Follow-Up of APSified–BMO-Based Retinal Microcirculation in Patients with Post-COVID-19 Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Optical Coherence Tomography Angiography (OCT-A)
2.3. Statistical Methods
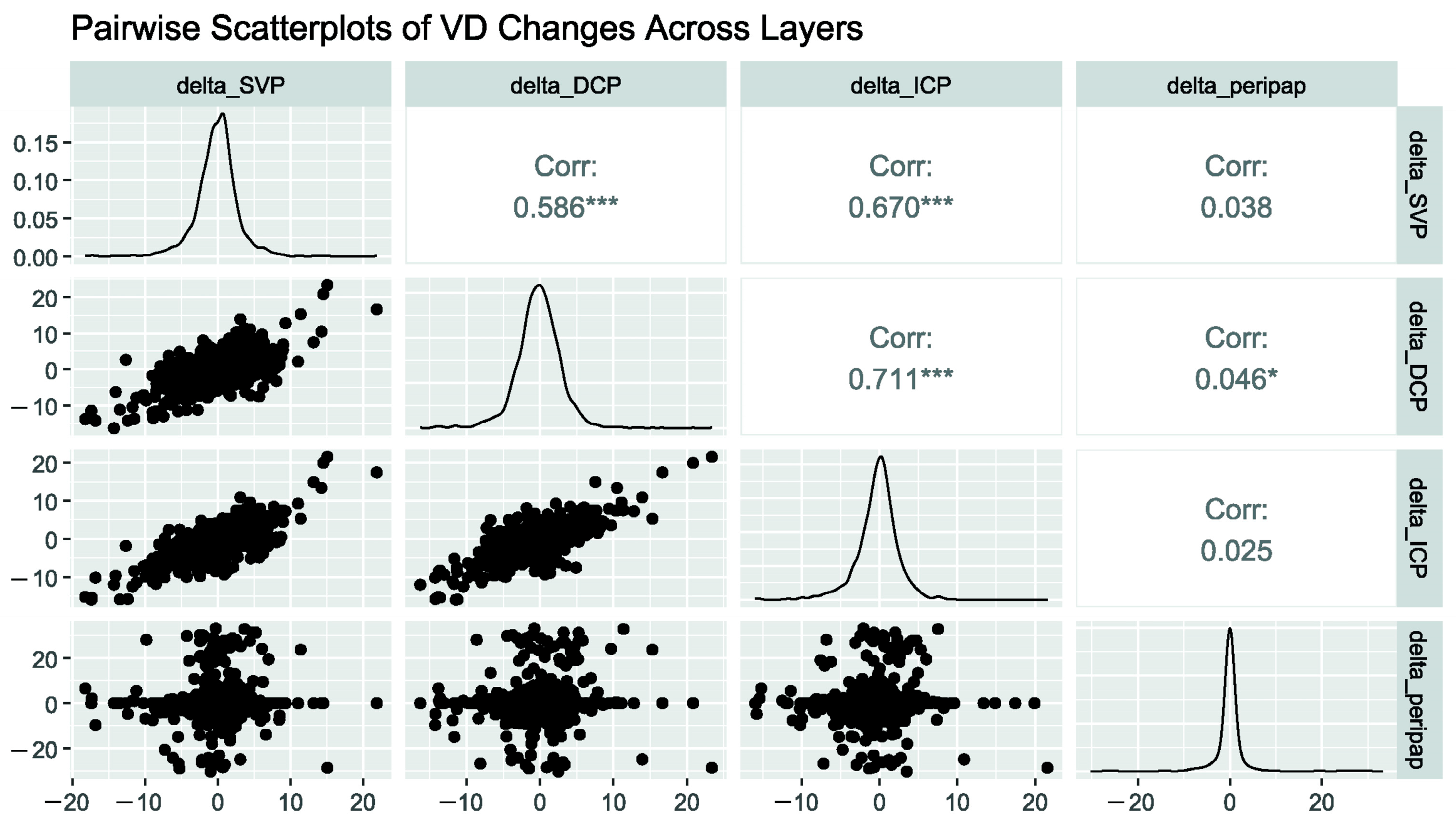
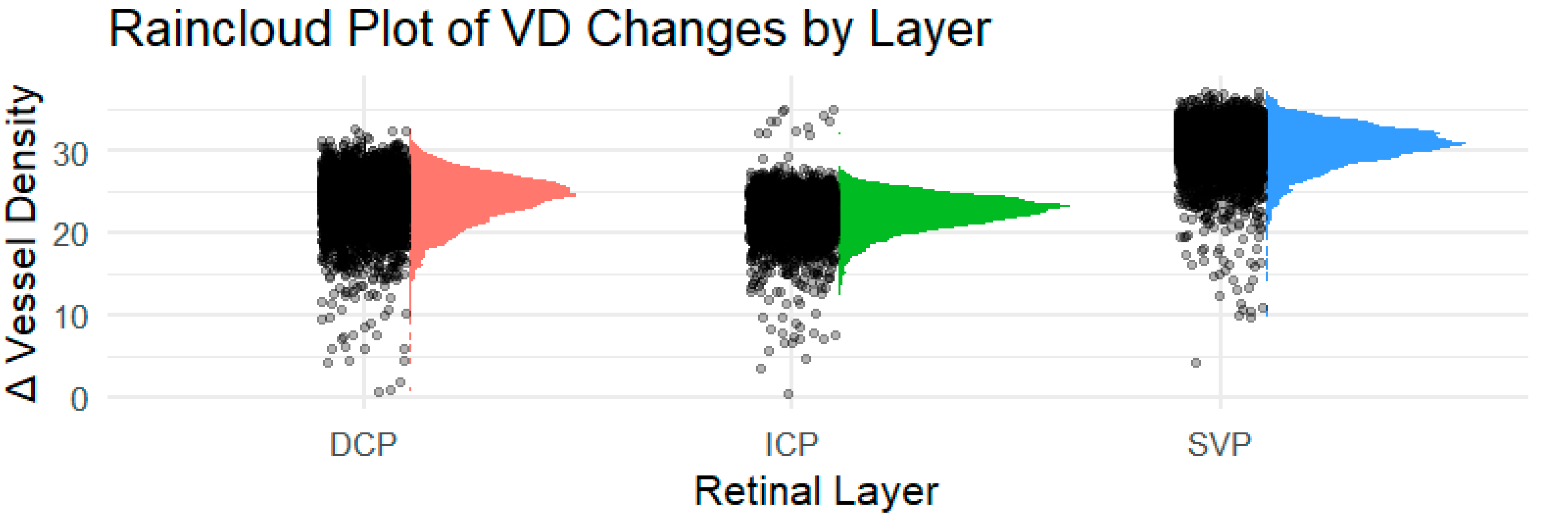
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE2 | Angiotensin-converting enzyme 2 |
| BCVA | Best-corrected visual acuity |
| BMO | Bruch’s membrane opening |
| COVID-19 | Coronavirus disease 2019 |
| DCP | Deep capillary plexus |
| ED | Endothelial dysfunction |
| FoBMOC | Fovea-to-Bruch’s membrane opening center |
| GCL | Ganglion cell layer |
| ICP | Intermediate capillary plexus |
| INL | Inner nuclear layer |
| IOP | Intraocular pressure |
| IPL | Inner plexiform layer |
| LC | Long COVID-19 syndrome |
| LS-Mean | Least-squares mean |
| ME/CFS | Myalgic encephalomyelitis/chronic fatigue syndrome |
| OPL | Outer plexiform layer |
| PASC | Post-Acute Sequelae of SARS-CoV-2 Infection |
| PCS | Post-COVID-19 syndrome |
| PCR | Reverse transcription polymerase chain reaction |
| PEM | Post-exertional malaise |
| POTS | Postural orthostatic tachycardia syndrome |
| RAAS | Renin–angiotensin–aldosterone system |
References
- World Health Organization (WHO). Coronavirus (COVID-19) Dashboard. 2025. Available online: https://data.who.int/dashboards/covid19/cases?n=c (accessed on 3 October 2025).
- Koczulla, A.R.; Ankermann, T.; Behrends, U.; Böing, S.; Berlit, P.; Brinkmann, F.; Franke, C.; Glöckl, R.; Gogoll, C.; Hummel, T.; et al. S1-Leitlinie Long/Post-COVID—Living Guideline. Pneumologie 2024, 75, 869–900. [Google Scholar]
- Greenhalgh, T.; Sivan, M.; Perlowski, A.; Nikolich, J. Long COVID: A clinical update. Lancet 2024, 404, 10453. [Google Scholar] [CrossRef]
- Jamies Daniell, J.B.; Paessler, D.; Heydecke, J.; Schoening, S.; McLennan, A. The rising cost of Long COVID and ME/CFS in Germany. 2025. Available online: https://mecfs-research.org/wp-content/uploads/2025/05/The-rising-cost-of-Long-COVID-and-MECFS-in-Germany.pdf (accessed on 15 June 2025).
- Frommhold, J.S.P.O. Post-COVID-Syndrom und Long-COVID Diagnostik, Therapie und Verlauf; Medizinisch Wissenschaftliche Verlagsgesellschaft: Berlin, Germany, 2023. [Google Scholar]
- Casagrande, M.; Fitzek, A.; Püschel, K.; Aleshcheva, G.; Schultheiss, H.-P.; Berneking, L.; Spitzer, M.S.; Schultheiss, M. Detection of SARS-CoV-2 in Human Retinal Biopsies of Deceased COVID-19 Patients. Ocul. Immunol. Inflamm. 2020, 28, 721–725. [Google Scholar] [CrossRef] [PubMed]
- Bonaventura, A.; Vecchié, A.; Dagna, L.; Martinod, K.; Dixon, D.L.; Van Tassell, B.W.; Dentali, F.; Montecucco, F.; Massberg, S.; Levi, M.; et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat. Rev. Immunol. 2021, 21, 319–329. [Google Scholar] [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023, 21, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Hosari, S.; Hohberger, B.; Theelke, L.; Sari, H.; Lucio, M.; Mardin, C.Y. OCT Angiography: Measurement of Retinal Macular Microvasculature with Spectralis II OCT Angiography—Reliability and Reproducibility. Ophthalmologica 2020, 243, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Szewczykowski, C.; Mardin, C.; Lucio, M.; Wallukat, G.; Hoffmanns, J.; Schröder, T.; Raith, F.; Rogge, L.; Heltmann, F.; Moritz, M.; et al. Long COVID: Association of Functional Autoantibodies against G-Protein-Coupled Receptors with an Impaired Retinal Microcirculation. Int. J. Mol. Sci. 2022, 23, 7209. [Google Scholar] [CrossRef] [PubMed]
- Cennamo, G.; Reibaldi, M.; Montorio, D.; D′ANdrea, L.; Fallico, M.; Triassi, M. Optical Coherence Tomography Angiography Features in Post-COVID-19 Pneumonia Patients: A Pilot Study. Arch. Ophthalmol. 2021, 227, 182–190. [Google Scholar] [CrossRef] [PubMed]
- Jerratsch, H.; Beuse, A.; Spitzer, M.S.; Grohmann, C. The Current Status of OCT and OCTA Imaging for the Diagnosis of Long COVID. J. Clin. Transl. Ophthalmol. 2024, 2, 113–130. [Google Scholar] [CrossRef]
- Fu, X.; Ren, X.; Chen, W.; Chen, D. Reduced macular thickness and vascular density in abnormal glucose metabolism patients: A meta-analysis of optical coherence tomography (OCT) and OCT angiography studies. Chin. Med J. 2024, 137, 1054–1068. [Google Scholar] [CrossRef] [PubMed]
- Anjos, R.; Ferreira, A.; Barkoudah, E.; Claggett, B.; Pinto, L.A.; Miguel, A. Application of Optical Coherence Tomography Angiography Macular Analysis for Systemic Hypertension. A Systematic Review and Meta-analysis. Am. J. Hypertens. 2022, 35, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Ganslmayer, M.; Lucio, M.; Kruse, F.; Hoffmanns, J.; Moritz, M.; Rogge, L.; Heltmann, F.; Szewczykowski, C.; Fürst, J.; et al. Retinal Microcirculation as a Correlate of a Systemic Capillary Impairment After Severe Acute Respiratory Syndrome Coronavirus 2 Infection. Front. Med. 2021, 8, 676554. [Google Scholar] [CrossRef]
- Schlick, S.; Lucio, M.; Wallukat, G.; Bartsch, A.; Skornia, A.; Hoffmanns, J.; Szewczykowski, C.; Schröder, T.; Raith, F.; Rogge, L.; et al. Post-COVID-19 Syndrome: Retinal Microcirculation as a Potential Marker for Chronic Fatigue. Int. J. Mol. Sci. 2022, 23, 13683. [Google Scholar] [CrossRef]
- Hohberger, B.; Harrer, T.; Mardin, C.; Kruse, F.; Hoffmanns, J.; Rogge, L.; Heltmann, F.; Moritz, M.; Szewczykowski, C.; Schottenhamml, J.; et al. Neutralization of Autoantibodies Targeting G-Protein Coupled Receptors Improves Capillary Impairment and Fatigue Symptoms after COVID-19 Infection. Front. Med. 2021, 8, 754667. Available online: https://ssrn.com/abstract=3879488 (accessed on 11 March 2025). [CrossRef]
- Lyons, C.E.; Alhalel, J.; Busza, A.; Suen, E.; Gill, N.; Decker, N.; Suchy, S.; Orban, Z.; Jimenez, M.; Perez Giraldo, G.; et al. Non-Hospitalized Long COVID Patients Exhibit Reduced Retinal Capillary Perfusion: A Prospective Cohort Study. J. Imaging 2025, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Noor, M.; McGrath, O.; Drira, I.; Aslam, T. Retinal Microvasculature Image Analysis Using Optical Coherence Tomography Angiography in Patients with Post-COVID-19 Syndrome. J. Imaging 2023, 9, 234. [Google Scholar] [CrossRef] [PubMed]
- Ozturk, M.; Guler, D.K.; Oskan, E.E.; Onder, F. Long-Term Effects of COVID-19 on Optic Disc and Retinal Microvasculature Assessed by Optical Coherence Tomography Angiography. Diagnostics 2025, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Rocholz, R.; Teussink, M.; Dolz-Marco, R.; Holzhey, C.; Dechent, J.F.; Tafreshi, A.; Schulz, S. SPECTRALIS Optical Coherence Tomography Angiography (OCTA): Principles and Clinical Applications Heidelberg Engineering Academy. 2018. Available online: https://www.heidelbergengineering.com/int/news/spectralis-optical-coherence-tomography-angiography-principles-and-clinical-applications-69710055/ (accessed on 12 April 2025).
- Kuchler, T.; Günthner, R.; Ribeiro, A.; Hausinger, R.; Streese, L.; Wöhnl, A.; Kesseler, V.; Negele, J.; Assali, T.; Carbajo-Lozoya, J.; et al. Persistent endothelial dysfunction in post-COVID-19 syndrome and its associations with symptom severity and chronic inflammation. Angiogenesis 2023, 26, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Kubánková, M.; Hohberger, B.; Hoffmanns, J.; Fürst, J.; Herrmann, M.; Guck, J.; Kräter, M. Physical phenotype of blood cells is altered in COVID-19. Biophys. J. 2021, 120, 2838–2847. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, E.; Vlok, M.; Venter, C.; Bezuidenhout, J.A.; Laubscher, G.J.; Steenkamp, J.; Kell, D.B. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021, 20, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Motlagh, M.; Singh, G. Anatomy, Head and Neck, Eye Arteries. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Orgül, S.; Gugleta, K.; Flammer, J. Physiology of perfusion as it relates to the optic nerve head. Surv. Ophthalmol. 1999, 43, S17–S26. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Pattathil, N.; Choudhry, N. OCTA: Essential or Gimmick? Ophthalmol. Ther. 2024, 13, 2293–2302. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Chen, C.-L.; Gahm, J.K.; Zheng, F.; Richter, G.M.; Rosenfeld, P.J.; Shi, Y.; Wang, R.K. Optical coherence tomography angiography: A comprehensive review of current methods and clinical applications. Prog. Retin. Eye Res. 2017, 60, 66–100. [Google Scholar] [CrossRef] [PubMed]
- Hohberger, B.; Mardin, C.Y. OCT Angiography as an Interdisciplinary Diagnostic Tool for Systemic Diseases. Klin. Monbl. Augenheilkd 2021, 238, 1294–1298. [Google Scholar] [PubMed]
- Bilbao-Malavé, V.; González-Zamora, J.; de Viteri, M.S.; de la Puente, M.; Gándara, E.; Casablanca-Piñera, A.; Boquera-Ventosa, C.; Zarranz-Ventura, J.; Landecho, M.F.; García-Layana, A. Persistent Retinal Microvascular Impairment in COVID-19 Bilateral Pneumonia at 6-Months Follow-Up Assessed by Optical Coherence Tomography Angiography. Biomedicines 2021, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Jevnikar, K.; Meglič, A.; Lapajne, L.; Logar, M.; Valentinčič, N.V.; Petrovič, M.G.; Mekjavić, P.J. The Comparison of Retinal Microvascular Findings in Acute COVID-19 and 1-Year after Hospital Discharge Assessed with Multimodal Imaging—A Prospective Longitudinal Cohort Study. Int. J. Mol. Sci. 2023, 24, 4032. [Google Scholar] [CrossRef] [PubMed]
- Abrishami, M.; Hassanpour, K.; Hosseini, S.; Emamverdian, Z.; Ansari-Astaneh, M.-R.; Zamani, G.; Gharib, B.; Abrishami, M. Macular vessel density reduction in patients recovered from COVID-19: A longitudinal optical coherence tomography angiography study. Graefes Arch. Clin. Exp. Ophthalmol. 2022, 260, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Blasco, B.; Güemes-Villahoz, N.; Vidal-Villegas, B.; Martinez-De-La-Casa, J.M.; Garcia-Feijoo, J.; Donate-Lopez, J.; Martin-Sanchez, F.J.; Gonzalez-Armengol, J.J.; Mendez-Hernandez, C.D. One-Year Changes in Optic Nerve Head Parameters in Recovered COVID-19 Patients. Neuro-Ophthalmology 2022, 42, 476–482. [Google Scholar] [CrossRef]
- García, S.B.; Aragón, D.; Azarfane, B.; Trejo, F.; Garrell-Salat, X.; Sánchez-Montalvá, A.; Otero-Romero, S.; Garcia-Arumi, J.; Zapata, M.A. Persistent reduction of retinal microvascular vessel density in patients with moderate and severe COVID-19 disease. BMJ Open Ophthalmol. 2022, 7, e000867. [Google Scholar] [CrossRef]
- Castellino, N.; Longo, A.; Russo, A.; Bonfiglio, V.; Fallico, M.; Toro, M.D.; Cappellani, F.; Grillo, M.; Gaudio, A.; Cicero, L.L.; et al. COVID-19-related retinal microvasculopathy and systemic implications in patients with severe disease: Results from the Methuselah study. Front. Med. 2024, 11, 1294432. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.P.; Zhang, M.; Hwang, T.S.; Bailey, S.T.; Wilson, D.J.; Jia, Y.; Huang, D. Detailed Vascular Anatomy of the Human Retina by Projection-Resolved Optical Coherence Tomography Angiography. Sci. Rep. 2017, 7, srep42201. [Google Scholar] [CrossRef] [PubMed]
- Cosmo, E.; Frizziero, L.; Schiavon, S.; Cattelan, A.M.; Leoni, D.; Capizzi, A.; Torresin, T.; Midena, G.; Roveri, E.A.S.D.; Parrozzani, R.; et al. The neurovascular retinal involvement in a large population of patients recovered from COVID-19: An OCT and OCT angiography study. Eye 2024, 38, 1674–1680. [Google Scholar] [CrossRef] [PubMed]
- Lavia, C.; Couturier, A.; Erginay, A.; Dupas, B.; Tadayoni, R.; Gaudric, A. Reduced vessel density in the superficial and deep plexuses in diabetic retinopathy is associated with structural changes in corresponding retinal layers. PLoS ONE 2019, 14, e0219164. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Balaratnasingam, C.; Yu, P.K.; Morgan, W.H.; McAllister, I.L.; Cringle, S.J.; Yu, D.-Y. Quantitative morphometry of perifoveal capillary networks in the human retina. Investig. Opthalmol. Vis. Sci. 2012, 53, 5502–5514. [Google Scholar] [CrossRef]
- Park, J.J.B.; Soetikno, B.T.B.; Fawzi, A.A. CHARACTERIZATION OF THE MIDDLE CAPILLARY PLEXUS USING OPTICAL COHERENCE TOMOGRAPHY AN-GIOGRAPHY IN HEALTHY AND DIABETIC EYES. Retina 2016, 36, 2039–2050. [Google Scholar] [CrossRef]
- Choudhary, R.; Kapoor, M.S.; Singh, A.; Bodakhe, S.H. Therapeutic targets of renin-angiotensin system in ocular disorders. J. Curr. Ophthalmol. 2017, 29, 7–16. [Google Scholar] [CrossRef] [PubMed]
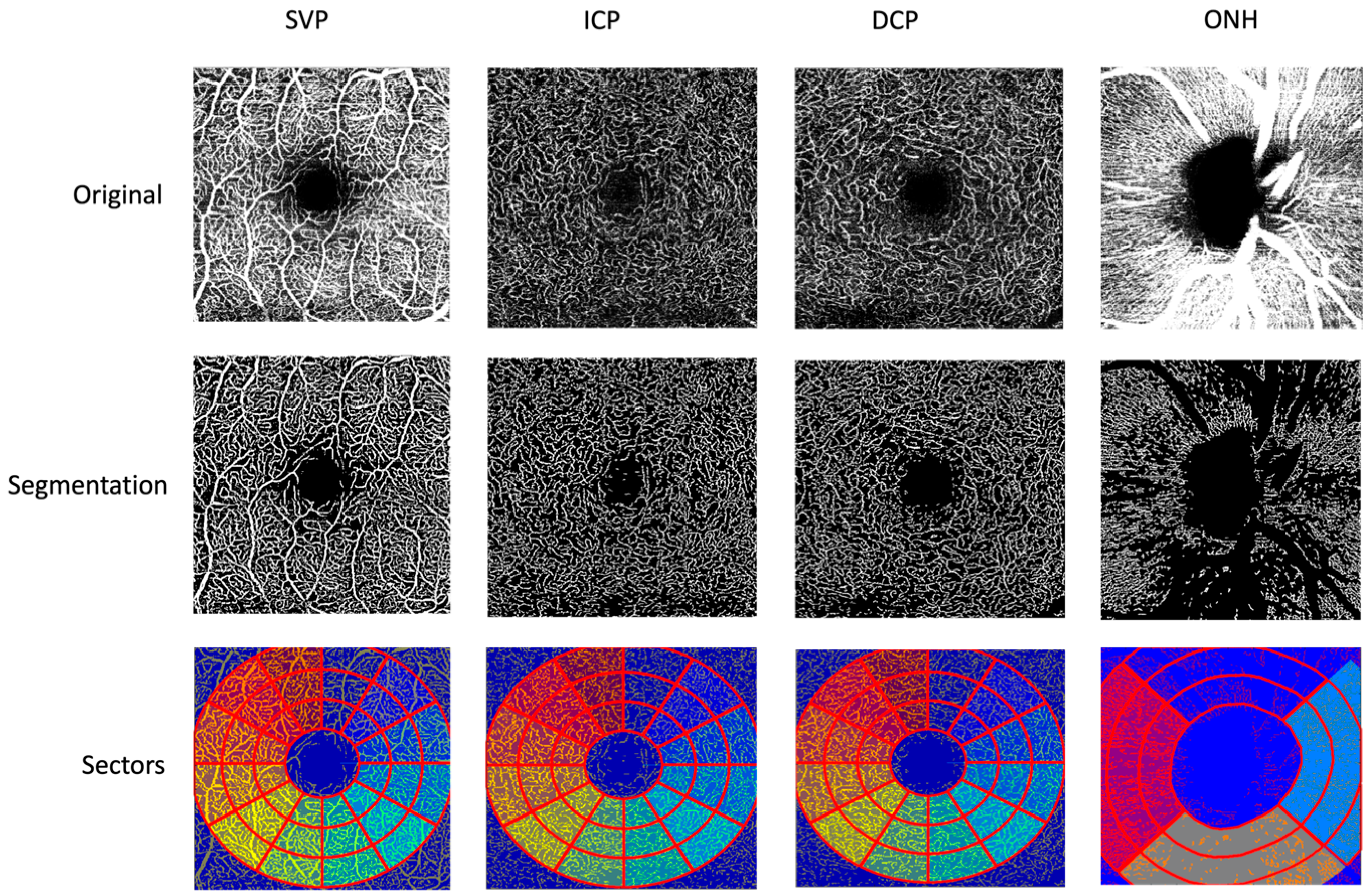
| Number | Age | Gender (f/m) | BCVA | IOP [mmHg] | Axial Length [mm] | |
|---|---|---|---|---|---|---|
| Total | 80 | 40.33 ± 12 | 45/35 | 0.995 ± 0 | 14.6 ± 3 | 24.02 ± 1 |
| Female | 45 | 40.35 ± 12 | 45 | 0.991 ± 0 | 14.91 ± 3 | 23.67 ± 1 |
| Male | 35 | 40.31 ± 11 | 35 | 1.0 ± 0 | 14.20 ± 3 | 24.47 ± 1 |
| Group | LS-Mean | SE | DF | Lower.CL | Upper.CL | p-Value | |
|---|---|---|---|---|---|---|---|
| SVP | PCS visit1 | 30.4 | 0.168 | 313 | 30 | 30.7 | |
| PCS visit2 | 30.3 | 0.166 | 313 | 29.9 | 30.6 | 0.677 | |
| ICP | PCS visit1 | 22.4 | 0.143 | 313 | 22.2 | 22.7 | |
| PCS visit2 | 22.2 | 0.141 | 313 | 21.9 | 22.5 | 0.263 | |
| DCP | PCS visit1 | 23.9 | 0.186 | 314 | 23.5 | 24.3 | |
| PCS visit2 | 23.8 | 0.185 | 314 | 23.4 | 24.2 | 0.725 | |
| Peripapillary Region | PCS visit1 | 27.4 | 0.226 | 313 | 27 | 27.9 | |
| PCS visit2 | 27 | 0.224 | 313 | 26.5 | 27.4 | 0.142 |
| Parameter | Estimate | SE | DF | t-Value | p-Value |
|---|---|---|---|---|---|
| (Intercept) | 26.74 | 0.51 | 480 | 52.35 | <0.0001 |
| Visit | 0.09 | 0.12 | 480 | 0.74 | 0.4606 |
| ICP | −1.89 | 0.30 | 320 | −6.28 | <0.0001 |
| SVP | 6.41 | 0.30 | 320 | 21.32 | <0.0001 |
| Gender | −0.38 | 0.24 | 158 | −1.56 | 0.1208 |
| Age | −0.07 | 0.01 | 158 | −6.56 | <0.0001 |
| Visit–ICP | 0.24 | 0.18 | 480 | 1.39 | 0.1662 |
| Visit–SVP | 0.04 | 0.18 | 480 | 0.26 | 0.7986 |
| Contrast | Estimate | SE | DF | t-Value | p-Value |
| DCP-ICP | 1.52 | 0.145 | 320 | 10.486 | <0.0001 |
| DCP-SVP | −6.47 | 0.145 | 320 | −44.598 | <0.0001 |
| ICP-SVP | −7.99 | 0.145 | 320 | −55.084 | <0.0001 |
| Study | Cohort Size | COVID-19 Severity/Inclusion Criteria | Device | Segmentation Approach | Duration | Main Findings |
|---|---|---|---|---|---|---|
| Ozturk, M. et al. [20] | n = 40; PCS patients | Mild to moderate SARS-CoV-2-induced pneumonia | AngioVue Imaging System version 2017.1, Optovue. Inc., Fremont, CA, USA | SCP and DCP, FAZ, 4.5 × 4.5 mm2 | 4 and 12 months | Progressive decrease in foveal SVP and DCP and an increased FAZ |
| Noor et al. [19] | n = 40; PCS patients | Post-COVID-19 syndrome (persistent symptoms ≥ 12 weeks) | Canon Xephilio OCTA-1 machine (Canon Medical Systems Europe B.V©, Amstelveen, The Netherlands) | SCP, FAZ, 10 × 10 mm2 and 4 × 4 mm2 | ≈15 months | No significant differences in VD of SCP and FAZ |
| Lyons et al. [18] | n = 30; Neuro-PASC patients | Neuro-PASC (long COVID-19 with persistent neurological symptoms) | RTVue-XR Avanti system (Optovue, Inc., Fremont, CA, USA) | SCP and DCP, FAZ, 3 × 3 mm2 scans | ~12 months | No significant differences in VD of SCP, DCP, and FAZ |
| Bilbao-Malavé, V. et al. [30] | n = 17; COVID-19 patients | Bilateral pneumonia | DRI OCT Triton SS-OCT Angio (Topcon Medical Systems, Inc., Oakland, NJ, USA) | SCP and DCP, FAZ area, 4.5 × 4.5 mm2 and 6 × 6 mm2 | 6 months | No differences at baseline, a reduced VD of SCP and DCP, and enlarged FAZ |
| Jevnikar, K. et al. [31] | n = 30; COVID-19 patients | Recovered | Topcon DRI OCT Triton (Topcon Corp., Tokyo, Japan) | SCP and DCP, FAZ, 3 × 3 mm2 | 12-month follow-up | No alterations in VD after one-year follow-up |
| Abrishami, M. et al. [32] | n = 18; COVID-19 patients | Recovered from mild to moderate COVID-19 | AngioVue system (RTVue XR Avanti, Optovue, Fremont, CA, USA) | SCP and DCP, FAZ, 3 × 3 mm2 | 1 and 3 months | Progressive reduction in VD in DCP over 3 months; FAZ stable |
| Burgos-Blasco, B. et al. [32,33] | n = 90; COVID-19 patients | Hospitalized with moderate to severe COVID-19 | Cirrus HD-OCT 5000 with AngioPlex OCTA | Peripapillary and macular SCP, 4.5 × 4.5 mm2 | 3 and 12 months | Increased VD at 12 months |
| Banderas García, S. et al. [34] | n = 75; COVID-19 patients | Moderate and severe COVID-19 (with demand for hospitalization) | DRI OCT Triton Swept Source equipment (Topcon Corporation, Tokyo, Japan) | SCP and DCP, 4.5 × 4.5 mm2 | 8 months | Persistent reduction in VD of SCP and DCP |
| Castellino, N. et al. [35] | n = 25; COVID-19 patients | Hospitalization due to severe COVID-19 | AngioVue XR Avanti (Optovue Inc., Fremont, CA, USA) | SCP and DCP, FAZ area, 6 × 6 mm2 | 1 and 12 months | No reversion of the reduced SCP and enlarged FAZ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosenkranz, C.; Lucio, M.; Ganslmayer, M.; Harrer, T.; Hoffmanns, J.; Szewczykowski, C.; Schröder, T.; Raith, F.; Zellinger, S.; Abelardo, D.; et al. Follow-Up of APSified–BMO-Based Retinal Microcirculation in Patients with Post-COVID-19 Syndrome. Biophysica 2025, 5, 46. https://doi.org/10.3390/biophysica5040046
Rosenkranz C, Lucio M, Ganslmayer M, Harrer T, Hoffmanns J, Szewczykowski C, Schröder T, Raith F, Zellinger S, Abelardo D, et al. Follow-Up of APSified–BMO-Based Retinal Microcirculation in Patients with Post-COVID-19 Syndrome. Biophysica. 2025; 5(4):46. https://doi.org/10.3390/biophysica5040046
Chicago/Turabian StyleRosenkranz, Cornelius, Marianna Lucio, Marion Ganslmayer, Thomas Harrer, Jakob Hoffmanns, Charlotte Szewczykowski, Thora Schröder, Franziska Raith, Stephanie Zellinger, Denzel Abelardo, and et al. 2025. "Follow-Up of APSified–BMO-Based Retinal Microcirculation in Patients with Post-COVID-19 Syndrome" Biophysica 5, no. 4: 46. https://doi.org/10.3390/biophysica5040046
APA StyleRosenkranz, C., Lucio, M., Ganslmayer, M., Harrer, T., Hoffmanns, J., Szewczykowski, C., Schröder, T., Raith, F., Zellinger, S., Abelardo, D., Schumacher, J., Flecks, M., Lakatos, P., Mardin, C., & Hohberger, B. (2025). Follow-Up of APSified–BMO-Based Retinal Microcirculation in Patients with Post-COVID-19 Syndrome. Biophysica, 5(4), 46. https://doi.org/10.3390/biophysica5040046







