and TrC2 trends remained stable throughout the study period. A scatter plot of the yield versus the mTrL is shown in Figure 4; definite segregation of the period 1980–1988 was identified with high yield values (>6.00 t/km2) and low mTrL range values (2.81–3.08), whereas in the second sub-period (1989–2020), there were lower yield values as well as a wider fluctuating range of mTrL values (2.39–3.09).
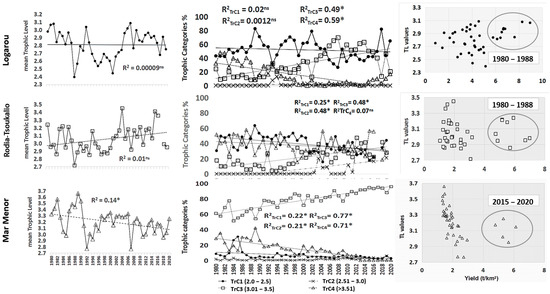
Figure 4.
Mean trophic level trend, trophic category trends and TL values versus yield plot for the period 1980–2020 in the studied systems. *: statistically significant correlation at 0.05 level. ns: no statistically significant trend at 0.05 level.
The FiB index (Figure 5) showed a negative trend (p < 0.01) and exhibited negative values in almost the total of the time series (positive values were recorded only in the first two years of the study period), ranging from −1.13 (in 2020) to 0.21 in 1981 (mean value −0.39 ± 0.42). According to the Gordon–Schaefer model (Figure 5), the maximum sustainable yield value (MSY), was estimated as 9.01 t/km2 while the mean number of fishermen for the study period (90.95 ± 28.63) is considered very low in relation to the maximum estimated number of fishermen (317 persons). According to the FiB index versus the CPUE plot, the total CPUE values of the time series (0.73–2.60 t/fisherman) were over the maximum CPUE value (0.73 t/fisherman) predicted by the Gordon–Schaefer equilibrium model (Figure 5).
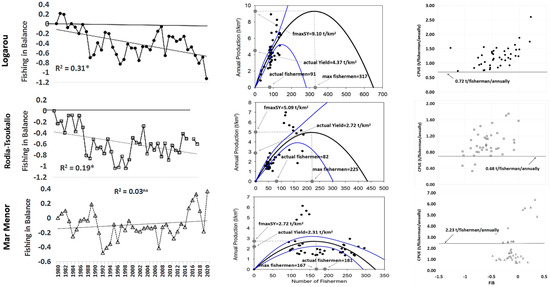
Figure 5.
Fishing in Balance index, the Gordon–Schaefer model and Catch per Unit Effort versus FiB values for the period 1980–2020 in the studied systems. *: statistically significant correlation at 0.05 level. ns: no statistically significant trend at 0.05 level. (the blue lines in Gordon-Schaefer graphs denote the confidence intervals of the model).
3.3. Rodia-Tsoukalio Lagoon
The fishing yield of this lagoon indicated a clear decreasing trend (p < 0.05) for the period 1980–1997, followed by a period with stable but low fishing yield values (less than 3 t/km2) (Figure 2). Values ranged from 1.05 t/km2 (in 1997) to 6.97 t/km2 (in 1982), with a mean value of 2.72 t/km2 (±1.70). The landing trend of sea bream was the only one among the target species (Figure 3) that was characterized by not being statistically significant (p > 0.05).
The mTrL index (Figure 4) also showed a stable trend (p > 0.05), ranging from 2.72 (in 1989) to 3.45 (2005), with a mean value of 3.05 (±0.16). As seen in Figure 4, the contribution of TrC1 (p < 0.05, 39.35 ± 11.05) decreased throughout the time series and was gradually replaced by TrC2 (p < 0.05, 5.82 ± 10.43) and TrC3 (p < 0.05, 21.29 ± 11.82). The percentage of TrC4 was high and stable throughout the study period (p > 0.05, 37.17 ± 11.53). Figure 4 shows the scatter plot of yield versus mTrL, where a defined segregation of the period 1980–1988 with high yield values (>4.70 t/km2) was identified, while in the second sub-period (1989–2020), lower yield values were observed without significant changes in the range of mTrL values (2.85–3.25).
The FiB index also showed a significant decreasing trend (p < 0.05). It is noticeable that only negative values were obtained throughout the study period (Figure 5), ranging from 0 (in 1980) to −1.04 in 1997 and 2000 (mean value −0.58 ± 0.27). According to the Gordon–Schaefer model (Figure 5), the maximum sustainable yield value (MSY) was estimated as 5.09 t/km2 while the mean number of fishermen for the study period (81.60 ± 46.90) was low relative to the estimated maximum number of fishermen (225 persons). Plotting the FiB index versus the CPUE reveals that the CPUE values of the time series (0.33–1.75 t/fisherman) were over the maximum predicted CPUE value from the model (0.68 t/fisherman), except for the years 1997, 1989–1990 (Figure 5).
3.4. Mar Menor Lagoon
The fishing yield of the Mar Menor Lagoon showed a clear increasing trend (p < 0.05), especially from the year 2015 and onwards when it reached more than 2.5-fold higher values than in the previous time series period (Figure 2). Values ranged from 1.34 t/km2 (in 1987) to 6.14 t/km2 (in 2017), with a mean value of 2.31 t/km2 (±1.26).
The mTrL index (Figure 4) showed a significant decreasing trend (p < 0.05). Maximum values reached 3.66 in 1989. Minimum values of 2.76 were observed in 1992 and 2007. During the study period, the mean value was 3.20 (±0.20). The high contribution of TrC3 (Figure 4), showing a significant upward trend (p < 0.05, 78.21% ± 12.36), indicates the dominance of fish groups in high-trophic categories. Even though TrC1 showed a significant decreasing trend (p < 0.05, 6.37% ± 5.53), the overall average trophic level was shaped by a decrease in TrC4 (p < 0.05, 13.92% ± 10.55), which counteracted the increase in the TrC3. Meanwhile, the contribution of TrC2 (p > 0.05, 1.49% ± 1.36) remained negligible. The scatter plot of yield versus mTrL (Figure 4) highlights two distinct periods. The 1980–2014 subperiod was characterized by low yield values (1.34–2.96 t/km2) and a wide range of trophic level values (2.76–3.66). In contrast, the 2015–2020 subperiod showed higher yield values (4.80–6.14 t/km2) and a narrower trophic level range (2.95–3.25), primarily influenced by increased landings of sea bream, sardines, and anchovy.
In the FiB index (Figure 5), values ranged from 0.37 (2020) to −0.48 (1992), with a mean value of −0.10 (±0.19). Even though the values were below zero during almost the time series period, the index nevertheless showed a stable trend accompanied by recorded positive values due to the last five years’ extreme increase in fishing yield. According to the Gordon–Schaefer model (Figure 5), the maximum sustainable yield (MSY) was estimated as 2.72 t/km2, close to the average annual yield value of 2.31 t/km2, while the mean number of fishermen for the study period (180.64 ± 62.57) was higher than the estimated maximum potential number of fishermen (167 persons). The CPUE values for the period 1980–2006 (0.67–1.91 t/fisherman) fluctuated below the maximum CPUE value predicted by the model (2.23 t/fisherman), while a rational fisheries activity was recorded for the 2007–2020 period (2.53–3.83 t/fisherman). However, the maximum values recorded referred to the last five years of the time series, in which the productivity of the system was related to the increase in the stock of specific migratory species (Figure 5).
Table 2 reports the average values of yield, mTrL, and FiB indicators for the period 1980–2020, as well as the trends of the ecological indicators and the interpretations that emerge from the combined results.

Table 2.
Average, standard deviation, and minimum and maximum values for the yield, mTL, and FiB indices of the studied systems in the time series.
3.5. Comparison of the Systems Studied
Statistical analysis (one-way ANOVA with Tukey and Games–Howell post hoc tests) revealed significant differences in fishing yields and trophic structure among the three lagoon systems (p < 0.05). Logarou exhibited the highest average yields, followed by Rodia-Tsoukalio and Mar Menor, which presented comparable lower values. Similarly, the mean trophic level (mTrL) was significantly higher in Mar Menor, with intermediate values in Rodia-Tsoukalio, and the lowest in Logarou (p < 0.01). These differences were primarily attributed to shifts in the relative abundance of key trophic categories: detritivores (TrC1), mid-level carnivores (TrC3), and others. Specifically, Logarou had a significantly higher proportion of TrC1 species, while Mar Menor was dominated by TrC3 species. Rodia-Tsoukalio showed distinct patterns in intermediate trophic categories (TrC2 and TrC4), setting it apart from the other two systems [29].
Time series analysis for the FiB index showed that the mean values for the studied systems (Table 2) were negative, reaching +0.37 for the Mar Menor lagoon and as low as −1.13 for the Logarou lagoon, both in 2020, while the lowest values in the Rodia-Tsoukalio lagoon (−1.04) were recorded in 1997 and 2000. FiB values among the lagoons were significantly different (one-way ANOVA, Tukey post hoc test p < 0.01, FiBMar Menor > FiBLogarou > FiBRodia-Tsoukalio). In the Greek lagoons, the reduction in the intensity of fishing efforts was similar, coinciding with the corresponding reduction in fisheries production, while in the Mar Menor Lagoon (except during the last five years of the time series), the stable FiB index trend was related to the almost stable trend of yield production.
The Supplementary Table S1 shows the environmental indicators (physical, chemical, sedimentological, mean and range values) in the studied systems. For the Greek lagoons, the data on the environmental variables of the water column were considered minimal, for the period studied, especially in the 1980s, but the frequency of the recorded parameters became periodic in the last decade of the time series. Seasonal measurements were taken (in the spring and autumn) at two stations in each lagoon in 2012, 2013, and 2015, as part of the Water Frame Directive (WFD). In the case of Mar Menor, the dataset comprised over 6600 samples collected biweekly at 27 locations distributed across the lagoon. Sediment data were obtained from the same sampling stations on a seasonal basis, as well as from numerous additional, more heterogeneous coastal sites, depending on the specific projects conducted during the study period.
The sediment type differentiation of the studied systems was also evident in the BTLS of the food web (Figure 6A), which revealed different dominant trophic categories in the studied systems (TrC1, TrC4, and TrC3 in the Logarou Lagoon, the Rodia-Tsoukalio Lagoon, and the Mar Menor Lagoon, respectively). In general, it is worth mentioning that the trophic pattern of the Mar Menor Lagoon coincided with the Rodia-Tsoukalio Lagoon, with trophic values between 3.13 and 3.30, and with the Logarou Lagoon, with trophic values between 3.42–3.85.
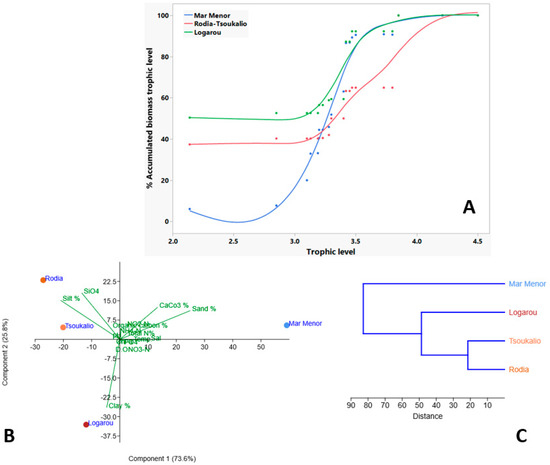
Figure 6.
Meta-analysis of indicators statistical method in the form of (A) biomass trophic level spectra (BTLS), (Β) principal component analysis (PCA), and (C) cluster analysis (CA) for studied periods for the period 1980–2020 (see also Supplementary Table S1).
In the PCA (Figure 6B), the first two axes explained 73.55% and 25.81%, respectively, meaning that together, these two components captured nearly all the variation in the dataset. Sand% and CaCO3% were strongly associated with the x-axis and aligned with the Mar Menor Lagoon. In contrast, the Rodia-Tsoukalio Lagoon was more closely linked to higher silt% and SiO4, indicating finer sediment composition, whereas the Logarou Lagoon, located lower on the y-axis, exhibited unique environmental conditions due to the clay% in the sediment. Cluster analysis in all studied systems generated three main clusters, at a cut-off linkage distance of 84, in which the Mar Menor Lagoon was clearly separated from the Greek lagoonal systems (Figure 6C).
4. Discussion
4.1. Fishing Yield and Studied Lagoon Characteristics
In our study, the fishing yields were considered low, with landings below the average annual landings reported for both the Mediterranean region (12.89 t/km2) and lagoons worldwide (10.10 t/km2) [12,20] (Supplementary Table S1). Understanding the mechanisms that regulate fish production in these unique environments is a complex task due to the distinct environmental and geomorphological characteristics of each lagoon, varying anthropogenic pressures, and the diverse fishing strategies employed.
Although several studies have explored the relationship between fishing yield and the geomorphological and environmental characteristics of Mediterranean and global coastal lagoons [12,20,36], few have compared the temporal dynamics of catches across lagoons with differing environmental conditions.
Primary production and ecological balance in lagoon ecosystems are largely influenced by salinity levels, which can vary significantly depending on hydrological isolation, freshwater inflows, and evaporation dynamics [37,38]. Salinity not only shapes the structure and function of phytoplankton communities but also determines zooplankton composition and benthic organism distribution, all of which are key elements in the fish food chain and affect trophic transfer efficiency [39,40,41]. In the present study, salinity-linked variables such as silicate concentrations, calcium carbonate content (%), and sediment type emerged as significant components in the PCA meta-analysis, highlighting their ecological importance. The lagoons analyzed in this study exhibited different salinity regimes: the Mar Menor Lagoon showed salinity levels ranging from 42 to 47 PSU, characteristic of hypersaline systems, while Logarou and Rodia-Tsoukalio typically fluctuated between 18 and 30 PSU, influenced by freshwater inputs and exchange with the open sea. It is important to emphasize that “hypersaline” systems encompass a wide range of salinities, each producing distinct ecological outcomes. Sharp or prolonged salinity changes can trigger major shifts in ichthyocenosis, altering both species composition and trophic interactions. This has also occurred in hypersaline systems like Sivash Bay and Bardawil Lake, where human-induced changes in salinity caused major ecosystem shifts [42,43,44]. These cases reinforce the idea that salinity regimes must be considered as dynamic and site-specific drivers when assessing productivity and resilience in lagoon ecosystems. Future monitoring in the studied lagoons should incorporate high-resolution salinity data to better link trophic changes and fisheries dynamics to environmental variability.
In the Logarou Lagoon, the high clay concentration in the sediment indicates calm hydrodynamic conditions, low water flow, and increased retention of organic matter and nutrients. These factors affect water circulation and evaporation [45], while promoting the growth of plankton and benthic organisms that serve as food for fish [46]. In contrast, the Rodia–Tsoukalio Lagoon is characterized by relatively low salinity values, supporting higher concentrations of silicates (SiO4). This favors the rapid growth of diatoms, influencing the N:P ratio and nutrient availability in the system, factors indirectly linked to productivity and the structure of the fish assemblage [47]. In the Mar Menor Lagoon, the high percentage of calcium carbonate (CaCO3%) in the sediment, an essential compound for benthic foraminifera, suggests a limitation in primary production due to phosphorus adsorption onto carbonate sediments. At the same time, it indicates a lagoon with intense biological activity and a well-developed food chain.
It has also been reported that the sediment granulometry significantly affects macrophyte communities, as well as the diversity, composition, and abundance indices of benthic communities [39,48,49]. In the Greek lagoons, the deterioration in water quality of the Arachthos River due to nutrient enrichment [50] and the lower water outflow quantity of the Louros River [27] appear to be responsible for significant habitat loss and/or habitat degradation in the Amvrakikos ecosystem. This is evidenced by the gradual decrease in the presence of rooted angiosperms such as Zostera noltei Hornemann 1832, Ruppia maritima Linnaeus 1753, Ruppia cirrhosa (Petagna) Grande 1918, and Cymodocea nodosa (Ucria) Ascherson 1870 [39,51,52], along with a corresponding shift toward opportunistic green macroalgae [51,53]. Changes in vegetation cover have altered the benthic community structure, while increasing content of organic matter in the muddy bottoms and reducing the levels of dissolved oxygen have negatively influenced the yield trends of the lagoons, without ever reaching hypoxic or anoxic conditions [51,54]. The decline of species richness in benthic communities is also associated with changes in vegetation in Amvrakikos lagoons [51]. Vegetated areas, especially seagrass beds, support higher community diversity and may combine different transfer pathways of organic matter, sustaining more successful elemental recycling [39,55]. Moreover, hydroelectric dams on the Louros (1954) and Arachthos (1981) rivers regulate the supply and timing of fresh water to the Amvrakikos Gulf [56], contributing to reduced abiotic parameter footprints in the Ionian Sea and subsequently diminishing fish attraction to the Gulf and the Logarou and Rodia-Tsoukalio Lagoons [57]. Due to the semi-enclosed character of the Amvrakikos Gulf, water quality is strongly influenced by organic load and pollutant inputs from these rivers, which carry runoff from agriculture, livestock, and sewage from coastal towns and villages. These pressures, coupled with the limited physical connection of the Gulf to the Ionian Sea, have altered environmental variables and fostered hypoxic conditions, ultimately reducing fisheries productivity in the Gulf [58].
In the Mar Menor Lagoon, excluding 2015–2020, fishing yield trends have remained stable but lower than in the oligotrophic pre-1980 period. In the early 1970s, the dredging and widening of one of the main inlets, El Estacio, for the construction of a navigable canal and a marina brought about drastic changes in the hydrology of the lagoon, with a decrease in salinity and a softening of extreme temperatures. This Mediterraneisation of the lagoon conditions led to the colonization of new species, including the algae Caulerpa prolifera (Forsskål) J.V.Lamouroux 1809. The reduced intensity of the salinity gradient [59] supports the hypothesis that weaker gradients decrease lagoon productivity [11]. On the other hand, the colonization of Caulerpa prolifera, which accumulates organic matter and induces anoxia in the sediments [60], was related to a strong reduction in the fishing yields of grey mullets and sea bream.
Assessing the effects of environmental status and coastal fishing activity in the Amvrakikos Gulf on lagoon yields is challenging due to limited historical data. Notably, data gaps from the 1980s onward coincide with major ecosystem changes in its environmental conditions. Available data span larger areas (e.g., sub-area 04: Lefkada Island and Amvrakikos Gulf; Figure 1) and are insufficient for reliably characterizing production trends specific to the Gulf [61]. In the broader Ionian Sea region (central Mediterranean, subdivision 2.2 according to the General Fisheries Commission for the Mediterranean Sea-GFCM), total catches increased over 25 years (1970–1995) before declining at a slower rate [62]. However, in the Greek marine sub-area 04, a statistically significant increase in catches was recorded from 1928 to 2010 [63]. These trends do not align with the studied lagoons, whose fisheries production is influenced by the Gulf’s environmental changes and changes in fisheries activity, with minimal impact from the open sea. A recent ecological status study classified the South Amvrakikos Gulf and the Arachthos River estuary as being in poor condition, while the Louros River estuary and studied lagoons were considered moderate [21].
Comparing fishing trends among the studied systems revealed two patterns. The Rodia-Tsoukalio and Logarou Lagoons exhibited long-term fluctuations in fishing yield (1980–2000), followed by stabilization (2001–2020) but at minimal values. The Mar Menor Lagoon showed stable trends (1980–2015), similar to the Greek lagoons’ second sub-period, reflecting the different timing of pressures, such as the El Estacio channel widening in the 1970s versus the Arachthos dam operation in 1981. Before the studied time series, much higher yields were also recorded in the Mar Menor Lagoon [64], suggesting that the studied systems are transitioning from increased productivity within carrying capacity to a new environmental equilibrium with reduced productivity.
4.2. Species Landing Analysis
The recorded species are characteristic of warm, temperate, and semi-tropical lagoons [65], particularly in the southern European region [66,67]. Multivariate analyses (PCA and CA) largely explained the difference in species composition in landings between lagoons, with sediment type emerging as an important variable, primarily affecting macrobenthic communities. In the Greek lagoons, significant long-term declines were observed in the yields of dominant species, except for gilthead sea bream landings in the Rodia-Tsoukalio Lagoon. For mullets, a decreasing trend—especially after the first decade of the time series—coincided with increased macroalgae colonization, which could be attributed to the limited net primary production available for fisheries. This trend can be partially explained by the high mean trophic level of the catch (2.81 in Logarou and 3.05 in the Rodia-Tsoukalio Lagoon) and the high proportion of primary production diverted to the refractory detritus compartment. This reduced oxygen concentration in the lagoons’ sediments made detritus unavailable to most feeders, particularly mullets [64].
The fluctuations in sea bream landings could also be linked to changes in vegetation cover and, to some extent, efforts at juvenile enrichment during 1991–2000 [27]. However, the stable production trend in the Rodia-Tsoukalio Lagoon indicates these enrichment attempts may not have yielded spectacular results [68]. The dramatic decline in gobiid abundance could be attributed to the loss of seagrass habitats [69], increased organic matter in sediments [70], and overlapping nutritional requirements with sea bream [32]. For eels, the annual rate of decline was steeper in Logarou Lagoon, although it mirrored the European decline of eel landings from 1980–2001, which reached a historical minimum of 1–2% of pre-1980 levels due to widespread environmental changes [57,71].
Fisheries production reports in the Gulf noted have relative stability in cuttlefish and prawn landings, whereas stocks of Sparidae species and striped red mullet have either decreased or disappeared [61].
In contrast, the fisheries landing patterns from the Mar Menor Lagoon reflect the increasing human pressures in recent decades. These pressures include a progressive rise in organic matter content in sediments covered by new meadows, significant changes in faunal assemblages due to inlet enlargement [64], and intensified agricultural practices and urbanization that discharge nutrient-rich waters into the lagoon [72]. In this lagoon, since 1995, changes in agricultural practices in the watershed have led to eutrophication processes [73]. The new environmental state has kept relevant species yields low, probably exacerbated by the increased presence of dinoflagellates, which, under eutrophic conditions, reduce nutrient flow to lower trophic levels [74]. The stable landing trend of the Mugilidae species appears to be influenced by the distribution of Caulerpa prolifera-dominated meadows. The production/biomass ratio (F/B) has decreased since the inlet enlargements, causing accumulation of organic matter and hypoxic conditions rather than availability for benthic fauna and microphytobenthos-feeding fish like mullets [64]. Similarly, sand smelt yields declined sharply, while the initial decline in sand steenbras production was interrupted during the early years of agricultural management changes (1996).
Conversely, significant increases were observed in the yields of sea bream, anchovy, and sardine during the critical eutrophic period of 2016–2020, which included the two major eutrophic crises [75]. The 2016 crisis led to a drastic reduction in the meadows dominated by Caulerpa prolifera in the deep areas of the lagoon (>2 m deep) that make up almost 80% of the lagoon surface, which allowed for reoxygenation of the surface sediments and their refaunation, favoring species such as sea bream that feed on benthic invertebrates. The increasing trend in sea bream production may be also associated with an increase in the temperature of the system as well as the recruitment rate of these species influenced by the fish farming activities in the wider marine area of Murcia, which reported annual mean production of approximately 3000 tons in the last five years of the time series [60,76]. Concerning anchovy and sardine, the increase in planktonic primary production due to the eutrophication process favors planktophagous pelagic fish in the first instance, as they control the phytoplankton production and help the recovery of water quality by exerting top-down control over the pelagic system. After the 2016 crisis, in 2018, the lagoon began a consolidated process of water quality recovery promoted by the top-down control exerted by trophic networks, such as the increase in planktophagous species that regulate the pelagic system, despite the maintenance in nutrient inputs. Sardines, anchovies, and sardinella were the dominant catches in the western Mediterranean area, representing 26%, 13%, and 7% of total landings, respectively [77]. The reasons for the sharp decline in eel production are related to a combination of pressures such as overfishing, habitat loss, and climate change, exerted across Europe in all lagoon systems [78], but global effects associated with the influence of climate change on the biology and larval life span of this species throughout its journey across the Atlantic cannot be ruled out.
4.3. BTLS, Mean Trophic Level (mTrL), and Trophic Category (TrC)
We employed the BTLS index to compare the studied systems over the entire study period, describing the trophic patterns of fish assemblages rather than tracking changes between sub-periods of the time series. The analysis results (Figure 6C) revealed differences in the trophic structure among the lagoons, which were attributed to the synergistic effects of various pressures [17]. A deeper examination of the trophic levels, grouped into trophic categories, showed significant differentiation between the Mar Menor Lagoon and the Greek lagoons, with smaller differences observed between the Logarou and Rodia-Tsoukalio Lagoons. The different geomorphologies and habitat types of the lagoons affect the community structure and composition of the benthic fauna that may act as prey for fish.
In the Mar Menor lagoon, the trophic structure during the study period showed some changes, but on average was dominated by omnivorous species (TL: 2.8–3.1), comprising 78.21% of the catch composition. The colonization of Caulerpa prolifera from 1980 to 1995 impacted sediment properties, increasing organic matter content in muddy bottoms and reducing dissolved oxygen levels [59]. From 1995 onward, these changes further intensified due to starting a eutrophication process, increasing the participation of mid-level carnivorous species (TrC3; TL 3.01–3.50). Additionally, the period marked by the eutrophic crisis that dramatically manifested in the lagoon in 2016 was perfectly differentiated by the corresponding recorded high yield values and the narrower trophic level range.
The BTLS patterns in the Logarou and Rodia-Tsoukalio Lagoons revealed contrasting trophic structures. The Logarou Lagoon was dominated by herbivorous–detritivorous species (TrC1; TL: 2.00–2.50), comprising 51.97% of the catch. In contrast, the Rodia-Tsoukalio Lagoon showed a more balanced trophic composition, with 37.17% detritivorous species and 35.01% high-level carnivorous species (TL: >3.5), influenced by the Louros River’s direct effects on the trophic web. Regarding mean trophic level (mTrL), values in the studied systems were high but consistent with those observed in other Mediterranean lagoons [64].
The stable mTrL trends in the Greek lagoons suggest an absence of the three primary fishing mechanisms: fishing down the food web (replacing high-trophic-level species with lower ones as abundance decreases; [14]), fishing through (maintaining high-trophic-level species despite a decline in TL values; [79]), and fishing up (increasing the TL of catches over time). This stability implies either a maintained ecosystem structure or a consistent fishing strategy [13,80].
However, the trophic category analysis indicated that the Logarou Lagoon experienced restricted trophic flow due to increased sedimentary organic material, stabilizing herbivorous–detritivorous species (>50%), and declining high-level carnivorous species (eels). This led to a significant drop in lagoon productivity. The Rodia Lagoon, influenced by its proximity to the Louros River, exhibited a more uniform trophic category distribution. Despite this, the significant loss of herbivorous–detritivorous species contributed to a decline in the lagoon system’s overall yield.
In contrast, in the Ionian Sea (Mediterranean subdivision 2.2 as defined by the General Fisheries Commission for the Mediterranean Sea-GFCM), the mTL remained stable during 1980–1990 but began declining significantly after 2010. In sub-area 04 (Lefkada Island and Amvrakikos Gulf), mTL values declined notably from 1982 to 1997. These trends did not align with those observed in the Greek lagoons.
In the Mar Menor Lagoon, a “fishing through the food web” mechanism was observed, where landings of high-trophic-level species were maintained or grew despite a significant decline in mTrL throughout the study period [79]. However, this occurred through the sequential reduction of lower-trophic-level fisheries within the lagoonal system, mainly related to the existing pressures and the impacts caused by loss of biodiversity and ecosystem services and to a lesser extent by fisheries management and new policies. A pattern of abrupt change was observed between 1980–1998, followed by more uniform trends in subsequent periods, indicating constant changes in the lagoon’s structure and function, negatively affecting herbivorous–detritivorous (TrC1) and high-level carnivorous (TrC4) species.
The mean trophic level of the Mar Menor Lagoon’s catches over the time series (3.2) and its declining trend mirrored trends in the Balearic Islands during 1970–2017. The mean weighted trophic level of marine catches in this region fluctuated around 3.3, with stability in structure and function despite a decline after the late 2000s [62,81]. This highlights the influence of the sea on fishing composition and yields in the Mar Menor Lagoon.
Observed changes in trophic structure in the studied lagoons are likely to have resulted from loss of submerged vegetation, environmental status, and fishing strategies in neighboring marine areas. Yield versus TL plots (Figure 4) clearly distinguished the first decade in the Greek lagoons and the last five years in the Mar Menor Lagoon, showing no correlation between fluctuations in landings and mTL variations.
4.4. Fishing Pressure (FiB and CPUE)
The fishing pressure parameter, a critical factor influencing the fishing yield of the studied systems, was analyzed using FiB and CPUE indices. Fisheries in the Greek lagoons rely on highly efficient barrier fish traps that capture migratory fish on their return to the sea. These traps are considered optimal for resource exploitation in terms of yield per recruit analysis [82]. By contrast, in the Mar Menor, this type of fishing system, called Encañizadas, fell into disuse after the dredging and widening of the El Estacio channel in the 1970s, leading to the use of smaller nets such as trawls and paranzas. The type of gear used in each lagoon or in the different periods must be considered when analyzing indicators of fishing efforts. While the quantities of net gear and other minor gear such as traps or hook are directly related to the number of fishermen and the number of vessels that operate using them, systems based on palisades in the inlets can be relatively independent of fishing efforts since they act passively, their numbers are stable, and they catch the same amount regardless of the number of fishermen involved in their maintenance and in the collection of the catch [12,60]. However, we can use the number of fishermen operating in the studied systems as a measure of fishing effort because this records annual changes in numbers of employed persons in relation to the quantities of fisheries production.
The declining FiB index in the Greek lagoons reflects contracting fisheries, attributed to impaired system functioning caused by excessive catchment removal [83] and environmental changes in both the lagoons and the Amvrakikos Gulf. The Gordon–Schaefer model results indicated that both Greek lagoons are under-exploited, as the average actual yield and the number of fishermen is below the estimated sustainable levels. Additionally, the density values (3.60 and 2.84 fishermen/km2) are significantly lower than the Mediterranean regional average (8.72 ± 2.3 fishermen/km2; [12]), confirming the absence of overfishing effects. Plotting CPUE against FiB confirmed that fishing pressure in these lagoons corresponds to the exploitation level.
In the Ionian Sea, the FiB index increased sharply from 1970 to the mid-1980s, remained stable for the next three decades, and then declined sharply after 2010, reaching a minimum in 2017. This contrasts with the significant upward trend reported for sub-area 04 [57] and the negative trends observed in the Greek lagoons.
In the Mar Menor Lagoon, periods of rational activity and periods with overfishing characteristics alternated in the exploited fisheries time series. The Gordon–Schaefer model identified an almost over-fished state during 1980–2006, as the average actual yield and numbers of fishermen fluctuated between the estimated MSY levels. Plotting CPUE against FiB revealed frequent changes in the lagoon’s ecological status, highlighting the influence of exploitation levels. Despite eutrophication, the lagoon has demonstrated a robust homeostatic mechanism, enabling recovery from human impacts and maintaining fishing yields close to the maximum sustainable yield (MSY-[60]), while the stable FiB index trend reflects a proportional shift between landings and trophic level changes, as the average fishermen density (1.34 fishermen/km2) indicates balanced exploitation. However, in the western Mediterranean Sea, the FiB index for the Balearic Islands was stable from 1980 to 2010 but has declined consistently since 2010, turning negative in recent years [62]. This pattern aligns with the influence of the sea on the fishing composition and yield of the Mar Menor Lagoon.
5. Conclusions
The long-term analysis of these Mediterranean lagoon systems underscores the critical role of integrated ecological indicators—BTLS, TrL, and TrC—alongside fishing-related metrics such as FiB, MSY, and CPUE in unraveling the complex interplay between ecological status and fisheries productivity. While habitat degradation and shifting energy pathways have led to reduced yields in the Greek lagoons, the Mar Menor case illustrates how eutrophication can reshape trophic dynamics without causing fisheries collapse—provided that traditional, low-impact fishing practices are preserved.
In the Greek lagoons, the indicators suggest a generally stable trophic structure resulting from under-exploitation. The observed decline in fisheries productivity appears primarily driven by resource depletion and habitat alteration, rather than by reduced primary productivity. Conversely, Mar Menor demonstrates a resilient system where fisheries remain sustainable due to a proportional adjustment between landings and trophic structure, even under increasing eutrophication.
These findings highlight the limitations of relying on single indicators to evaluate ecosystem health, emphasizing the necessity of multi-metric frameworks that consider local pressures (e.g., habitat loss, nutrient loading) and their synergistic effects. The comparative approach developed here provides a transferable template for assessing other semi-enclosed coastal lagoons and transitional waters globally, particularly those experiencing similar ecological stressors and management challenges.
Looking ahead, adaptive management strategies must integrate consideration of both site-specific drivers (e.g., seagrass loss, riverine nutrient inputs) and broader environmental shifts such as climate change. The interaction between habitat restoration, nutrient control, and low-intensity fishing practices emerges as a key mechanism for balancing conservation and productivity in vulnerable lagoon systems.
We believe this study contributes to the global understanding of coastal lagoon fishery dynamics and provides a replicable model for ecosystem-based management in diverse geographic contexts—particularly in Mediterranean-type and other temperate or subtropical semi-enclosed coastal systems undergoing rapid change. Such an approach may support policy development and sustainability efforts in fisheries in transitional coastal zones beyond the Mediterranean region.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ecologies6020035/s1, Table S1. Mean physical, chemical and sedimentological parameters (in bold) and range values (in parenthesis) in the systems studied. T = Temperature, DO = Dissolved oxygen, pH = potential of Hydrogen, S‰ = Salinity in parts per thousand, chl-a = chlorophyll-a, NH4-N = Ammonia, NO2-N = Nitrites, NO3-N = Nitrates, PO4 = Phosphates, SiO4 = Silicates, Total N% = % percentage of Total Nitrogen, CaCo3 % = % percentage of Calcium Carbonate. Sources: [20,21,39,50,51,84,85,86,87].
Author Contributions
Conceptualization: T.Z.; methodology: T.Z.; software: T.Z., D.K., and A.C.; validation: A.P.-R., C.M., A.C., S.R., D.V., and D.K.; formal analysis: T.Z., A.C., and D.K.; investigation: T.Z.; resources: T.Z. and A.P.-R.; data curation: T.Z.; writing—original draft preparation: T.Z.; writing—review and editing: A.P.-R., C.M., A.C., S.R., and D.K.; visualization: T.Z. and D.K.; supervision: D.K.; project administration: T.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The datasets analyzed from the current study are available from the corresponding author upon reasonable request.
Acknowledgments
The Greek research team wishes to thank V. Petaniti (Senior Officer of Fisheries Department of Arta), Ch. Gkanaras (fisherman in Logarou Lagoon, president of ‘Koronisia-Kalogeriko’ fisheries co-operative), and N. Fotou (fisherman in Rodia-Tsoukalio complex, president of fisheries co-operative of Aneza ‘Agios Nikolaos’), for their kind and analytic provision of the fisheries landing data and for providing information about technical and management issues in the Greek systems studied in this paper. The Murcia research team would like to thank the fishermen, the Fishermen’s Association of San Pedro del Pinatar, and the Fisheries Service of the Regional Government of Murcia for providing data and information used in this work. We would also like to acknowledge the project funding “Monitoring and modelling of the water quality and ecological status of the Mar Menor and prevention of impacts” from the Regional Ministry of Environment, Universities, Research and Mar Menor of the Autonomous Government of the Region of Murcia, which provides a support framework for multiple research activities that our team carries out in the lagoon.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pérez-Ruzafa, A.; Pérez-Ruzafa, I.M.; Newton, A.; Marcos, C. Coastal Lagoons: Environmental Variability, Ecosystem Complexity, and Goods and Services Uniformity. In Coasts and Estuaries; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–276. [Google Scholar]
- Rodrigues-Filho, J.L.; Macêdo, R.L.; Sarmento, H.; Pimenta, V.R.A.; Alonso, C.; Teixeira, C.R.; Pagliosa, P.R.; Netto, S.A.; Santos, N.C.L.; Daura-Jorge, F.G. From Ecological Functions to Ecosystem Services: Linking Coastal Lagoons Biodiversity with Human Well-Being. Hydrobiologia 2023, 850, 2611–2653. [Google Scholar] [CrossRef]
- Cataudella, S.; Crosetti, D.; Massa, F. Mediterranean Coastal Lagoons: Sustainable Management and Interactions among Aquaculture, Capture Fisheries and the Environment. Gen. Fish. Comm. Mediterr. Stud. Rev. 2015, 95, 278. [Google Scholar]
- Mariani, S. Can Spatial Distribution of Ichthyofauna Describe Marine Influence on Coastal Lagoons? A Central Mediterranean Case Study. Estuar. Coast. Shelf Sci. 2001, 52, 261–267. [Google Scholar] [CrossRef]
- Coleman, J.M.; Huh, O.K.; Braud Jr, D. Wetland Loss in World Deltas. J. Coast. Res. 2008, 24, 1–14. [Google Scholar] [CrossRef]
- de Leiva Moreno, J.I.; Agostini, V.N.; Caddy, J.F.; Carocci, F. Is the Pelagic-Demersal Ratio from Fishery Landings a Useful Proxy for Nutrient Availability? A Preliminary Data Exploration for the Semi-Enclosed Seas around Europe. ICES J. Mar. Sci. 2000, 57, 1091–1102. [Google Scholar] [CrossRef]
- Garrison, L.P.; Link, J.S. Fishing Effects on Spatial Distribution and Trophic Guild Structure of the Fish Community in the Georges Bank Region. ICES J. Mar. Sci. 2000, 57, 723–730. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Walters, C. Ecopath, Ecosim, and Ecospace as Tools for Evaluating Ecosystem Impact of Fisheries. ICES J. Mar. Sci. 2000, 57, 697–706. [Google Scholar] [CrossRef]
- Oczkowski, A.; Nixon, S. Increasing Nutrient Concentrations and the Rise and Fall of a Coastal Fishery; a Review of Data from the Nile Delta, Egypt. Estuar. Coast. Shelf Sci. 2008, 77, 309–319. [Google Scholar] [CrossRef]
- Erzini, K.; Sadat, Z.; Bentes, L.; Coelho, R.; Lino, P.G.; Monteiro, P.; Oliveira, F.; Ribeiro, J.; Gonçalves, J.M.S. The Potential Fish Provisioning Services of Vegetated and Unvegetated Habitat in a Lagoon Nursery. Fish. Res. 2024, 278, 107115. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Molina-Cuberos, G.J.; García-Oliva, M.; Umgiesser, G.; Marcos, C. Why Coastal Lagoons Are so Productive? Physical Bases of Fishing Productivity in Coastal Lagoons. Sci. Total Environ. 2024, 922, 171264. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Marcos, C. Fisheries in Coastal Lagoons: An Assumed but Poorly Researched Aspect of the Ecology and Functioning of Coastal Lagoons. Estuar. Coast. Shelf Sci. 2012, 110, 15–31. [Google Scholar] [CrossRef]
- Libralato, S.; Pranovi, F.; Raicevich, S.; Da Ponte, F.; Giovanardi, O.; Pastres, R.; Torricelli, P.; Mainardi, D. Ecological Stages of the Venice Lagoon Analysed Using Landing Time Series Data. J. Mar. Syst. 2004, 51, 331–344. [Google Scholar] [CrossRef]
- Pauly, D.; Christensen, V.; Dalsgaard, J.; Froese, R.; Torres Jr, F. Fishing down Marine Food Webs. Science 1998, 279, 860–863. [Google Scholar] [CrossRef] [PubMed]
- Pinnegar, J.K.; Jennings, S.; O’brien, C.M.; Polunin, N.V.C. Long-Term Changes in the Trophic Level of the Celtic Sea Fish Community and Fish Market Price Distribution. J. Appl. Ecol. 2002, 39, 377–390. [Google Scholar] [CrossRef]
- Pauly, D.; Palomares, M.-L. Fishing down Marine Food Web: It Is Far More Pervasive than We Thought. Bull. Mar. Sci. 2005, 76, 197–212. [Google Scholar]
- Sosa-López, A.; Mouillot, D.; Do Chi, T.; Ramos-Miranda, J. Ecological Indicators Based on Fish Biomass Distribution along Trophic Levels: An Application to the Terminos Coastal Lagoon, Mexico. ICES J. Mar. Sci. 2005, 62, 453–458. [Google Scholar] [CrossRef]
- Caddy, J.F.; Garibaldi, L. Apparent Changes in the Trophic Composition of World Marine Harvests: The Perspective from the FAO Capture Database. Ocean Coast. Manag. 2000, 43, 615–655. [Google Scholar] [CrossRef]
- Schaefer, M.B. Some Aspects of the Dynamics of Populations Important to the Management of the Commercial Marine Fisheries. Bull. Math. Biol. 1991, 53, 253–279. [Google Scholar] [CrossRef]
- Joyeux, J.-C.; Ward, A.B. Constraints on Coastal Lagoon Fisheries. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1998; Volume 34, pp. 73–199. ISBN 0065-2881. [Google Scholar]
- HCMR. Coastal and Transitional Waters Monitoring Program According to Article 8 of the Directive 2000/60/EC. Annual Report of the 14 Water Districts of Greece for the Year 2014; HCMR: Anavissos, Greece, 2015. [Google Scholar]
- HCMR. “State-of-the-Lagoon Report” for Amvrakikos Lagoon Complex, Western Greece. In ARCH Project (282748) Work Package Report; Conides, A.J., Klaoudatos, D.S., Eds.; Hellenic Center for Marine Research: Anavyssos, Greece, 2012; p. 186. [Google Scholar]
- García-Oliva, M.; Pérez-Ruzafa, Á.; Umgiesser, G.; McKiver, W.; Ghezzo, M.; De Pascalis, F.; Marcos, C. Assessing the Hydrodynamic Response of the Mar Menor Lagoon to Dredging Inlets Interventions through Numerical Modelling. Water 2018, 10, 959. [Google Scholar] [CrossRef]
- Umgiesser, G.; Ferrarin, C.; Cucco, A.; De Pascalis, F.; Bellafiore, D.; Ghezzo, M.; Bajo, M. Comparative Hydrodynamics of 10 Mediterranean Lagoons by Means of Numerical Modeling. J. Geophys. Res. Ocean. 2014, 119, 2212–2226. [Google Scholar] [CrossRef]
- HCMR. Study of Fisheries Management of Lakes (Natural and Artificial), Utilization of Water Resources of Mountainous and Disadvantaged Areas of the Prefectures of Aitoloakarnania. Evritania, Karditsa, Boeotia, Arcadia, Ilia and Achaia. In Final Published Technical Report, in Greek, Hellenic Center for Marine Research—Institute of Inland Waters—Greek Ministry of Agriculture; EU PESCA Programme. Main Document; Hellenic Center for Marine Research: Anavyssos, Greece, 2001; 599p, ISBN 960-86651-2-4. [Google Scholar]
- MAPA. Anuario de Pesca Marítima [Maritime Fisheries Yearbook]; Dirección General de Pesca Marítima. Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1986. [Google Scholar]
- Katselis, G.N.; Moutopoulos, D.K.; Dimitriou, E.N.; Koutsikopoulos, C. Long-Term Changes of Fisheries Landings in Enclosed Gulf Lagoons (Amvrakikos Gulf, W Greece): Influences of Fishing and Other Human Impacts. Estuar. Coast. Shelf Sci. 2013, 131, 31–40. [Google Scholar] [CrossRef]
- Chou, Y.-L. Statistical Analysis: With Business and Economic Applications; Holt, Rinehart and Winston: New York, NY, USA, 1963; ISBN 0039100618. [Google Scholar]
- Pauly, D.; Christensen, V. Primary Production Required to Sustain Global Fisheries. Nature 1995, 374, 255–257. [Google Scholar] [CrossRef]
- Vivekanandan, E.; Srinath, M.; Kuriakose, S. Fishing the Marine Food Web along the Indian Coast. Fish. Res. 2005, 72, 241–252. [Google Scholar] [CrossRef]
- Stergiou, K.I.; Karpouzi, V.S. Feeding Habits and Trophic Levels of Mediterranean Fish. Rev. Fish Biol. Fish. 2002, 11, 217–254. [Google Scholar] [CrossRef]
- FishBase: World Wide Web Electronic Publication. Available online: https://www.fishbase.org (accessed on 24 April 2025).
- Verdiell-Cubedo, D.; Torralva, M.; Ruiz-Navarro, A.; Oliva-Paterna, F.J. Fish Assemblages in Different Littoral Habitat Types of a Hypersaline Coastal Lagoon (Mar Menor, Mediterranean Sea). Ital. J. Zool. 2013, 80, 104–116. [Google Scholar] [CrossRef]
- Sparre, P.; Venema, S.C. Introduction to Fish Stock Assessment. Part 1: Manual; FAO Fisheries Technical Paper; FAO: Rome, Italy, 1998; Volume 306. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Pearson Education: Uttar Pradesh, India, 1999. [Google Scholar]
- Pérez-Ruzafa, A.; Mompeán, M.C.; Marcos, C. Hydrographic, Geomorphologic and Fish Assemblage Relationships in Coastal Lagoons. In Proceedings of the Lagoons and Coastal Wetlands in the Global Change Context: Impacts and Management Issues: Selected papers of the International Conference “CoastWetChange”, Venice, Italy, 26–28 April 2004; Springer: Berlin/Heidelberg, Germany, 2007; pp. 107–125. [Google Scholar]
- Andrisoa, A.; Stieglitz, T.C.; Rodellas, V.; Raimbault, P. Primary Production in Coastal Lagoons Supported by Groundwater Discharge and Porewater Fluxes Inferred from Nitrogen and Carbon Isotope Signatures. Mar. Chem. 2019, 210, 48–60. [Google Scholar] [CrossRef]
- Al-Khalidy, H.I.; Al-Haidarey, M.J.S. Impact of Salinity on Primary Production in the Marshes. Indian J. Ecol. 2019, 46, 614–618. [Google Scholar]
- Reizopoulou, S.; Nicolaidou, A. Benthic Diversity of Coastal Brackish-water Lagoons in Western Greece. Aquat. Conserv. Mar. Freshw. Ecosyst. 2004, 14, S93–S102. [Google Scholar] [CrossRef]
- Ligorini, V.; Garrido, M.; Malet, N.; Simon, L.; Alonso, L.; Bastien, R.; Aiello, A.; Cecchi, P.; Pasqualini, V. Response of Phytoplankton Communities to Variation in Salinity in a Small Mediterranean Coastal Lagoon: Future Management and Foreseen Climate Change Consequences. Water 2023, 15, 3214. [Google Scholar] [CrossRef]
- Ersoy, Z.; Abril, M.; Cañedo-Argüelles, M.; Espinosa, C.; Vendrell-Puigmitja, L.; Proia, L. Experimental Assessment of Salinization Effects on Freshwater Zooplankton Communities and Their Trophic Interactions under Eutrophic Conditions. Environ. Pollut. 2022, 313, 120127. [Google Scholar] [CrossRef]
- Shadrin, N.V.; Anufriieva, E.V.; Kipriyanova, L.M.; Kolesnikova, E.A.; Latushkin, A.A.; Romanov, R.E.; Sergeeva, N.G. The Political Decision Caused the Drastic Ecosystem Shift of the Sivash Bay (the Sea of Azov). Quat. Int. 2018, 475, 4–10. [Google Scholar] [CrossRef]
- Anufriieva, E.V.; El-Shabrawy, G.M.; Shadrin, N.V. Copepoda in the Shallow Hypersaline Bardawil Coastal Lake (Egypt): Are There Long-Term Changes in Composition and Abundance? Oceanol. Hydrobiol. Stud. 2018, 47, 219–229. [Google Scholar] [CrossRef]
- Anufriieva, E.; Kolesnikova, E.; Revkova, T.; Latushkin, A.; Shadrin, N. Human-Induced Sharp Salinity Changes in the World’s Largest Hypersaline Lagoon Bay Sivash (Crimea) and Their Effects on the Ecosystem. Water 2022, 14, 403. [Google Scholar] [CrossRef]
- Meredith, W.; Casamitjana, X.; Quintana, X.D.; Menció, A. Effects of Morphology and Sediment Permeability on Coastal Lagoons’ Hydrological Patterns. J. Hydrol. 2022, 612, 128259. [Google Scholar] [CrossRef]
- Jin, H.; van Leeuwen, C.H.A.; Van de Waal, D.B.; Bakker, E.S. Impacts of Sediment Resuspension on Phytoplankton Biomass Production and Trophic Transfer: Implications for Shallow Lake Restoration. Sci. Total Environ. 2022, 808, 152156. [Google Scholar] [CrossRef]
- Franco, T.P.; Neves, L.M.; Araújo, F.G. Better with More or Less Salt? The Association of Fish Assemblages in Coastal Lagoons with Different Salinity Ranges. Hydrobiologia 2019, 828, 83–100. [Google Scholar] [CrossRef]
- Gray, J.S. Animal-Sediment Relationships. Oceanogr. Mar. Biol. Ann. Rev. 1974, 12, 223–261. [Google Scholar]
- Mouillot, D.; Laune, J.; Tomasini, J.-A.; Aliaume, C.; Brehmer, P.; Dutrieux, E.; Do Chi, T. Assessment of Coastal Lagoon Quality with Taxonomic Diversity Indices of Fish, Zoobenthos and Macrophyte Communities. Hydrobiologia 2005, 550, 121–130. [Google Scholar] [CrossRef]
- Kormas, K.A.; Nicolaidou, A.; Reizopoulou, S. Temporal Variations of Nutrients, Chlorophyll a and Particulate Matter in Three Coastal Lagoons of Amvrakikos Gulf (Ionian Sea, Greece). Mar. Ecol. 2001, 22, 201–213. [Google Scholar] [CrossRef]
- Nicolaidou, A.; Petrou, K.; Kormas, K.A.; Reizopoulou, S. Inter-Annual Variability of Soft Bottom Macrofaunal Communities in Two Ionian Sea Lagoons. Mar. Biodivers. Patterns Process. Assess. Threat. Manag. Conserv. 2006, 183, 89–98. [Google Scholar]
- Guelorget, O.; Perthuisot, J.P. PARALIC ECOSYSTEMS Biological Organization and Functionning. Vie Milieu/Life Environ. 1992, 42, 215–251. [Google Scholar]
- Valiela, I.; McClelland, J.; Hauxwell, J.; Behr, P.J.; Hersh, D.; Foreman, K. Macroalgal Blooms in Shallow Estuaries: Controls and Ecophysiological and Ecosystem Consequences. Limnol. Oceanogr. 1997, 42, 1105–1118. [Google Scholar] [CrossRef]
- Vasileiadou, K.; Pavloudi, C.; Kalantzi, I.; Apostolaki, E.T.; Chatzigeorgiou, G.; Chatzinikolaou, E.; Pafilis, E.; Papageorgiou, N.; Fanini, L.; Konstas, S. Environmental Variability and Heavy Metal Concentrations from Five Lagoons in the Ionian Sea (Amvrakikos Gulf, W Greece). Biodivers. Data J. 2016, 1, e8233. [Google Scholar] [CrossRef] [PubMed]
- Mazzella, L.; Buia, M.C.; Gambi, M.C.; Lorenti, M.; Russo, G.F.; Scipione, M.B.; Zupo, V. Plant-Animal Trophic Relationships in the Posidonia Oceanica Ecosystem of the Mediterranean Sea: A Review. In Plant-Animal Interactions in the Marine Benthos; John, D.M., Howkins, S.J., Price, J.H., Eds.; Claredon Press: Oxford, UK, 1992; pp. 165–187. [Google Scholar]
- Panagopoulos, I.; Mimikou, M. Assessment of the Changes in the Arachtos River Flow and Sediment DISCHARGES Due to Anthropogenic Interventions. In Proceedings of the Protection and Restoration of the Environment VIII, Chania, Greece, 3–7 July 2006. [Google Scholar]
- Moutopolos, D.K.; Ramfos, A.; Spala, K.; Koutsikopoulos, C.; Katselis, G. Long Term Changes of Fisheries Landings Patterns of Most Important Species in Amvrakikos Lagoonal System. In Proceedings of the 9th Symposium on Oceanography and Fisheries, Patra, Greece, 13–16 May 2009; Volume 2, pp. 995–1000. [Google Scholar]
- Ferentinos, G.; Papatheodorou, G.; Geraga, M.; Iatrou, M.; Fakiris, E.; Christodoulou, D.; Dimitriou, E.; Koutsikopoulos, C. Fjord Water Circulation Patterns and Dysoxic/Anoxic Conditions in a Mediterranean Semi-Enclosed Embayment in the Amvrakikos Gulf, Greece. Estuar. Coast. Shelf Sci. 2010, 88, 473–481. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Marcos-Diego, C.; Ros, J.D. Environmental and Biological Changes Related to Recent Human Activities in the Mar Menor (SE of Spain). Mar. Pollut. Bull. 1991, 23, 747–751. [Google Scholar] [CrossRef]
- Marcos, C.; Torres, I.; López-Capel, A.; Pérez-Ruzafa, A. Long Term Evolution of Fisheries in a Coastal Lagoon Related to Changes in Lagoon Ecology and Human Pressures. Rev. Fish Biol. Fish. 2015, 25, 689–713. [Google Scholar] [CrossRef]
- Giovos, I.; Gonzalvo, J.; Ciprian, M.; Gaentlich, M.; Gavriel, E.; Konstas, S.; Kordopatis, P.; Koutsikopoulos, C.; MaGiovo, I.; Gonzalvo, J.; et al. Amvrakikos Gulf: Biodiversity and Threats. Project “Contributing to the Effective Management of the Amvrakikos Gulf National Park. Available online: https://amvrakikosalliance.net/about/reports/ (accessed on 24 April 2025).
- Dimarchopoulou, D.; Keramidas, I.; Sylaios, G.; Tsikliras, A.C. Ecotrophic Effects of Fishing across the Mediterranean Sea. Water 2021, 13, 482. [Google Scholar] [CrossRef]
- Moutopoulos, D.K.; Libralato, S.; Solidoro, C.; Erzini, K.; Stergiou, K.I. Effect of Landings Data Disaggregation on Ecological Indicators. Mar. Ecol. Prog. Ser. 2014, 509, 27–38. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Morkune, R.; Marcos, C.; Pérez-Ruzafa, I.M.; Razinkovas-Baziukas, A. Can an Oligotrophic Coastal Lagoon Support High Biological Productivity? Sources and Pathways of Primary Production. Mar. Environ. Res. 2020, 153, 104824. [Google Scholar] [CrossRef]
- Costa, M.J.; Cabral, H.N.; Drake, P.; Economou, A.N.; Fernandez-Delgado, C.; Gordo, L.; Marchand, J.; Thiel, R. Recruitment and Production of Commercial Species in Estuaries. In Fishes in Estuaries; Wiley: Hoboken, NJ, USA, 2002; pp. 54–123. [Google Scholar] [CrossRef]
- Pombo, L.; Elliott, M.; Rebelo, J.E. Changes in the Fish Fauna of the Ria de Aveiro Estuarine Lagoon (Portugal) during the Twentieth Century. J. Fish Biol. 2002, 61, 167–181. [Google Scholar] [CrossRef]
- Malavasi, S.; Fiorin, R.; Franco, A.; Franzoi, P.; Granzotto, A.; Riccato, F.; Mainardi, D. Fish Assemblages of Venice Lagoon Shallow Waters: An Analysis Based on Species, Families and Functional Guilds. J. Mar. Syst. 2004, 51, 19–31. [Google Scholar] [CrossRef]
- Zoulias, T.; Papadopoulos, A.; Conides, A. An Ecological Evaluation Using Fisheries Landings Time-Series Data: A Case Study of Two Coastal Lagoons in the Ionian Sea (E. Mediterranean, Greece). Estuar. Coast. Shelf Sci. 2019, 216, 229–239. [Google Scholar] [CrossRef]
- Malavasi, S.; Franco, A.; Fiorin, R.; Franzoi, P.; Torricelli, P.; Mainardi, D. The Shallow Water Gobiid Assemblage of the Venice Lagoon: Abundance, Seasonal Variation and Habitat Partitioning. J. Fish Biol. 2005, 67, 146–165. [Google Scholar] [CrossRef]
- Ribeiro, J.; Monteiro, C.C.; Monteiro, P.; Bentes, L.; Coelho, R.; Gonçalves, J.M.S.; Lino, P.G.; Erzini, K. Long-Term Changes in Fish Communities of the Ria Formosa Coastal Lagoon (Southern Portugal) Based on Two Studies Made 20 Years Apart. Estuar. Coast. Shelf Sci. 2008, 76, 57–68. [Google Scholar] [CrossRef]
- ICES. Report of the 2006 Session of the Joint European Inland Fisheries Advisory Commision/International Council for the Exploration of the Sea; EIFAC Occasional Paper, 38; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Álvarez-Rogel, J.; Barberá, G.G.; Maxwell, B.; Guerrero-Brotons, M.; Díaz-García, C.; Martínez-Sánchez, J.J.; Sallent, Á.; Martínez-Ródenas, J.; González-Alcaraz, M.N.; Jiménez-Cárceles, F.J. The Case of Mar Menor Eutrophication: State of the Art and Description of Tested Nature-Based Solutions. Ecol. Eng. 2020, 158, 106086. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Gilabert, J.; Gutiérrez, J.M.; Fernández, A.I.; Marcos, C.; Sabah, S. Evidence of a Planktonic Food Web Response to Changes in Nutrient Input Dynamics in the Mar Menor Coastal Lagoon, Spain. In Proceedings of the Nutrients and Eutrophication in Estuaries and Coastal Waters: Proceedings of the 31st Symposium of the Estuarine and Coastal Sciences Association (ECSA), Bilbao, Spain, 3–7 July 2000; Springer: Berlin/Heidelberg, Germany, 2002; pp. 359–369. [Google Scholar]
- Yunev, O.A.; Carstensen, J.; Moncheva, S.; Khaliulin, A.; Ærtebjerg, G.; Nixon, S. Nutrient and Phytoplankton Trends on the Western Black Sea Shelf in Response to Cultural Eutrophication and Climate Changes. Estuar. Coast. Shelf Sci. 2007, 74, 63–76. [Google Scholar] [CrossRef]
- Zamora-López, A.; Guerrero-Gómez, A.; Torralva, M.; Zamora-Marín, J.M.; Guillén-Beltrán, A.; Oliva-Paterna, F.J. Shallow Waters as Critical Habitats for Fish Assemblages under Eutrophication-Mediated Events in a Coastal Lagoon. Estuar. Coast. Shelf Sci. 2023, 291, 108447. [Google Scholar] [CrossRef]
- Apromar. Aquaculture in Spain; Apromar: Cádiz, Spain, 2020. [Google Scholar]
- FAO. The State of Mediterranean and Black Sea Fisheries; General Fisheries Commission for the Mediterranean: Rome, Italy, 2016; Volume 511. [Google Scholar]
- Dekker, W. Did Lack of Spawners Cause the Collapse of the European Eel, Anguilla Anguilla? Fish. Manag. Ecol. 2003, 10, 365–376. [Google Scholar] [CrossRef]
- Essington, T.E.; Beaudreau, A.H.; Wiedenmann, J. Fishing through Marine Food Webs. Proc. Natl. Acad. Sci. USA 2006, 103, 3171–3175. [Google Scholar] [CrossRef]
- Steneck, R.S. Human Influences on Coastal Ecosystems: Does Overfishing Create Trophic Cascades? Trends Ecol. Evol. 1998, 13, 429–430. [Google Scholar] [CrossRef]
- Pinnegar, J.K.; Polunin, N.V.C.; Badalamenti, F. Long-Term Changes in the Trophic Level of Western Mediterranean Fishery and Aquaculture Landings. Can. J. Fish. Aquat. Sci. 2003, 60, 222–235. [Google Scholar] [CrossRef]
- Pauly, D.; Yáñez-Arancibia, A. Fisheries in Coastal Lagoons. In Elsevier Oceanography Series; Elsevier: Amsterdam, The Netherlands, 1994; Volume 60, pp. 377–399. ISBN 0422-9894. [Google Scholar]
- Kleisner, K.; Pauly, D. The Marine Trophic Index (MTI), the Fishing in Balance (FIB) Index. Fish. Cent. Res. Rep. 2011, 19, 41. [Google Scholar]
- Karageorgis, A.; Krasakopoulou, E.; Pappas, G.; Papageorgiou, A.; Taxiarchi, M. Geochemical Characteristics of Surface Sediments from the Lagoons of Amvrakikos Gulf, Ionian Sea. In Proceedings of the 8th Hellenic Symposium in Oceanography and Fisheries, Thessaloniki, Greece, 4–8 June 2006; pp. 793–799. [Google Scholar]
- Pérez-Ruzafa, A.; Marcos, C.; Bernal, C.M.; Quintino, V.; Freitas, R.; Rodrigues, A.M.; García-Sánchez, M.; Pérez-Ruzafa, I.M. Cymodocea Nodosa vs. Caulerpa Prolifera: Causes and Consequences of a Long Term History of Interaction in Macrophyte Meadows in the Mar Menor Coastal Lagoon (Spain, Southwestern Mediterranean). Estuar. Coast. Shelf Sci. 2012, 110, 101–115. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Campillo, S.; Fernández-Palacios, J.M.; García-Lacunza, A.; García-Oliva, M.; Ibañez, H.; Navarro-Martínez, P.C.; Pérez-Marcos, M.; Pérez-Ruzafa, I.M.; Quispe-Becerra, J.I. Long-Term Dynamic in Nutrients, Chlorophyll a, and Water Quality Parameters in a Coastal Lagoon during a Process of Eutrophication for Decades, a Sudden Break and a Relatively Rapid Recovery. Front. Mar. Sci. 2019, 6, 26. [Google Scholar] [CrossRef]
- Pérez-Ruzafa, A.; Dezileau, L.; Martínez-Sánchez, M.J.; Pérez-Sirvent, C.; Pérez-Marcos, M.; von Grafenstein, U.; Marcos, C. Long-Term Sediment Records Reveal over Three Thousand Years of Heavy Metal Inputs in the Mar Menor Coastal Lagoon (SE Spain). Sci. Total Environ. 2023, 902, 166417. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).