Climatic and Biological Factors Related with Goat Grazing Management in the Arid Grassland of the Coquimbo Region (Northern Chile)
Abstract
1. Introduction
2. Experimental Section
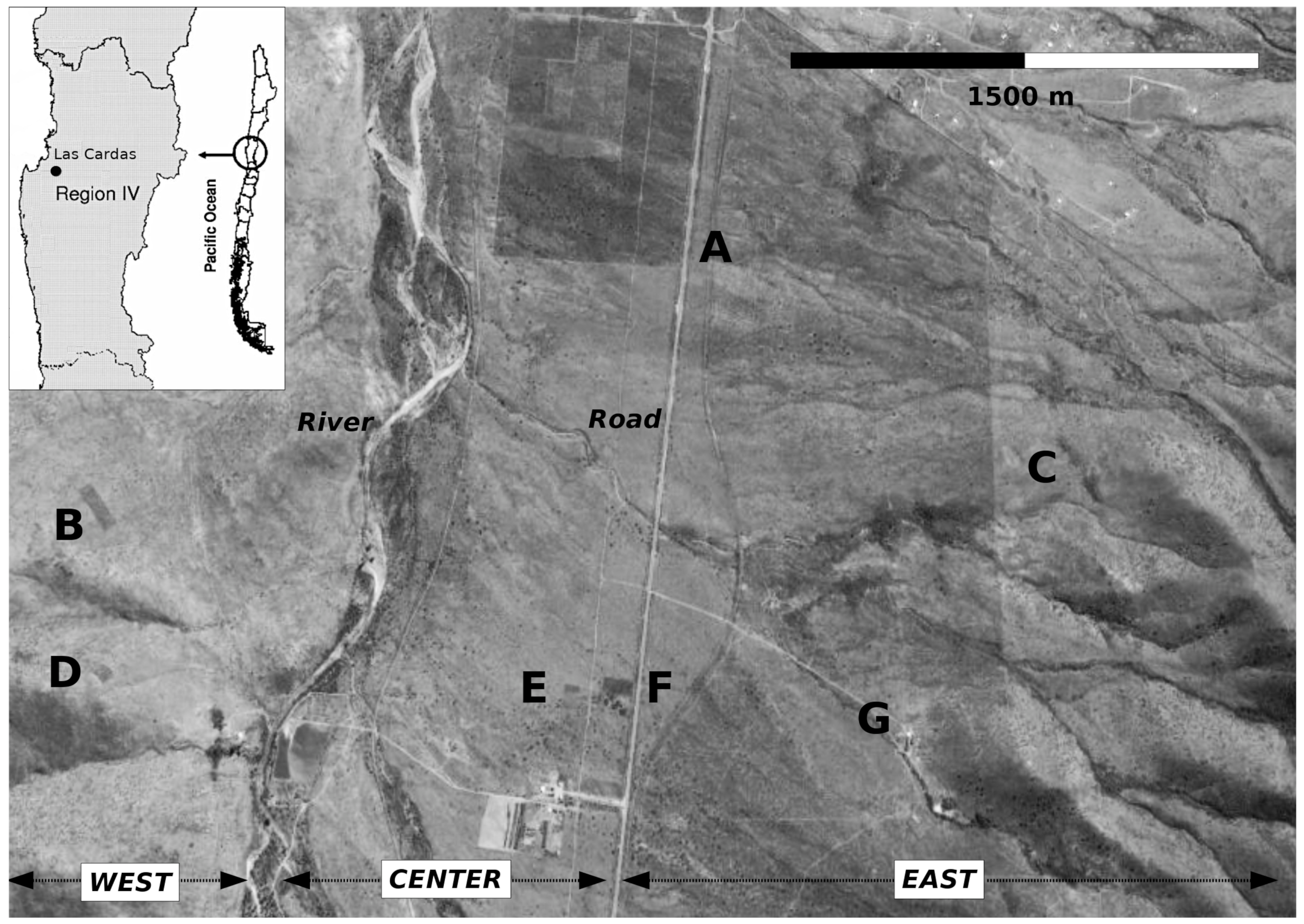
2.1. Sampling Area
2.2. Management Systems
2.3. Sampling Method
2.4. Statistical Analysis
3. Results
3.1. Botanical Composition of Grassland Community
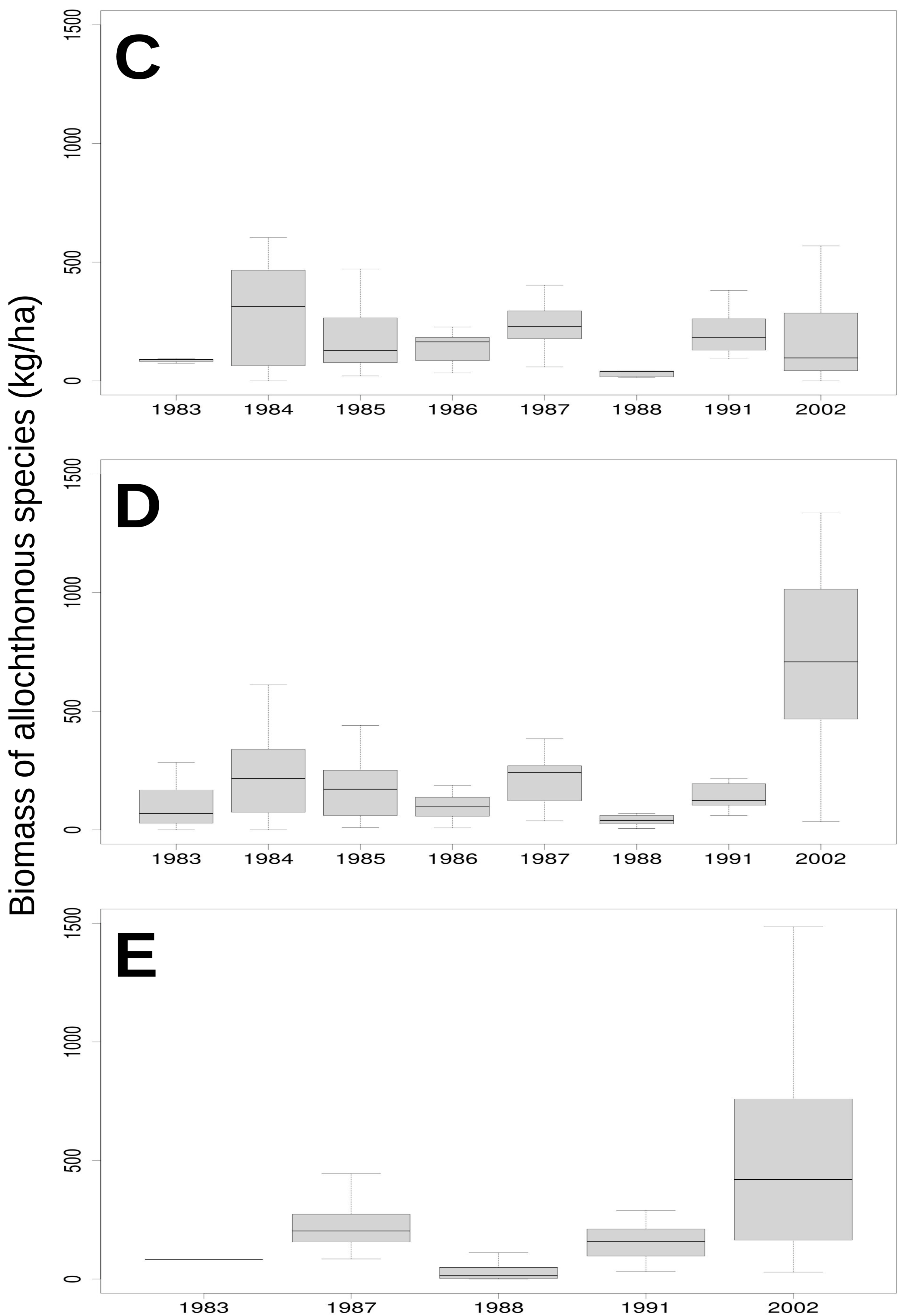
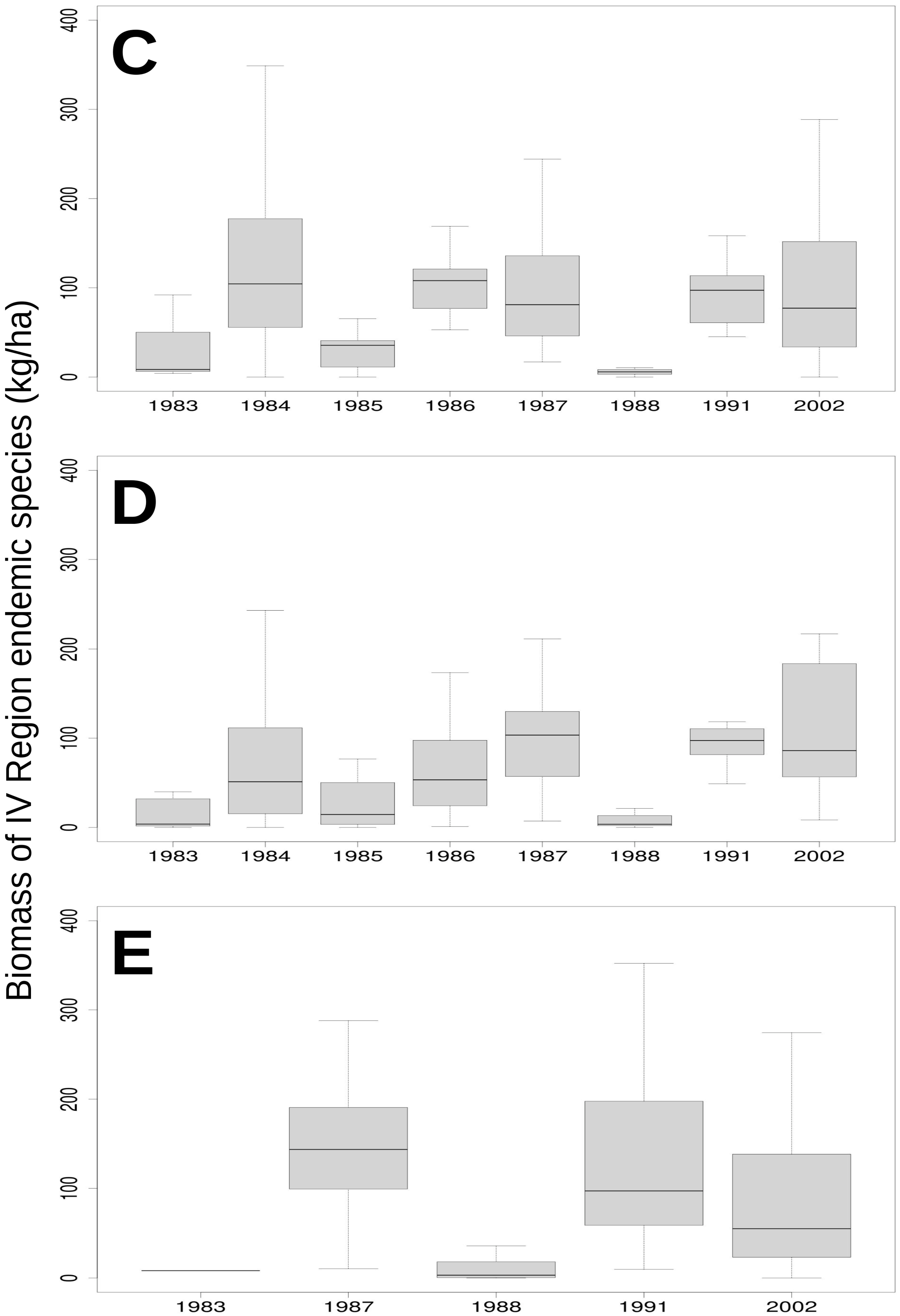
3.2. Relationship of Rainfall, Season and Grazing System with Biomass and Botanical Composition
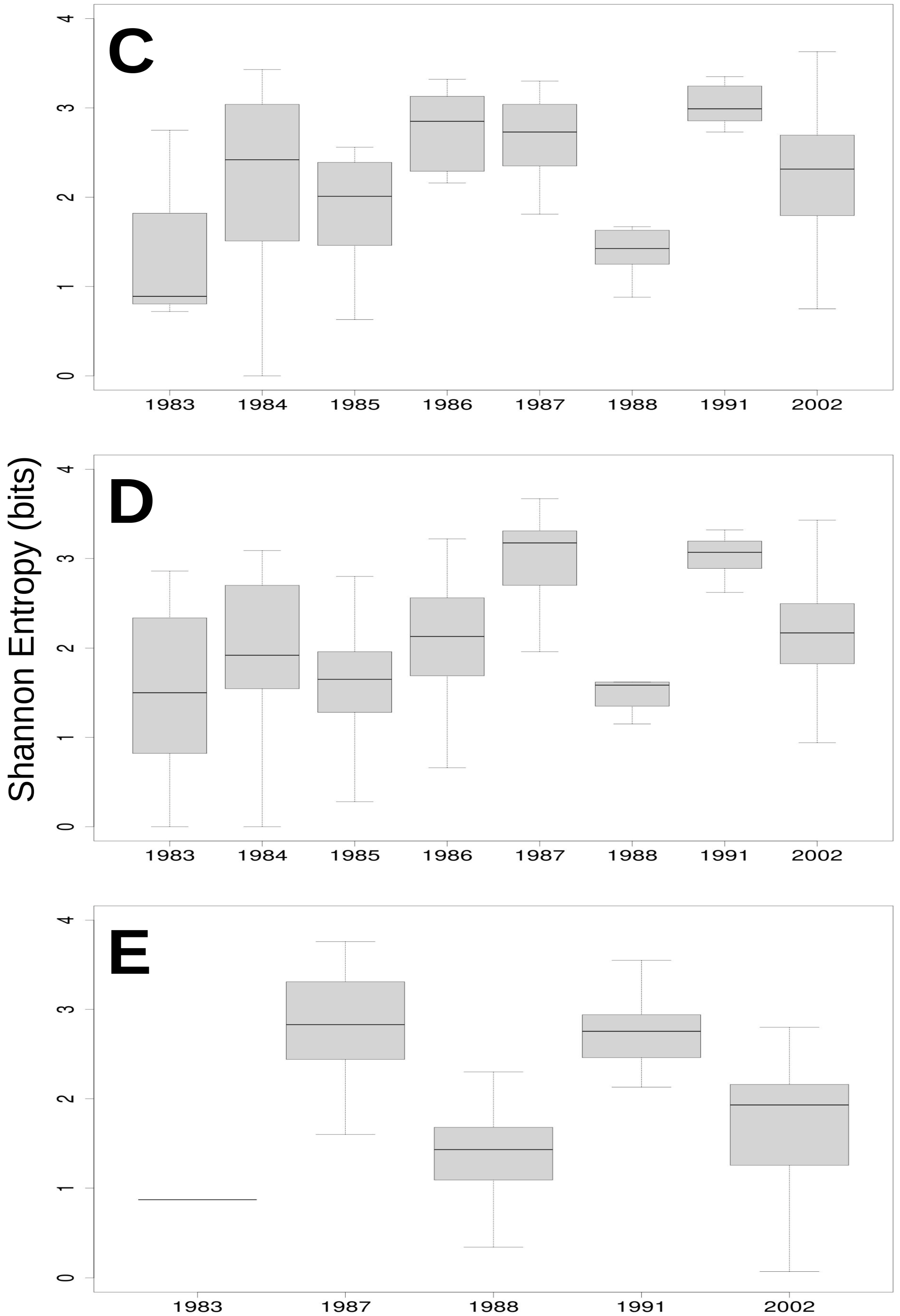
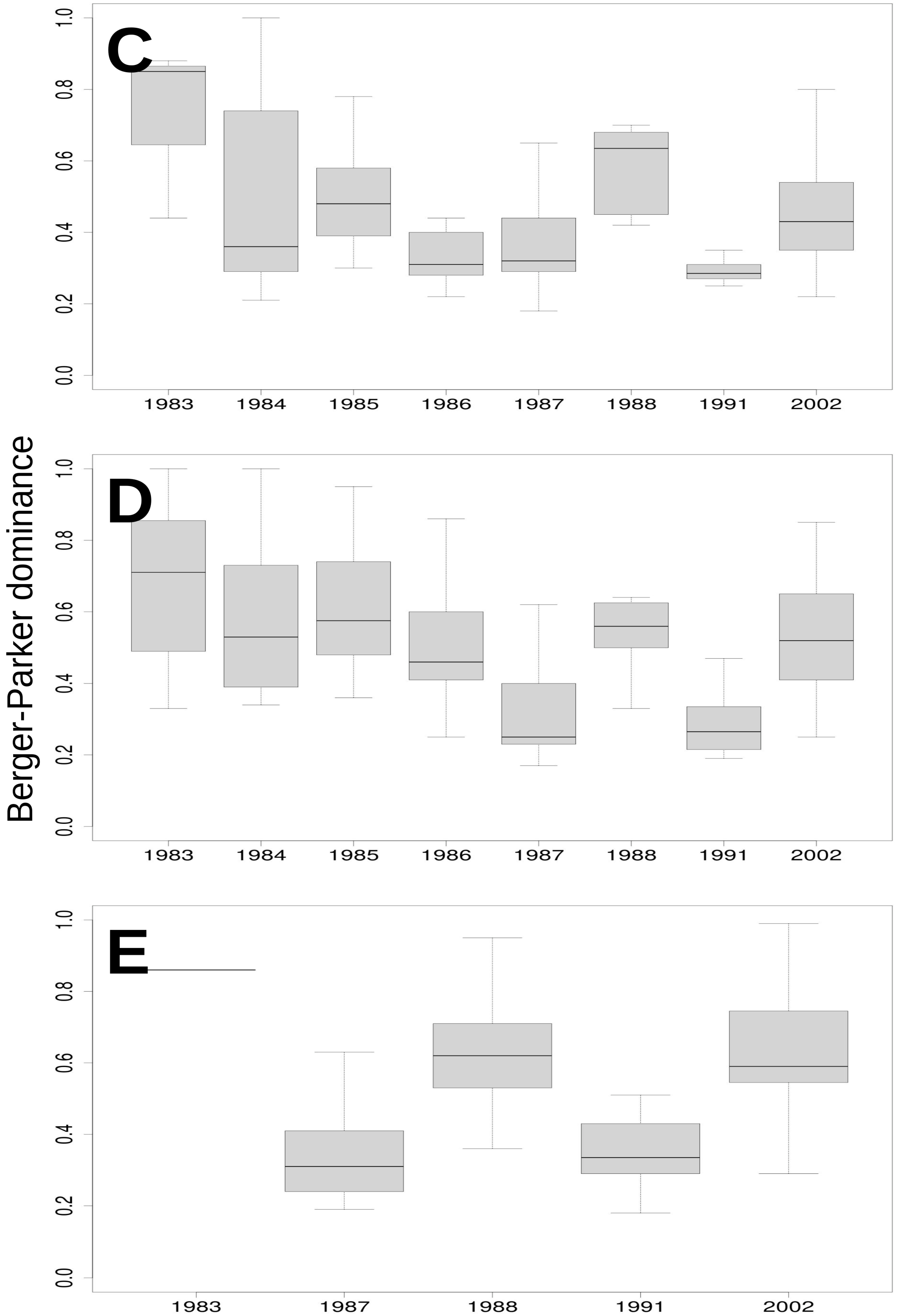
3.3. Relationship of Rainfall, Season and Grazing System with Richness, Dominance and Diversity
3.4. Overall Differences between Grazing Treatments
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Conflicts of Interest
References
- Burrell, A.L.; Evans, J.P.; De Kauwe, M.G. Anthropogenic climate change has driven over 5 million km2 of drylands towards desertification. Nat. Commun. 2020, 11, 3853. [Google Scholar] [CrossRef]
- Akhtar-Schuster, M.; Schmiedel, U.; Jürgens, N. Biodiversity and Desertification. In Global Change: Enough Water for All? Lozán, J.L., Grassl, H., Hupfer, P., Menzel, L., Schönwiese, C.D., Eds.; Wissenschaftliche Auswertungen: Hamburg, Germany, 2007; pp. 224–226. [Google Scholar]
- Zhang, R.; Jinsong, W.; Shuli, N. Toward a sustainable grazing management based on biodiversity and ecosystem multifunctionality in drylands. Curr. Opin. Environ. Sustain. 2021, 48, 36–43. [Google Scholar] [CrossRef]
- López, B.C.; Rodríguez, R.; Gracia, C.A.; Sabaté, S. Climatic signals in growth and its relation to ENSO events of two Prosopis species following a latitudinal gradient in South America. Glob. Chang. Biol. 2006, 12, 897–906. [Google Scholar] [CrossRef]
- Müller, F.L.; Samuels, M.I.; Cupido, C.F.; Swarts, M.B.V.; Hattas, D.; Morris, C.; Cyster, L.F.; Boatwright, J.S.; Amary, N.M. The impacts of season and livestock management strategy on the quality of diets selected by goats and sheep in the semi-arid rangelands of Namaqualand, South Africa. Afr. J. Range Forage Sci. 2019, 36, 1–10. [Google Scholar] [CrossRef]
- El Aich, A.; Waterhouse, A. Small ruminants in environmental conservation. Small Rumin. Res. 1999, 34, 271–287. [Google Scholar] [CrossRef]
- Azócar, P.; Patón, D. Influencia del sistema de pastoreo y de la variabilidad interanual sobre la biodiversidad del pastizal mediterráneo árido de la IV Región de Chile. In Proceedings of the 49th Symposium of the Agronomic Society of Chile (SACH), Santiago, Chile, 30 November–3 December 1998. [Google Scholar]
- Bahre, C.J. Destruction of the Natural Vegetation of North-Central Chile; University of California Publications in Geography: Berkeley, CA, USA, 1979; Volume 23, pp. 1–118. [Google Scholar]
- Salinas, C.X.; Mendieta, J. Mitigation and adaptation investments for desertification and climate change: An assessment of the socioeconomic return. Mitig. Adapt. Strateg. Glob. Chang. 2013, 18, 659–672. [Google Scholar] [CrossRef]
- Azócar, P.; Lailhacar, S. Bases ecológicas para el desarrollo de la zona de clima mediterráneo árido de Chile. Terra Arida 1990, 8, 221–301. [Google Scholar]
- Kiringe, J.; Okello, M.M. Degradation of Rangelands: Causes, Ecological, Wildlife Conservation and Socio-Economic Implications; Nova Science Publishers: New York, NY, USA, 2010. [Google Scholar]
- Bedunah, D.J.; Angerer, J.P. Rangeland degradation, poverty, and conflict: How can rangeland scientists contribute to effective responses and solutions? Rangel. Ecol. Manag. 2012, 65, 606–612. [Google Scholar] [CrossRef]
- Thokchom, B.; Qiu, P.; Singh, P.; Iyer, P. Water Conservation in the Era of Global Climate Change; Elsevier: New Delhi, India, 2021. [Google Scholar]
- O’Connor, D.; Ford, D. Increasing the effectiveness of the “Great Green Wall” as an adaptation to the effects of climate change and desertification in the Sahel. Sustainability 2014, 6, 7142–7154. [Google Scholar] [CrossRef]
- Squeo, F.A.; Aravena, R.; Aguirre, E.; Pollastri, A.; Jorquera, C.B.; Ehleringer, J.R. Groundwater dynamics in a coastal aquifer in north-central Chile: Implications for groundwater recharge in an arid ecosystem. J. Arid Environ. 2006, 67, 240–254. [Google Scholar] [CrossRef]
- Holmgren, M.; López, B.C.; Gutiérrez, J.R.; Squeo, F.A. Herbivory and plant growth rate determine the success of El Niño Southern Oscillation-driven tree establishment in semiarid South America. Glob. Chang. Biol. 2006, 12, 2263–2271. [Google Scholar] [CrossRef]
- Torres, R.; Squeo, F.A.; Jorquera, C.; Aguirre, E.; Ehleringer, J.R. Evaluación de la capacidad estacional de utilizar eventos de precipitación en tres especies de arbustos nativos de Chile con distintos sistemas radiculares. Rev. Chil. De Hist. Nat. 2002, 75, 737–749. [Google Scholar] [CrossRef][Green Version]
- Contreras, D.; Azocar, P.; Covarrabias, G.; Soto, G. Use of Forage Shrubs in the Arid-Land of Chile; General Technical Report INT-172; US Department of Agriculture, Forest Service: Washington, DC, USA, 1984; pp. 237–242. [Google Scholar]
- Tracol, Y.; Gutiérrez, J.R.; Squeo, F.A. Plant Area Index and microclimate underneath shrub species from a Chilean semiarid community. J. Arid Environ. 2011, 75, 1–6. [Google Scholar] [CrossRef]
- Thibault, K.M.; Ernest, S.K.M.; White, E.P.; Nrown, J.H.; Goheen, J.R. Long-term insights into the influence of precipitation on community dynamics in desert rodents. J. Mammal. 2010, 91 Pt 4, 787–797. [Google Scholar] [CrossRef]
- Dotterweich, M. The history of human-induced soil erosion: Geomorphic legacies, early descriptions and research, and the development of soil conservation. A global synopsis. Geomorphology 2013, 201, 1–34. [Google Scholar] [CrossRef]
- Azócar, P. Hábitos de pastoreo y de consumo de especies forrajeras del ganado caprino en zonas áridas. Av. Prod. Anim. 1987, 12, 3–9. [Google Scholar]
- Azócar, P.; D‒Herbes, J.M.; Díaz, J. Estudio de sistemas de pastoreo con caprinos para el secano árido de la IV Región de Coquimbo. 1: Dieta e índices de aceptabilidad relativa de arbustos. Av. Prod. Anim. 1987, 12, 35–47. [Google Scholar]
- Shackleton, C.M. Comparison of plant diversity in protected and communal lands in the Bushbuckridge low veld savanna, South Africa. Biol. Conserv. 2000, 94, 273–285. [Google Scholar] [CrossRef]
- Stafford-Smith, D.M.; Campbell, B.D.; Archer, S.; Steffen, W. Pasture and Rangeland Network: An Implementation Plan. In Global Change and Terrestrial Ecosystems; Report no 3; CSRIO, Division of Wildlife and Ecology: Canberra, Australia, 1995. [Google Scholar]
- Holecheck, J.L.; Pieper, R.D.; Herbel, C.H. Range Management. Principles and Practices; Pearson: New York, NY, USA, 2011. [Google Scholar]
- Al-Rowaily, S.L.; El-Bana, M.I.; Al-Bakre, D.A.; Assaeed, A.M.; Hegazy, A.K.; Ali, M.A. Effects of open grazing and livestock exclusion on floristic composition and diversity in natural ecosystem of Western Saudi Arabia. Saudi J. Biol. Sci. 2015, 22, 430–437. [Google Scholar] [CrossRef]
- Fabio, G.; Merlo, M.; Tosi, V. Silvicultural management in maintaining biodiversity and resistance of forests in Europe-the Mediterranean Region. J. Environ. Manag. 2003, 67, 67–76. [Google Scholar] [CrossRef]
- Peacock, J.M.; Ferguson, M.E.; Alhadrami, G.A.; McCann, I.R.; Al Hajoj, A.; Saleh, A.; Karnik, R. Conservation through utilization: A case study of the indigenous forage grasses of the Arabian Peninsula. J. Arid Environ. 2003, 54, 15–28. [Google Scholar] [CrossRef]
- Polley, H.W.; Johson, H.B.; Tischler, C.R. Woody invasion of grasslands: Evidence that CO2 enrichment indirectly promotes establishment of Prosopis Glandulosa. Plant. Ecol. 2003, 164, 85–94. [Google Scholar] [CrossRef]
- Na, Y.; Li, J.; Hoshino, B.; Bao, S.; Qin, F.; Myagmartseren, P. Effects of Different Grazing Systems on Aboveground Biomass and Plant Species Dominance in Typical Chinese and Mongolian Steppes. Sustainability 2018, 10, 4753. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, X.; Chen, Y.; Luo, Y.; Wang, S. Effects of grazing exclusion on carbon sequestration and the associated vegetation and soil characteristics at a semi-arid desertified sandy site in Inner Mongolia, Northern China. Can. J. Soil Sci. 2012, 92, 807–819. [Google Scholar] [CrossRef]
- Zeng, H.; Wu, B.; Zhang, M.; Zhang, N.; Elnashar, A.; Zhu, L.; Zhu, W.; Wu, F.; Yan, N.; Liu, W. Dryland ecosystem dynamic change and its drivers in Mediterranean region. Curr. Opin. Environ. Sustain. 2021, 48, 59–67. [Google Scholar] [CrossRef]
- Ormazábal, C.S. Silvopastoral systems in arid and semiarid zones of Northern Chile. Agrofor. Syst. 1991, 14 Pt 3, 207–217. [Google Scholar] [CrossRef]
- Fuentes, N.; Pauchard, A.; Sánchez, P.; Esquivel, J.; Marticorena, A. A new comprehensive database of alien plant species in Chile based on herbarium records. Biol. Invasions 2013, 15, 847–858. [Google Scholar] [CrossRef]
- Collier, S.; Sater, W.F. A History of Chile 1802–2002; Cambridge University Press: Cambridge, UK, 2004; 454p. [Google Scholar]
- Squeo, F.A.; Loayza, A.P.; López, R.P.; Gutiérrez, J.A. Vegetation of Bosque Fray Jorge National Park and its surrounding matrix in the Coastal Desert of north-central Chile. J. Arid Environ. 2016, 126, 12–22. [Google Scholar] [CrossRef]
- Menestrey-Schwieger, D.A.; Mbidzo, M. Socio-historical and structural factors linked to land degradation and desertification in Namibia‒s former Herero ‘homelands’. J. Arid Environ. 2020, 178, 104151. [Google Scholar] [CrossRef]
- Montecinos, S.; Gutiérrez, J.R.; López-Cortés, F.; López, D. Climatic characteristics of the semi-arid Coquimbo Region in Chile. J. Arid Environ. 2016, 126, 7–11. [Google Scholar] [CrossRef]
- Verbist, K.; Robertson, A.W.; Cornelis, W.M.; Gabriels, D. Seasonal predictability of daily rainfall characteristics in central northern Chile for dry-land management. J. Appl. Meteorol. Climatol. 2010, 49, 1938–1955. [Google Scholar] [CrossRef][Green Version]
- Poveda, G.; Waylen, P.R.; Pulwarty, R.S. Annual and inter-annual variability of the present climate in northern South America and southern Mesoamerica. Paleogeogr. Paleoclimatol. Paleoecol. 2006, 234, 3–27. [Google Scholar] [CrossRef]
- Azócar, P.; Patón, D.; Osorio, R.; Le Floc’h, E. Efecto de la poda sobre la produción de forraje del Rumpiato (Bridgesia incisifolia, Bert ex Cambess). Av. Prod. Anim. 2001, 1–2, 97–106. [Google Scholar]
- Schulz, N.; Aceituno, P.; Richter, M. Phytogeographic divisions, climate change and plant dieback along the coastal desert of Northern Chile. Erdkunde 2011, 65, 169–187. [Google Scholar] [CrossRef]
- Soto, M.V.; Märker, M.; Arriagada, J.; Castro, C.P.; Rodolfi, G. Evaluación de la amenaza natural en ambiente semiárido, sustentada en la geomorfología y el modelamiento de índices topográficos. Salamanca, Región de Coquimbo, Chile. Investig. Geogr. 2010, 42, 19–36. [Google Scholar] [CrossRef][Green Version]
- Rojo, H. Efecto del Sistema y de la Época de Pastoreo sobre la Disponibilidad de Forraje del Incienso (Flourensia thurifera (Mol.) DC.); Memoria de Título Profesional de Ingeniero Agrónomo; Facultad de Ciencias Agrarias y Forestales, Universidad de Chile: Santiago, Chile, 1989. [Google Scholar]
- Díaz, J. Recursos Forrajeros y Comportamiento del Ganado Caprino en dos Localidades del Secano Mediterráneo Árido; Memoria de Título Profesional de Ingeniero Agrónomo; Facultad de Ciencias Agrarias y Forestales, Universidad de Chile: Santiago, Chile, 1988. [Google Scholar]
- Squeo, F.A.; Martínez, K.; Letelier, L.; Rentería, L.; Figueroa, M. Libro Rojo de la Flora Nativa y de los Sitios Prioritarios para su Conservación, Región de Coquimbo; Ediciones Universidad de La Serena: La Serena, Chile, 2008; 451p. [Google Scholar]
- Rojas, P.; González, M.; Benedetti, S.; Yates, P.; Sotomayor, A.; Dube, F. Silvopastoral Systems in Arid and Semiarid Zones of Chile. In Silvopastoral Systems in Southern South America; Peri, P.L., Dube, F., Varella, A., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 169–181. [Google Scholar]
- Patón, D.; Osorio, R.; Le Floc’ h, P.; Azócar, P.; Portilla, L. Analysis of a multivariate model for detecting goat overbrowsing in the arid zone of Northern Chile. Application to Bridgesia incisifolia (Bert ex Cambess) shrublands. J. Arid Environ. 1999, 43, 197–204. [Google Scholar] [CrossRef]
- Azor, P.J.; Monteagudo, L.V.; Luque, M.; Tejedor, M.T.; Rodero, E.; Sierra, I.; Herrera, M.; Rodero, A.; Arruga, M.V. Phylogenetic relationships between Spanish goat breeds. Anim. Genet. 2005, 36, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Legendre, P.; Legendre, L. Numerical Ecology; Elsevier: Amsterdam, The Netherlands, 2012; 1006p. [Google Scholar]
- Sutherland, W.J. Ecological Census Techniques: A Handbook; Cambridge University Press: Cambridge, UK, 2006; 432p. [Google Scholar]
- Poissonet, P.S.; Daget, P.M.; Poissonet, J.A.; Long, G.A. Rapid point survey by bayonet blade. J. Range Manag. 1972, 25, 313. [Google Scholar] [CrossRef]
- Greig-Smith, P. Quantitative Plant Ecology; Academic Press: New York, NY, USA, 1983; 359p. [Google Scholar]
- Balzter, H.; Braun, P.; Köhler, W. Detection of Spatial Discontinuities in Vegetation Data by a Moving Window Algorithm. In From Data to Knowledge: Theoretical and Practice Aspects of Classification, Data Analysis and Knowledge Organization; Gaul, W., Pfeifer, D., Eds.; Springer: Berlin, Germany, 1995; pp. 243–252. [Google Scholar]
- Buyolo, T.; Patón, D.; Fernández, l.; Cabezas, J. Estimación de la biomasa de pastos mediterráneos mediante modelos de algoritmos genéticos sobre medidas de point-quadrat. In XLIV Reunión Científica de la SEEP. Pastos y Ganadería Extensiva; SEEP: Salamanca, Spain, 2004; pp. 105–109. [Google Scholar]
- Patón, D.; Bermejo, L.; Camacho, A.; De Nascimento, L.; Bethencourt, L.; Mata, J. A biomass prediction model for grasslands of Canary Islands. In Grassland Science in Europe. Sustainable Grassland Productivity; EGF: Badajoz, Spain, 2006; pp. 523–525. [Google Scholar]
- Magurran, A.E. Measuring Biological Diversity; Blackwell Publishing: Oxford, UK, 2013; 266p. [Google Scholar]
- Mac Nally, R. Multiple regression and inference in ecology and conservation biology: Further comments on identifying important predictor variables. Biodivers. Conserv. 2002, 11, 1397–1401. [Google Scholar] [CrossRef]
- Chevan, A.; Sutherland, M. Hierarchical Partitioning. Am. Stat. 1991, 45, 90–96. [Google Scholar]
- Jobson, J.D. Applied Multivariate Data Analysis. Volume I: Regression and Experimental Design; Springer: Berlin, Germany, 1991; 544p. [Google Scholar]
- Hatt, B.E.; Fletcher, T.D.; Walsh, C.J.; Taylor, S.L. The influence of urban density and drainage infrastructure on the concentrations and loads of pollutants in small streams. Environ. Manag. 2004, 34, 112–124. [Google Scholar] [CrossRef]
- Walsh, C.J.; Papas, P.J.; Crowther, D.; Sim, P.T.; Yoo, J. Stormwater drainage pipes as a threat to a stream-dwelling amphipod of conservation significance, Austrogammarus australis, in South-Eastern Australia. Biodivers. Conserv. 2004, 13, 781–793. [Google Scholar] [CrossRef]
- Olea, P.; Mateo-Tomás, P.; De Frutos, A. Estimating and modeling bias of the Hierarchical Partitioning public-domain software: Implications in environmental management and conservation. PLoS ONE 2010, 5, 1–7. [Google Scholar] [CrossRef]
- Mac Nally, R. Regression and model building in conservation biology, biogeography and ecology: The distinction between and reconciliation of predictive and explanatory models. Biodivers. Conserv. 2000, 9, 655–671. [Google Scholar] [CrossRef]
- Oliver, I.; Mac Nally, R.; York, A. Identifying performance indicators of the effects of forest management on ground-active arthropod biodiversity using hierarchical partitioning and partial canonical correspondence analysis. For. Ecol. Manag. 2000, 139, 21–40. [Google Scholar] [CrossRef]
- Logan, M. Biostatistical Design and Analysis Using R; Wiley-Blackwell: Oxford, UK, 2010; 546p. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
- Holzapfel, C.; Tielbörger, K.; Parag, H.A.; Kigel, J.; Sternberg, M. Annual plant-shrub interactions along an aridity gradient. Basic Appl. Ecol. 2006, 7, 268–279. [Google Scholar] [CrossRef]
- Patón, D. Elaboration of a multivariate model for the determination of the metabolizable energy of Mediterranean bushes based on chemical parameters. J. Arid Environ. 2003, 53, 271–280. [Google Scholar] [CrossRef]
- Patón, D.; Salvado, A.; Venegas, F. Relationships between metabolizable energy and chemical parameters from forest fruits using a parallel genetic algorithm worldwide model. In New Research in Forest Ecology; Scaggs, A.K., Ed.; Nova Science Publishers: New York, NY, USA, 2007; pp. 129–140. [Google Scholar]
- Hohnwald, S.; Trautwein, J.; Camarão, A.P.; Wollny, C.B. Relative palatability and growth performance of capoeira species as supplementary forages in the NE-Amazon. Agric. Ecosyst. Environ. 2016, 218, 107–115. [Google Scholar] [CrossRef]
- Montserrat, P.; Fillat, F. The systems of grassland management in Spain. In Managed Grasslands; Breymeyer, A., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 1990; pp. 37–70. [Google Scholar]
- Malo, J.E.; Suarez, F. The dispersal of a dry-fruited shrub by red deer in a Mediterranean ecosystem. Ecography 1998, 21, 204–211. [Google Scholar] [CrossRef]
- Van Rheede, K.; Van Rhooyen, M.W. Dispersal Biology of Desert Plants; Springer: Berlin/Heidelberg, Germany, 1999. [Google Scholar]
- Squeo, F.A.; Olivares, N.; Olivares, S.; Pollastri, A.; Aguirre, E.; Aravena, R.; Jorquera, C.; Ehleringer, J.R. Functional groups in North Chilean desert shrubs species based on the water sources used. Gayana Bot. 1999, 56, 1–15. [Google Scholar]
- De Soyza, A.G.; Van Zee, J.W.; Whitford, W.G.; Neale, A.; Tallent-Hallsel, N.; Herrick, J.E.; Havstad, K.M. Indicators of Great Basin rangeland health. J. Arid Environ. 2000, 45, 289–304. [Google Scholar] [CrossRef]
- Tilman, D.; Wedin, D.; Knops, J. Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 1996, 379, 718–720. [Google Scholar] [CrossRef]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef]
- Tilman, D.; Reich, P.B.; Knops, J.M.H. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature 2006, 441, 629–631. [Google Scholar] [CrossRef] [PubMed]
- Cepeda, J. Geoecología de los Andes Desérticos. La Alta Montaña del Valle de Elqui; Ediciones Universidad de la Serena: La Serena, Chile, 2006; 551p. [Google Scholar]
- Pucheta, E.; Vendramini, F.; Cabido, M.; Díaz, S. Estructura y funcionamiento de un pastizal de montaña bajo pastoreo y su respuesta luego de la exclusión. Rev. Fac. Agron. La Plata 1998, 103, 77–92. [Google Scholar]
- Costello, D.A.; Lunt, I.A.; Williams, J.E. Effects on invasion by the indigenous shrub Acacia sophorae on plant composition on coastal grasslands in south-eastern Australia. Biol. Conserv. 2000, 96, 113–121. [Google Scholar] [CrossRef]
- Homewood, K.M. Policy, environment and development in Africa rangelands. Environ. Sci. Policy 2004, 7, 125–143. [Google Scholar] [CrossRef]
- Allen-Diaz, B.; Jackson, R.D. Grazing effects on spring ecosystem vegetation of California‒s hardwood rangelands. J. Range Manag. 2000, 53, 215–220. [Google Scholar] [CrossRef]
- Patón, D.; Muñoz, A.; Tovar, J. Impacto medioambiental del vacuno Retinto sobre pastizales mediterráneos. In Proceedings of the I Congreso Internacional de Veterinaria y Medioambiente, Cáceres, Spain, 24–26 April 1997; pp. 482–489. [Google Scholar]
- Patón, D.; Muriel, J.; Nuñez, J. Determination of population diversity using Von Bertalanffy‒s regression model. Application to cumulative sampling sizes of grazing grasslands by Retinto cattle in Extremadura region (SW Spain). In Proceedings of the First International Conference on Applied Science and the Environment (ASE 98), Cádiz, Spain, 5–8 October 1998; pp. 23–24. [Google Scholar]
- Patón, D.; Muriel, J.; Tovar, J. Cambios en la vegetación después de la exclusión de áreas pastoreadas por Retinto en Extremadura. In Actas del Congreso Europeo de Agricultura Sostenible en Ambientes Mediterráneos, Proceedings of the Congreso Europeo de Agricultura Sostenible en Ambientes Mediterráneos, Badajoz-Mérida, Spain, 22–25 March 1999; Consejería de Agricultura y Comercio, Junta de Extremadura: Merida, Spain, 1999; pp. 152–156. [Google Scholar]
- Volesky, J.D.; De Achaval, F.; Ellis, W.C.; Kothmann, M.M.; Horn, F.P.; Phillips, W.A.; Coleman, S.W. A comparison of frontal, continuous and rotational grazing systems. J. Range Manag. 1994, 47, 210–214. [Google Scholar] [CrossRef][Green Version]
- Duru, M.; Hubert, B. Management of grazing systems: From decision and biophysical models to principles for action. In Sustainable Agriculture; Litchfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Amsterdam, The Netherlands, 2009; pp. 823–842. [Google Scholar]
- Whitson, R.E.; Heitschmidt, R.K.; Kothmann, M.M.; Lundgren, G.K. The impact of grazing systems on the magnitude and stability of ranch income in the rolling plains of Texas. J. Range Manag. 1982, 35, 526–532. [Google Scholar] [CrossRef]
- Alados, C.L.; El Aich, A.; Papanastasis, V.P.; Ozbek, H.; Navarro, T.; Freitas, H.; Mihalis, V.; Larrosi, D.; Cabezudo, B. Change in plant spatial patterns and diversity along the successional gradient of Mediterranean grazing ecosystems. Ecol. Model. 2004, 180, 523–535. [Google Scholar] [CrossRef]
- Alhamad, M.N. Ecological and species diversity of arid Mediterranean grazing land vegetation. J. Arid Environ. 2006, 66, 698–715. [Google Scholar] [CrossRef]
- Bai, Y.; Abouguendia, Z.; Recmann, R.E. Relationship between plant species diversity and grassland condition. J. Range Manag. 2001, 54, 177–183. [Google Scholar] [CrossRef]
- Adler, P.B.; Morales, J.M. Influence of environmental factors and sheep grazing on an Andean grasslands. J. Range Manag. 1999, 52, 471–480. [Google Scholar] [CrossRef]
- Altieri, M.A. Agroecology: The science of natural resource management for poor farmers in marginal environments. Agric. Ecosyst. Environ. 2002, 1971, 1–24. [Google Scholar] [CrossRef]
- Aguilar, C.; Vera, R.; Allende, R.; Toro, P. Supplementation, stocking rates and economic performance of lamb production systems in the Mediterranean-type region of Chile. Small Rumin. Res. 2006, 66, 108–115. [Google Scholar] [CrossRef]
- Gutiérrez-Peña, R.; Mena, Y.; Batalla, I.; Mancilla-Leytón, J.M. Carbon footprint of dairy goat production systems: A comparison of three contrasting grazing levels in the Sierra de Grazalema Natural Park (Southern Spain). J. Environ. Manag. 2019, 232, 993–998. [Google Scholar] [CrossRef] [PubMed]




| Season | 1983 | 1984 | 1985 | 1986 | 1987 | 1988 | 1991 | 2002 |
|---|---|---|---|---|---|---|---|---|
| Spring | 11 | 33 | 33 | 24 | 55 | 0 | 54 | 0 |
| Autumn | 12 | 31 | 24 | 0 | 0 | 29 | 0 | 0 |
| Winter | 11 | 33 | 33 | 33 | 56 | 56 | 0 | 115 |
| Parameter | Type of Year | Season | Grazing System |
|---|---|---|---|
| Allochthonous species (Kg ha−1) | 19.89 (5.83 *) | 73.07 (10.27 *) | 7.05 (0.07 ns) |
| Native species (Kg ha−1) | 32.17 (18.99 *) | 54.57 (24.29 *) | 13.25 (7.15 *) |
| Endemic species (Kg ha−1) | 35.53 (13.16 *) | 31.94 (8.60 *) | 32.54 (7.85 *) |
| Richness (S) | 52.87 (67.91 *) | 44.62 (22.64 *) | 2.51 (0.53 ns) |
| Dominance (d) | 22.04 (9.91 *) | 70.42 (25.31 *) | 7.54 (1.92 *) |
| Diversity (H′) | 42.92 (31.17 *) | 54.49 (22.80 *) | 2.59 (0.19 ns) |
| Factor | Allochthonous−Native | Allochthonous−Endemic | Native−Endemic |
|---|---|---|---|
| Continuous grazing | 0.085 ns | 0.291 *** | 0.148 ns |
| Deferred grazing | 0.212 ** | 0.480 *** | 0.148 ns |
| Exclusion to grazing | 0.256 ** | 0.255 ** | 0.292 ** |
| Wet years | 0.046 ns | 0.179 ** | 0.088 ns |
| Dry years | 0.533 *** | 0.494 *** | 0.372 *** |
| Spring | −0.022 ns | 0.531 *** | −0.039 ns |
| Fall | 0.089 ns | 0.674 *** | 0.022 ns |
| Winter | 0.232 *** | 0.137 * | 0.279 *** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patón, D. Climatic and Biological Factors Related with Goat Grazing Management in the Arid Grassland of the Coquimbo Region (Northern Chile). Ecologies 2021, 2, 345-365. https://doi.org/10.3390/ecologies2040020
Patón D. Climatic and Biological Factors Related with Goat Grazing Management in the Arid Grassland of the Coquimbo Region (Northern Chile). Ecologies. 2021; 2(4):345-365. https://doi.org/10.3390/ecologies2040020
Chicago/Turabian StylePatón, Daniel. 2021. "Climatic and Biological Factors Related with Goat Grazing Management in the Arid Grassland of the Coquimbo Region (Northern Chile)" Ecologies 2, no. 4: 345-365. https://doi.org/10.3390/ecologies2040020
APA StylePatón, D. (2021). Climatic and Biological Factors Related with Goat Grazing Management in the Arid Grassland of the Coquimbo Region (Northern Chile). Ecologies, 2(4), 345-365. https://doi.org/10.3390/ecologies2040020






