Stem Diameter Decrement in Holm Oak (Quercus rotundifolia Lam.): Insights into Tree Decline Pathways in Endangered Woodlands of Southern Portugal
Abstract
1. Introduction
2. Materials and Methods
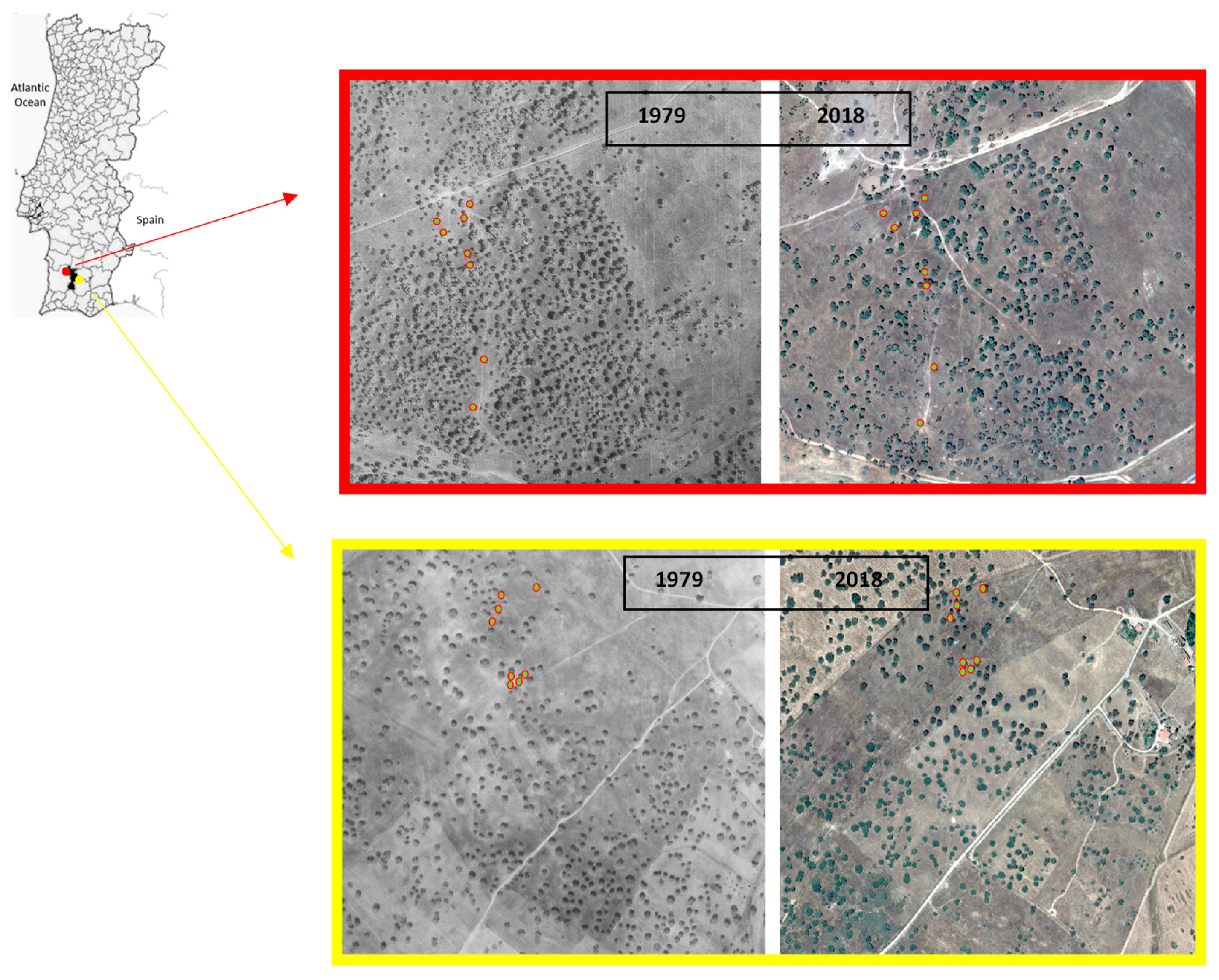
2.1. Study Area
2.2. Tree Selection and Field Work
2.3. Data Analysis
3. Results
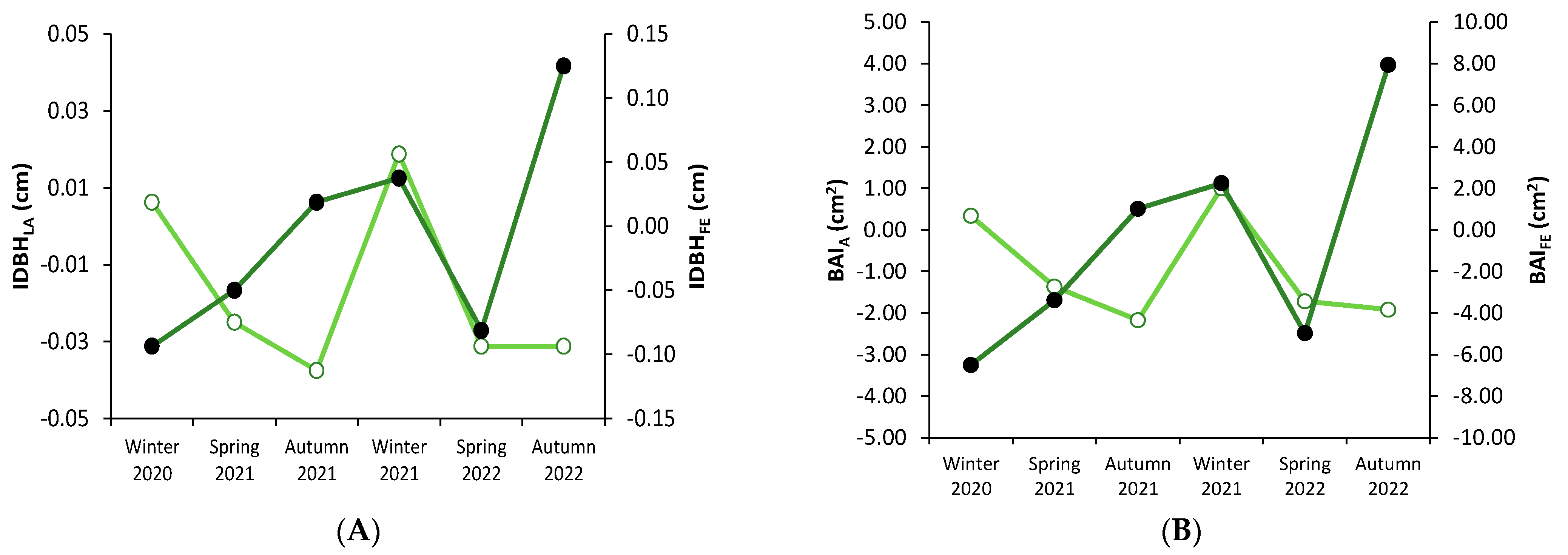
3.1. Stem Diameter Increase Variation
3.2. Tree Size Effect on Stem Radial Growth Variation
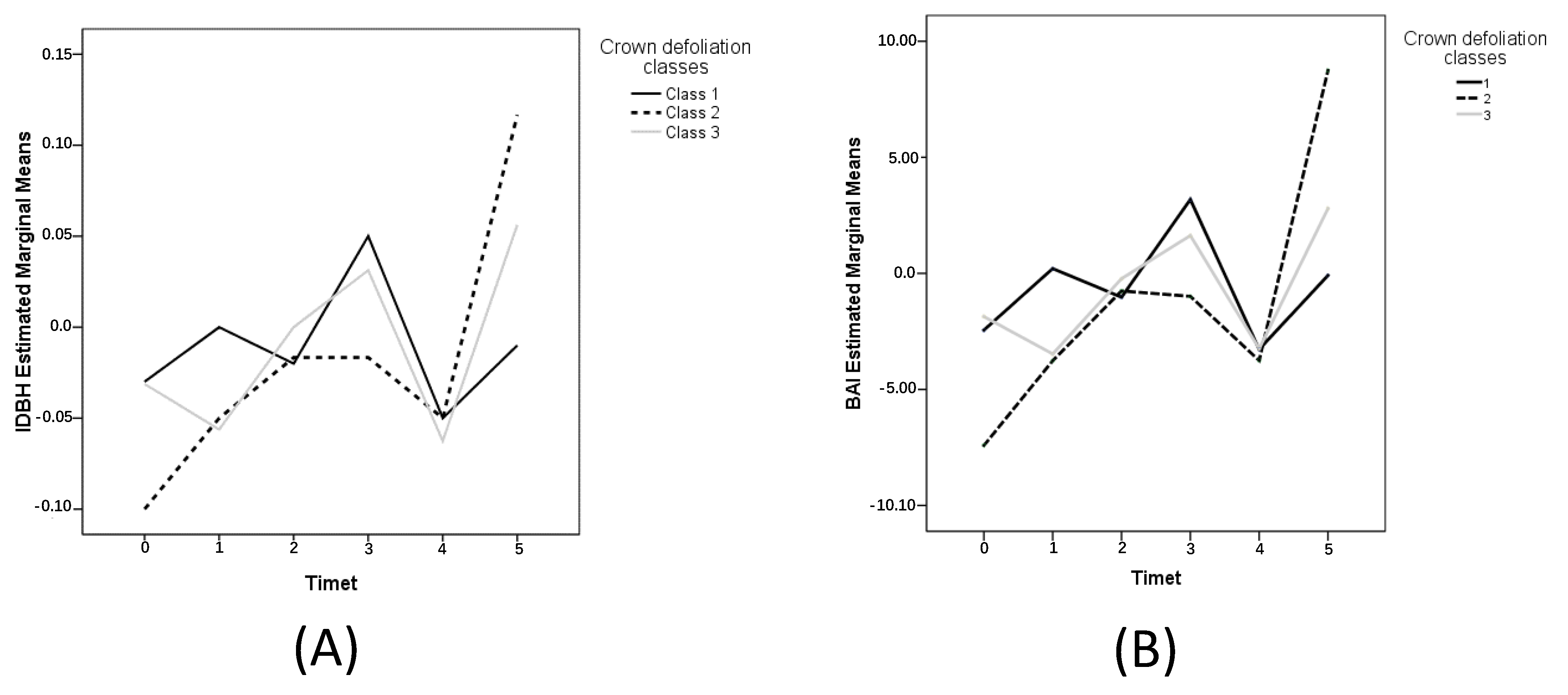
3.3. Crown Defoliation Effect on Stem Radial Growth
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pinto-Correia, T.; Muñoz-Rojas, J.; Thorsøe, M.H.; Noe, E.B. Governance discourses reflecting tensions in a multifunctional land use system in decay; Tradition versus modernity in the Portuguese montado. Sustainability 2019, 11, 3363. [Google Scholar] [CrossRef]
- Garbarino, M.; Bergmeier, E. Plant and vegetation diversity in European wood-pastures. In European Wood-Pastures in Transition: A Social-Ecological Approach; Hartel, T., Plieninger, T., Eds.; Taylor and Francis Inc.: London, UK, 2014; pp. 113–131. [Google Scholar] [CrossRef]
- Bugalho, M.N.; Caldeira, M.C.; Pereira, J.S.; Aronson, J.; Pausas, J. Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front. Ecol. Environ. 2011, 9, 278–286. [Google Scholar] [CrossRef]
- Plieninger, T.; Flinzberger, l.; Hetman, M.; Horstmannshoff, I.; Reinhard-Kolempas, M.; Topp, E.; Moreno, G.; Huntsinger, L. Dehesas as high nature value farming systems: A social-ecological synthesis of drivers, pressures, state, impacts, and responses. Ecol. Soc. 2021, 26, 23. [Google Scholar] [CrossRef]
- Costa, A.; Madeira, M.; Lima, J.S.; Oliveira, A. Change and dynamics in Mediterranean evergreen oak woodlands landscapes of Southwestern Iberian Peninsula. Landsc. Urban Plan. 2011, 102, 164–176. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M.; et al. Climate change impacts, adaptive capacity, and vulnerability of European forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Mendes, M.P.; Cherubini, P.; Plieninger, T.; Ribeiro, L.; Costa, A. Climate effects on stem radial growth of Quercus suber L.: Does tree size matter? Forestry 2019, 92, 73–84. [Google Scholar] [CrossRef]
- Gea-Izquierdo, G.; Natalini, F.; Cardillo, E. Holm oak death is accelerated but not sudden and expresses drought legacies. Sci. Total Environ. 2021, 754, 141793. [Google Scholar] [CrossRef]
- Besson, C.K.; Lobo-do-Vale, R.; Rodrigues, M.C.; Almeida, P.; Herd, A.; Grant, O.M.; David, T.S.; Schmidt, M.; Otieno, D.; Keenan, T.F.; et al. Cork oak physiological responses to manipulated water availability in a Mediterranean woodland. Agric. For. Meteorol. 2014, 184, 230–242. [Google Scholar] [CrossRef]
- Mamet, S.D.; Chun, K.P.; Metsaranta, J.M.; Barr, A.G.; Johnstone, J.F. Tree rings provide early warning signals of jack pine mortality across a moisture gradient in the southern boreal forest. Environ. Res. Lett. 2015, 10, 8. [Google Scholar] [CrossRef]
- Moreira, A.C.; Martins, J.M. Influence of site factors on the impact of Phytophthora cinnamomi in cork oak stands in Portugal. For. Patholol. 2005, 35, 145–162. [Google Scholar] [CrossRef]
- Camarero, J.J.; Gazol, A.; Sangüesa-Barreda, G.; Oliva, J.; Vicente-Serrano, S.M. To die or not to die: Early warnings of tree dieback in response to a severe drought. J. Ecol. 2015, 103, 44–57. [Google Scholar] [CrossRef]
- Cailleret, M.; Vasilis, D.; Steven, J.; Robert, E.M.R.; Aakala, T.; Amoroso, M.M.; Antos, J.A.; Bigler, C.; Bugmann, H.; Caccianaga, M.; et al. Early-Warning Signals of Individual Tree Mortality Based on Annual Radial Growth. Front. Plant Sci. 2019, 9, 1964. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.C.; Rodrigues, A. Effect of soil water content and soil texture on Phytophthora cinnamomi infection on cork and holm oak. Silva Lusit. 2021, 29, 133–160. [Google Scholar] [CrossRef]
- Pastur, G.M.; Lencinas, M.V.; Cellini, J.M.; Mundo, I. Diameter growth: Can live trees decrease? For. Int. J. For. Res. 2007, 80, 83–88. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources (WRB). A Framework for International Classification, Correlation and Communication, 2nd ed.; World Soil Reports n. 103; IUSS Working Group WRB: Wakerley, Australia; FAO: Rome, Italy, 2006. [Google Scholar]
- Costa, A.; Pereira, H.; Madeira, M. Landscape dynamics in endangered cork oak woodlands in Southwestern Portugal (1958–2005). Agrofor. Syst. 2009, 77, 83–96. [Google Scholar] [CrossRef]
- Costa, A.; Madeira, M.; Lima, J.S. Is cork oak (Quercus suber L.) woodland loss driven by eucalyptus plantation? A case-study in southwestern Portugal. iForest-Biogeosciences For. 2014, 7, 193–203. [Google Scholar] [CrossRef]
- Joffre, R.; Rambal, S.; Ratte, J.P. The dehesa system of southern Spain and Portugal as a natural ecosystem mimic. Agrofor. Syst. 1999, 45, 57–79. [Google Scholar] [CrossRef]
- Kottect, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Moreira, A.C.; Seita-Coelho, I.; Fernandes, L.; Martins, J.C. Cultural measures to control cork and holm oak decline in Southern of Portugal. In Book of Abstracts-1° Simpósio SCAP-Novos desafios na Protecção das Plantas-7° Congresso da SPF–20–21 November 2014; Auditório INIAV: Oeiras, Portugal, 2014. (In Portuguese) [Google Scholar]
- Manion, P.D. Tree Disease Concepts; Prentice-Hall Inc.: Englewoods Cliffs, NJ, USA, 1981. [Google Scholar]
- Thomas, F.M.; Blank, R.; Hartmann, G. Abiotic and biotic factors and their interactions as cause of oak decline in Central Europe. For. Pathol. 2002, 32, 277–307. [Google Scholar] [CrossRef]
- Corcobado, T.; Cubera, E.; Juárez, E.; Moreno, G.; Solla, A. Drought events determine performance of Quercus ilex seedlings and increase their susceptibility to Phytophthora cinnamomi. Agric. For. Meteorol. 2014, 192–193, 1–8. [Google Scholar] [CrossRef]
- Ruiz Gómez, F.J.; Perez-de-Luque, A.; Sanchez-Cuesta, R.; Quero, J.L.; Navarro-Cerrillo, R.M. Differences in the Response to Acute Drought and Phytophthora cinnamomi Rands Infection in Quercus ilex L. Seedlings. Forests 2018, 9, 634. [Google Scholar] [CrossRef]
- Bormann, F.H.; Kozlowski, T.T. Measurements of Tree Growth with Dial Gage Dendrometers and Vernier Tree Ring Bands. Ecology 1962, 43, 289–294. [Google Scholar] [CrossRef]
- West, P.W. Use of diameter increment and basal area increment in tree growth studies. Can. J. For. Res. 1980, 10, 71–77. [Google Scholar] [CrossRef]
- Gómez-Aparicio, L.; García-Valdés, R.; Ruiz-Benito, P.; Zavala, M.A. Disentangling the relative importance of climate, size and competition on tree growth in Iberian forests: Implications for forest management under global change. Glob. Chang. Biol. 2011, 17, 2400–2414. [Google Scholar] [CrossRef]
- Hernández-Lambraño, R.E.; de la Cruz, D.R.; Sánchez-Agudo, J.A. Spatial oak decline models to inform conservation planning in the Central-Western Iberian. For. Ecol. Manag. 2019, 441, 115–126. [Google Scholar] [CrossRef]
- Mendes, M.P.; Ribeiro, L.; David, T.S.; Costa, A. How dependent are cork oak (Quercus suber L.) woodlands on groundwater? A case study in southwestern Portugal. For. Ecol. Manag. 2016, 378, 122–130. [Google Scholar] [CrossRef]
- Carranca, C.; Castro, I.V.; Figueiredo, N.; Redondo, R.; Rodrigues, A.R.F.; Saraiva, I.; Maricato, R.; Madeira, M.A.V. Influence of tree canopy on N2 fixation by pasture legumes and soil rhizobial abundance in Mediterranean oak woodlands. Sci. Total Environ. 2015, 506–507, 86–94. [Google Scholar] [CrossRef]
- Oliveira, G.; Correia, O.; Martins-Loução, M.A.; Catarino, F. Phenological and growth patterns of the Mediterranean oak Quercus suber L. Trees 1994, 9, 41–46. [Google Scholar] [CrossRef]
- Campelo, F.; Ribas, M.; Gutiérrez, E. Plastic bimodal growth in a Mediterranean mixed-forest of Quercus ilex and Pinus halepensis. Dendrochronologia 2021, 67, 125836. [Google Scholar] [CrossRef]
- Skelton, R. Stem Diameter Fluctuations Provide a New Window into Plant Water Status and Function. Plant Physiol. 2020, 183, 1414–1415. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, M.; Bascompte, J.; Brock, W.A.; Brovkin, V.; Carpenter, S.R.; Dakos, V.; Held, H.; van Nes, E.H.; Rietkerk, M.; Sugihara, G. Early-warning signals for critical transitions. Nature 2009, 461, 53–59. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Cherubini, P. Is Cork Growth a Reliable Proxy for Stem Diameter Growth in Cork Oak (Quercus suber L.)? Implications for Forest Management under Climate Change in Mediterranean Regions. Appl. Sci. 2021, 11, 11998. [Google Scholar] [CrossRef]
- Ferretti, M.; Bacaro, G.; Brunialti, G.; Calderisi, M.; Crois, L.; Frati, L.; Nicolas, M. Tree canopy defoliation can reveal growth decline in mid-latitude temperate forests. Ecol. Indic. 2021, 127, 107749. [Google Scholar] [CrossRef]


| Local | Variable | December 2020 | December 2021 | December 2022 | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| LA | DBH (cm) | 35.48 ± 3.73 | 32.36–38.60 | 35.42 ± 3.72 | 32.31–38.53 | 35.38 ± 3.68 | 32.30–38.45 |
| BA (cm2) | 997.97 ± 208.71 | 823.49–1172.46 | 994.77 ± 207.82 | 821.03–1168.51 | 992.13 ± 205.18 | 820.60–1163.67 | |
| FE | DBH (cm) | 40.60 ± 9.77 | 32.43–48.77 | 40.48 ± 9.71 | 32.36–48.59 | 40.56 ± 9.71 | 32.44–48.67 |
| BA (cm2) | 1360.27 ± 575.39 | 879.24–1841.31 | 1351.40 ± 570.18 | 874.71–1828.08 | 1356.61 ± 571.48 | 878.84–1834.38 |
| Source of Variation | p.f. | SS | MS | F-Ratio | p-Value | G-G | H-F |
|---|---|---|---|---|---|---|---|
| Within-subjects | |||||||
| Timet (seasons) | 5 | 0.166 | 0.033 | 6.881 | 0.000 | 0.546 | 0.759 |
| Timet X Tree-size | 5 | 0.062 | 0.012 | 2.576 | 0.034 | ||
| Error | 70 | 0.337 | 0.005 | ||||
| Between-subjects | |||||||
| Tree-size | 1 | 0.000 | 0.000 | 0.004 | 0.948 | ||
| Error | 14 | 0.050 | 0.004 |
| Source of Variation | d.f. | SS | MS | F-Ratio | p-Value | G-G | H-F |
|---|---|---|---|---|---|---|---|
| Within-subjects | |||||||
| Timet (seasons) | 5 | 739.492 | 147.898 | 8.195 | 0.000 | 0.549 | 0.746 |
| Timet X Tree-size | 5 | 411.560 | 0.012 | 82.312 | 4.561 | ||
| Error | 70 | 1263.354 | 18.048 | ||||
| Between-subjects | |||||||
| Tree-size | 1 | 0.971 | 0.971 | 0.065 | 0.803 | ||
| Error | 14 | 210.317 | 15.023 |
| Source of Variation | d.f. | SS | MS | F-Ratio | p-Value | G-G | H-F |
|---|---|---|---|---|---|---|---|
| Within-subjects | |||||||
| Time (seasons) | 5 | 0.131 | 0.026 | 5.056 | 0.001 | 0.493 | 0.712 |
| Time X Tree-defoliation | 10 | 0.063 | 0.006 | 1.212 | 0.300 | ||
| Error | 65 | 0.101 | 0.008 | ||||
| Between-subjects | |||||||
| Tree-defoliation | 2 | 0.001 | 0.001 | 0.165 | 0.850 | ||
| Error | 13 | 0.049 | 0.004 |
| Source of Variation | d.f. | SS | MS | F-Ratio | p-Value | G-G | H-F |
|---|---|---|---|---|---|---|---|
| Within-subjects | |||||||
| Time (seasons) | 5 | 608.597 | 121.719 | 5.732 | 0.000 | 0.451 | 0.634 |
| Time X Tree-defoliation | 10 | 294.678 | 29.468 | 1.388 | 0.206 | ||
| Error | 65 | 1380.237 | 21.234 | ||||
| Between-subjects | |||||||
| Tree-defoliation | 2 | 6.676 | 3.338 | 0.212 | 0.812 | ||
| Error | 13 | 204.613 | 15.739 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Moreira, A.C. Stem Diameter Decrement in Holm Oak (Quercus rotundifolia Lam.): Insights into Tree Decline Pathways in Endangered Woodlands of Southern Portugal. Ecologies 2023, 4, 229-241. https://doi.org/10.3390/ecologies4020016
Costa A, Moreira AC. Stem Diameter Decrement in Holm Oak (Quercus rotundifolia Lam.): Insights into Tree Decline Pathways in Endangered Woodlands of Southern Portugal. Ecologies. 2023; 4(2):229-241. https://doi.org/10.3390/ecologies4020016
Chicago/Turabian StyleCosta, Augusta, and Ana Cristina Moreira. 2023. "Stem Diameter Decrement in Holm Oak (Quercus rotundifolia Lam.): Insights into Tree Decline Pathways in Endangered Woodlands of Southern Portugal" Ecologies 4, no. 2: 229-241. https://doi.org/10.3390/ecologies4020016
APA StyleCosta, A., & Moreira, A. C. (2023). Stem Diameter Decrement in Holm Oak (Quercus rotundifolia Lam.): Insights into Tree Decline Pathways in Endangered Woodlands of Southern Portugal. Ecologies, 4(2), 229-241. https://doi.org/10.3390/ecologies4020016










