Investigation of Cowpea (Vigna unguiculata (L.) Walp.)–Insect Pollinator Interactions Aiming to Increase Cowpea Yield and Define New Breeding Tools
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Growth Conditions
2.3. Floral Traits
2.4. Yield Related Traits
2.5. Pollinators Record and Investigation of Inter-Crossing
2.6. Statistical Analysis
3. Results
3.1. Flower Traits
3.2. Yield Related Traits
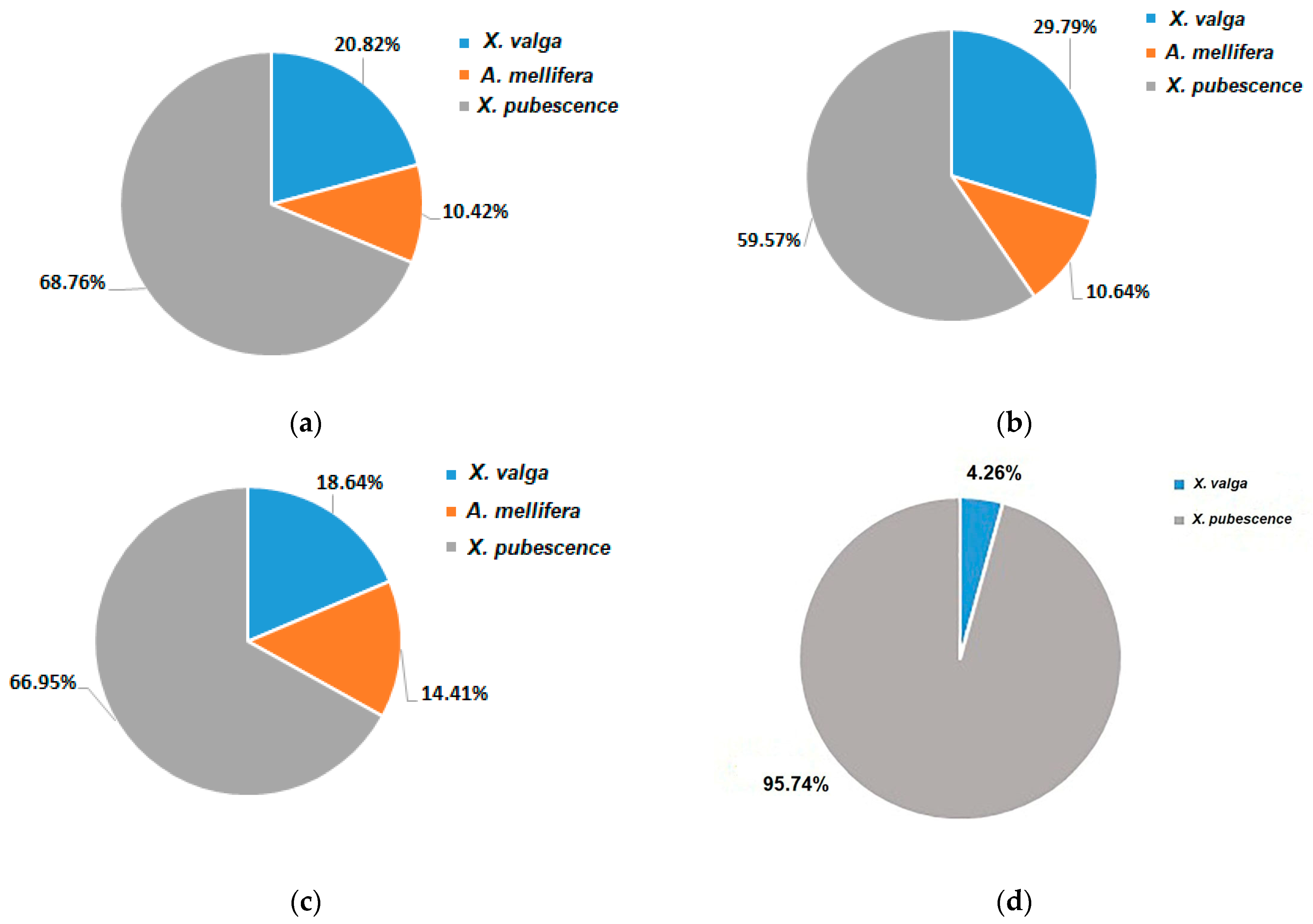
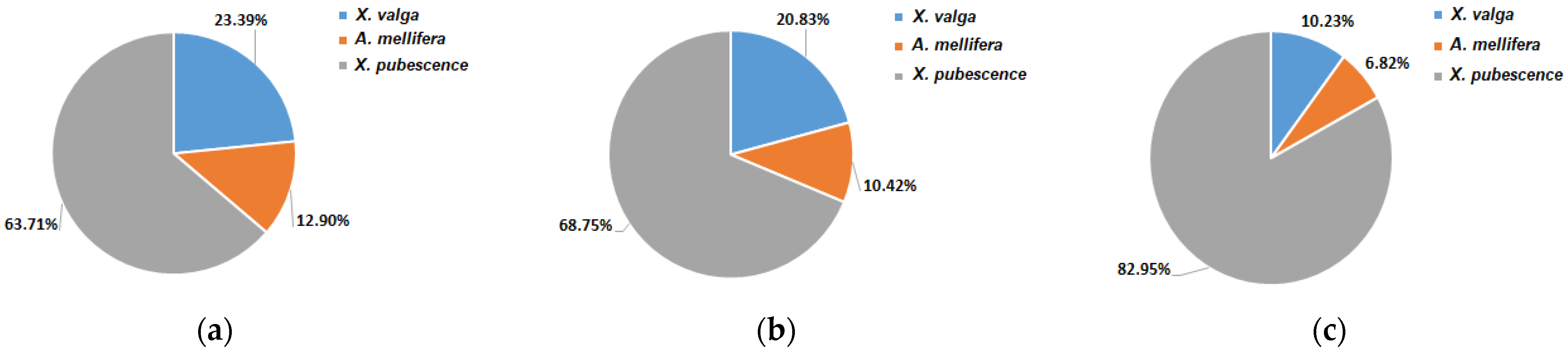
3.3. Pollinators Record
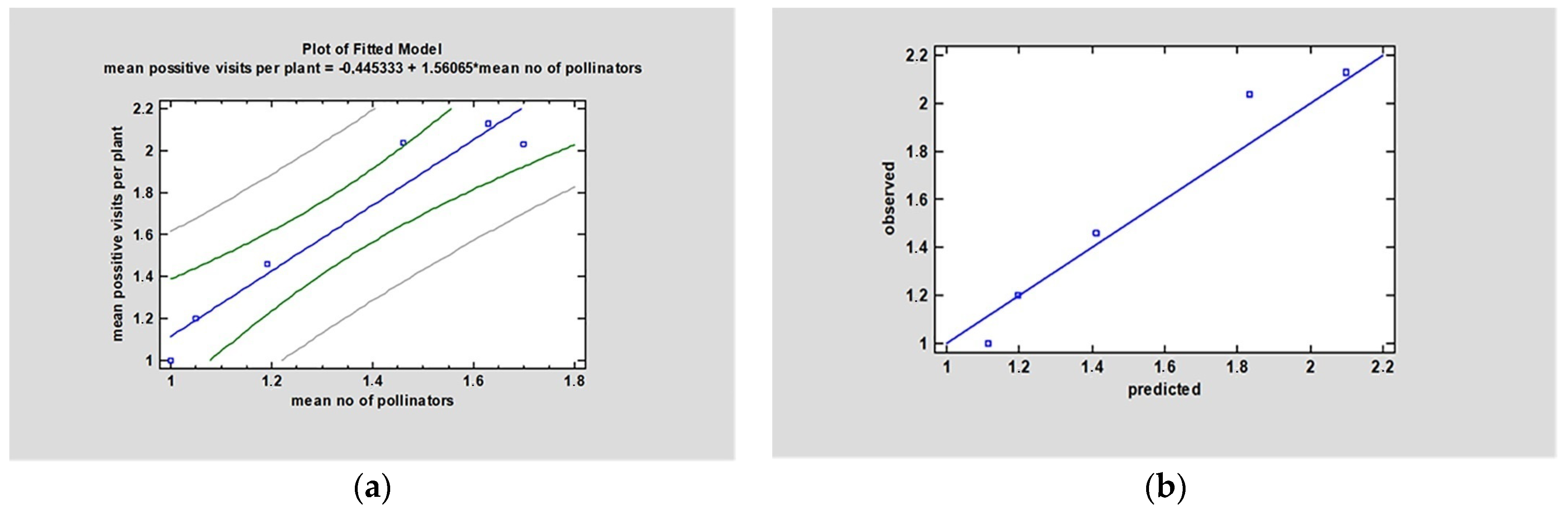
3.4. Floral Traits Relation with Pollinators Abundance and Foraging Activity
3.5. Pollinators Abundance and Foraging Activity Relation to Yield
4. Discussion
4.1. Plant Traits
4.2. Pollinators Observations
4.3. Floral Traits Relation with Pollinators Abundance and Foraging Activity
4.4. Pollinators Abundance and Foraging Activity in Relation to Yield
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Christmann, S.; Bencharki, Y.; Anougmar, S.; Rasmont, P.; Smaili, M.C.; Tsivelikas, A.; Aw-Hassan, A. Farming with Alternative Pollinators benefits pollinators, natural enemies, and yields, and offers transformative change to agriculture. Sci. Rep. 2021, 11, 18206. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.; Imperatriz-Fonseca, V.; Ngo, H.T.; Aizen, M.A.; Biesmeijer, J.C.; Breeze, T.D.; Dicks, L.V.; Garibaldi, L.A.; Hill, R.; Settele, J.; et al. Safeguarding pollinators and their values to human well-being. Nature 2016, 540, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Klein, A.-M.; Vaissiėre, B.E.; Cane, J.H.; Steffan-Dewenter, I.; Cunningham, S.A.; Kremen, C.; Tscharntke, T. Importance of pollinators in changing landscapes for world crops. Proc. R. Soc. B. 2007, 274, 303–313. [Google Scholar] [CrossRef]
- Gemmill-Herren, B.; Garibaldi, L.A.; Kremen, C.; Ngo, H.T. Building effective policies to conserve pollinators: Translating knowledge into policy. Curr. Opin. Insect Sci. 2021, 46, 64–71. [Google Scholar] [CrossRef]
- Majewska, A.A.; Altizer, S. Planting gardens to support insect pollinators. Conserv. Biol. 2018, 34, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Potts, S.G.; Roberts, S.P.M.; Dean, R.; Marris, G.; Brown, M.A.; Jones, R.; Neumann, P.; Settele, J. Declines of managed honeybees and beekeepers in Europe. J. Apic. Res. 2010, 49, 15–22. [Google Scholar] [CrossRef]
- Palmer, R.G.; Perez, P.T.; Ortiz-Perez, E.; Maalouf, F.; Suso, M.J. The role of crop-pollinator relationships in breeding for pollinator-friendly legumes: From a breeding perspective. Euphytica 2009, 170, 35–52. [Google Scholar] [CrossRef]
- Suso, M.J.; Harder, L.; Moreno, M.T.; Maalouf, F. New strategies for increasing heterozygosity in crops: Vicia faba mating system as a study case. Eyphytica 2005, 143, 51–65. [Google Scholar] [CrossRef]
- Suso, M.J.; Bebeli, P.J.; Christmann, S.; Mateus, C.; Negri, V.; Pinheiro de Carvalho, M.A.A.; Torricelli, R.; Veloso, M.M. Enhancing Legume Ecosystem Services through an Understanding of Plant–Pollinator Interplay. Front. Plant Sci. 2016, 7, 333. [Google Scholar] [CrossRef]
- Descamps, C.; Quinet, M.; Jacquemart, A.-L. The effects of drought on plant–pollinator interactions: What to expect? Env. Exp. Bot. 2021, 182, 104297. [Google Scholar] [CrossRef]
- Suso, M.J.; Pierre, J.; Moreno, M.; Esnault, R.; Le Guen, J. Variation in outcrossing levels in faba bean cultivars: Role of ecological factors. J. Agric. Sci. 2001, 136, 399–405. [Google Scholar] [CrossRef]
- Arista, M.; Talavera, M.; Berjano, R.; Ortiz, P.L. Abiotic factors may explain the geographical distribution of flower colour morphs and the maintenance of colour polymorphism in the scarlet pimpernel. J. Ecol. 2013, 101, 1613–1622. [Google Scholar] [CrossRef]
- Jiménez-López, F.J.; Ortiz, P.L.; Talavera, M.; Arista, M. Reproductive Assurance Maintains Red-Flowered Plants of Lysimachia arvensis in Mediterranean Populations Despite Inbreeding Depression. Front. Plant Sci. 2020, 11, 563110. [Google Scholar] [CrossRef] [PubMed]
- Gigord, L.D.B.; Macnair, M.R.; Smithson, A. Negative frequency-dependent selection maintains a dramatic flower color polymorphism in the rewardless orchid Dactylorhiza sambucina (L.) Soò. Proc. Natl. Acad. Sci. USA 2001, 98, 6253–6255. [Google Scholar] [CrossRef]
- Ige, O.E.; Olotuah, O.F.; Akerele, V. Floral Biology and Pollination Ecology of Cowpea (Vigna Unguiculata L. Walp). Mod. Appl. Sci. 2011, 5, 74–82. [Google Scholar] [CrossRef]
- Asiwe, J.A.N. Insect mediated outcrossing and geneflow in cowpea (Vigna unguiculata (L.) Walp): Implication for seed production and provision of containment structures for genetically transformed cowpea. Afr. J. Biotech. 2009, 8, 226–230. [Google Scholar]
- Safety Assessment of Transgenic Organisms in the Environment: OECD Consensus Documents Vol. 6, Chapter 5, 2016. Available online: www.oecd-ilibrary.org/docserver/9789264253421-8-en.pdf?expires=1568975050&id=id&accname=guest&checksum=D1AF6F1763B8F7F34E1EA7F71F5038EC (accessed on 29 November 2022).
- Fatokun, C.A.; Ng, Q. Outcrossing in cowpea. J. Food Agric. Environ. 2007, 5, 334–338. [Google Scholar]
- Lush, W.M. Floral morphology of wild and cultivated cowpeas. Econ. Bot. 1979, 33, 442–447. [Google Scholar] [CrossRef]
- deMooy, B.E.; deMooy, C.J.; Burke, D.W. Estimation of Percentage Natural Outcrossing in Cowpea (Vigna unguiculata (L.) Walp. in Botswana. USDA Publications, Reports of Bean Improvement Cooperative and National Dry Bean Council Research Conference, 1990; p. 122. Available online: https://naldc.nal.usda.gov/download/IND92013799/PDF (accessed on 25 November 2022).
- Kouam, E.B.; Pasquet, R.S.; Campagne, P.; Tignegre, J.-B.; Thoen, K.; Gaudin, R.; Quedraogo, J.T.; Salifu, A.B.; Muluvi, G.M.; Gepts, P. Genetic structure and mating system of wild cowpea populations in West Africa. BMC Plant Biol. 2012, 12, 113. [Google Scholar] [CrossRef]
- Rachie, K.O.; Silvestre, P. Grain legumes. In Food Crops of the Lowland Tropics; Lealey, C.L.A., Wills, J.B., Eds.; Oxford Univ. Press: Oxford, UK, 1977; pp. 41–74. [Google Scholar]
- Liu, Y.; Li, J.; Zhu, Y.; Jones, A.; Rose, R.J.; Song, Y. Heat Stress in Legume Seed Setting: Effects, Causes, and Future Prospects. Front. Plant Sci. 2019, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Phillips, B.B.; Shaw, R.F.; Holland, M.J.; Fry, E.L.; Bardgett, R.D.; Bullock, J.M.; Osborne, J.L. Drought reduces floral resources for pollinators. Glob. Change Biol. 2018, 24, 3226–3235. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, R.S.; Peltier, A.; Hufford, M.B.; Oudin, E.; Saulnier, J.; Paul, L.; Knudsen, J.T.; Herren, H.R.; Gepts, P. Long-distance pollen flow assessment through evaluation of pollinator foraging range suggests transgene escape distances. Proc. Natl. Acad. Sci. USA 2008, 105, 13456–13461. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, T.S. Pollination in the Agricultural Landscape: Best Management Practices for Crop Pollination; University of Guelph: Guelph, ON, USA, 2012. [Google Scholar]
- Dingha, B.N.; Jackai, L.E.; Amoah, B.A.; Akotsen-Mensah, C. Pollinators on Cowpea Vigna unguiculata: Implications for Intercropping to Enhance Biodiversity. Insects 2021, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Vaz, C.G.; de Oliveira, D.; Ohashi, O.S. Pollinator contribution to the production of cowpea in the Amazon. HortScience 1998, 33, 1157–1159. [Google Scholar] [CrossRef]
- Musa, A.K.; Liadi, M.T.; Adegbite, O.R. Impact of honeybees (Apis mellifera adansonii) (Hymenoptera: Apidae) pollination on pod and seed set of cowpea (Vigna unguiculata L. Walp) in Ilorin, Southern Guinea Savanna of Nigeria. WJAR 2013, 1, 83–87. [Google Scholar]
- Wousla, E.N.; Andargie, M.; Pasquet, R.S.; Mondon, M.; Menez, V.; Cochin, C.; Paul, L.; Pardon, L.; Roubaud, M. Is bigger better? Apidae (Xylocopinae), megachilidae and cowpea (Vigna unguiculata) pollination. Plant Breed. 2019, 139, 156–166. [Google Scholar] [CrossRef]
- Stefanie, K.B.; Albert, N.; Fernand-Nestor, T.F. Pollination and yield attributes of (cowpea) Vigna unguiculata L. Walp. (Fabaceae) as influenced by the foraging activity of Xylocopa olivacea Fabricius (Hymenoptera: Apidae) and inoculation with Rhizobium in Ngaoundere, Cameroon. IJAAR 2015, 6, 62–76. [Google Scholar]
- Susan, D.; Murungi, L.K.; Kioko, E. Diversity and abundance of insect pollinators and their effect on yield and quality of cowpea and cucumber in Makueni, Kenya. Afr. J. Hort. Sci. 2019, 16, 41–52. [Google Scholar]
- Fohouo, F.-N.T.; Ngakou, A.; Kengni, S. Pollination and yield responses of cowpea (Vigna unguiculata L. Walp.) to the foraging activity of Apis mellifera adansonii (Hymenoptera: Apidae) at Ngaoundéré (Cameroon). Afr. J. Biotech. 2009, 8, 1988–1996. [Google Scholar]
- Adamidis, G.C.; Cartar, R.V.; Melathopoulos, A.P.; Pernal, S.F.; Hoover, S.E. Pollinators enhance crop yield and shorten the growing season by modulating plant functional characteristics: A comparison of 23 canola varieties. Sci Rep. 2019, 9, 14208. [Google Scholar] [CrossRef]
- Suso, M.J.; Maalouf, F. Direct and correlated responses to upward and downward selection for outcrossing in Vicia faba. Field Crop Res. 2010, 116, 116–126. [Google Scholar] [CrossRef]
- Karapanos, I.; Papandreou, A.; Skouloudi, M.; Makrogianni, D.; Fernández, J.A.; Rosa, E.; Ntatsi, G.; Bebeli, P.J.; Savvas, D. Cowpea fresh pods—A new legume for the market: Assessment of their quality and dietary characteristics of 37 cowpea accessions grown in southern Europe. J. Sci. Food Agric. 2017, 97, 4343–4352. [Google Scholar] [CrossRef] [PubMed]
- IBPGR Descriptors for Cowpea; IBPGR Secretariat: Rome, Italy, 1983.
- Statgraphics Centurion XVII Version 17.2.0.0. 1982–2016; StatPoint, Inc.: Herndon, VA, USA, 2016.
- Papa, R.; Gepts, P. Gene Flow Between Crops and Their Wild Progenitors. In Encyclopedia of Plant and Crop Science, 1st ed.; Goodman, R.M., Ed.; CRC Press, Taylor & Francis Group LLC: New York, U.S.A, 2004; pp. 488–791. [Google Scholar]
- Purseglove, J.W. Tropical Crops; Dicotyledons: London, UK, 1968. [Google Scholar]
- Hall, A.E. Phenotyping cowpeas for adaptation to drought. Front Physiol. 2012, 3, 155. [Google Scholar] [CrossRef] [PubMed]
- Grindeland, J.M.; Sletvold, N.; Ims, R.A. Effects of floral display size and plant density on pollinator visitation rate in a natural population of Digitalis purpurea. Funct. Ecol. 2005, 19, 383–390. [Google Scholar] [CrossRef]
- Toji, T.; Ishimoto, N.; Egawa, S.; Nakase, Y.; Hattori, M.; Itino, T. Intraspecific convergence of floral size correlates with pollinator size on different mountains: A case study of a bumblebee-pollinated Lamium (Lamiaceae) flowers in Japan. BMC Ecol. Evo. 2021, 21, 64. [Google Scholar] [CrossRef] [PubMed]
- Lazaridi, E.; Ntatsi, G.; Fernández, J.A.; Karapanos, I.; Carnide, V.; Savvas, D.; Bebeli, P.J. Phenotypic diversity and evaluation of fresh pods of cowpea landraces from Southern Europe. J. Sci. Food Agric. 2017, 97, 4326–4333. [Google Scholar] [CrossRef]
- Warrag, M.O.A.; Hall, A.E. Reproductive responses of cowpea (Vigna unguiculata (L.) Walp) to heat stress. II. Responses to night air temperature. Field Crops Res. 1984, 8, 17–33. [Google Scholar] [CrossRef]
- Farooq, M.; Gogoi, N.; Barthakur, S.; Baroowa, B.; Bharadwaj, N.; Algahamdi, S.S.; Siddique, K.H.M. Drought Stress in Grain Legumes during Reproduction and Grain Filling. J. Agro. Crop Sci. 2017, 203, 81–102. [Google Scholar] [CrossRef]
- Olaji, A.A.; Ilori, C.O. Effects of Drought on Morphological Traits in Some Cowpea Genotypes by Evaluating Their Combining Abilities. Adv. Agric. 2017, 2017, 7265726. [Google Scholar] [CrossRef]
- Van Ginkel, M.; Flipphi, R.C.H. Why Self-fertilizing Plants Still Exist in Wild Populations: Diversity Assurance through Stress-Induced Male Sterility May Promote Selective Outcrossing and Recombination. Agronomy 2020, 10, 349. [Google Scholar] [CrossRef]
- Kandemir, İ.; Özkan, A.; Fuchs, S. Reevaluation of honeybee (Apis mellifera) microtaxonomy: A geometric morphometric approach. Apidologie 2001, 42, 618. [Google Scholar] [CrossRef]
- Bänsch, S.; Tscharntke, T.; Ratnieks, F.L.W.; Härtel, S.; Westphal, C. Foraging of honey bees in agricultural landscapes with changing patterns of flower resources. Agric. Ecosyst. Environ. 2020, 291, 106792. [Google Scholar] [CrossRef]
- Ghosh, S.; Jeon, H.; Jung, C. Foraging behaviour and preference of pollen sources by honey bee (Apis mellifera) relative to protein contents. J. Ecol. Environ. 2020, 44, 4. [Google Scholar] [CrossRef]
- Trianto, M.; Marisa, F. Diversity of Bees and Wasp (Hymenoptera) in Cowpea (Vigna sinensis L.) in Agricultural Area at Martapura District, Banjar Regency, South Kalimantan. J. Sci. Technol. 2020, 9, 29–33. [Google Scholar] [CrossRef]
- Westerkamp, C. The co-operation between the asymmetric flower of Lathyrus latifolius (Fabaceae-Vicieae) and its visitors. Phyton 1993, 33, 121–137. [Google Scholar]
- Watmough, R. The significance of alfalfa, Medicago sativa L. (Fabaceae), as a foodplant for Xylocopa caffra (Linnaeus) and Megachile gratiosa Cameron (Hymenoptera: Anthophoridae and Megachilidae) near Oudtshoorn, South Africa. Afr. Entomol. 1999, 7, 307–311. [Google Scholar]
- Bebeli, P.J.; Lazaridi, E.; Chatzigeorgiou, T.; Suso, M.-J.; Hein, W.; Alexopoulos, A.A.; Canha, G.; van Haren, R.J.F.; Jóhannsson, M.H.; Mateos, C.; et al. State and Progress of Andean Lupin Cultivation in Europe: A Review. Agronomy 2020, 10, 1038. [Google Scholar] [CrossRef]
- Pierre, J.; Suso, M.-J.; Moreno, M.T.; Esnault, R.; Le Guen, J. Diversité et efficacité de l’ entomofaune pollinisatrice (Hymenoptera: Apidae) de la féverole (Vicia faba L.) sur deux sites, en France et an Espagne. Ann. Soc. Entomol. Fr. 1999, 35, 312–318. [Google Scholar]
- Klecka, J.; Hadrava, J.; Koloušková, P. Vertical stratification of plant–pollinator interactions in a temperate grassland. Peer J. 2018, 6, e4998. [Google Scholar] [CrossRef]
- Ichimura, K.; Suto, K. Environmental Factors Controlling Flower Opening and Closing in a Portulaca Hybrid. Ann. Bot. 1998, 82, 67–70. [Google Scholar] [CrossRef]
- McCall, C.; Primack, R.B. Influence of flower characteristics, weather, time of day, and season on insect visitation rates in three plant communities. Am. J. Bot. 1992, 79, 434–442. [Google Scholar] [CrossRef]
- Gallagher, K.M.; Campbell, D.R. Shifts in water availability mediate plant-pollinator interactions. New Phytol. 2017, 215, 792–802. [Google Scholar] [CrossRef] [PubMed]
- Solís-Montero, L.; Vallejo-Marín, M. Does the morphological fit between flowers and pollinators affect pollen deposition? An experimental test in a buzz-pollinated species with anther dimorphism. Ecol. Evol. 2017, 7, 2706–2715. [Google Scholar] [CrossRef] [PubMed]
- Nzaramba, M.N.; Hale, A.L.; Scheuring, D.C.; Miller, J.C., Jr. Inheritance of antioxidant activity and its association with seedcoat color in Cowpea. J. Amer. Soc. Sci. 2005, 130, 386–391. [Google Scholar] [CrossRef]
- Xu, P.; Hu, T.; Yang, Y.; Wu, X.; Wang, B.; Liu, Y.; Qin, D.; Ehlers, J.; Close, T.; Lu, Z.; et al. Mapping Genes Governing Flower and Seedcoat Color in Asparagus Bean (Vigna unguiculata ssp. sesquipedalis) Based on Single Nucleotide Polymorphism and Simple Sequence Repeat Markers. HortSci 2011, 46, 1102–1104. [Google Scholar] [CrossRef]
- Amusa, O.D.; Ogunkanmi, A.L.; Adetumbi, J.A.; Akinyosoye, S.T.; Ogundipe, O.T. Morpho-genetic variability in F2 progeny cowpea genotypes tolerant to bruchid (Callosobruchus maculatus). J. Agric. Sci. 2015, 64, 53–68. [Google Scholar] [CrossRef]
- Ajayi, A.T.; Gbadamosi, A.E.; Olotuah, O.F.; David, E.A. Crossability and inheritance of seed coat colour in cowpea at F1 generation. Front. Life Sci. RT. 2020, 1, 58–62. [Google Scholar]
- Nwofia, G.E. Inheritance of Leaf Shape, Pod Shape, Pod Colour and Seed Coat Colour in Cowpea (Vigna unguiculata L. Walp). World J. Agric. Sci. 2014, 10, 178–184. [Google Scholar]
- Herniter, I.A.; Lo, R.; Muñoz-Amatriaín, M.; Lo, S.; Guo, Y.-N.; Huynh, B.-L.; Lucas, M.; Jia, Z.; Roberts, P.A.; Lonardi, S.; et al. Seed Coat Pattern QTL and Development in Cowpea (Vigna unguiculata [L.] Walp.). Front. Plant Sci. 2019, 10, 1346. [Google Scholar] [CrossRef]
- Xiong, H.; Shi, A.; Mou, B.; Qin, J.; Motes, D.; Lu, W.; Ma, J.; Weng, Y.; Yang, W.; Wu, D. Genetic Diversity and Population Structure of Cowpea (Vigna unguiculata L. Walp). PLoS ONE 2016, 11, e0160941. [Google Scholar] [CrossRef] [PubMed]
- Sita, K.; Sehgal, A.; Kumar, J.; Kumar, S.; Singh, S.; Siddique, K.H.; Nayyar, H. Identification of high-temperature tolerant lentil (Lens culinaris Medik.) genotypes through leaf and pollen traits. Front. Plant Sci. 2017, 8, 744. [Google Scholar] [CrossRef] [PubMed]
- Bishop, J.; Jones, H.E.; Lukac, M.; Potts, S.G. Insect pollination reduces yield loss following heat stress in faba bean (Vicia faba L.). Agric. Ecosyst. Environ. 2016, 220, 89–96. [Google Scholar] [CrossRef]
- Gasim, S.M.; Abdelmula, A.A. Impact of Bee Pollination on Yield of Faba Bean (Vicia faba L.) Grown under Semi-Arid Conditions. Agric. Sci. 2018, 9, 729–740. [Google Scholar] [CrossRef]
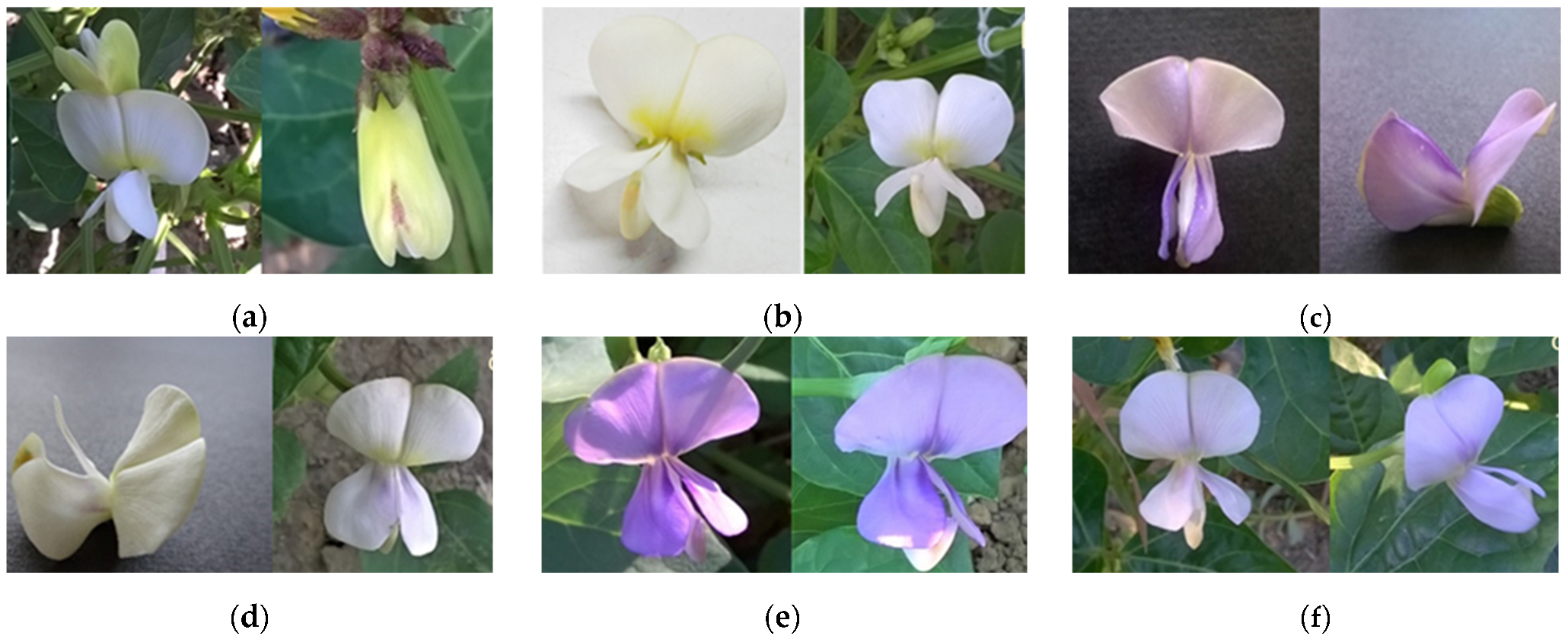




| Accession | Breeding Status | Country of Origin | Flower Color | Raceme Position | Seed Coat Color and Eye Color |
|---|---|---|---|---|---|
| IT97K-499-35 | Breeding line | Nigeria | White with purple pigmentation and a red tip on the back of the standard petal | Mostly above the canopy | White with black eye |
| VG2 | Landrace | Greece | White | On the upper canopy surface | White/cream |
| VG21 | Landrace | Greece | Violet | Mostly above the canopy | Brown |
| Vg60 | Landrace | Portugal | White with purple pigmentation | Mostly above the canopy | Cream with brown eye |
| BGE038478 | Landrace | Spain | Violet | Throughout canopy | Brown/beige |
| BGE038479 | Landrace | Spain | Light Violet | Throughout canopy | Brown/red |
| Accessions | Days to Flowering from Sowing (DFL) | Days to the Last Flower Open (DLFL) | Duration of Flowering (FDUR) |
|---|---|---|---|
| IT97K-499-35 | 74.94 ± 2.29 b | 104.62 ± 2.29 a | 29.68 ± 1.62 a |
| CV% | 17.81 | 12.74 | 31.91 |
| VG2 | 55.64 ± 0.72 c | 71.18 ± 0.92 c | 15.20 ± 1.16 c |
| CV% | 7.37 | 8.00 | 48.46 |
| VG21 | 57.62 ± 1.09 c | 82.53 ± 1.93 b | 22.35 ± 1.86 b |
| CV% | 11.49 | 14.77 | 52.54 |
| Vg60 | 55.23 ± 1.39 c | 79.00 ± 1.54 b | 22.78 ± 2.16 ab |
| CV% | 15.92 | 12.37 | 57.36 |
| BGE038478 | 85.15 ± 1.34 a | 103.80 ± 1.26 a | 18.65 ± 0.73 bc |
| CV% | 9.99 | 7.69 | 24.90 |
| BGE038479 | 82.80 ± 1.95 a | 103.85 ± 1.68 a | 21.05 ± 1.29 bc |
| CV% | 14.93 | 10.21 | 38.71 |
| Level of significance | *** | *** | *** |
| Accessions | Fresh Pod Weight (g) | Yield (kg ha−1) |
|---|---|---|
| IT97K-499-35 | 3.98 ± 0.16 a | 887.43 ± 82.23 a |
| CV% | 18.12 | 79.99 |
| VG2 | 2.41 ± 0.13 b | 598.76 ± 62.30 b |
| CV% | 24.11 | 65.81 |
| VG21 | 2.50 ± 0.19 b | 560.47 ± 57.94 b |
| CV% | 33.78 | 65.39 |
| Vg60 | 2.19 ± 0.17 b | 552.37 ± 62.98 b |
| CV% | 34.72 | 72.11 |
| BGE038478 | 1.96 ± 0.18 b | 543.24 ± 45.29 b |
| CV% | 40.83 | 52.74 |
| BGE038479 | 2.24 ± 0.13 b | 525.49 ± 53.01 b |
| CV% | 25.28 | 63.79 |
| Level of significance | *** | *** |
| Accessions | Pollinators Abundance | Pollinators Foraging Activity |
|---|---|---|
| IT97K-499-35 | 1.00 ± 0.01 c | 1.00 ± 0.01 b |
| CV% | 10.00 | 10.00 |
| VG2 | 1.63 ± 0.17 ab | 2.13 ± 0.29 a |
| CV% | 50.72 | 67.00 |
| VG21 | 1.70 ± 0.10 a | 2.03 ± 0.20 ab |
| CV% | 34.50 | 55.73 |
| Vg60 | 1.19 ± 0.08 bc | 1.46 ± 0.14 ab |
| CV% | 33.71 | 48.31 |
| BGE038478 | 1.05 ± 0.05 c | 1.20 ± 0.09 b |
| CV% | 21.30 | 34.20 |
| BGE038479 | 1.46 ± 0.12 abc | 2.04 ± 0.23 ab |
| CV% | 40.34 | 54.96 |
| Level of significance | *** | ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazaridi, E.; Suso, M.J.; Ortiz-Sánchez, F.J.; Bebeli, P.J. Investigation of Cowpea (Vigna unguiculata (L.) Walp.)–Insect Pollinator Interactions Aiming to Increase Cowpea Yield and Define New Breeding Tools. Ecologies 2023, 4, 124-140. https://doi.org/10.3390/ecologies4010010
Lazaridi E, Suso MJ, Ortiz-Sánchez FJ, Bebeli PJ. Investigation of Cowpea (Vigna unguiculata (L.) Walp.)–Insect Pollinator Interactions Aiming to Increase Cowpea Yield and Define New Breeding Tools. Ecologies. 2023; 4(1):124-140. https://doi.org/10.3390/ecologies4010010
Chicago/Turabian StyleLazaridi, Efstathia, María J. Suso, F. Javier Ortiz-Sánchez, and Penelope J. Bebeli. 2023. "Investigation of Cowpea (Vigna unguiculata (L.) Walp.)–Insect Pollinator Interactions Aiming to Increase Cowpea Yield and Define New Breeding Tools" Ecologies 4, no. 1: 124-140. https://doi.org/10.3390/ecologies4010010
APA StyleLazaridi, E., Suso, M. J., Ortiz-Sánchez, F. J., & Bebeli, P. J. (2023). Investigation of Cowpea (Vigna unguiculata (L.) Walp.)–Insect Pollinator Interactions Aiming to Increase Cowpea Yield and Define New Breeding Tools. Ecologies, 4(1), 124-140. https://doi.org/10.3390/ecologies4010010







