Susceptibility of Tidal Pool Fish Assemblages to Climate Change
Abstract
1. Introduction
2. Materials and Methods
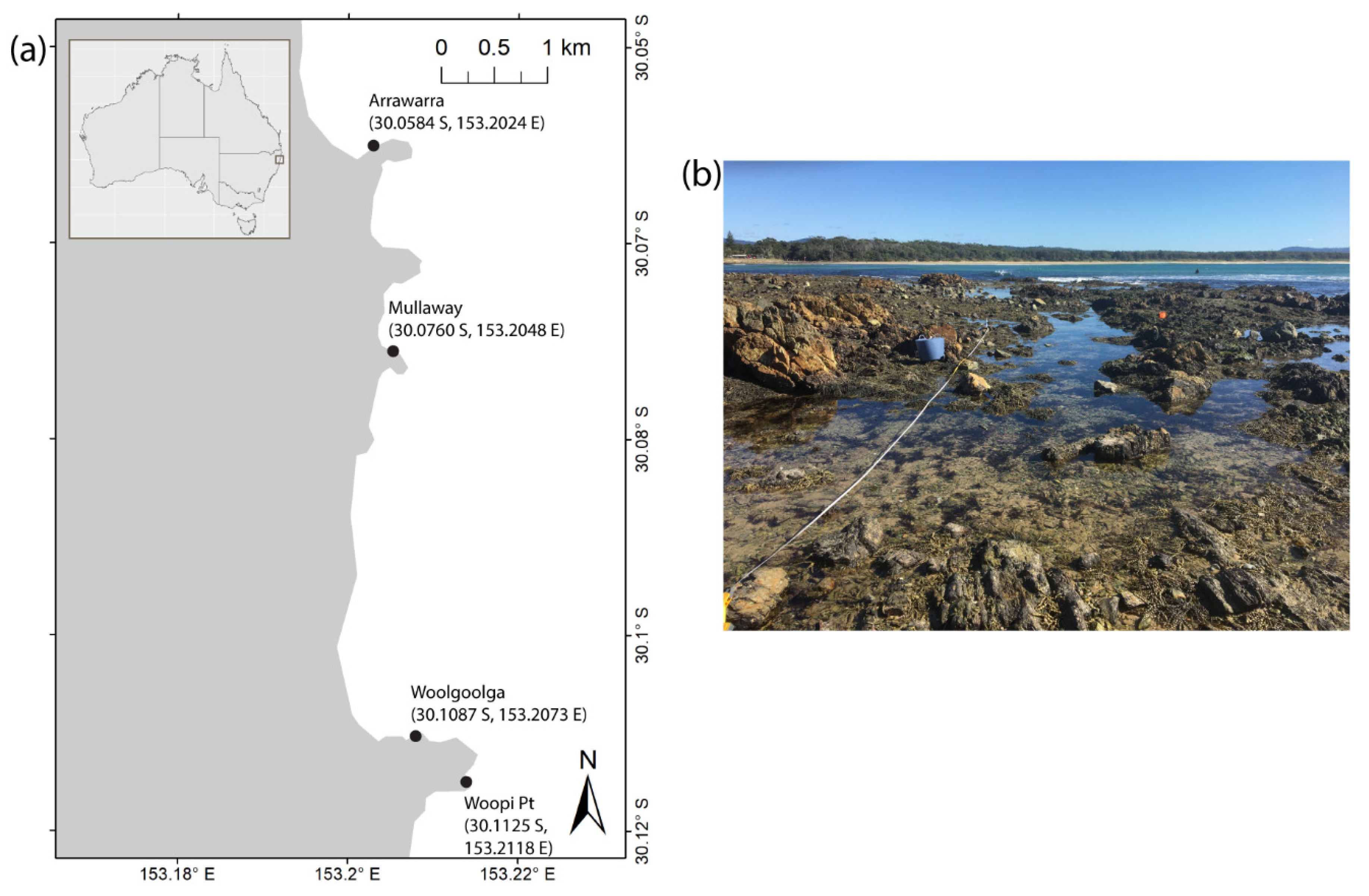
2.1. Study Site
2.2. Sampling Methods
2.3. Statistical Analysis
3. Results
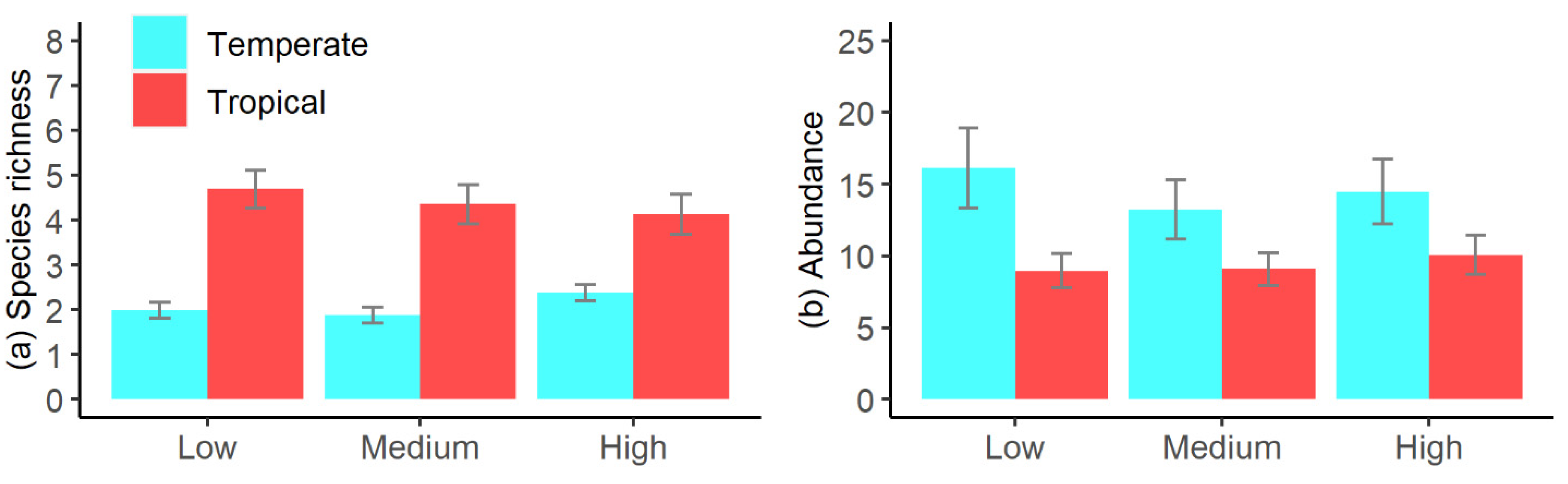
3.1. Influence of Explanatory Variables on Pool Fish Assemblages
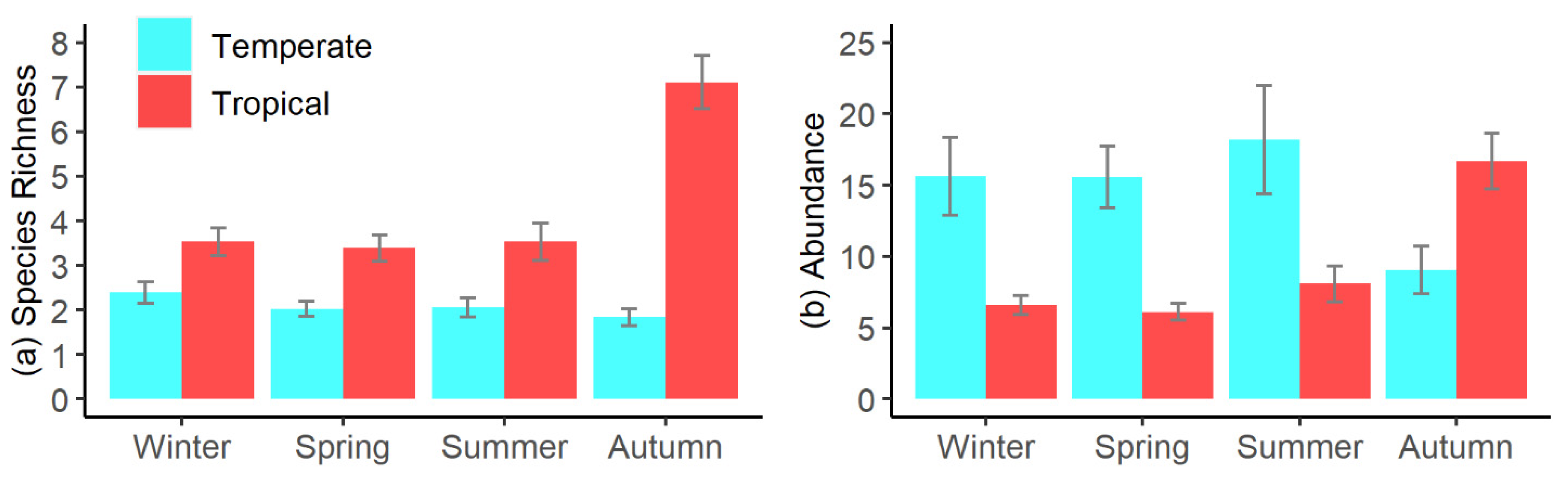
3.2. Seasonal Patterns in Pool Fish Assemblages
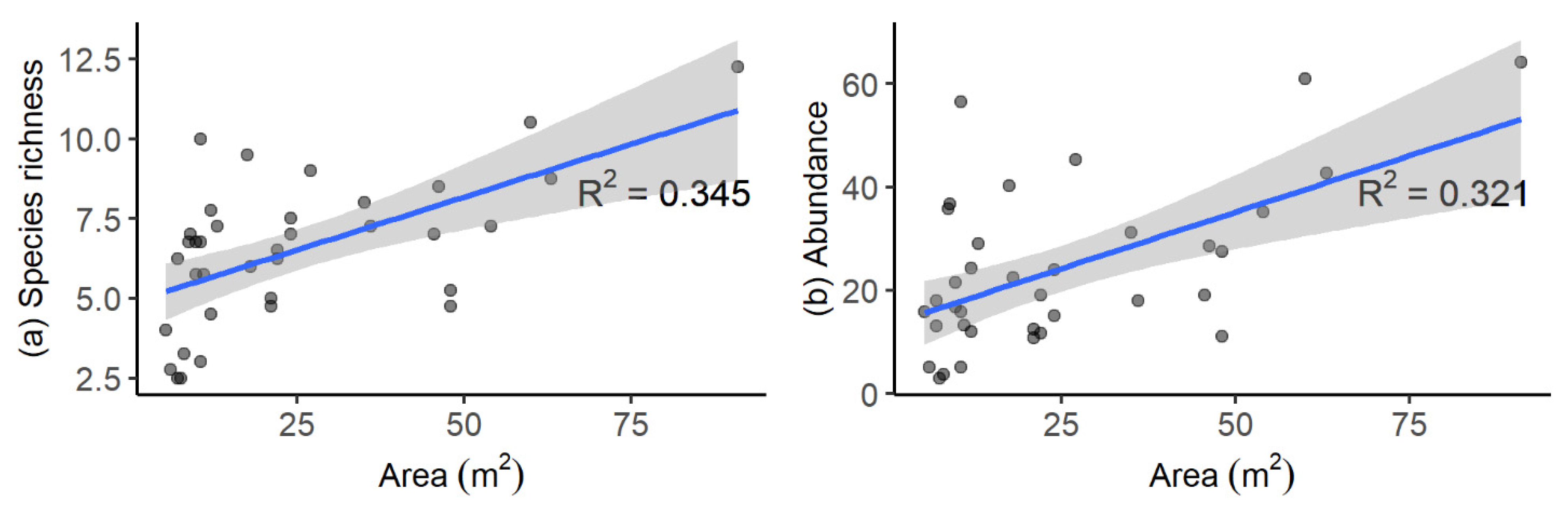
3.3. Influence of Elevation, Pool Area, and Pool Depth
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Helmuth, B.; Mieszkowska, N.; Moore, P.; Hawkins, S.J. Living on the edge of two changing worlds: Forecasting the responses of rocky intertidal ecosystems to climate change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 373–404. [Google Scholar] [CrossRef]
- Pitt, N.R.; Poloczanska, E.S.; Hobday, A.J. Climate-driven range changes in Tasmanian intertidal fauna. Mar. Freshw. Res. 2010, 61, 963–970. [Google Scholar] [CrossRef]
- Kaplanis, N.J.; Edwards, C.B.; Eynaud, Y.; Smith, J.E. Future sea-level rise drives rocky intertidal habitat loss and benthic community change. PeerJ 2020, 8, e9186. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, N.; Mayer-Pinto, M.; Griffin, K.J.; Johnston, E.L.; Glamore, W.; Dafforn, K.A. Predicting the impact of sea-level rise on intertidal rocky shores with remote sensing. J. Environ. Manag. 2020, 261, 110203. [Google Scholar] [CrossRef]
- Jackson, A.C.; McIlvenny, J. Coastal squeeze on rocky shores in northern Scotland and some possible ecological impacts. J. Exp. Mar. Biol. Ecol. 2011, 400, 314–321. [Google Scholar] [CrossRef]
- Thorner, J.; Kumar, L.; Smith, S.D.A. Impacts of climate-change-driven sea level rise on intertidal rocky reef habitats will be variable and site specific. PLoS ONE 2014, 9, e86130. [Google Scholar] [CrossRef]
- Underwood, A.J. Structure of a rocky intertidal community in New South Wales: Patterns of vertical distribution and seasonal changes. J. Exp. Mar. Biol. Ecol. 1981, 51, 57–85. [Google Scholar] [CrossRef]
- Chappuis, E.; Terradas, M.; Cefalì, M.E.; Mariani, S.; Ballesteros, E. Vertical zonation is the main distribution pattern of littoral assemblages on rocky shores at a regional scale. Estuar. Coast. Shelf Sci. 2014, 147, 113–122. [Google Scholar] [CrossRef]
- Davis, T.R.; Larkin, M.F.; Harasti, D. Application of non-destructive methods for assessing rock pool fish assemblages on Lord Howe Island, Australia. Reg. Stud. Mar. Sci. 2018, 24, 251–259. [Google Scholar] [CrossRef]
- Harasti, D.; McLuckie, C.; Gallen, C.; Malcolm, H.; Moltschaniwskyj, N. Assessment of rock pool fish assemblages along a latitudinal gradient. Mar. Biodivers. 2018, 48, 1147–1158. [Google Scholar] [CrossRef]
- Smith, S.D.A. Rapid assessment of invertebrate biodiversity on rocky shores: Where there’s a whelk there’s a way. Biodivers. Conserv. 2005, 14, 3565–3576. [Google Scholar] [CrossRef]
- Compaire, J.C.; Montes, J.; Gonçalves, J.M.S.; Soriguer, M.C.; Erzini, K. Site fidelity of fish on a rocky intertidal in the south of Portugal. J. Sea Res. 2022, 183, 102202. [Google Scholar] [CrossRef]
- Dias, M.; Roma, J.; Fonseca, C.; Pinto, M.; Cabral, H.N.; Silva, A.; Vinagre, C. Intertidal pools as alternative nursery habitats for coastal fishes. Mar. Biol. Res. 2016, 12, 331–344. [Google Scholar] [CrossRef]
- Mendonca, V.; Flores, A.A.V.; Silva, A.C.F.; Vinagre, C. Do marine fish juveniles use intertidal tide pools as feeding grounds? Estuar. Coast. Shelf Sci. 2019, 225, 106255. [Google Scholar] [CrossRef]
- Harasti, D.; Gallen, C.; Malcolm, H.; Tegart, P.; Hughes, B. Where are the little ones: Distribution and abundance of the threatened serranid Epinephelus daemelii (Günther, 1876) in intertidal habitats in New South Wales, Australia. J. Appl. Ichthyol. 2014, 30, 1007–1015. [Google Scholar] [CrossRef]
- Davis, J.L.D. Changes in a tidepool fish assemblage on two scales of environmental variation: Seasonal and El Nino Southern Oscillation. Limnol. Oceanogr. 2000, 45, 1368–1379. [Google Scholar] [CrossRef]
- Barreiros, J.P.; Bertoncini, Á.; Machado, L.; Hostim-Silva, M.; Santos, R.S. Diversity and seasonal changes in the ichthyofauna of rocky tidal pools from Praia Vermelha and São Roque, Santa Catarina. Braz. Arch. Biol. Technol. 2004, 47, 291–299. [Google Scholar] [CrossRef]
- Andrades, R.; Reis-Filho, J.A.; Macieira, R.M.; Giarrizzo, T.; Joyeux, J.-C. Endemic fish species structuring oceanic intertidal reef assemblages. Sci. Rep. 2018, 8, 10791. [Google Scholar] [CrossRef]
- Griffiths, S.P.; West, R.J.; Davis, A.R. Effects of intertidal elevation on the rockpool ichthyofaunas of temperate Australia. Environ. Biol. Fishes 2003, 68, 197–204. [Google Scholar] [CrossRef]
- Wong, M.Y.L.; Gordon, P.; Paijmans, K.C.; Rees, M.J. Finding rockpool fishes: A quantitative comparison of non-invasive and invasive methods for assessing abundance, species richness and assemblage structure. Environ. Biol. Fishes 2019, 102, 81–94. [Google Scholar] [CrossRef]
- Piggott, C.V.H.; Depczynski, M.; Gagliano, M.; Langlois, T.J. Remote video methods for studying juvenile fish populations in challenging environments. J. Exp. Mar. Biol. Ecol. 2020, 532, 151454. [Google Scholar] [CrossRef]
- Willis, T.J.; Millar, R.B.; Babcock, R.C. Detection of spatial variability in relative density of fishes: Comparison of visual census, angling, baited underwater video. Mar. Ecol. Prog. Ser. 2000, 198, 249–260. Available online: http://www.int-res.com/abstracts/meps/v198/p249-260/ (accessed on 20 July 2022). [CrossRef]
- Whitmarsh, S.K.; Fairweather, P.G.; Huveneers, C. What is Big BRUVver up to? Methods and uses of baited underwater video. Rev. Fish Biol. Fish. 2017, 27, 53–73. [Google Scholar] [CrossRef]
- Anderson, M.J.; Gorley, R.N.; Clarke, K.R. PERMANOVA+ for PRIMER: Guide to Software and Statistical Methods; PRIMER-E Ltd.: Plymouth, UK, 2008. [Google Scholar]
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J.; et al. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Clarke, K.R.; Gorley, R.N. PRIMER v7: User Manual/Tutorial; PRIMER-E Ltd.: Plymouth, UK, 2015. [Google Scholar]
- Beckley, L.E. The fish community of East Cape tidal pools and an assessment of the nursery function of this habitat. Afr. Zool. 1985, 20, 21–27. [Google Scholar] [CrossRef]
- Beckley, L.E. Species composition and recruitment of tidal pool fishes in KwaZulu-Natal, South Africa. Afr. Zool. 2000, 35, 29–34. [Google Scholar] [CrossRef][Green Version]
- Arakaki, S.; Tokeshi, M. Short-term dynamics of tidepool fish community: Diel and seasonal variation. Environ. Biol. Fishes 2006, 76, 221–235. [Google Scholar] [CrossRef]
- Paijmans, K.C.; Wong, M.Y.L. Linking animal contests and community structure using rockpool fishes as a model system. Funct. Ecol. 2017, 31, 1612–1623. [Google Scholar] [CrossRef]
- Vergés, A.; Doropoulos, C.; Malcolm, H.A.; Skye, M.; Garcia-Pizá, M.; Marzinelli, E.M.; Campbell, A.H.; Ballesteros, E.; Hoey, A.S.; Vila-Concejo, A.; et al. Long-term empirical evidence of ocean warming leading to tropicalization of fish communities, increased herbivory, loss of kelp. Proc. Natl. Acad. Sci. USA 2016, 113, 13791–13796. [Google Scholar] [CrossRef]
- Vergés, A.; Steinberg, P.D.; Hay, M.E.; Poore, A.G.B.; Campbell, A.H.; Ballesteros, E.; Heck, K.L.; Booth, D.J.; Coleman, M.A.; Feary, D.A.; et al. The tropicalization of temperate marine ecosystems: Climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B 2014, 281, 20140846. [Google Scholar] [CrossRef]
- Hobday, A.J.; Pecl, G.T. Identification of global marine hotspots: Sentinels for change and vanguards for adaptation action. Rev. Fish Biol. Fish. 2014, 24, 415–425. [Google Scholar] [CrossRef]
- Davis, J.L.D. Spatial and seasonal patterns of habitat partitioning in a guild of southern California tidepool fishes. Mar. Ecol. Prog. Ser. 2000, 196, 253–268. [Google Scholar] [CrossRef][Green Version]
- Cox, T.E.; Baumgartner, E.; Philippoff, J.; Boyle, K.S. Spatial and vertical patterns in the tidepool fish assemblage on the island of Oahu. Environ. Biol. Fishes 2011, 90, 329–342. [Google Scholar] [CrossRef]
- Mahon, R.; Mahon, S.D. Structure and resilience of a tidepool fish assemblage at Barbados. Environ. Biol. Fishes 1994, 41, 171–190. [Google Scholar] [CrossRef]
- White, G.E.; Hose, G.C.; Brown, C. Influence of rock-pool characteristics on the distribution and abundance of inter-tidal fishes. Mar. Ecol. 2015, 36, 1332–1344. [Google Scholar] [CrossRef]
- González-Murcia, S.; Erdmann, S.; Larios, R.A. Is this rock pool suitable habitat? Fish diversity in intertidal rock pools of El Zonte, El Salvador. Rev. Mex. Biodivers. 2020, 91, e913099. [Google Scholar] [CrossRef]


| Site | Arrawarra | Mullaway | Woolgoolga | Woopi Point |
|---|---|---|---|---|
| Area range (m2) | 9.8–91.0 | 7.0–27.0 | 5.3–63.0 | 6.0–48.0 |
| Depth range (m) | 0.20–0.55 | 0.20–0.50 | 0.21–0.69 | 0.22–0.89 |
| Pool substrates | Rock, sand | Rock, pebbles, sand | Rock, pebbles, sand | Rock, pebbles, sand |
| Dominant habitats | Algae, seagrass | Algae | Algae, coral | Algae |
| Variable | S.S. | Pseudo-F | p | Prop. |
|---|---|---|---|---|
| Elevation (m) | 13,455 | 5.469 | 0.001 | 3.71% |
| Ocean temperature (°C) | 33,127 | 14.269 | 0.001 | 9.13% |
| Pool temperature (°C) | 10,444 | 4.209 | 0.001 | 2.88% |
| Pool depth (m) | 12,426 | 5.036 | 0.001 | 3.43% |
| Pool area (m2) | 15,776 | 6.455 | 0.001 | 4.35% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Davis, T.R.; Nimbs, M. Susceptibility of Tidal Pool Fish Assemblages to Climate Change. Ecologies 2022, 3, 510-520. https://doi.org/10.3390/ecologies3040037
Davis TR, Nimbs M. Susceptibility of Tidal Pool Fish Assemblages to Climate Change. Ecologies. 2022; 3(4):510-520. https://doi.org/10.3390/ecologies3040037
Chicago/Turabian StyleDavis, Tom R., and Matt Nimbs. 2022. "Susceptibility of Tidal Pool Fish Assemblages to Climate Change" Ecologies 3, no. 4: 510-520. https://doi.org/10.3390/ecologies3040037
APA StyleDavis, T. R., & Nimbs, M. (2022). Susceptibility of Tidal Pool Fish Assemblages to Climate Change. Ecologies, 3(4), 510-520. https://doi.org/10.3390/ecologies3040037






