Asexual Regeneration Response of Ilex canariensis Poir. to Management of the Canopy of Pinus radiata D.Don
Abstract
1. Introduction
2. Material and Methods
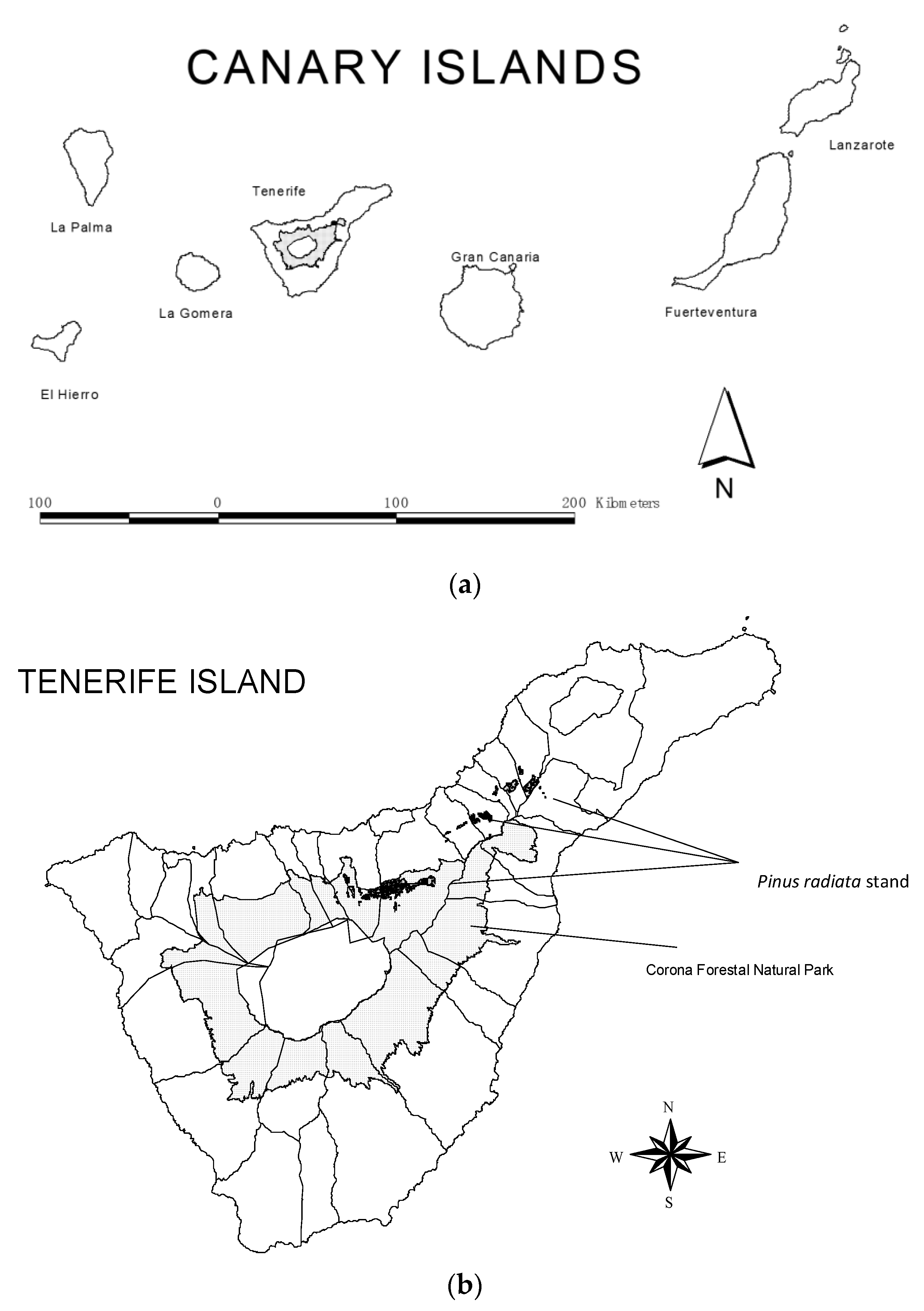
2.1. Study Site
2.2. Design of the Experiment
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Palmer, M.A.; Zedler, J.B.; Falk, D.A. Foundations of Restoration Ecology; Island Press: Washington, DC, USA, 2016. [Google Scholar]
- Arévalo, J.R.; Delgado, J.D.; Fernández-Palacios, J.M. Regeneration of potential laurel forest under a native canopy and an exotic canopy, Tenerife (Canary Islands). For. Syst. 2011, 20, 255–265. [Google Scholar] [CrossRef]
- Geldenhuys, C.J. The Blakwood Group system, its relevance for sustainable forest management in the southern Cape. S. Afr. For. J. 1996, 178, 15–24. [Google Scholar]
- Parrotta, J.A. Influence of overstory composition on understory colonization by native species in plantations on a degraded tropical site. J. Veg. Sci. 1995, 6, 627–636. [Google Scholar] [CrossRef]
- Fimbel, A.R.; Fimbel, C.C. The role of exotic conifer plantations in rehabilitating degraded tropical forest lands, i.c. A case study from the Kivale Forest in Uganda. For. Ecol. Manag. 1996, 81, 215–226. [Google Scholar] [CrossRef]
- Loumeto, J.J.; Huttel, C. Understory vegetation in fast growing tree plantations on savanna soils in Congo. For. Ecol. Manag. 1997, 99, 65–81. [Google Scholar] [CrossRef]
- Bruno, J.F.; Stachowicz, J.J.; Bertness, M.D. Inclusion of facilitation into ecological theory. Trends Ecol. Evol. 2003, 18, 119–125. [Google Scholar] [CrossRef]
- Parrotta, J.A.; Turnbull, J.W.; Jones, N. Catalyzing native forest regeneration on degraded tropical lands. For. Ecol. Manag. 1997, 99, 1–7. [Google Scholar] [CrossRef]
- De Nascimento, L.; Willis, K.J.; Fernández-Palacios, J.M.; Criado, C.; Whittaker, R.J. The long-term ecology of the lost forests of La Laguna, Tenerife (Canary Islands). J. Biogeogr. 2009, 36, 499–514. [Google Scholar] [CrossRef]
- Höllermann, P. The impact of fire in Canarian ecosystems 1983–1998. Erdkunde 2000, 54, 70–75. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Fernández-Palacios, J.M.; Jiménez, M.J.; Gil, P. The effect of fire intensity on the understory species composition of two Pinus canariensis reforested stands in Tenerife (Canary Islands). For. Ecol. Manag. 2001, 148, 21–29. [Google Scholar] [CrossRef]
- Arévalo, J.R.; Naranjo-Cigala, A. Wildfire impact and the FIRE PARADOX in a natural and endemic pine forest stand and shrubland. Fire 2018, 1, 44. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Arévalo, J.R.; González-Delgado, G.; Delgado, J.D.; Otto, R. Estrategias de regeneración en la laurisilva. Makaronesia 2004, 6, 90–101. [Google Scholar]
- Pretzsch, H. Forest Dynamics, Growth, and Yield; Springer: Berlin/Heidelberg, Germany, 2009. [Google Scholar]
- Del Arco, M.J.; Wildpret, W.; Pérez de Paz, P.L.; Rodríguez-Delgado, O.; Acebes, J.R.; García-Gallo, A.; Martín, V.E.; Reyes-Betancort, J.A.; Salas, M.; Bermejo, J.A.; et al. Mapa de Vegetación de Canarias; GRAFCAN: Santa Cruz de Tenerife, Spain, 2006. [Google Scholar]
- Del Arco, M.J.; Pérez de Paz, P.L.; Salas, M.; Wildpret, W. Atlas Cartográfico de los Pinares Canaries: II Tenerife; Viceconsejería de Medio Ambiente: Santa Cruz de Tenerife, Spain, 1992. [Google Scholar]
- Ceballos, L.; Ortuño, F. Vegetación y Flora Forestal de las Islas Occidentales, 2nd ed.; Cabildo Insular de Tenerife: S/C de Tenerife, Spain, 1974. [Google Scholar]
- Kämmer, F. Klima und Vegetation auf Tenerife, besonders in Hinblick auf den Nebelniederschlag. Scripta Geobot. 1974, 7, 1–78. [Google Scholar]
- Fernández-Caldas, E.; Tejedor, M.; Quantin, P. Los Suelos Volcánicos de Canarias; Servicio de Publicaciones de La Universidad de La Laguna: La Laguna, Spain, 1985. [Google Scholar]
- Acebes, J.R.; del Arco, M.; García, A.; León, M.C.; Pérez de Paz, P.L.; Rodríguez, O.; Wildpret, W.; Martín, V.E.; Marrero, M.C.; Rodríguez, L. Pteridophyta, Spermatophyta. In Lista de Especies Silvestres de Canarias (Hongos, Plantas y Animales Terrestres); Izquierdo, I., Martín, J.L., Zurita, N., Arechavaleta, M., Eds.; Consejería de Medio Ambiente y Ordenación Territorial, Gobierno de Canarias: S/C de Tenerife, Spain, 2004; pp. 96–143. [Google Scholar]
- Arévalo, J.R.; García, G.; Montes de Oca, Y.; Fernández, S. Differences in Carbon Sequestration in Native vs. Exotic Pine Species (Tenerife, Canary Islands). Bull. UASVM Ser. Agric. 2013, 70, 299–303. [Google Scholar]
- Lemmon, P.E. A new instrument for measuring forest overstory density. J. For. 1957, 55, 667–668. [Google Scholar]
- Anderson, M.; Gorley, R.N.; Clarke, R.K. Permanova+ for Primer: Guide to Software and Statistical Methods; PRIMER-E: Plymouth, UK, 2008. [Google Scholar]
- Ren, H.; Long, Y.; Nan, L. Nurse plant theory and its application in ecological restoration in lower subtropics of China. Prog. Nat. Sci. 2008, 18, 137–142. [Google Scholar] [CrossRef]
- Fernández-Palacios, J.M.; Arévalo, J.R.; Balguerías, E.; Barone, R.; De Nascimento, L.; Elias, R.B.; Delgado, J.D.; Fernández-Lugo, S.; Méndez, J.; Menezes de Sequeira, M.; et al. La Laurisilva. Canarias, Madeira y Azores; Editorial Macaronesia: SC de Tenerife, Spain, 2017. [Google Scholar]
- Arévalo, J.R.; Fernández-Palacios, J.M. Natural regeneration of Pinus canariensis Chr. Sm. ex DC in Buch in forest plantations after thinning. Open For. Sci. J. 2008, 1, 54–60. [Google Scholar] [CrossRef]
- Santos, A. Bosques de Laurisilva en la Región Macaronésica; Colección Naturaleza y Medio ambiente, 49; Council of Europe: Strasbourg, France, 1990. [Google Scholar]
- Onaindia, M.; Mitxelena, A. Potential use of pine plantations to restore native forests in a highly fragmented river basin. Ann. For. Sci. 2009, 66, 1–11. [Google Scholar] [CrossRef]
- Adam, F.S.; Norton, D.A.; Carswell, F.E. Artificial canopy gaps accelerate restoration within an exotic Pinus radiata plantation. Restor. Ecol. 2016, 24, 336–345. [Google Scholar]
- Lameira, L.L.; Ferreira, F.C.; Esteves, R.A.; Queiroz, J. Plant-canopy Effects on Natural Regeneration in Sites Under Restoration: Do Tree Species Matter? Floresta Ambiente 2019, 26. [Google Scholar] [CrossRef]


| Plot | Treatment | Slope (°) | Aspect | Altitude (m) | A | B | C | D | Avg. |
|---|---|---|---|---|---|---|---|---|---|
| CON1 | Control | 25 | N | 1100 | 90 | 80 | 85 | 85 | 85 |
| CON2 | Control | 12 | NO | 1045 | 85 | 85 | 80 | 90 | 85 |
| LI1 | Low Intensity | 8 | N | 920 | 70 | 65 | 70 | 75 | 70 |
| LI2 | Low Intensity | 10 | N | 910 | 65 | 80 | 75 | 75 | 74 |
| HI1 | High Intensity | 10 | NO | 950 | 80 | 80 | 80 | 85 | 80 |
| HI2 | High Intensity | 10 | NO | 960 | 70 | 75 | 75 | 70 | 72 |
| SC | Stand Clear Cut | 10 | NO | 1090 | – | – | – | – |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arévalo, J.R. Asexual Regeneration Response of Ilex canariensis Poir. to Management of the Canopy of Pinus radiata D.Don. Ecologies 2020, 1, 14-21. https://doi.org/10.3390/ecologies1010003
Arévalo JR. Asexual Regeneration Response of Ilex canariensis Poir. to Management of the Canopy of Pinus radiata D.Don. Ecologies. 2020; 1(1):14-21. https://doi.org/10.3390/ecologies1010003
Chicago/Turabian StyleArévalo, José Ramón. 2020. "Asexual Regeneration Response of Ilex canariensis Poir. to Management of the Canopy of Pinus radiata D.Don" Ecologies 1, no. 1: 14-21. https://doi.org/10.3390/ecologies1010003
APA StyleArévalo, J. R. (2020). Asexual Regeneration Response of Ilex canariensis Poir. to Management of the Canopy of Pinus radiata D.Don. Ecologies, 1(1), 14-21. https://doi.org/10.3390/ecologies1010003





