Abstract
We present a corrosion-resistant quadrupole ion trap mass spectrometer (QIT-MS) for the direct detection of biosignature gasses in chemically reactive planetary atmospheres, such as Venusian clouds. The system employs a Paul trap with hyperbolic titanium alloy electrodes and alumina spacers for chemical durability and precise ion confinement. An yttria-coated iridium filament serves as the thermionic emitter within a modular electron gun capable of axial and radial ionization. Analytes are introduced through fused silica capillaries and crescent inlets into a miniature pressure cell. The testbed integrates high-voltage RF electronics, pressure-regulated sample delivery, and FPGA-based control for real-time tuning. Continuous operation in 98% sulfuric acid vapor for over three months demonstrated no degradation in emitter or sensor performance. Mass spectra revealed H2SO4 fragmentation and thermally induced decomposition up to 425 K. Spectral variations with filament current and electron energy highlight thermal and electron-induced dissociation dynamics. Operational modes include high-resolution scans and selective ion ejection (e.g., CO2+, N2+) to enhance the detection of PH3+, H2S+, and daughter ions. The compact QIT-MS platform is validated for future missions targeting corrosive atmospheres, enabling in situ astrobiological investigations through the detection of biosignature gasses such as phosphine and hydrogen sulfide.
1. Introduction
In molecular biology, mass spectrometry (MS) plays a crucial role in characterizing biomolecules such as nucleic acids, proteins, lipids, and metabolites. While volatile acids like formic acid (HCOOH) or trifluoroacetic acid (TFA) are more commonly used, sulfuric acid (H2SO4) finds specialized applications in sample preparation due to its powerful protonating, dehydrating, and oxidative properties. Sulfuric acid is primarily utilized in “soft” electrospray ionization (ESI), chemical ionization (CI), and matrix-assisted laser desorption/ionization (MALDI), where it enhances ion formation and stabilizes reactive species. The efficient protonation of analytes leads to improved ionization efficiency in both positive and negative ion modes. Particularly valuable in the analysis of highly polar, ionic, and thermally labile compounds, H2SO4 is an essential component in applications such as environmental monitoring, bioanalytical chemistry, and petroleum analysis. Electron impact ionization (EI) is, on the other hand, a “hard” ionization technique that causes the extensive fragmentation of molecules. When H2SO4 vapors are ionized in vacuum using EI, the resulting mass spectrum primarily consists of fragment ions rather than a strong molecular ion (H2SO4+). EI-MS requires samples to be volatile and thermally stable, and sulfuric acid is used in pre-treatment steps (hydrolysis of biomolecules, destruction of matrix components, and oxidation of organic compounds) to modify or prepare samples.
In applications of the hydrolysis of biomolecules, H2SO4 is used to hydrolyze DNA and RNA into nucleotides, allowing for the analysis of nucleobases and phosphate modifications via liquid chromatography–mass spectrometry (LC-MS). In an LC-MS analysis of nucleotides, mild sulfuric acid hydrolysis (0.1–1 M H2SO4) helps break phosphodiester bonds, releasing nucleobases for characterization. Mild sulfuric acid hydrolysis is also used in conjunction with MALDI-MS to analyze glycosylation patterns in proteins. For biofuel research, the biomass degradation caused by sulfuric acid enables breakdown products to be analyzed via gas chromatography–mass spectrometry (GC-MS). H2SO4 is often employed in protein hydrolysis for keratin analysis in forensic and biochemical studies. In the destruction of matrix components present in complex biological or environmental samples, sulfuric acid can remove unwanted organic matter that may interfere with EI. In glycomics, mild sulfuric acid hydrolysis aids in breaking glycosidic bonds, enabling the MS analysis of glycans and carbohydrate structures. Through the oxidation of organic compounds, H2SO4 facilitates the oxidative decomposition of complex molecules into smaller, more analyzable fragments. Some nonvolatile or thermally labile compounds require derivatization before EI-MS analysis, and for those, sulfuric acid assists in chemical modifications that improve analyte volatility and stability. For example, acid aids in breaking down lipid membranes for the study of phospholipids and fatty acids, commonly esterified to their methyl esters, making them more volatile and detectable in EI-MS. Alcohols and phenols can be derivatized into sulfate esters, yielding distinct fragmentation patterns, enhancing their detectability, and aiding in structural elucidation. Sulfuric acid-treated compounds containing hydroxyl or amine groups may show stronger signals of molecular ions due to the stabilization of charge sites.
Recently, Bains et al. [1] modeled the stability of several sets of chemicals in concentrated sulfuric acid (CSA) as a solvent for supporting life on Venus. Often regarded as Earth’s twin due to its similar size and composition, Venus has an extremely harsh surface environment with temperatures exceeding 450 °C and a dense, CO2-rich atmosphere. However, its cloud layers, particularly between 48 and 70 km in altitude, present a more temperate environment where sulfuric acid aerosols dominate the atmospheric composition. These aerosols have been proposed as potential habitats for microbial life due to their unique chemistry and persistence in the Venusian atmosphere. Venus’s atmosphere contains high concentrations of sulfur dioxide (SO2), which undergoes photochemical reactions, leading to the formation of sulfuric acid droplets: (1) SO2 + hv (UV radiation) → SO + O; (2) SO + O2 → SO3; and (3) SO3 + H2O → H2SO4 (sulfuric acid formation). These droplets condense to form Venusian cloud layers, with varying concentrations and droplet sizes depending on altitude. The cloud layers are stratified into three distinct regions: (a) the upper cloud deck (~70 km altitude) with high levels of UV radiation and thin acidic haze; (b) the middle cloud deck (~58–65 km altitude) with stable sulfuric acid droplets, moderate temperatures (~30–60 °C), and potential for microbial life; and (c) the lower cloud deck (~48–55 km altitude) with larger droplets, higher acidity, and transition to denser atmospheric layers. Despite the extreme acidity and oxidizing nature of Venus’s clouds, there are three key factors that suggest the potential for life: (i) Moderate temperatures in the cloud layers are comparable to Earth’s extreme microbial habitats. (ii) There is the presence of water activity (though very low), which is a fundamental requirement for biological processes. (iii) Earth-based extremophiles (acidophilic microbes) thrive in highly acidic environments, suggesting that life forms with adaptations to sulfuric acid could theoretically exist. Microbial life in Venus’s clouds would need to withstand high acidity, limited water availability, and exposure to UV radiation. Hypothetical life forms could possess acid-resistant membranes composed of sulfur-based or polymeric compounds; use chemical energy sources, such as the oxidation of sulfur compounds, rather than photosynthesis; and exist in a dormant or spore-like state, surviving in sulfuric acid droplets and becoming metabolically active under favorable conditions.
Several missions have investigated Venus’s atmosphere, but the direct detection of life remains unconfirmed. Upcoming missions aim to analyze Venusian aerosols in greater detail: DAVINCI+ (NASA, 2031) will measure sulfuric acid and chemical signatures of habitability [2]; VERITAS (NASA, late 2020s) will focus on mapping Venus’s geology but may provide indirect insights into atmospheric chemistry [3]; and Venera-D (Russia, future concept) aims to explore Venus’s clouds with advanced instrumentation [4]. The possibility of microbial life within Venus’s sulfuric acid aerosols remains an open question, but the presence of stable liquid droplets, moderate temperatures, and chemical energy sources suggests that life could persist in this extreme environment. If life exists within Venus’s sulfuric acid aerosols, it would redefine the limits of habitability and suggest that acidic environments on other planets (e.g., Europa, Titan, exoplanets) could also support life. It would also have profound implications for understanding Earth’s early acidic environments and microbial evolution.
A Gas and Aerosol Mass Spectrometer (GAMS) is currently being developed at the Jet Propulsion Laboratory (JPL) as an instrument for the autonomous in-flight mass spectrometry analysis of gas, aerosol, and liquid samples. It utilizes a quadrupole ion trap mass spectrometer (QIT-MS), which has the International Space Station (ISS) flight heritage [5], which was first used in the Vehicle Cabin Atmosphere Monitor (VCAM) [6] in 2010 and upgraded in 2019 as a Spacecraft Atmosphere Monitor (S.A.M.) [7]. This study summarizes the progress made on the technology development efforts in terms of the QIT-MS sensor’s deployment on a Venus aerobot and use in characterizing the chemical composition of sulfuric acid aerosols. Section 2.1 describes the QIT-MS sensor and operation modes it supports in detail, followed by Section 2.2 where we describe the experimental setup. In Section 3, we present the mass spectra of the 98% sulfuric acid eluted into the QIT-MS through capillaries. The capillaries are either kept at room temperature or heated to 425 K to induce the thermal decomposition of H2SO4 during the capillary transport, Thermal decomposition is observed through the reduction in signals from H2SO4+ parent ion fragments and the simultaneous increase in signals for H2O+ and SO3+ daughter ion fragments.
The direct detection of potential biosignature gasses is a key objective in the search for life beyond Earth. Molecules such as PH3 and H2S, which have been proposed as indicators of microbial activity, are challenging to measure in situ due to their low abundance and chemical reactivity in oxidizing or corrosive environments. This work addresses these challenges by introducing a QIT-MS sensor engineered for operation in extreme atmospheres like that of Venus, where high concentrations of sulfuric acid and thermal decomposition processes complicate traditional analyses. The sensor’s robustness and trace detection capability make it particularly well suited for future life detection missions.
2. Materials and Methods
2.1. Sensor Design and Operation
The principle on which the QIT-MS sensor operates is illustrated in Figure 1 and is that of a Paul ion trap using the time-dependent r.f. electric field. The ion confinement volume is defined by (1) top cap, (2) ring, and (3) bottom cap electrodes with hyperbolic interior surfaces. Electrodes are made of a Ti alloy, machined to within 0.0002” tolerance, and electrically insulated from each other with two alumina spacer rings. In this architecture, the QIT-MS sensor acts as a pressure cell because end cap (bottom and top) electrodes have a small hole along the central z-axis direction, and the ring electrode has four small holes along the radial x-axis direction. These holes serve as pumping apertures, inlets, and outlets for injecting the electron beam and the ejection of trapped ions. The samples to be analyzed are introduced into the trap through the holes in the top cap electrode and the alumina spacer ring. An additional crescent cut-out in the top cap electrode (see Figure 2a, item 6) has been used in the experimental simulations [8] of Enceladus plume flyby and served for the injection of hypervelocity water ice particles into the QIT-MS sensor.
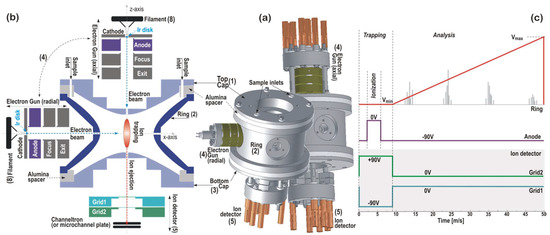
Figure 1.
(a) A schematic of the quadrupole ion trap mass spectrometer (QIT-MS) sensor showing its three main electrodes: (1) top cap, (2) ring, and (3) bottom cap. The sensor supports two interchangeable ionization configurations: radial ionization, where the electron gun (4) is mounted on the ring electrode, and axial ionization, where the electron gun is mounted on the top cap electrode. The ion detector (5) is mounted on the bottom cap electrode. (b) A cutaway view of the QIT-MS sensor geometry illustrating the electrode arrangement and ion trajectories. (c) The scan function illustrating the nominal operating mode, which consists of sequential trapping, ionization, and mass-selective ejection phases. During the ejection phase, the amplitude of the RF voltage on the ring electrode is linearly ramped, enabling the time-resolved detection of ions in order of an increasing mass-to-charge (m/z) ratio. Heavier ions are ejected later, consistent with the linearity of the voltage ramp.
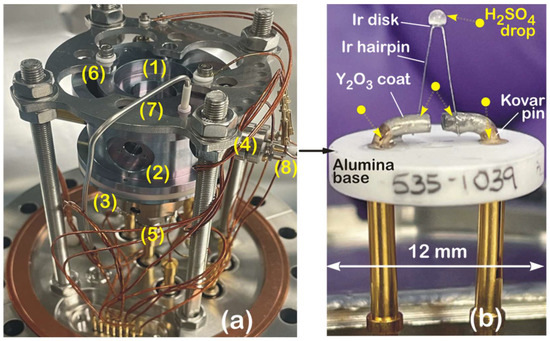
Figure 2.
(a) Photograph of QIT-MS sensor mounted a 6-inch ConFlat flange, highlighting key components: (1) top cap, (2) ring, and (3) bottom cap electrodes; (4) electron gun housing with integrated thermionic filament (8); (5) ion detector mounted on bottom cap; (6) cut-out for aerosol introduction; and (7) inlet tube for delivery of liquid sulfuric acid (H2SO4). (b) Close-up view of thermionic filament (8) within the gun assembly (4), showing exposure to 98% H2SO4 droplets at several electrical contact points and across surface of iridium disk thermionic emitter. See main text for discussion of filament degradation and operational implications.
The outer diameter of the end cap electrodes is 6 cm, and the compact design is preserved by running all electrical connections to the electrodes through mounting posts (see Figure 1a). The electron gun assembly (4) has a filament (8) with a mounted iridium (Ir) disk as the electron emitter. Four electrodes (cathode, anode, focus, and exit) deliver the electron beam along the x-axis (radial ionization) or the z-axis (axial ionization) and focus it in the center of the trap. The Ir disk electron emitter is an equipotential planar surface that is an indirectly heated (by 1.55 A d.c.) point source with a low (~0.5 eV) energy spread of emitted electrons. The cathode is housed in the equipotential electrode (cathode holder), followed by a simple A/D = 1 three-electrode lens. The exit electrode has the same potential as the bottom cap electrode. The focus electrode defines the position of the cathode image inside the trap. By changing the potential of the focus electrode, the transmission of the electron beam through the ring or the top cap electrode can be optimized for the maximum ion production rate at a given filament current.
The ion detector assembly (5) consists of either the Channeltron or the z-stacked microchannel plates (shown) operated in a pulse counting mode at 2.5 kV, which integrates the charge from the plates into one output signal. The ion detector is placed in a holder such that the central axis of the collection area is aligned with the z-axis of the trap. In front of the ion detector are two repelling grids: one for electrons (Grid 1) and one for ions (Grid 2). Both grids are biased such that electrons and ions are unable to pass through to the detector during the ionization phase (when the anode is grounded) and are kept at ground potential during the analysis phase, as illustrated in Figure 1c. Low mass cut-off and trapping properties are tuned with the trapping voltage Vmin. In contrast, the mass range is set with the maximum ramping voltage Vmax. In the nominal mode of operations, as shown in Figure 1c, the QIT-MS sensor is operated with electrically grounded top cap and bottom cap electrodes. For the measurements of 98% concentrated sulfuric acid samples, the ring electrode is driven by Vmin = 106 V and 882,832 Hz r.f. voltage with a 50 ms duty cycle (20 full mass spectra per second), and the maximum ejection voltage amplitude is Vmax = 876 V. The duration of the ionization phase is shortened to 1.25 ms to reduce the protonation effects when operating at higher sulfuric acid vapor pressures (~5 × 10−8 Torr) and prolonged to 8 ms to improve the counting statistics at lower sulfuric acid vapor pressures (~1 × 10−9 Torr).
The QIT-MS sensor, shown in Figure 2a, is rugged for continuous operations in sulfuric acid vapors, which is the most stressful test for the corrosive resistance of its components. For three months, starting on 1 December 2022, the QIT-MS sensor continuously acquired the mass spectra of the 98% concentrated sulfuric acid eluted into the ion trap via chemically inert capillaries. Shown are the QIT-MS sensor’s three electrically insulated electrodes: (1) the top cap, (2) ring, and (3) bottom cap; an electron gun assembly (4) in radial ionization configuration; and a Channeltron electron multiplier assembly (5) mounted on the bottom cap. The top cap has the crescent cut-out (6) previously used to inject microscopic water ice particles [8]. The QIT-MS sensor was operated with electrically grounded top and bottom electrodes. The ring electrode was driven by a voltage scan function illustrated in Figure 1c. The filament (8) of the electron gun assembly is detailed in Figure 2b, and it consists of an yttrium oxide-coated iridium disk (ES-535 from Kimball Physics Inc., Wilton, NH, USA) that is heated by conduction from an iridium hairpin and mounted on an alumina insulator base. The filament was exposed to droplets of 98% concentrated H2SO4 and kept enclosed in the vacuum chamber under laboratory conditions for a month to allow for the corrosion of electrical contacts. The vacuum chamber was then evacuated to ultra-high vacuum (UHV), and the filament current was slowly ramped up to 1.45 A, yielding a normal operating temperature of 1200 K and 1–2 μA electron emission current. Repeated tests showed no functional damage to the Ir disk, Ir hairpins, or Kovar pins, confirming the corrosive resistance of the electron gun assembly to the sulfuric acid. The electrons emitted from the hot surface of the Ir disk were extracted as a 70 eV electron beam from the electron gun assembly, focused in the center of the ion trapping region (see Figure 1b), and used for the ionization of sulfuric acid vapors.
We used the stainless steel tube (7) on the vacuum side to guide the 15 μm inner diameter chemically inert fused silica capillary into the QIT-MS sensor interior. Via the Valco fitting in the 6” ConFlat flange, the air side of a 60 cm long capillary was immersed in 98% concentrated liquid sulfuric acid. Liquid propagated through the capillary from atmospheric pressure into the QIT-MS sensor, where flash evaporation occurred in UHV. Acid vapors were electron impact-ionized during the first 9 ms of the duty cycle (see Figure 1c), during which ion fragments were confined by driving the ring electrode with an RF voltage of constant amplitude Vmin. By linearly ramping the ring electrode RF voltage amplitude during the 41 ms, confined ion fragments were subsequently ejected from the QIT-MS interior and detected by the Channeltron electron multiplier according to ion fragment mass-to-charge ratios (m/q). The results obtained are presented in Section 3.
Two additional modes of operation of the QIT-MS sensor, both relevant to this study, are shown in Figure 3. These are as follows: (a) the high-resolution low-sensitivity mode in Figure 3a and (b) the low-resolution high-sensitivity mode in Figure 3b. By slowing down the ring voltage ramp in Figure 3a, we isolated the narrow mass range from m/q = 31 Th to m/q = 36 Th in the search for the parent (PH3+, H2S+) and daughter ion fragments (P+, S+, PH+, HS+, PH2+). We pulsed the grids the same way as in the study of H2SO4 (see Figure 1c), with grounded top cap and bottom cap electrodes. The anode was pulsed during the 9 ms of the ionization phase at a higher filament current, I = 1.48 A, to increase the overall ionization rate. The main differences in the PH3 / H2S study are the higher vacuum chamber pressure, p = 7.1 × 10−7 Torr, and slower voltage ramp function (190 ms analysis phase). The results for the high-resolution low-sensitivity mode will be published elsewhere.
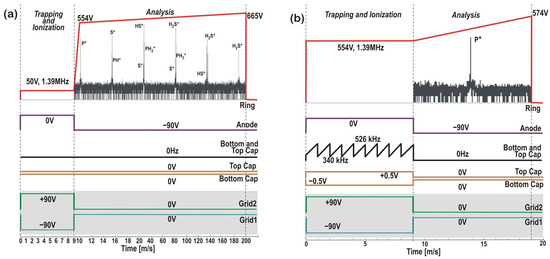
Figure 3.
Scan functions for (a) the high-resolution low-sensitivity mode and (b) the secular low-resolution high-sensitivity mode of operation at ring frequency νr = 1394363 Hz. Both end cap electrodes were grounded in the high-resolution mode, and the ring electrode voltage was driven at 5 duty cycles per second. Low-mass (30.45 Th) and high-mass (36.55 Th) cut-off voltages were set to 554 V and 665 V, respectively. In the secular mode (b), the cut-off voltages were set at 554 V and 574 V to detect only the trace P+ ions in the m/q = 30.45–31.55 Th mass range. At a repetition rate of 50 duty cycles per second, the top and bottom cap electrodes were phase-inverted and driven at 0.5 V under 334–514 kHz dipole frequency sweeps. Sweeps ensure that in the 9 ms ionization phase, all ions in the m/q = 32–44 Th mass range are removed from the ion trap before the analysis phase of P+ ions begins.
On the other hand, the low-resolution, high-sensitivity secular mode proposed in Figure 3b is designed to detect phosphorus species in Venus clouds. This particular mode of QIT-MS sensor operation was not used in this study, as both the top and bottom cap electrodes must be driven with a separate set of electronic boards. However, this mode was previously developed for collisional ionization studies at ~2 × 10−5 Torr pressures and used in MS-MS operations of the QIT-MS sensor to detect nonvolatile organics on Ocean Worlds [9]. One advantage of the secular mode is the enhanced signal from trace species (e.g., PH3 and H2S) concerning the dominant signal coming from one or more major species (e.g., CO2 and N2). Namely, higher operating pressures, longer ionization phases, and higher electron emission currents will proportionally produce more ions, the majority of which are CO2+, and will obstruct the detection of trace ions, as illustrated in Figure 4. If, during the ionization phase, both the top and bottom cap electrodes are driven with r.f. voltages composed of two or more secular frequencies, major species ions can be eliminated from the trap the instant they are created. Applied secular frequencies do not disturb the trapping of trace species that remain trapped and accumulated during the ionization phase and are detected in the analysis phase with increased precision.
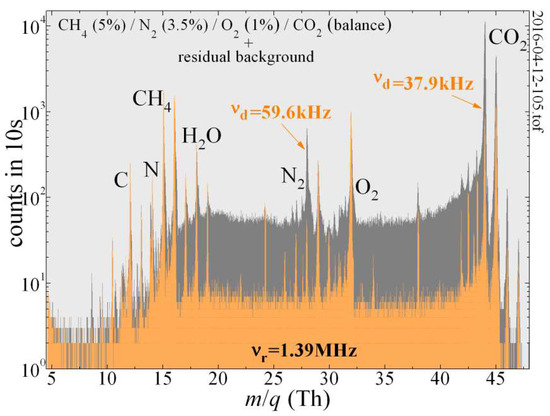
Figure 4.
The QIT-MS can selectively suppress major species in the Venus atmosphere and increase the signal-to-noise ratio for the detection of the trace species present in the residual background that are otherwise obscured. For the trapping amplitude of Vmin = 67.7 V and ring electrode frequency of νr = 1,394,363 Hz, the secular frequencies to suppress N2 and CO2 were νd = 59,617 Hz and νd = 37,891 Hz, respectively, with the 520 mV drive being active during the ionization phase.
The dual-frequency secular mode illustrated in Figure 4 demonstrates how the QIT-MS can detect trace gas components in the presence of major constituents CO2 and N2 when the pressure in the vacuum chamber was held at 4 × 10−6 Torr. The mass spectrum obtained by the QIT-MS while operating in the “nominal” mode (with grounded end cap electrodes; see Figure 1c) is given in a dark gray color. When operating in the “secular suppression” mode (orange color), a ~0.5 V auxiliary phase-inverted r.f. drive (similar to that in Figure 3b) is used during the ionization phase to selectively suppress CO2 and N2 signals from the mass spectrum. The exception in this case is that instead of the frequency sweep, the superposition of specific secular frequencies (59.6 kHz and 37.9 kHz) was applied simultaneously to the end cap electrodes during the entire ionization phase. Notable reductions in the measured N2 and CO2 peak strengths (and their collision-induced low-mass tails) were achieved. The secular suppression of these two major species improved the signal-to-noise ratio of the trace species present in the residual gas background that are otherwise indistinguishable in CO2 and N2 low-mass tails. Because we suppressed dominant ion species during their creation, the space charge effects were also reduced, and we observed the improved trapping efficiency of residual gas background ions that the presence of CO2+ and N2+ ion clouds would otherwise cause them to become destabilized and prevent them from being efficiently trapped.
2.2. Testbed Setup
The experimental testbed contains three sets of hardware modules with components presented as a block diagram in Figure 5a. The vacuum module consists of a vacuum chamber housing the QIT-MS sensor and supporting turbomolecular and roughing pumps.
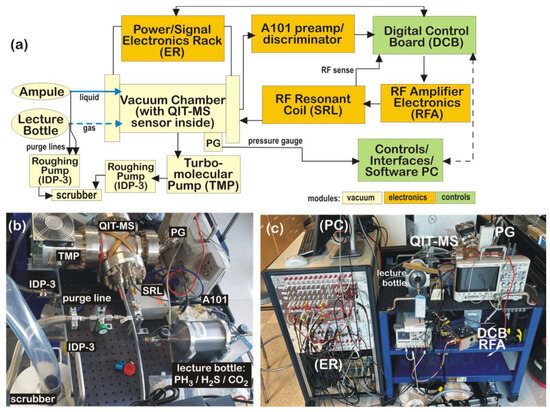
Figure 5.
(a) Functional diagram of main components of experimental setup; (b) sample inlet system used to elute liquid 98% concentrated sulfuric acid and calibrated PH3/H2S/CO2 gas mixture; (c) portable experimental testbed with interfaced components.
The electronics module comprises custom boards to drive the top, bottom, and central ring electrodes of the QIT-MS sensor; power supplies; and pulsers as well as readout boards to monitor the quality of the RF drives and vacuum integrity. The control module consists of a custom Digital Control Board (DCB) with a dualcore ARM Cortex A9 (Arm Limited, San Jose, CA, USA) processor and the Xilinx Zynq 7010 (Advanced Micro Devices Inc, Santa Clara, CA, USA) Field-Programmable Gate Array (FPGA) system-on-chip logic that allows for firmware and hardware programming. The DCB runs on embedded Linux to remotely control all aspects of QIT-MS operations, instrument health telemetry, and the acquisition of mass spectrum data. These components are identified in Figure 5b,c. Power/Signal supporting equipment is confined in the electronics rack (ER), which at the bottom has eight Kepco MST programmable power supplies in an RA 55 rack adapter (Kepco, Inc., Flushing, NY, USA) operated remotely via a GPIB controller (Kepco, Inc., Flushing, NY, USA). The next vertical component in the rack is the safety box, which monitors the pressure in the vacuum chamber. It turns off the Kepco power supply to the electron gun filament and shuts down the PHOTONIS high-voltage 3 kV feed to the ion detector (Channeltron/MAGNUM Electron Multiplier 5900 Series, Photonis Inc, Fiskdale, MA, USA). Above the safety box is a deck of National Instruments cards used to record signals in an analog manner. This is followed by a deck of voltage dividers, as well as a deck of counters, used to record ion detector signals via an Amptek A101 charge sensitive preamplifier/discriminator (Amptek Inc, Bedford, NH, USA) and a deck of pulsers used to drive the anode electrode in the Einzel lens of the electron gun assembly and protective grids in the ion detector assembly. The experimental setup has a remotely controlled oscilloscope for visualizing RF drives to QIT-MS electrodes and all pulser drives for the electron gun and detector assemblies. A PBR 260 Pirani/Bayard-Alpert gauge (PG, Pfeiffer Vacuum, Inc., Nashua, NY, USA) measures residual background pressure in the vacuum chamber. It is maintained at 5 × 10−11 Torr by the turbomolecular pump (TMP, Agilent Technologies, Inc., Santa Clara, CA, USA) backed by the Agilent IDP-3 roughing pump. An RF tank (SRL) is inductively coupled to the QIT-MS sensor and resonantly amplifies the RF drive to the central electrode generated by the DCB and RFA. The SRL is a basket-weave of low-loss Litz wire on a 3D-printed polyetherimide mandrel (5.1 cm × 12.7 cm × 12.7 cm). The experimental testbed is controlled and operated by a Linux workstation (PC) using a suite of programs developed for QIT-MS operations, monitoring and controlling equipment, data acquisition, and analysis [5].
3. Results and Discussion
Capillaries transported the liquid 98% H2SO4 acid from sealed glass ampoules into the QIT-MS sensor, where droplets boil off in vacuum, and vapors are ionized and analyzed. Capillary lengths and diameters were chosen so that the rate of sulfuric acid elution into the QIT-MS vacuum chamber would not yield vapor pressures above 1 × 10−7 Torr. The recorded mass spectra are shown in Figure 6 such that red, green, and blue spectra denote accumulations during the first day of each month, and the orange spectrum is the final measurement for 28 February 2023. Capillaries were held at room temperature during the first three weeks of December. In the first week, capillary conductance was chosen to yield vapor pressures above 4 × 10−8 Torr to study protonation and water attachment under enhanced ionization rates (prolonged ionization phase with increased electron gun filament current). During the second and third weeks, we used capillaries with reduced conductance to lower the vapor pressure and suppress water attachment. We operated the QIT-MS sensor at shorter ionization pulses with reduced filament currents to suppress protonation. We also started to leak CO2 into the vacuum chamber to compare the production rate of atomic oxygen ions at m/q = 16 Th due to the electron impact fragmentation of CO2 and H2O. During the last week of December, we gradually heated capillaries to 425 K to induce the thermal decomposition of H2SO4 and SO3 and observe the increased production of oxygen ions.
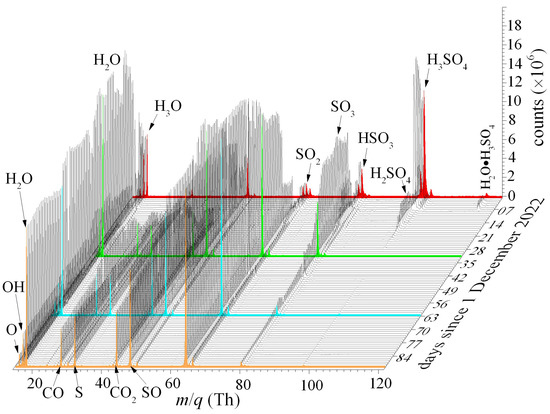
Figure 6.
The mass spectra of the 98% H2SO4 eluted through capillaries into the QIT-MS sensor, recorded over a three-month period of continuous operation. The red, green, and blue spectra correspond to measurements taken on the first day of each respective month. The orange spectrum represents the final measurement acquired on 28 February 2023. Progressive spectral changes over time reflect sensor response evolution under prolonged exposure to concentrated sulfuric acid.
In Figure 7a, we show an example of a QIT-MS sensor being operated at a nominal sensitivity of ~2 × 1012 cps/Torr, with the mass spectrum recorded during the 10 min elution of 98% H2SO4 at room temperature and 1.2 × 10−9 Torr vapor pressure. We detect all major ion fragments of H2SO4 with peak intensities in qualitative agreement with the previous results of Snow and Thomas [10]. The largest difference is between our measurement of the HSO3+/H2SO4+ = 1.66 ion fragment ratio, for which Snow and Thomas [10] reported a 1.27 value.
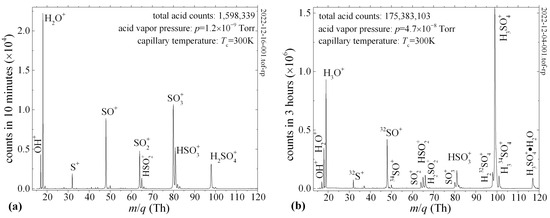
Figure 7.
Mass spectrum of 98% H2SO4 eluted through capillary at room temperature into QIT-MS sensor. (a) Major ion fragments detected during 10 min. (b) At vapor pressures above 4 × 10−8 Torr, trapped ion fragments experience protonation and attachment of water molecules (H3SO4•H2O).
Besides the differences in electron impact energies, this discrepancy is due to the enhanced protonation of SO3+ (see Figure 7b) and twice as many protonated water ions. Namely, we used 70 eV electron beams [11] with capillaries held at 300 K, whereas Snow and Thomas [10] used 50 eV electron beams and a 351 K sample temperature. Thus, the main causes of the differences in the measured fragmentation patterns are the higher sample temperatures and lower electron impact energies reported by Snow and Thomas [10]. For example, at electron impact energies of 70 eV, we measure the ratio SO3+/SO2+ = 2.2 at room temperature, whereas Briggs et al. [12] report a value of 0.83 for a sample temperature of 743 K. For this same ratio at the sample temperature of 351 K, Snow and Thomas [10] report the values of 6.7 and 3.6 at electron impact energies of 20 eV and 50 eV, respectively. These examples indicate that sulfuric acid’s thermal decomposition and protonation are as important as electron impact energies and in classifying electron impact fragmentation patterns. For spaceflight applications [5], the vacuum chambers house a CAPACITorr® Z200 NEG (Milan, Italy) non-evaporative getter pump with a pumping speed of 200 L/s for hydrogen, which will suppress the protonation of sulfuric acid ion fragments.
We also observed the thermal decomposition of H2SO4 and SO3 in the mass spectrum by heating the capillary wall to a temperature of Tc = 425 K. Figure 8a shows that at this temperature, the gas-phase relaxation of H2SO4 to the new equilibrium (SO3 + H2O) inside the capillary is complete, demonstrated by the disappearance of the parent H2SO4+ ion and the suppression of the daughter SO3+ ion due to SO2 + O equilibrium. As a result of the thermal decomposition of H2SO4, before it is subjected to electron impact fragmentation, the water peaks at m/q = 17 Th (OH+) and m/q = 18 Th (H2O+) had increased intensities. In addition, the electron impact fragmentation of SO3 [12] contributed significantly to the intensities of the SO+ and SO2+ daughter ion fragments. Figure 8b shows the effects of the thermal decomposition of SO3 (SO2 + O) that reduce the SO3+/SO2+ ratio from 0.58 to 0.05. The intensity of the O+ peak at m/q = 16 Th is 45% due to the thermal decomposition of SO3 and 58% due to the electron impact fragmentation of CO2, which we introduced as a micro-leak into the vacuum chamber through a separate capillary.
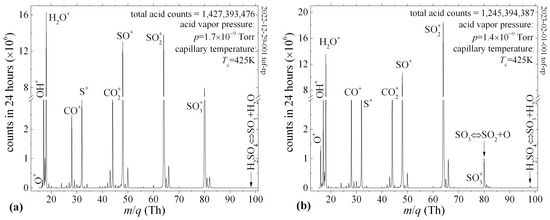
Figure 8.
The mass spectrum of the 98% H2SO4 eluted through a heated capillary into the QIT-MS sensor. Capillary temperature Tc favored the shift in the gas-phase equilibrium toward the thermal decomposition of H2SO4 (a) and SO3 (b) as a source of increased oxygen ion production.
4. Conclusions
This study presents the development, modification, and validation of a quadrupole ion trap mass spectrometer (QIT-MS) sensor system engineered for atmospheric analysis in extreme planetary environments, such as the cloud layers of Venus. The sensor is based on a Paul trap design with hyperbolic titanium alloy electrodes and alumina insulators, ensuring mechanical precision and corrosion resistance. A rugged electron gun assembly supports both axial and radial ionization, featuring an yttria-coated iridium filament that maintained stable performance during continuous three-month exposure to 98% sulfuric acid vapor.
The portable experimental testbed integrates three key modules: (1) a vacuum module with turbomolecular and roughing pumps; (2) an electronics module with custom RF drives, voltage ramps, and detector pulsing circuits; and (3) a control module using a Linux-based Digital Control Board with ARM + FPGA logic for remote operation, telemetry, and real-time mass spectral acquisition.
The mass spectra obtained under varying vapor pressures and capillary temperatures revealed characteristic sulfuric acid fragmentation patterns influenced by both thermal decomposition and electron impact ionization. Elevated temperatures promoted the full dissociation of H2SO4 and SO3, increasing the intensity of oxygen-bearing fragments (e.g., O+, OH+, SO2+). Secular frequency suppression modes enabled the selective ejection of dominant species (CO2+, N2+), enhancing the trace detection of astrobiologically relevant gasses such as PH3 and H2S. These molecules are considered candidate biosignatures, and their unambiguous detection in situ is essential for missions targeting the direct detection of life in chemically extreme planetary environments. The QIT-MS sensor demonstrated a nominal sensitivity of ~2 × 1012 cps/Torr, confirming its readiness for integration into future spaceflight missions aimed at biosignature gas analysis and astrobiological exploration.
Author Contributions
Conceptualization, D.N.; methodology, D.N. and S.M.M.; software, S.M.M. and D.N.; validation, D.N. and S.M.M.; formal analysis, D.N.; investigation, D.N.; resources, S.M.M.; data curation, D.N.; writing—original draft preparation, D.N. writing—review and editing, D.N.; visualization, D.N.; supervision, D.N.; project administration, D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data presented in this study are available on request from the corresponding author due to export control restrictions. Access will be granted on a case-by-case basis, subject to compliance with applicable regulations.
Acknowledgments
The authors would like to thank Julio Urrutia (JPL) for his help in preparing capillary inlets for sulfuric acid measurements and designing the gas manifold for phosphine introduction, as well as late Frank Maiwald (JPL) for his programmatic support. This research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with the National Aeronautics and Space Administration (80NM0018D0004). Government sponsorship is acknowledged. © 2025 California Institute of Technology.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bains, W.; Jurand Petkowski, J.; Zhan, Z.; Seager, S. Evaluating Alternatives to Water as Solvents for Life: The Example of Sulfuric Acid. Life 2021, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- NASA to Explore Divergent Fate of Earth’s Mysterious Twin with Goddard’s DAVINCI+. Available online: https://www.nasa.gov/solar-system/nasa-to-explore-divergent-fate-of-earths-mysterious-twin-with-goddards-davinci (accessed on 21 April 2025).
- NASA. VERITAS Overview. Available online: https://science.nasa.gov/mission/veritas/overview/ (accessed on 21 April 2025).
- Zasova, L.; Gregg, T.; Burdanov, A.; Economou, T.; Eismont, N.; Gerasimov, M.; Gorinov, D.; Hall, J.; Ignatiev, N.; Ivanov, M.; et al. Venera-D Mission Concept to Study Atmosphere, Surface and Plasma Environment: Phase II Report of the Venera-D Joint Science Definition Team. 2019. Available online: https://ntrs.nasa.gov/citations/20190001913 (accessed on 21 April 2025).
- Madzunkov, S.M.; Nikolić, D.; Simcic, J.; Belousov, A.; Gonzalez, M.P.; Darrach, M.R. Data analysis and isotopic ratios measured onboard the Spacecraft Atmosphere Monitor. Int. J. Mass Spectrom. 2022, 477, 116847. [Google Scholar] [CrossRef]
- Darrach, M.R.; Chutjian, A.; Bornstein, B.J.; Croonquistet, A.P.; Garkanian, V.; Hofman, J.; Karmon, J.; Kenny, J.; Kidd, R.D.; Lee, S.; et al. Trace chemical and Major Constituents Measurements of the International Space Station Atmosphere by the Vehicle Cabin Atmosphere Monitor. In Proceedings of the 42nd International Conference on Environmental Systems, San Diego, CA, USA, 15–19 July 2012. [Google Scholar] [CrossRef]
- Darrach, M.; Madzunkov, S.; Kidd, R.; Bae, B.; Zhong, F.; Simic, J.; Malone, C.; Belousov, A.; Maiwald, F.; Gonzales, M. Update on the Spacecraft Atmosphere Monitor Technology Demonstration Project. In Proceedings of the 50th International Conference on Environmental Systems, Virtual, 31 June 2020; Available online: https://hdl.handle.net/2346/86313 (accessed on 21 April 2025).
- Belousov, A.; Miller, M.; Continetti, R.; Madzunkov, S.; Simcic, J.; Nikolic, D.; Maiwald, F.; Waller, S.; Malaska, M.; Cable, M. Sampling Accelerated Micron Scale Ice Particles with a Quadrupole Ion Trap Mass Spectrometer. J. Am. Soc. Mass Spectrom. 2021, 32, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Waller, S.E.; Belousov, A.; Kidd, R.D.; Nikolić, D.; Madzunkov, S.M.; Wiley, J.S.; Darrach, M.R. Chemical Ionization Mass Spectrometry: Applications for the In Situ Measurement of Nonvolatile Organics at Ocean Worlds. Astrobiology 2019, 19, 1196–1210. [Google Scholar] [CrossRef] [PubMed]
- Snow, K.B.; Thomas, T.F. Mass spectrum, ionization potential, and appearance potentials for fragment ions of sulfuric acid vapor. Int. J. Mass Spectrom. Ion Process. 1990, 96, 49–68. [Google Scholar] [CrossRef]
- NIST. Standard Reference Database 1A, Software Version: 3.0; National Institute of Standards and Technology: Gaithersburg, MD, USA, 2023. [CrossRef]
- Briggs, J.P.; Hudgins, R.R.; Silveston, P.L. The fragmentation of SO3 in electron impact ionization sources. Int. J. Mass Spectrom. Ion Phys. 1976, 20, 1–5. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).