The Effect of Age on Survival Is Similar in Males and Females of an Aquatic Insect Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Capture–Mark–Recapture Protocol
2.3. Capture–Mark–Recapture Modeling
2.4. Lifespan
2.5. Statistical Analyses
3. Results
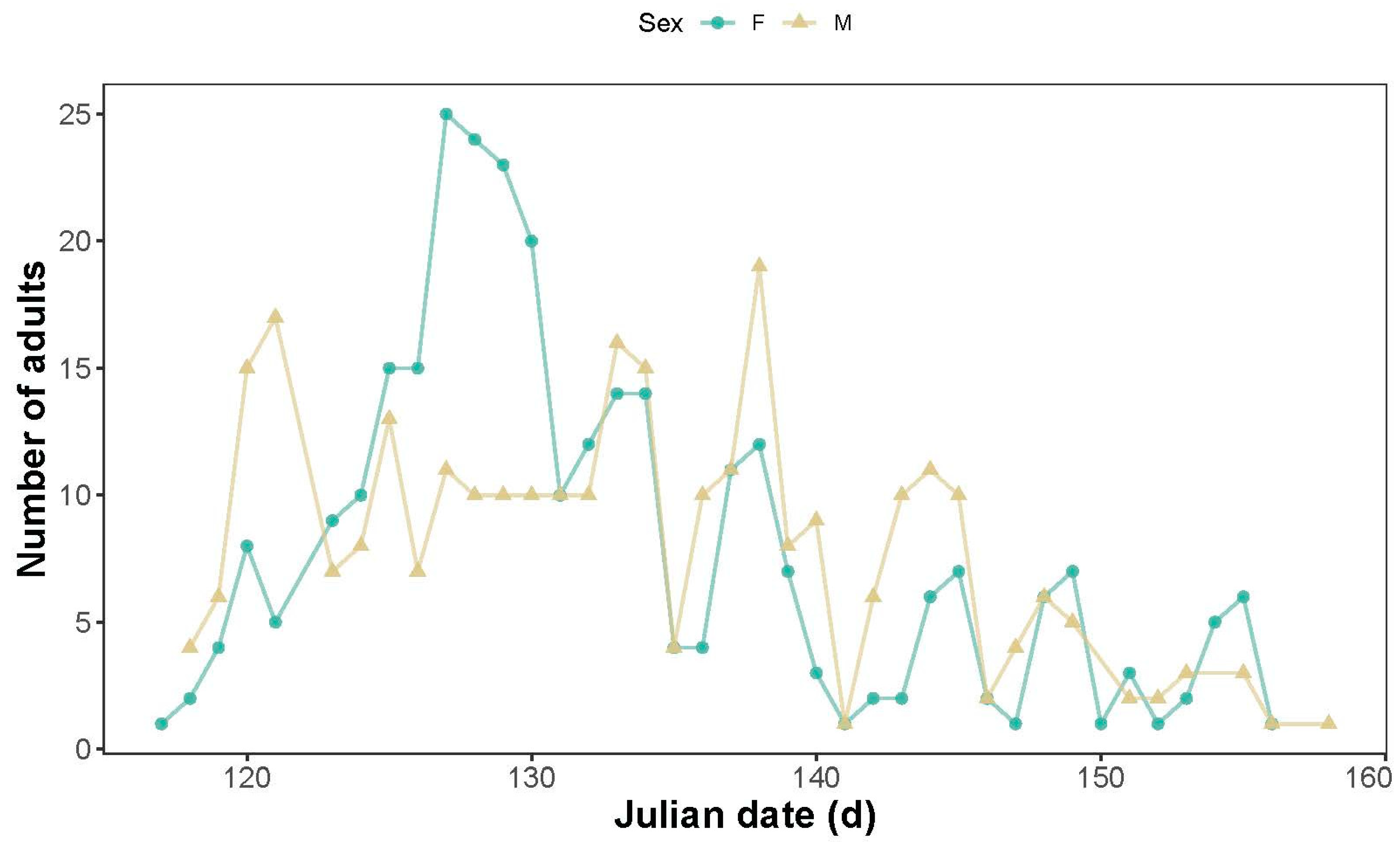
3.1. Seasonal Pattern of Abundance
3.2. Cormack–Jolly–Seber Model
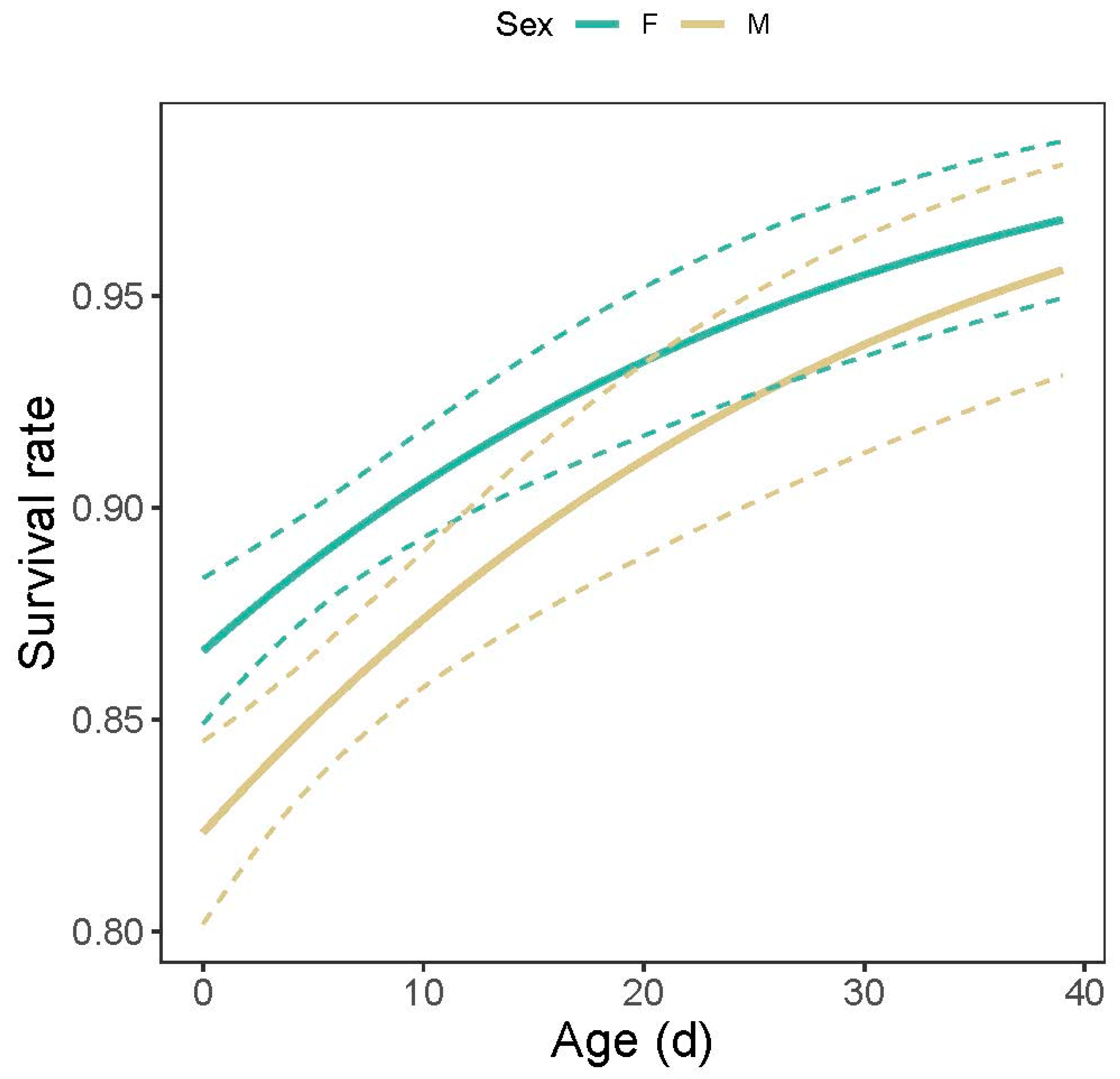
3.3. Lifespan
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sherratt, T.N.; Wilkinson, D.M. Big Questions in Ecology and Evolution; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Stearns, S.C. The Evolution of Life Histories; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Péron, G.; Crochet, P.A.; Choquet, R.; Pradel, R.; Lebreton, J.D.; Gimenez, O. Capture–recapture models with heterogeneity to study survival senescence in the wild. Oikos 2010, 119, 524–532. [Google Scholar] [CrossRef]
- Brunet-Rossinni, A.K.; Austad, S.N. Senescence in wild populations of mammals and birds. In Handbook of the Biology of Aging; Austad, S.N., Masoro, E.J., Eds.; Academic Press: Burlington, MA, USA, 2006; pp. 243–266. [Google Scholar]
- Kirkwood, T.B.; Austad, S.N. Why do we age? Nature 2000, 408, 233–238. [Google Scholar] [CrossRef]
- Bonduriansky, R.; Brassil, C. Reproductive ageing and sexual selection on male body size in a wild population of antler flies (Protopiophila litigata). J. Evol. Biol. 2005, 18, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Brassil, C.E.; Brooks, R.C.; Bonduriansky, R. Environmental effects on the expression of life span and aging: An extreme contrast between wild and captive cohorts of Telostylinus angusticollis (Diptera: Neriidae). Am. Nat. 2008, 172, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Dukas, R. Mortality rates of honey bees in the wild. Insectes Sociaux 2008, 55, 252–255. [Google Scholar] [CrossRef]
- Zajitschek, F.; Bonduriansky, R.; Zajitschek, S.R.; Brooks, R.C. Sexual dimorphism in life history: Age, survival, and reproduction in male and female field crickets Teleogryllus commodus under seminatural conditions. Am. Nat. 2009, 173, 792–802. [Google Scholar] [CrossRef]
- Sherratt, T.; Laird, R.; Hassall, C.; Lowe, C.; Harvey, I.; Watts, P.; Cordero-Rivera, A.; Thompson, D. Empirical evidence of senescence in adult damselflies (Odonata: Zygoptera). J. Anim. Ecol. 2010, 79, 1034–1044. [Google Scholar] [CrossRef]
- Khelifa, R.; Mahdjoub, H.; Aouaouche, M.S.; Houhamdi, M. Reproductive behaviour of a North African endemic damselfly, Platycnemis subdilatata (Odonata: Platycnemididae) and probable senescence effects. Int. J. Odonatol. 2016, 19, 157–167. [Google Scholar] [CrossRef]
- Hassall, C.; Sherratt, T.N.; Watts, P.C.; Thompson, D.J. Live fast, die old: No evidence of reproductive senescence or costs of mating in a damselfly (Odonata: Zygoptera). J Anim. Ecol. 2015, 84, 1542–1554. [Google Scholar] [CrossRef]
- Zajitschek, F.; Brassil, C.E.; Bonduriansky, R.; Brooks, R.C. Sex effects on life span and senescence in the wild when dates of birth and death are unknown. Ecology 2009, 90, 1698–1707. [Google Scholar] [CrossRef]
- Stoks, R. Male-biased sex ratios in mature damselfly populations: Real or artefact? Ecol. Entomol. 2001, 26, 181–187. [Google Scholar] [CrossRef]
- Beirinckx, K.; Van Gossum, H.; Lajeunesse, M.J.; Forbes, M.R. Sex biases in dispersal and philopatry: Insights from a meta-analysis based on capture-mark-recapture studies of damselflies. Oikos 2006, 113, 539–547. [Google Scholar] [CrossRef]
- Cordero-Rivera, A.; Stoks, R. Mark-recapture studies and demography. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Córdoba-Aguilar, A., Ed.; Oxford University Press: Oxford, UK, 2008; pp. 7–20. [Google Scholar]
- Stoks, R.; Córdoba-Aguilar, A. Evolutionary ecology of Odonata: A complex life cycle perspective. Annu. Rev. Entomol. 2012, 57, 249–265. [Google Scholar] [CrossRef]
- Corbet, P.S. Biology of odonata. Annu. Rev. Entomol. 1980, 25, 189–217. [Google Scholar] [CrossRef]
- Waller, J.T.; Svensson, E.I. Body size evolution in an old insect order: No evidence for Cope’s Rule in spite of fitness benefits of large size. Evolution 2017, 71, 2178–2193. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín-Villar, I.; Cordero-Rivera, A. Odonata survival: Insights from mark-recapture experiments. In Dragonflies and Damselflies: Model Organisms for Ecological and Evolutionary Research; Córdoba-Aguilar, A., Beatty, C., Bried, J., Eds.; Oxford University Press: Oxford, UK, 2022; pp. 129–140. [Google Scholar]
- Khelifa, R.; Mahdjoub, H.; Baaloudj, A.; Cannings, R.A.; Samways, M.J. Remarkable population resilience in a North African endemic damselfly in the face of rapid agricultural transformation. Insects 2021, 12, 353. [Google Scholar] [CrossRef]
- La Porta, G.; Goretti, E. Investigation on the declining Southern Damselfly (Coenagrion mercuriale, Odonata) in a Mediterranean population: Survival rate and population size. J. Insect Conserv. 2019, 23, 667–675. [Google Scholar] [CrossRef]
- Conrad, K.; Willson, K.; Harvey, I.; Thomas, C.; Sherratt, T. Dispersal characteristics of seven odonate species in an agricultural landscape. Ecography 1999, 22, 524–531. [Google Scholar] [CrossRef]
- Clausnitzer, V. Calopteryx haemorrhoidalis. The IUCN Red List of Threatened Species 2018: E.T158695A75085099. 2009. Available online: https://www.iucnredlist.org/species/158695/75085099 (accessed on 17 March 2024).
- Dijkstra, K.-D.; Schröter, A. Field Guide to the Dragonflies of Britain and Europe; Bloomsbury Publishing: London, UK, 2020. [Google Scholar]
- Maynou, X.; Martín, R. Phenology of the Odonata assemblage in a Mediterranean stream in the north-eastern Iberian Peninsula. Odonatologica 2019, 48, 27–48. [Google Scholar]
- Jacquemin, G.; Boudot, J.P. Les libellules (odonates) du Maroc; Société Francaise d’Odonatologie: Bois d’Arcy, France, 1999. [Google Scholar]
- Khelifa, R.; Youcefi, A.; Kahlerras, A.; Al Farhan, A.; Al-Rasheid, K.A.; Samraoui, B. L’odonatofaune (Insecta: Odonata) du bassin de la Seybouse en Algérie: Intérêt pour la biodiversité du Maghreb. Revue d’écologie 2011, 66, 55–66. [Google Scholar] [CrossRef]
- Cordero, A. Estructura de tres comunidades de Calopteryx (Odonata: Calopterygidae) con diferente composición específica. Limnética 1989, 5, 83–91. [Google Scholar] [CrossRef]
- Heymer, A. Comportements Social et Territorial des Calopterygidae [Odon. Zygoptera]. Ann. Soc. Entomol. Fr. 1972, 8, 3–53. [Google Scholar] [CrossRef]
- Khelifa, R. Flight period, apparent sex ratio and habitat preferences of the Maghribian endemic Calopteryx exul Selys, 1853 (Odonata: Zygoptera). Revue d’Ecologie (La Terre et La Vie) 2013, 68, 37–45. [Google Scholar] [CrossRef]
- Khelifa, R. Partial bivoltinism and emergence patterns in the North African endemic damselfly Calopteryx exul: Conservation implications. Afr. J. Ecol. 2017, 55, 145–151. [Google Scholar] [CrossRef]
- Mellal, M.K.; Bensouilah, M.; Houhamd, M.; Khelifa, R. Reproductive habitat provisioning promotes survival and reproduction of the endangered endemic damselfly Calopteryx exul. J. Insect Conserv. 2018, 22, 563–570. [Google Scholar] [CrossRef]
- Benchalel, W.; Beddiar, M.; Boucetta, S.; Bouslama, Z. Bioecology of calopteryx haemorrhoidalis (Zygoptera, odonata) in response to environmental factors in the brabtia sector streams, el-kala, algeria: Implications for ecohydrological biomonitoring. Studia Universitatis” Vasile Goldis” Arad. Seria Stiintele Vietii (Life Sci. Ser.) 2020, 30, 21–32. [Google Scholar]
- Córdoba-Aguilar, A. Wing pigmentation in territorial male damselflies, Calopteryx haemorrhoidalis: A possible relation to sexual selection. Anim. Behav. 2002, 63, 759–766. [Google Scholar] [CrossRef]
- Cordero, A. Forced copulations and female contact guarding at a high male density in a calopterygid damselfly. J. Insect Behav. 1999, 12, 27–37. [Google Scholar] [CrossRef]
- Heymer, A. Étude du Comportement Reproducteur et Analyse des Mécanismes Déclencheurs Innés (Mdi) Optiques chez les Calopterygidae [Odon. Zygoptera]. Ann. Soc. Entomol. Fr. 1973, 9, 219–255. [Google Scholar] [CrossRef]
- Khelifa, R.; Mellal, M.K.; Zouaimia, A.; Amari, H.; Zebsa, R.; Bensouilah, S.; Laouar, A.; Houhamdi, M. On the restoration of the last relict population of a dragonfly Urothemis edwardsii Selys (Libellulidae: Odonata) in the Mediterranean. J. Insect Conserv. 2016, 20, 797–805. [Google Scholar] [CrossRef]
- Cooch, E.; White, G. Program MARK: A Gentle Introduction. 2006. Available online: http://www.phidot.org/software/mark/docs/book (accessed on 26 April 2024).
- Lebreton, J.-D.; Burnham, K.P.; Clobert, J.; Anderson, D.R. Modeling survival and testing biological hypotheses using marked animals: A unified approach with case studies. Ecol. Monogr. 1992, 62, 67–118. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multimodel inference: Understanding AIC and BIC in model selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Kahalerras, A.; Boucenna, N.; Bensakhri, Z.; Boukhamza, M.; Houhamdi, M. No evidence of body size effects on the reproductive behaviour of a non-territorial damselfly Chalcolestes viridis. Zool. Ecol. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Laake, J.L. RMark: An R Interface for Analysis of Capture-Recapture Data with MARK. Alaska Fish. Sci. Cent., NOAA, Natl. Mar. Fish. Serv. 2013, 25. Available online: http://www.afsc.noaa.gov/Publications/ProcRpt/PR2013-01.pdf (accessed on 20 January 2024).
- Venables, W.N.; Ripley, B.D. Random and mixed effects. In Modern Applied Statistics with S; Springer: New York, NY, USA, 2002; pp. 271–300. [Google Scholar]
- Corbet, P.S. Dragonflies: Behaviour and Ecology of Odonata; Harley Books: Colchester, UK, 1999. [Google Scholar]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models. R Package Version 0.4.6. 2022. Available online: https://cran.r-project.org/web/packages/DHARMa/vignettes/DHARMa.html (accessed on 20 January 2024).
- Choquet, R.; Lebreton, J.D.; Gimenez, O.; Reboulet, A.M.; Pradel, R. U-CARE: Utilities for performing goodness of fit tests and manipulating capture–recapture data. Ecography 2009, 32, 1071–1074. [Google Scholar] [CrossRef]
- Jödicke, R.; Arlt, J.; Kunz, B.; Lopau, W.; Seidenbusch, R. The Odonata of Tunisia. Int. J. Odonatol. 2000, 3, 41–71. [Google Scholar] [CrossRef]
- Zebsa, R.; Khelifa, R.; Kahalerras, A. Emergence pattern, microhabitat choice, and population structure of the Maghribian endemic Gomphus lucasii Selys, 1849 (Odonata: Gomphidae) in northeastern Algeria. Aquat. Insects 2014, 36, 245–255. [Google Scholar] [CrossRef]
- Cordero-Rivera, A.; Núñez, J.; Suriel, C. Let’s wait for the evening: Nocturnal copulation in a tropical damselfly Phylolestes ethelae (Odonata, Synlestidae). Anim. Biodivers. Conserv. 2024, 47, 19–32. [Google Scholar] [CrossRef]
- Mahdjoub, H.; Zebsa, R.; Kahalerras, A.; Amari, H.; Bensouilah, S.; Samways, M.J.; Khelifa, R. Condition-dependent survival and movement behavior in an endangered endemic damselfly. Sci. Rep. 2023, 13, 21819. [Google Scholar] [CrossRef]
- Córdoba-Aguilar, A.; Cordero-Rivera, A. Evolution and ecology of Calopterygidae (Zygoptera: Odonata): Status of knowledge and research perspectives. Neotrop. Entomol. 2005, 34, 861–879. [Google Scholar] [CrossRef]
- Khelifa, R.; Mahdjoub, H.; Baaloudj, A.; Cannings, R.A.; Samways, M.J. Effects of both climate change and human water demand on a highly threatened damselfly. Sci. Rep. 2021, 11, 7725. [Google Scholar] [CrossRef]
- Khelifa, R.; Zebsa, R.; Amari, H.; Mellal, M.K.; Mahdjoub, H. Field estimates of fitness costs of the pace-of-life in an endangered damselfly. J. Evol. Biol. 2019, 32, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Anholt, B.R.; Vorburger, C.; Knaus, P. Mark-recapture estimates of daily survival rates of two damselflies (Coenagrion puella and Ischnura elegans). Can. J. Zool. 2001, 79, 895–899. [Google Scholar] [CrossRef]
- Johnson, C. Polymorphism and natural selection in Ischnuran damselflies. Evol. Theory 1975, 1, 81–90. [Google Scholar]
- Waage, J.K. Reproductive behavior and its relation to territoriality in Calopteryx maculata (Beauvois)(Odonata: Calopterygidae). Behaviour 1973, 47, 240–256. [Google Scholar] [CrossRef]
- Higashi, K. Length of maturation period and daily food consumption of immature damselfly, Mnais pruinosa pruinosa Selys (Zygoptera: Calopterygidae). Tombo 1982, 25, 23–26. [Google Scholar]
- Dijkstra, K.-D.B.; Monaghan, M.T.; Pauls, S.U. Freshwater biodiversity and aquatic insect diversification. Annu. Rev. Entomol. 2014, 59, 143–163. [Google Scholar] [CrossRef]


| Model | npar | AICc | ΔAICc | Weight | Deviance |
|---|---|---|---|---|---|
| Phi(~.)p(~Sex + time) | 41 | 2012.9 | 0.000 | 0.563 | 1580.2 |
| Phi(~.)p(~Sex + time + Tempc) | 42 | 2015.2 | 2.317 | 0.177 | 1580.2 |
| Phi(~.)p(~Sex + time + Age_class) | 43 | 2016.1 | 3.182 | 0.115 | 1578.8 |
| Phi(~.)p(~time) | 40 | 2016.2 | 3.263 | 0.110 | 1585.8 |
| Phi(~.)p(~Sex + Tempc + time + Age_class) | 44 | 2018.4 | 5.517 | 0.036 | 1578.8 |
| Phi(~.)p(~Sex * time) | 79 | 2046.3 | 33.427 | 0.000 | 1519.2 |
| Phi(~.)p(~Sex * time + Tempc) | 80 | 2049.0 | 36.102 | 0.000 | 1519.2 |
| Phi(~.)p(~Sex * Time) | 5 | 2071.0 | 58.060 | 0.000 | 1716.4 |
| Phi(~.)p(~Sex + Time) | 4 | 2073.2 | 60.315 | 0.000 | 1720.7 |
| Phi(~.)p(~Time) | 3 | 2074.3 | 61.362 | 0.000 | 1723.8 |
| Phi(~.)p(~Sex) | 3 | 2081.1 | 68.214 | 0.000 | 1730.6 |
| Phi(~.)p(~.) | 2 | 2082.2 | 69.267 | 0.000 | 1733.7 |
| Phi(~.)p(~Sex + Tempc) | 4 | 2083.0 | 70.058 | 0.000 | 1730.5 |
| Phi(~.)p(~Tempc) | 3 | 2084.1 | 71.157 | 0.000 | 1733.6 |
| Phi(~.)p(~Sex + Age_class) | 5 | 2085.1 | 72.197 | 0.000 | 1730.6 |
| Phi(~.)p(~Age_class) | 4 | 2086.0 | 73.079 | 0.000 | 1733.5 |
| Phi(~.)p(~Sex + Age_class + Tempc) | 6 | 2086.9 | 74.038 | 0.000 | 1730.4 |
| Model | npar | AICc | ΔAICc | Weight | Deviance |
|---|---|---|---|---|---|
| Phi(~Sex * Age)p(~Sex + time) | 44 | 2005.6 | 0.000 | 0.453 | 1565.9 |
| Phi(~Sex + Age)p(~Sex + time) | 43 | 2007.6 | 1.989 | 0.168 | 1570.2 |
| Phi(~Sex * Age + Sex * Age2)p(~Sex + time) | 46 | 2007.9 | 2.343 | 0.140 | 1563.6 |
| Phi(~Sex * Age_class + Sex * Age)p(~Sex + time) | 48 | 2009.0 | 3.461 | 0.080 | 1559.9 |
| Phi(~Sex + Age_class + Age)p(~Sex + time) | 45 | 2009.4 | 3.817 | 0.067 | 1567.4 |
| Phi(~Sex + Age + Age2)p(~Sex + time) | 44 | 2009.8 | 4.207 | 0.055 | 1570.1 |
| Phi(~Sex)p(~Sex + time) | 42 | 2011.7 | 6.113 | 0.021 | 1576.7 |
| Phi(~Sex * Age_class + Age)p(~Sex + time) | 47 | 2012.4 | 6.821 | 0.015 | 1565.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Youcefi, A.; Mahdjoub, H.; Zebsa, R.; Kahalerras, A.; Amari, H.; Zouaimia, A.; Bensouilah, S.; Khelifa, R. The Effect of Age on Survival Is Similar in Males and Females of an Aquatic Insect Species. Ecologies 2024, 5, 491-501. https://doi.org/10.3390/ecologies5030030
Youcefi A, Mahdjoub H, Zebsa R, Kahalerras A, Amari H, Zouaimia A, Bensouilah S, Khelifa R. The Effect of Age on Survival Is Similar in Males and Females of an Aquatic Insect Species. Ecologies. 2024; 5(3):491-501. https://doi.org/10.3390/ecologies5030030
Chicago/Turabian StyleYoucefi, Abdeldjalil, Hayat Mahdjoub, Rabah Zebsa, Amin Kahalerras, Hichem Amari, Abdelheq Zouaimia, Soufyane Bensouilah, and Rassim Khelifa. 2024. "The Effect of Age on Survival Is Similar in Males and Females of an Aquatic Insect Species" Ecologies 5, no. 3: 491-501. https://doi.org/10.3390/ecologies5030030
APA StyleYoucefi, A., Mahdjoub, H., Zebsa, R., Kahalerras, A., Amari, H., Zouaimia, A., Bensouilah, S., & Khelifa, R. (2024). The Effect of Age on Survival Is Similar in Males and Females of an Aquatic Insect Species. Ecologies, 5(3), 491-501. https://doi.org/10.3390/ecologies5030030







