Linking Rhizosphere Soil Aggregates with Belowground and Aboveground Plant Traits
Abstract
1. Introduction
2. Materials and Methods
2.1. Belowground and Aboveground Plant Data Collection
2.2. Profiling of Different Soil Aggregate-Size Classes
2.3. Analysis of Organic Carbon Contents of Different Soil Aggregate-Size Classes
2.4. Data Analysis
3. Results
3.1. Composition and Properties of Soil Aggregate Size-Classes

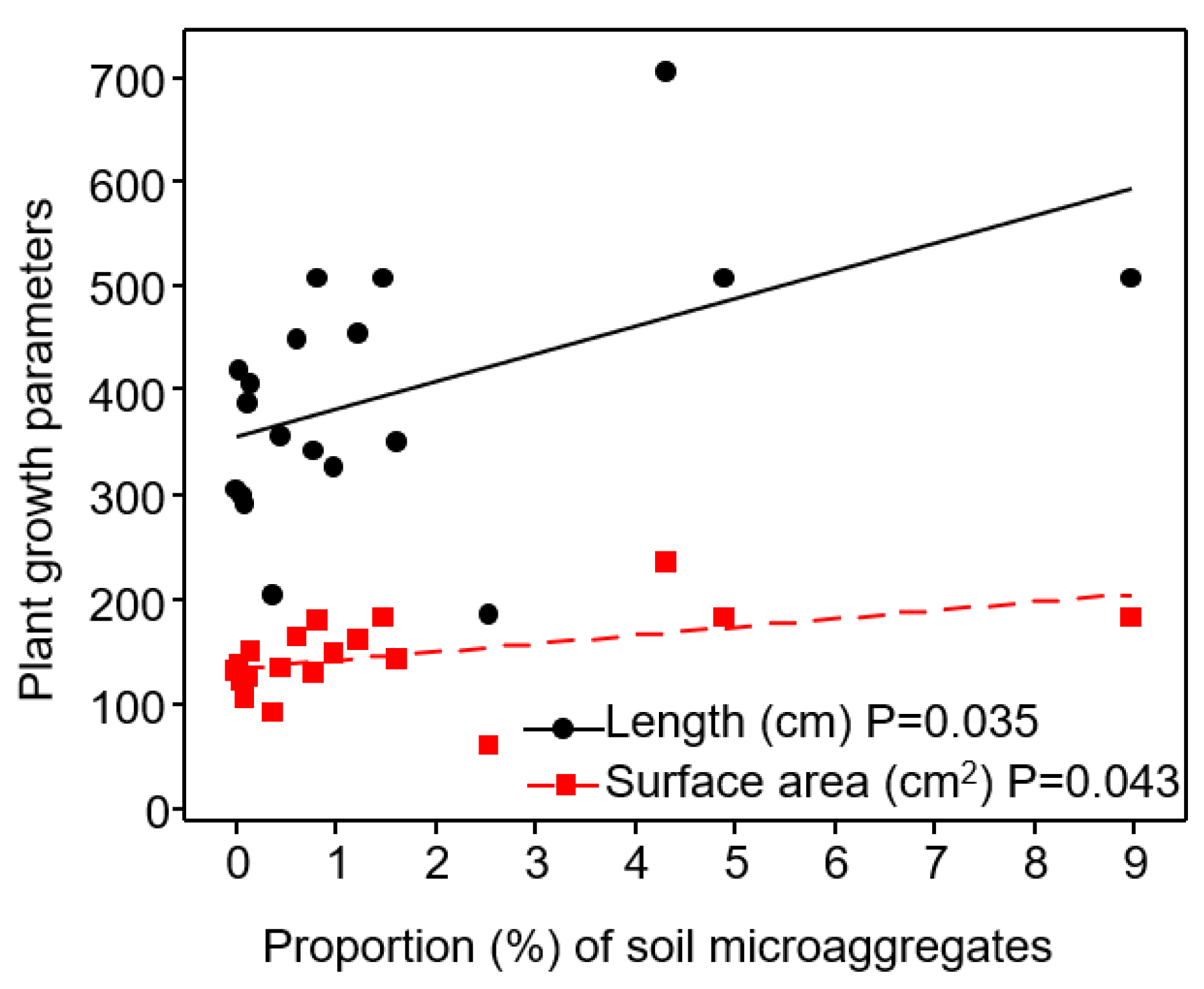
3.2. Relationship of Soil Aggregate-Size Classes with Plant Growth Parameters
3.3. Relationship of Large-Macroaggregates (>2000 μm) with Aboveground and Belowground Traits
| Large Aggregate (>2000 μm) | Linear Regression Coefficient (R2) | Probability Value (P) | Relationship |
|---|---|---|---|
| Shoot traits | |||
| Up trichome | R2 = 0.34 | p = 0.006 | + |
| Down trichome | R2 = 0.25 | p = 0.023 | + |
| Fine root traits | |||
| Fine root P contents (%) | R2 = 0.18 | p = 0.056 | + |
| Fine root K contents (%) | R2 = 0.26 | p = 0.02 | + |
| Fine root Ca contents (%) | R2 = 0.33 | p = 0.0008 | - |
| Fine root Zn contents (%) | R2 = 0.38 | p = 0.004 | - |
| Fine root Fe contents (%) | R2 = 0.30 | p = 0.012 | - |
| Fine root Mn contents (%) | R2 = 0.32 | p = 0.008 | - |
| Fine root Cu contents (%) | R2 = 0.27 | p = 0.017 | - |
| Large root traits | |||
| Large root K contents (%) | R2 = 0.38 | p = 0.004 | + |
| Large root Ca contents (%) | R2 = 0.21 | p = 0.038 | - |
| Large root Mn contents (%) | R2 = 0.27 | p = 0.019 | - |
| Large root Cu contents (%) | R2 = 0.18 | p = 0.059 | - |
3.4. Relationship of Small Macroaggregates (<2000–500 μm) with Plants Traits
| Small Macro-Aggregates (<2000–500 μm) | Linear Regression Coefficient (R2) | Probability Value (P) | Relationship |
|---|---|---|---|
| Plant traits | |||
| Up trichome | R2 = 0.28 | p = 0.024 | - |
| Down trichome | R2 = 0.24 | p = 0.023 | - |
| Shoot traits | |||
| Shoot S content (%) | R2 = 0.21 | p = 0.043 | + |
| Shoot Mn content (%) | R2 = 0.46 | p = 0.017 | + |
| Fine root traits | |||
| Fine root P contents (%) | R2 = 0.40 | p = 0.003 | - |
| Fine root K contents (%) | R2 = 0.25 | p = 0.029 | - |
| Fine root Ca contents (%) | R2 = 0.33 | p = 0.008 | + |
| Fine root Zn contents (%) | R2 = 0.22 | p = 0.035 | + |
| Fine root Fe contents (%) | R2 = 0.23 | p = 0.034 | + |
| Fine root Mn contents (%) | R2 = 0.22 | p = 0.035 | + |
| Fine root Cu contents (%) | R2 = 0.30 | p = 0.012 | + |
| Large root traits | |||
| Large root K contents (%) | R2 = 0.41 | p = 0.002 | - |
| Large root Cu (ppm) | R2 = 0.20 | p = 0.047 | + |
| Large root B content (%) | R2 = 0.56 | p = 0.0001 | - |
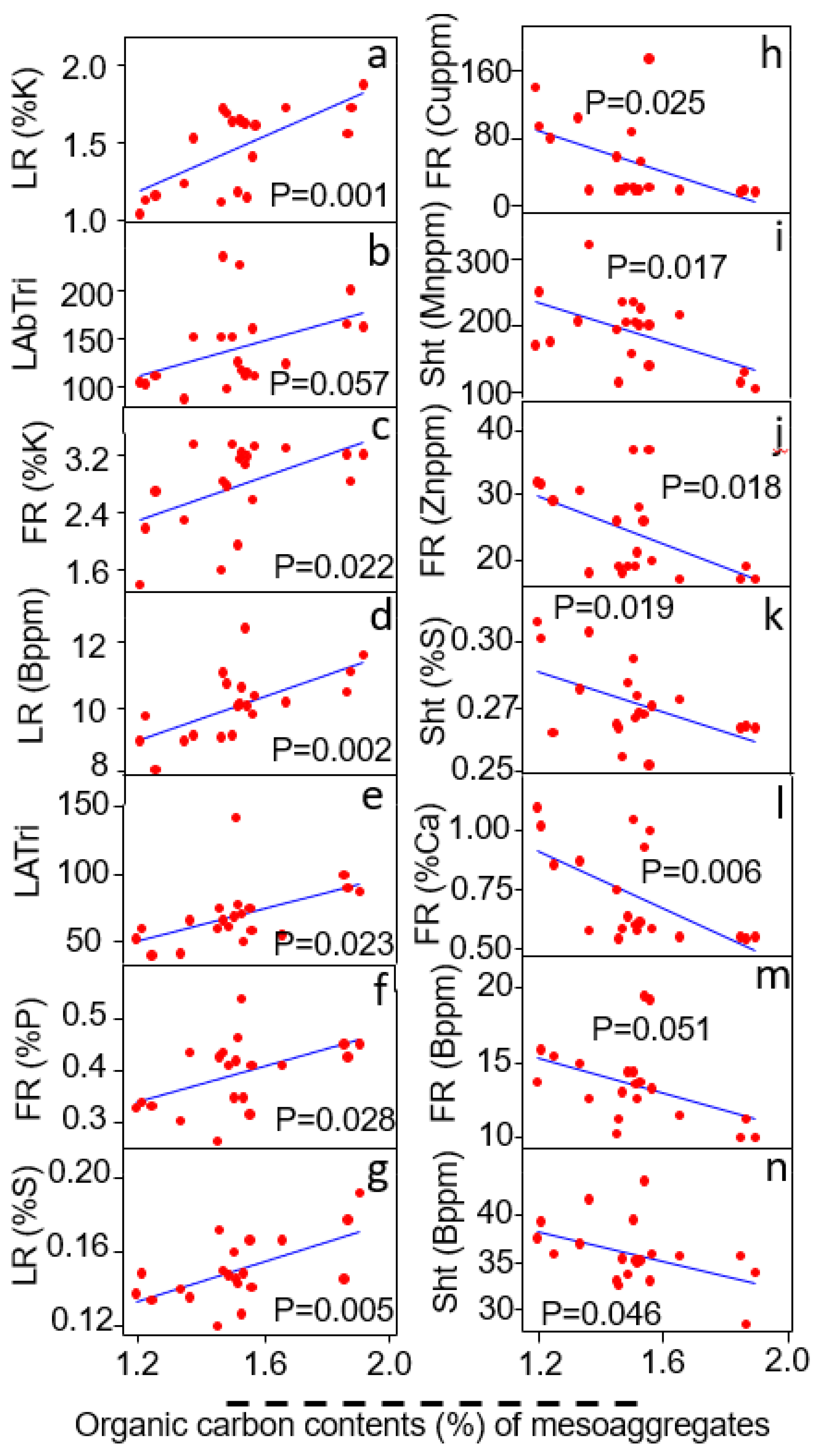
3.5. Relationship of Mesoaggregates (<500–250 μm) to Plant Traits
| Meso-Aggregates (<500–250 μm) | Linear Regression Coefficient (R2) | Probability Value (P) | Relationship |
|---|---|---|---|
| Shoot traits | |||
| Shoot Mn content (ppm) | R2 = 0.18 | p = 0.08 | - |
| Fine root traits | |||
| Fine root P content (%) | R2 = 0.19 | p = 0.07 | + |
| Large root traits | |||
| Large root P content (%) | R2 = 0.16 | p = 0.087 | + |
| Large root B content (%) | R2 = 0.47 | p = 0.0001 | + |
| Large root Mo content (ppm) | R2 = 0.45 | p = 0.0001 | - |
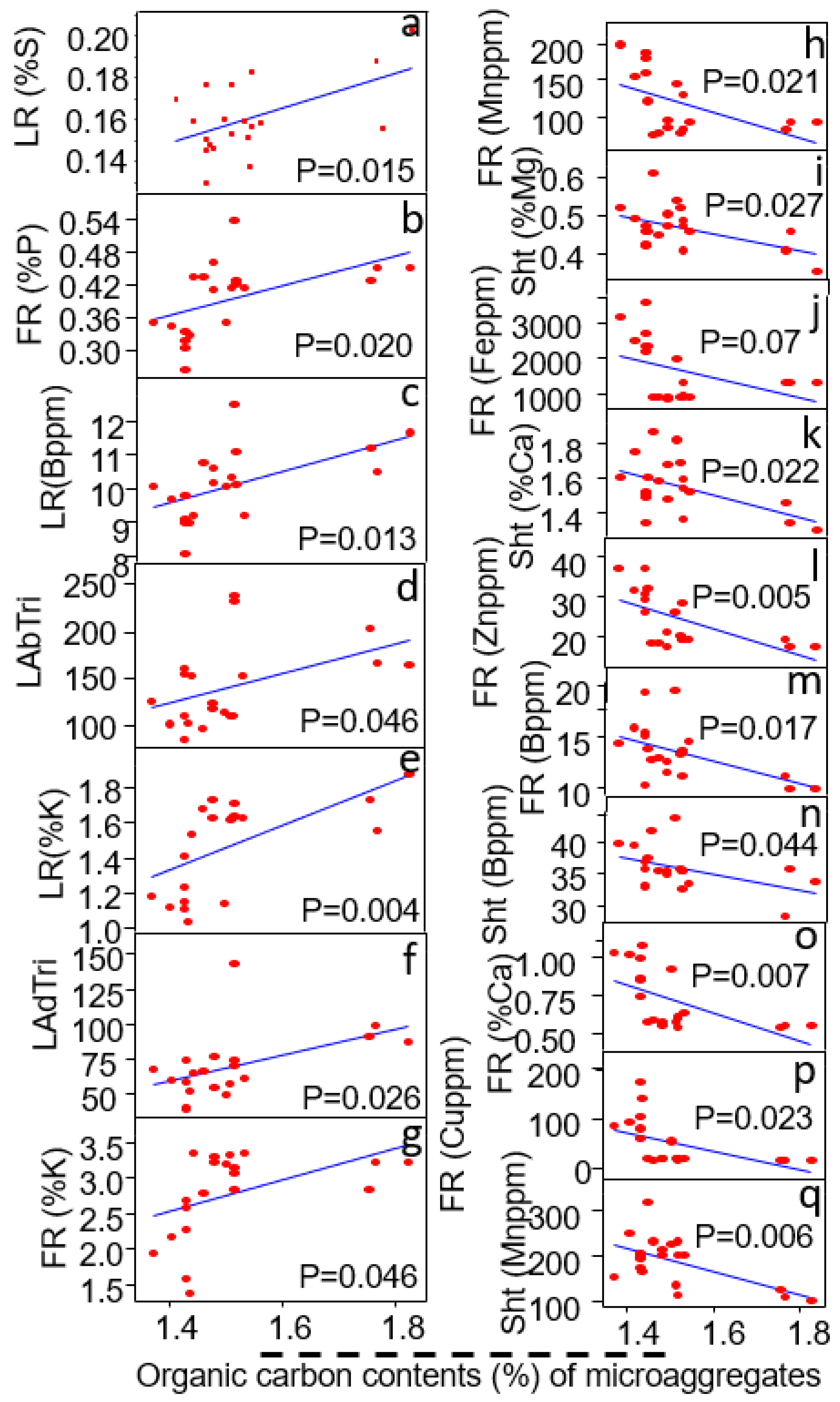
3.6. Relationship of Microaggregates (<250 μm) to Plant Traits
| Micro-Aggregates (<500–250 μm) | Linear Regression Coefficient (R2) | Probability Value (P) | Relationship |
|---|---|---|---|
| Shoot traits | |||
| Shoot Zn content (%) | R2 = 0.39 | p = 0.036 | - |
| Shoot B content (%) | R2 = 0.36 | p = 0.038 | - |
| Fine root traits | |||
| Fine root Ca content (%) | R2 = 0.35 | p = 0.034 | - |
| Fine root Zn content (%) | R2 = 0.28 | p = 0.067 | + |
| Fine root Fe content (%) | R2 = 0.33 | p = 0.09 | - |
| Fine root Mn content (%) | R2 = 0.33 | p = 0.037 | - |
| Fine root Cu content (%) | R2 = 0.34 | p = 0.058 | - |
| Large root traits | |||
| Large root K content (%) | R2 = 0.35 | p = 0.031 | + |
| Large root Cu content (%) | R2 = 0.30 | p = 0.053 | - |
| Large root B content (%) | R2 = 0.41 | p = 0.024 | + |
4. Discussion
5. Conclusions and Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saleem, M.; Pervaiz, Z.H.; Contreras, J.; Lindenberger, J.H.; Hupp, B.M.; Chen, D.; Zhang, Q.; Wang, C.; Iqbal, J.; Twigg, P. Cover Crop Diversity Improves Multiple Soil Properties via Altering Root Architectural Traits. Rhizosphere 2020, 16, 100248. [Google Scholar] [CrossRef]
- Topps, D.; Khabir, M.I.U.; Abdelmagid, H.; Jackson, T.; Iqbal, J.; Robertson, B.K.; Pervaiz, Z.H.; Saleem, M. Impact of Cover Crop Monocultures and Mixtures on Organic Carbon Contents of Soil Aggregates. Soil Syst. 2021, 5, 43. [Google Scholar] [CrossRef]
- Fang, X.; Zhou, G.; Li, Y.; Liu, S.; Chu, G.; Xu, Z.; Liu, J. Warming Effects on Biomass and Composition of Microbial Communities and Enzyme Activities within Soil Aggregates in Subtropical Forest. Biol. Fertil. Soils 2016, 52, 353–365. [Google Scholar] [CrossRef]
- Edwards, L.M. The Effect of Alternate Freezing and Thawing on Aggregate Stability and Aggregate Size Distribution of Some Prince Edward Island Soils. J. Soil Sci. 1991, 42, 193–204. [Google Scholar] [CrossRef]
- Kou, T.J.; Zhu, P.; Huang, S.; Peng, X.X.; Song, Z.W.; Deng, A.X.; Gao, H.J.; Peng, C.; Zhang, W.J. Effects of Long-Term Cropping Regimes on Soil Carbon Sequestration and Aggregate Composition in Rainfed Farmland of Northeast China. Soil Tillage Res. 2012, 118, 132–138. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Jin, J.; Xing, B. Some Concepts of Soil Organic Carbon Characteristics and Mineral Interaction from a Review of Literature. Soil Biol. Biochem. 2016, 94, 107–121. [Google Scholar] [CrossRef]
- da Silva, A.P.; Babujia, L.C.; Franchini, J.C.; Ralisch, R.; Hungria, M.; de Fátima Guimarães, M. Soil Structure and Its Influence on Microbial Biomass in Different Soil and Crop Management Systems. Soil Tillage Res. 2014, 142, 42–53. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, L.; Zhuang, Q.; Yu, D.; Shi, X.; Xing, S.; Xiong, D.; Liu, Y. Quantification of the Soil Organic Carbon Balance in the Tai-Lake Paddy Soils of China. Soil Tillage Res. 2016, 155, 95–106. [Google Scholar] [CrossRef]
- Gregorich, E.G.; Liang, B.C.; Drury, C.F.; Ellert, B.H. Fertilization Effects on Physically Protected Light Fraction Organic Matter. Soil Sci. Soc. Am. J. 1997, 61, 482–484. [Google Scholar] [CrossRef]
- Jiang, X.-J.; Xie, D.-T. Combining Ridge with No-Tillage in Lowland Rice-Based Cropping System: Long-Term Effect on Soil and Rice Yield. Pedosphere 2009, 19, 515–522. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Lü, Y.; Zhang, X.; Liang, W. Contributions of Soil Biota to C Sequestration Varied with Aggregate Fractions under Different Tillage Systems. Soil Biol. Biochem. 2013, 62, 147–156. [Google Scholar] [CrossRef]
- Wang, X.; Qi, J.-Y.; Zhang, X.-Z.; Li, S.-S.; Latif Virk, A.; Zhao, X.; Xiao, X.-P.; Zhang, H.-L. Effects of Tillage and Residue Management on Soil Aggregates and Associated Carbon Storage in a Double Paddy Cropping System. Soil Tillage Res. 2019, 194, 104339. [Google Scholar] [CrossRef]
- Rahman, M.T.; Guo, Z.C.; Zhang, Z.B.; Zhou, H.; Peng, X.H. Wetting and Drying Cycles Improving Aggregation and Associated C Stabilization Differently after Straw or Biochar Incorporated into a Vertisol. Soil Tillage Res. 2018, 175, 28–36. [Google Scholar] [CrossRef]
- Pires, L.F.; Auler, A.C.; Roque, W.L.; Mooney, S.J. X-ray Microtomography Analysis of Soil Pore Structure Dynamics under Wetting and Drying Cycles. Geoderma 2020, 362, 114103. [Google Scholar] [CrossRef] [PubMed]
- Diel, J.; Vogel, H.-J.; Schlüter, S. Impact of Wetting and Drying Cycles on Soil Structure Dynamics. Geoderma 2019, 345, 63–71. [Google Scholar] [CrossRef]
- Bathke, G.R.; Cassel, D.K.; Hargrove, W.L.; Porter, P.M. Modification of Soil Physical Properties and Root Growth Response. Soil Sci. 1992, 154, 316–329. [Google Scholar] [CrossRef]
- Letey, J. Relationship between Soil Physical Properties and Crop Production. In Advances in Soil Science; Stewart, B.A., Ed.; Advances in Soil Science; Springer: New York, NY, USA, 1985; pp. 277–294. ISBN 978-1-4612-5046-3. [Google Scholar]
- Xiu, L.; Zhang, W.; Sun, Y.; Wu, D.; Meng, J.; Chen, W. Effects of Biochar and Straw Returning on the Key Cultivation Limitations of Albic Soil and Soybean Growth over 2 years. Catena 2019, 173, 481–493. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, S.; Saleem, M.; Ali, M.Y.; Xiang, J. Biochar and Earthworms Synergistically Improve Soil Structure, Microbial Abundance, Activities and Pyraclostrobin Degradation. Appl. Soil Ecol. 2021, 168, 104154. [Google Scholar] [CrossRef]
- Kohler, J.; Caravaca, F.; Roldán, A. An AM Fungus and a PGPR Intensify the Adverse Effects of Salinity on the Stability of Rhizosphere Soil Aggregates of Lactuca Sativa. Soil Biol. Biochem. 2010, 42, 429–434. [Google Scholar] [CrossRef]
- Reinhart, K.O.; Vermeire, L.T. Soil Aggregate Stability and Grassland Productivity Associations in a Northern Mixed-Grass Prairie. PLoS ONE 2016, 11, e0160262. [Google Scholar] [CrossRef]
- Murungu, F.S.; Nyamugafata, P.; Chiduza, C.; Clark, L.J.; Whalley, W.R. Effects of Seed Priming, Aggregate Size and Soil Matric Potential on Emergence of Cotton (Gossypium hirsutum L.) and Maize (Zea mays L.). Soil Tillage Res. 2003, 74, 161–168. [Google Scholar] [CrossRef]
- Nichols, K.A.; Toro, M. A Whole Soil Stability Index (WSSI) for Evaluating Soil Aggregation. Soil Tillage Res. 2011, 111, 99–104. [Google Scholar] [CrossRef]
- Six, J.; Bossuyt, H.; Degryze, S.; Denef, K. A History of Research on the Link between (Micro)Aggregates, Soil Biota, and Soil Organic Matter Dynamics. Soil Tillage Res. 2004, 79, 7–31. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Iqbal, J.; Zhang, Q.; Chen, D.; Wei, H.; Saleem, M. Continuous Cropping Alters Multiple Biotic and Abiotic Indicators of Soil Health. Soil Syst. 2020, 4, 59. [Google Scholar] [CrossRef]
- Adesemoye, A.; Pervaiz, Z.H.; Parikh, L.; Kodati, S.; Zhang, Q.; Stepanović, S.; Saleem, M. Rhizobacterial, Fusarium Complex, and Fungicide Seed Treatments Regulate Shoot and Root Traits of Soybean Plants. J. Soil Sci. Plant Nutr. 2021, 21, 3502–3513. [Google Scholar] [CrossRef]
- Pervaiz, Z.H.; Contreras, J.; Hupp, B.M.; Lindenberger, J.H.; Chen, D.; Zhang, Q.; Wang, C.; Twigg, P.; Saleem, M. Root Microbiome Changes with Root Branching Order and Root Chemistry in Peach Rhizosphere Soil. Rhizosphere 2020, 16, 100249. [Google Scholar] [CrossRef]
- Jiménez, J.J.; Lal, R.; Leblanc, H.A.; Russo, R.O.; Raut, Y. The Soil C Pool in Different Agroecosystems Derived from the Dry Tropical Forest of Guanacaste, Costa Rica. Ecol. Eng. 2008, 34, 289–299. [Google Scholar] [CrossRef]
- Wang, B.; Brewer, P.E.; Shugart, H.H.; Lerdau, M.T.; Allison, S.D. Soil Aggregates as Biogeochemical Reactors and Implications for Soil–Atmosphere Exchange of Greenhouse Gases—A Concept. Glob. Change Biol. 2019, 25, 373–385. [Google Scholar] [CrossRef]
- Sainju, U.M. Carbon and Nitrogen Pools in Soil Aggregates Separated by Dry and Wet Sieving Methods. Soil Sci. 2006, 171, 937–949. [Google Scholar] [CrossRef]
- Kibblewhite, M.G.; Ritz, K.; Swift, M.J. Soil Health in Agricultural Systems. Philos. Trans. R. Soc. B Biol. Sci. 2008, 363, 685–701. [Google Scholar] [CrossRef]
- Menon, M.; Mawodza, T.; Rabbani, A.; Blaud, A.; Lair, G.J.; Babaei, M.; Kercheva, M.; Rousseva, S.; Banwart, S. Pore System Characteristics of Soil Aggregates and Their Relevance to Aggregate Stability. Geoderma 2020, 366, 114259. [Google Scholar] [CrossRef]
- Al-Maliki, S.; Ebreesum, H. Changes in Soil Carbon Mineralization, Soil Microbes, Roots Density and Soil Structure Following the Application of the Arbuscular Mycorrhizal Fungi and Green Algae in the Arid Saline Soil. Rhizosphere 2020, 14, 100203. [Google Scholar] [CrossRef]
- Jahangir, M.M.R.; Islam, S.; Nitu, T.T.; Uddin, S.; Kabir, A.K.M.A.; Meah, M.B.; Islam, R. Bio-Compost-Based Integrated Soil Fertility Management Improves Post-Harvest Soil Structural and Elemental Quality in a Two-Year Conservation Agriculture Practice. Agronomy 2021, 11, 2101. [Google Scholar] [CrossRef]
- Qaswar, M.; Jing, H.; Ahmed, W.; Dongchu, L.; Shujun, L.; Lu, Z.; Cai, A.; Lisheng, L.; Yongmei, X.; Jusheng, G.; et al. Yield Sustainability, Soil Organic Carbon Sequestration and Nutrients Balance under Long-Term Combined Application of Manure and Inorganic Fertilizers in Acidic Paddy Soil. Soil Tillage Res. 2020, 198, 104569. [Google Scholar] [CrossRef]
- Rillig, M.C.; Muller, L.A.; Lehmann, A. Soil Aggregates as Massively Concurrent Evolutionary Incubators. ISME J. 2017, 11, 1943–1948. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Bergmann, J.; Verbruggen, E.; Veresoglou, S.D.; Lehmann, A. Plant Root and Mycorrhizal Fungal Traits for Understanding Soil Aggregation. New Phytol. 2015, 205, 1385–1388. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ren, K.; Su, J.; He, X.; Zhao, G.; Hu, B.; Chen, Y.; Xu, Z.; Jin, Y.; Zou, C. Rotation and Organic Fertilizers Stabilize Soil Water-Stable Aggregates and Their Associated Carbon and Nitrogen in Flue-Cured Tobacco Production. J. Soil Sci. Plant Nutr. 2020, 20, 192–205. [Google Scholar] [CrossRef]
- Wright, A.L.; Hons, F.M. Soil Carbon and Nitrogen Storage in Aggregates from Different Tillage and Crop Regimes. Soil Sci. Soc. Am. J. 2005, 69, 141–147. [Google Scholar] [CrossRef]
- Li, Y.; Xie, Z.; Zhou, M.; Liu, C.; Yu, Z.; Wu, J.; Jin, J.; Chen, Y.; Zhang, X.; Liu, X. Soybean Yield and Quality Relative to Mollisols Fertility with 7-Year Consecutive Cattle Manure Application under Maize-Soybean Rotation. Land Degrad. Dev. 2021, 32, 4740–4754. [Google Scholar] [CrossRef]
- Anothai, J.; Chairin, T. Soil Physicochemical Properties Closely Associated with Fungal Enzymes and Plant Defense Enzymes in Ganoderma-Infected Oil Palm Orchards. Plant Soil 2020, 456, 99–112. [Google Scholar] [CrossRef]
- El-Naggar, A.; Lee, M.-H.; Hur, J.; Lee, Y.H.; Igalavithana, A.D.; Shaheen, S.M.; Ryu, C.; Rinklebe, J.; Tsang, D.C.W.; Ok, Y.S. Biochar-Induced Metal Immobilization and Soil Biogeochemical Process: An Integrated Mechanistic Approach. Sci. Total Environ. 2020, 698, 134112. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Zhou, H.; Chen, L.; Yuan, Y.; Fang, H.; Luan, L.; Chen, Y.; Wang, X.; Liu, M.; Li, H.; et al. Nematodes and Microorganisms Interactively Stimulate Soil Organic Carbon Turnover in the Macroaggregates. Front. Microbiol. 2018, 9, 2803. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.M.; Jastrow, J.D. The Role of Mycorrhizal Fungi in Soil Conservation. In Mycorrhizae in Sustainable Agriculture; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1992; pp. 29–44. ISBN 978-0-89118-320-4. [Google Scholar]
- Xiong, L.; Liu, X.; Vinci, G.; Sun, B.; Drosos, M.; Li, L.; Piccolo, A.; Pan, G. Aggregate Fractions Shaped Molecular Composition Change of Soil Organic Matter in a Rice Paddy under Elevated CO2 and Air Warming. Soil Biol. Biochem. 2021, 159, 108289. [Google Scholar] [CrossRef]
- Singh, M.K.; Singh, S.; Ghoshal, N. Impact of Land Use Change on Soil Aggregate Dynamics in the Dry Tropics. Restor. Ecol. 2017, 25, 962–971. [Google Scholar] [CrossRef]
- Cavalcante, D.M.; de Castro, M.F.; Chaves, M.T.L.; da Silva, I.R.; de Oliveira, T.S. Effects of Rehabilitation Strategies on Soil Aggregation, C and N Distribution and Carbon Management Index in Coffee Cultivation in Mined Soil. Ecol. Indic. 2019, 107, 105668. [Google Scholar] [CrossRef]
- Zhong, Z.; Wu, S.; Lu, X.; Ren, Z.; Wu, Q.; Xu, M.; Ren, C.; Yang, G.; Han, X. Organic Carbon, Nitrogen Accumulation, and Soil Aggregate Dynamics as Affected by Vegetation Restoration Patterns in the Loess Plateau of China. Catena 2021, 196, 104867. [Google Scholar] [CrossRef]
- Totsche, K.U.; Amelung, W.; Gerzabek, M.H.; Guggenberger, G.; Klumpp, E.; Knief, C.; Lehndorff, E.; Mikutta, R.; Peth, S.; Prechtel, A.; et al. Microaggregates in Soils. J. Plant Nutr. Soil Sci. 2018, 181, 104–136. [Google Scholar] [CrossRef]
- Edwards, A.P.; Bremner, J.M. Microaggregates in Soils1. J. Soil Sci. 1967, 18, 64–73. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khabir, M.I.u.; Topps, D.; Jhumur, J.F.; Adesemoye, A.; Brown, J.; Newman, A.; Robertson, B.K.; Iqbal, J.; Saleem, M. Linking Rhizosphere Soil Aggregates with Belowground and Aboveground Plant Traits. Ecologies 2023, 4, 74-87. https://doi.org/10.3390/ecologies4010007
Khabir MIu, Topps D, Jhumur JF, Adesemoye A, Brown J, Newman A, Robertson BK, Iqbal J, Saleem M. Linking Rhizosphere Soil Aggregates with Belowground and Aboveground Plant Traits. Ecologies. 2023; 4(1):74-87. https://doi.org/10.3390/ecologies4010007
Chicago/Turabian StyleKhabir, Md Imam ul, Daphne Topps, Jannatul Ferdous Jhumur, Anthony Adesemoye, Jasmine Brown, Antoine Newman, Boakai K. Robertson, Javed Iqbal, and Muhammad Saleem. 2023. "Linking Rhizosphere Soil Aggregates with Belowground and Aboveground Plant Traits" Ecologies 4, no. 1: 74-87. https://doi.org/10.3390/ecologies4010007
APA StyleKhabir, M. I. u., Topps, D., Jhumur, J. F., Adesemoye, A., Brown, J., Newman, A., Robertson, B. K., Iqbal, J., & Saleem, M. (2023). Linking Rhizosphere Soil Aggregates with Belowground and Aboveground Plant Traits. Ecologies, 4(1), 74-87. https://doi.org/10.3390/ecologies4010007






