Insights into Cysteine Protease Complexes with Grafted Chitosan–Poly(N-vinylpyrrolidone) Copolymers: Catalytic Activity and Storage Stability
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Molecular Docking
2.3. Cysteine Protease Complexation with the Cs-g-PVP Copolymers
2.4. Fourier-Transform Infrared Spectroscopy
2.5. Protein Content Assay
2.6. Enzyme Activity Assay
2.7. Statistical Assay
3. Results and Discussions
3.1. In Silico Study of the Interaction Between Cysteine Proteases and the Cs-g-PVP Copolymers
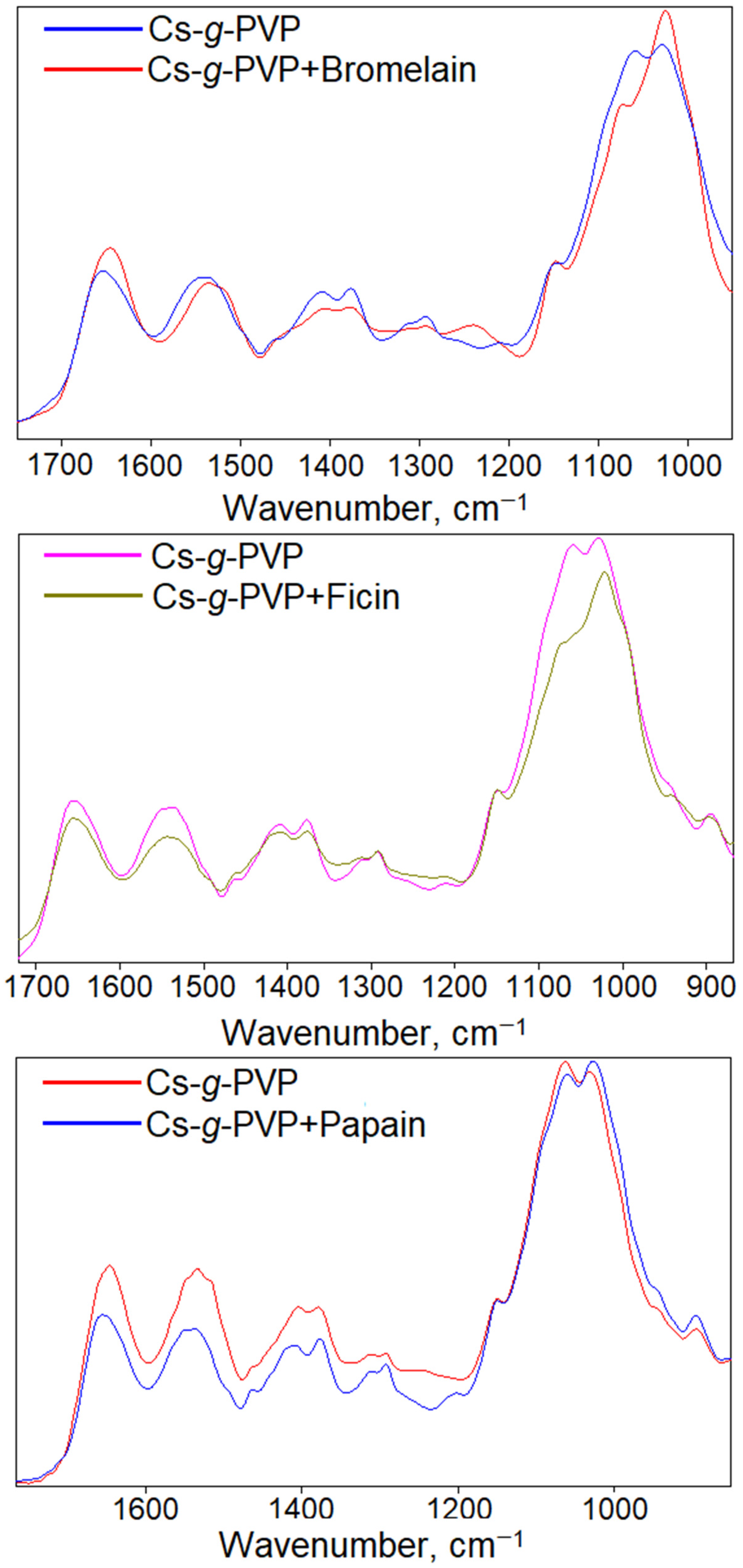
3.2. Spectroscopic Investigation of the Interaction Between Cysteine Proteases and Cs-g-PVP Copolymers
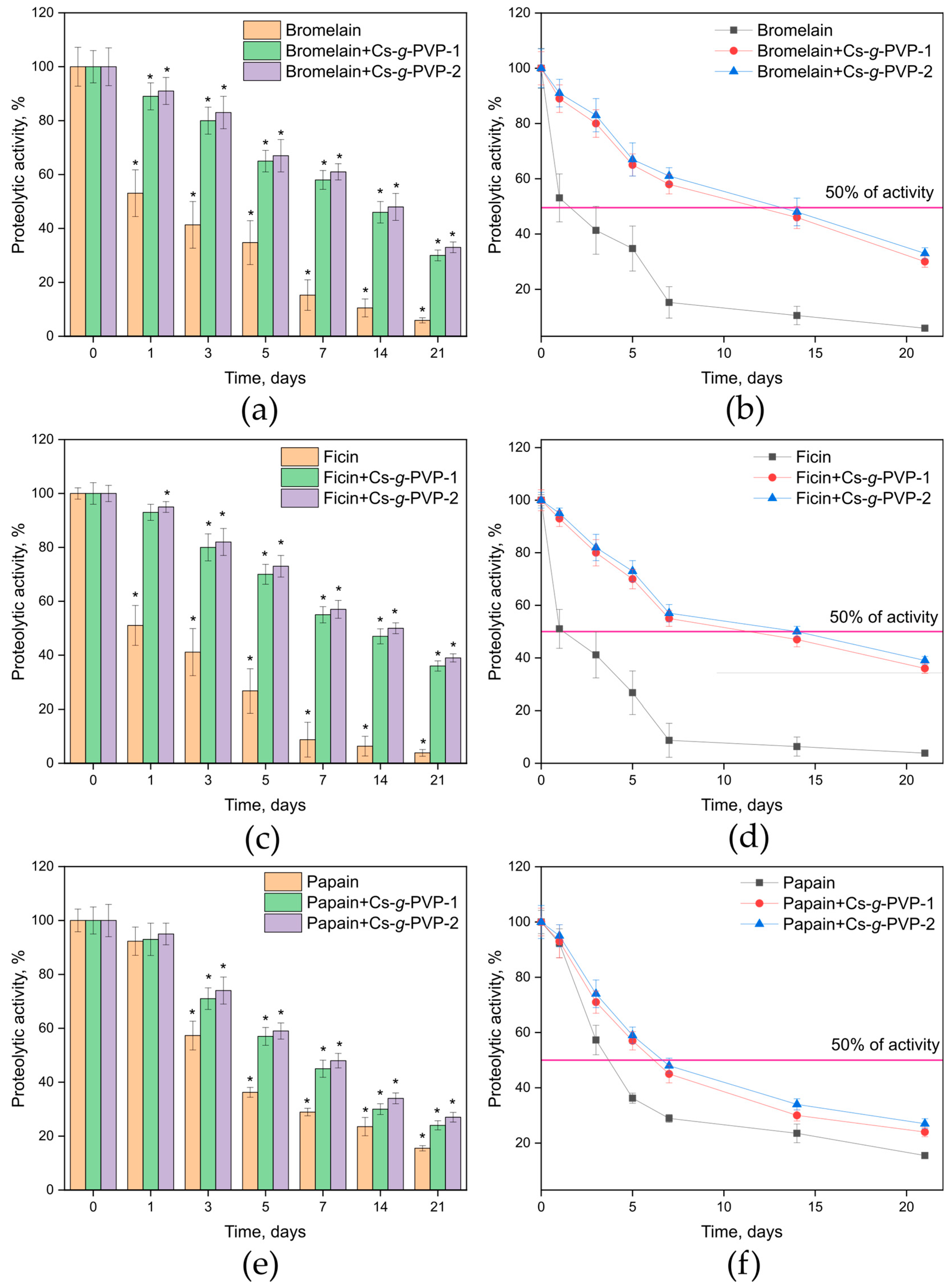
3.3. In Vitro Studies of Cysteine Protease Complexes with the Cs-g-PVP Copolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prabantu, V.M.; Gadiyaram, V.; Vishveshwara, S.; Srinivasan, N. Understanding Structural Variability in Proteins Using Protein Structural Networks. Curr. Res. Struc. Biol. 2022, 4, 134–145. [Google Scholar] [CrossRef]
- Accardo, F.; Leni, G.; Tedeschi, T.; Prandi, B.; Sforza, S. Structural and Chemical Changes Induced by Temperature and pH Hinder the Digestibility of Whey Proteins. Food Chem. 2022, 387, 132884. [Google Scholar] [CrossRef]
- Śledź, P.; Caflisch, A. Protein Structure-Based Drug Design: From Docking to Molecular Dynamics. Curr. Opin. Struct. Biol. 2018, 48, 93–102. [Google Scholar] [CrossRef]
- Garcia-Galan, C.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R.; Rodrigues, R.C. Potential of Different Enzyme Immobilization Strategies to Improve Enzyme Performance. Adv. Synth. Catal. 2011, 353, 2885–2904. [Google Scholar] [CrossRef]
- Rodrigues, R.C.; Berenguer-Murcia, Á.; Carballares, D.; Morellon-Sterling, R.; Fernandez-Lafuente, R. Stabilization of Enzymes via Immobilization: Multipoint Covalent Attachment and Other Stabilization Strategies. Biotechnol. Adv. 2021, 52, 107821. [Google Scholar] [CrossRef]
- Cipolatti, E.P.; Manoel, E.A.; Fernandez-Lafuente, R.; Freire, D.M.G. Support Engineering: Relation between Development of New Supports for Immobilization of Lipases and Their Applications. Biotechnol. Res. Innov. 2017, 1, 26–34. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Goncharova, S.S.; Sorokin, A.V.; Lavlinskaya, M.S.; Redko, Y.A.; Faizullin, D.A.; Baidamshina, D.R.; Zuev, Y.F.; Kondratyev, M.S.; Kayumov, A.R.; et al. Novel Biocatalysts Based on Bromelain Immobilized on Functionalized Chitosans and Research on Their Structural Features. Polymers 2022, 14, 5110. [Google Scholar] [CrossRef] [PubMed]
- Sorokin, A.V.; Goncharova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Zuev, Y.F.; Kondratyev, M.S.; Artyukhov, V.G. Complexation of Bromelain, Ficin, and Papain with the Graft Copolymer of Carboxymethyl Cellulose Sodium Salt and N-Vinylimidazole Enhances Enzyme Proteolytic Activity. Int. J. Mol. Sci. 2023, 24, 11246. [Google Scholar] [CrossRef]
- Aruna, V.; Chandrakala, V.; Angajala, G.; Nagarajan, E.R. Proteases: An Overview on Its Recent Industrial Developments and Current Scenario in the Revolution of Biocatalysis. Mater. Today Proceed. 2023, 92, 565–573. [Google Scholar] [CrossRef]
- Lalmanach, G.; Saidi, A.; Bigot, P.; Chazeirat, T.; Lecaille, F.; Wartenberg, M. Regulation of the Proteolytic Activity of Cysteine Cathepsins by Oxidants. Int. J. Mol. Sci. 2020, 21, 1944. [Google Scholar] [CrossRef]
- Holyavka, M.G.; Goncharova, S.S.; Redko, Y.A.; Lavlinskaya, M.S.; Sorokin, A.V.; Artyukhov, V.G. Novel Biocatalysts Based on Enzymes in Complexes with Nano- and Micromaterials. Biophys. Rev. 2023, 15, 1127–1158. [Google Scholar] [CrossRef] [PubMed]
- Kansakar, U.; Trimarco, V.; Manzi, M.V.; Cervi, E.; Mone, P.; Santulli, G. Exploring the Therapeutic Potential of Bromelain: Applications, Benefits, and Mechanisms. Nutrients 2024, 16, 2060. [Google Scholar] [CrossRef]
- Morellon-Sterling, R.; El-Siar, H.; Tavano, O.L.; Berenguer-Murcia, Á.; Fernández-Lafuente, R. Ficin: A Protease Extract with Relevance in Biotechnology and Biocatalysis. Int. J. Biol. Macromol. 2020, 162, 394–404. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Castañeda-Valbuena, D.; Morellon-Sterling, R.; Tavano, O.; Berenguer-Murcia, Á.; Vela-Gutiérrez, G.; Rather, I.A.; Fernandez-Lafuente, R. Bioactive Peptides from Fisheries Residues: A Review of Use of Papain in Proteolysis Reactions. Int. J. Biol. Macromol. 2021, 184, 415–428. [Google Scholar] [CrossRef]
- Baidamshina, D.R.; Koroleva, V.A.; Trizna, E.Y.; Pankova, S.M.; Agafonova, M.N.; Chirkova, M.N.; Vasileva, O.S.; Akhmetov, N.; Shubina, V.V.; Porfiryev, A.G.; et al. Anti-Biofilm and Wound-Healing Activity of Chitosan-Immobilized Ficin. Int. J. Biol. Macromol. 2020, 164, 4205–4217. [Google Scholar] [CrossRef]
- Hu, R.; Chen, G.; Li, Y. Production and Characterization of Antioxidative Hydrolysates and Peptides from Corn Gluten Meal Using Papain, Ficin, and Bromelain. Molecules 2020, 25, 4091. [Google Scholar] [CrossRef]
- Mohd Azmi, S.; Kumar, P.; Sharma, N.; Sazili, A.; Lee, S.-J.; Ismail-Fitry, M. Application of Plant Proteases in Meat Tenderization: Recent Trends and Future Prospects. Foods 2023, 12, 1336. [Google Scholar] [CrossRef]
- Fernández-Lucas, J.; Castañeda, D.; Hormigo, D. New Trends for a Classical Enzyme: Papain, a Biotechnological Success Story in the Food Industry. Trends Food Sci. Technol. 2017, 68, 91–101. [Google Scholar] [CrossRef]
- Aider, M. Potential Applications of Ficin in the Production of Traditional Cheeses and Protein Hydrolysates. JDS Commun. 2021, 2, 233–237. [Google Scholar] [CrossRef]
- Guarnieri, A.; Triunfo, M.; Scieuzo, C.; Ianniciello, D.; Tafi, E.; Hahn, T.; Zibek, S.; Salvia, R.; De Bonis, A.; Falabella, P. Antimicrobial Properties of Chitosan from Different Developmental Stages of the Bioconverter Insect Hermetia Illucens. Sci. Rep. 2022, 12, 8084. [Google Scholar] [CrossRef]
- Muthu, M.; Gopal, J.; Chun, S.; Devadoss, A.J.P.; Hasan, N.; Sivanesan, I. Crustacean Waste-Derived Chitosan: Antioxidant Properties and Future Perspective. Antioxidants 2021, 10, 228. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-Solubility of Chitosan and Its Antimicrobial Activity. Carbohyd. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Lavlinskaya, M.S.; Sorokin, A.V.; Mikhaylova, A.A.; Kuznetsov, E.I.; Baidamshina, D.R.; Saranov, I.A.; Grechkina, M.V.; Holyavka, M.G.; Zuev, Y.F.; Kayumov, A.R.; et al. The Low-Waste Grafting Copolymerization Modification of Chitosan Is a Promising Approach to Obtaining Materials for Food Applications. Polymers 2024, 16, 1596. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sorokin, A.V.; Goncharova, S.S.; Lavlinskaya, M.S.; Holyavka, M.G.; Faizullin, D.A.; Kondratyev, M.S.; Kannykin, S.V.; Zuev, Y.F.; Artyukhov, V.G. Carboxymethyl Cellulose-Based Polymers as Promising Matrices for Ficin Immobilization. Polymers 2023, 15, 649. [Google Scholar] [CrossRef]
- Tu, M.; Zheng, X.; Liu, P.; Wang, S.; Yan, Z.; Sun, Q.; Liu, X. Typical Organic Pollutant-Protein Interactions Studies through Spectroscopy, Molecular Docking and Crystallography: A Review. Sci. Total Environ. 2021, 763, 142959. [Google Scholar] [CrossRef]
- Vavra, O.; Damborsky, J.; Bednar, D. Fast Approximative Methods for Study of Ligand Transport and Rational Design of Improved Enzymes for Biotechnologies. Biotechnol. Adv. 2022, 60, 108009. [Google Scholar] [CrossRef]
- Secundo, F. Conformational Changes of Enzymes upon Immobilisation. Chem. Soc. Rev. 2013, 42, 6250. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Salvesen, G. Handbook of Proteolytic Enzymes, 3rd ed.; Academic Press: Boston, MA, USA; London, UK, 2013; ISBN 9780123822192. [Google Scholar]
- Beveridge, A.J. A Theoretical Study of the Active Sites of Papain and S195C Rat Trypsin: Implications for the Low Reactivity of Mutant Serine Proteinases. Protein Sci. 1996, 5, 1355–1365. [Google Scholar] [CrossRef]
- Bahamondes, C.; Illanes, A.; Pouchucq, L. Effect of External Diffusional Restrictions in Immobilized Enzymes in Stirred Reactors. Biocatal. Biotransfor. 2025, 1–17. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Woodley, J.M.; Fernandez-Lafuente, R. Is Enzyme Immobilization a Mature Discipline? Some Critical Considerations to Capitalize on the Benefits of Immobilization. Chem. Soc. Rev. 2022, 51, 6251–6290. [Google Scholar] [CrossRef] [PubMed]
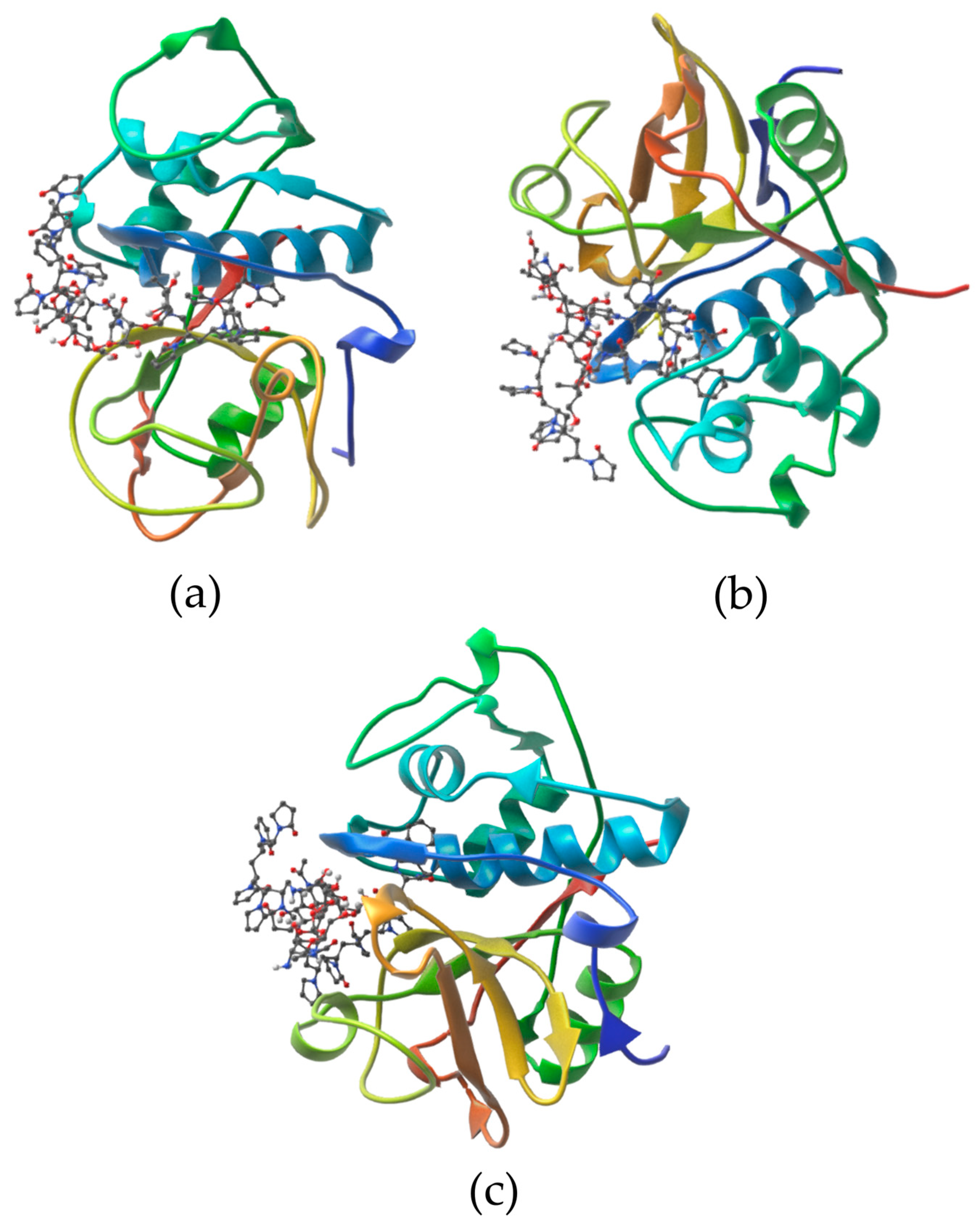

| Copolymer | PVP Content (% wt.) | PVP Molecular Weight | Z-Average Dh (nm) | PDI |
|---|---|---|---|---|
| Cs-g-PVP-1 | 51 | 10,000 | 189 ± 16 | 0.359 ± 0.02 |
| Cs-g-PVP-2 | 24 | 8000 | 136 ± 11 | 0.405 ± 0.03 |
| Affinity (kcal/mol) | Amino Acid Residues Forming | |
|---|---|---|
| H-bonds, Length, (Å) | Other Physical Interactions | |
| Bromelain | ||
| −8.1 | Asn19, 2.97; Asn21, 3.08; Gly66, 2.98 and 3.03; Ala136, 3.05; Gln141, 2.94 | Thr15, Ser16, Val17, Lys18, Asn19, Gln20, Asn21, Pro22, Gly24, Cys26 (αL1), Phe29 (αL1), Ala33 (αL1), Glu51 (αL2), Cys63, Lys64, Gly65, Gly66, Ala136, Phe140, Gln141, Leu156, Asn157, His158 (βR), Ala159 (βR), Thr161 (βR), Ile163 (βR), Ala178, Lys179, Trp180, Gly184, Trp185 |
| Ficin | ||
| −7.3 | Gly20, 2.87; Cys22, 2.95 and 3.25; Gly23, 2.90; Glu145, 3.09 and 3.23; Trp184, 3.11; His162 (βR), 3.30; Cys65, 2.85; Ser66, 2.89 and 3.27 | Gln19, Gly20; Arg21, Cys22, Gly23, Cys25 (αL1), Tyr60, Leu63, Cys65, Ser66, Gly68, Trp69, Met70 (αL3), Lys94, Lys95, Glu145 (αR2), Leu160, Asp161, His162 (βR), Trp184, Asn187, Trp188 |
| Papain | ||
| −6.1 | Gly20, 2.68 and 3.07; Cys22, 2.93 and 3.00; His159 (βR), 3.04; Trp177, 2.70 | Gln19, Gly20, Ser21, Cys22, Gly23, Cys63, Asn64, Gly65, Val133 (βR), Ala137, Gln142, Leu143 (αR2), Lys156, Asp158 (βR), His159 (βR), Ala160 (βR), Trp177, Gly180, Trp181 |
| Enzyme/Complex | Enzyme Content (mg g−1) * | Enzyme Complexation Efficiency (%) | Proteolytic Activity (U·mL−1) ** | Proteolytic Activity Complexation Efficiency (%) | Amidase Activity (U·mL−1) ** | Amidase Activity Complexation Efficiency (%) |
|---|---|---|---|---|---|---|
| Bromelain | − | − | 97.2 ± 7.2 | 100 | 7.1 ± 0.2 | 100 |
| Bromelain + Cs-g-PVP-1 | 20.1 ± 2.1 | 100 | 77.8 ± 4.5 a | 80 | 6.8 ± 0.2 | 95 |
| Bromelain + Cs-g-PVP-2 | 18.2 ± 1.2 | 90 | 84.5 ± 3.1 a | 87 | 6.9 ± 0.3 | 97 |
| Ficin | − | − | 96.5 ± 2.3 | 100 | 2.1 ± 0.2 | 100 |
| Ficin + Cs-g-PVP-1 | 13.3 ± 1.1 | 65 | 60.7 ± 3.7 b | 63 | 1.9 ± 0.1 | 89 |
| Ficin + Cs-g-PVP-2 | 9.1 ± 2.1 | 45 | 72.9 ± 6.1 b | 76 | 2.0 ± 0.1 | 93 |
| Papain | − | − | 95.4 ± 3.8 | 100 | 4.4 ± 0.4 | 100 |
| Papain + Cs-g-PVP-1 | 17.2 ± 2.3 | 85 | 76.2 ± 5.9 c | 80 | 4.1 ± 0.3 | 94 |
| Papain + Cs-g-PVP-2 | 10.5 ± 2.1 | 53 | 96.8 ± 4.2 | 100 | 4.3 ± 0.1 | 97 |
| Enzyme/Complex | Half-Life Time t1/2 (Days) |
|---|---|
| Bromelain | 1.53 |
| Bromelain + Cs-g-PVP-1 | 11.68 |
| Bromelain + Cs-g-PVP-2 | 12.95 |
| Papain | 3.69 |
| Papain + Cs-g-PVP-1 | 6.18 |
| Papain + Cs-g-PVP-2 | 6.65 |
| Ficin | 1.18 |
| Ficin + Cs-g-PVP-1 | 11.38 |
| Ficin + Cs-g-PVP-2 | 14.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lavlinskaya, M.S.; Sorokin, A.V.; Dubovitskaya, A.N.; Yutkina, A.I.; Kondratyev, M.S.; Holyavka, M.G.; Zuev, Y.F.; Artyukhov, V.G. Insights into Cysteine Protease Complexes with Grafted Chitosan–Poly(N-vinylpyrrolidone) Copolymers: Catalytic Activity and Storage Stability. Biophysica 2025, 5, 18. https://doi.org/10.3390/biophysica5020018
Lavlinskaya MS, Sorokin AV, Dubovitskaya AN, Yutkina AI, Kondratyev MS, Holyavka MG, Zuev YF, Artyukhov VG. Insights into Cysteine Protease Complexes with Grafted Chitosan–Poly(N-vinylpyrrolidone) Copolymers: Catalytic Activity and Storage Stability. Biophysica. 2025; 5(2):18. https://doi.org/10.3390/biophysica5020018
Chicago/Turabian StyleLavlinskaya, Maria S., Andrey V. Sorokin, Anastasia N. Dubovitskaya, Anastasia I. Yutkina, Maxim S. Kondratyev, Marina G. Holyavka, Yuriy F. Zuev, and Valeriy G. Artyukhov. 2025. "Insights into Cysteine Protease Complexes with Grafted Chitosan–Poly(N-vinylpyrrolidone) Copolymers: Catalytic Activity and Storage Stability" Biophysica 5, no. 2: 18. https://doi.org/10.3390/biophysica5020018
APA StyleLavlinskaya, M. S., Sorokin, A. V., Dubovitskaya, A. N., Yutkina, A. I., Kondratyev, M. S., Holyavka, M. G., Zuev, Y. F., & Artyukhov, V. G. (2025). Insights into Cysteine Protease Complexes with Grafted Chitosan–Poly(N-vinylpyrrolidone) Copolymers: Catalytic Activity and Storage Stability. Biophysica, 5(2), 18. https://doi.org/10.3390/biophysica5020018









