Abstract
Neurodegenerative disorders include Alzheimer’s and Parkinson’s, both of which lead to progressive loss of neurons resulting in the severe loss of cognitive and motor functions. These diseases are among the heavy burdens on global healthcare systems largely because there is no cure, and current treatments apply almost entirely to controlling symptoms rather than disease progression. Recent advances in 3D printing and bioprinting technologies now open the way to overcome these challenges and form patient-specific models and therapeutical tools closely simulating the complex environment of the human brain. It then further illustrates how this technological integration with the aid of 3D printing, coupled with microfabrication and biosensing technologies, transforms drug-screening platforms as well as develops customization in medicine. For example, one can form highly intricate and multi-materially composed structures to better facilitate one’s study or test into some new therapeutic possibilities using methodologies of stereolithography and selective laser sintering. Moreover, 3D printing allows the creation of organ-on-a-chip models that simulate brain-like conditions, which may help identify specific biomarkers and evaluate new options of therapy. On the other hand, bioprinting methods based on neural cells combined with scaffolds mimicking native tissue dramatically transform regenerative medicine. New pathways in neural tissue development and implantable devices are now being brought forth, which can be tailored to the needs of individual patients. These advances bring not only greater precision in terms of the therapy that can be delivered but also 3D printing of implantable microelectrodes able to determine real-time biomarkers responsible for neurodegenerative diseases. Thus, this review highlights the robust impact that might be brought forth on the diagnosis and treatment of these neurodegenerative diseases via 3D printing technologies toward more effective management and personal solutions for healthcare.
1. Introduction
Neurodegenerative disorders cause progressive loss of function and eventual death of nerve cells in the brain or peripheral nervous system. Gradual degeneration of brain cells and the connection that hold them together is primarily due to the accumulation of proteins like amyloid beta, tau, or alpha-synuclein [1]. Neurodegenerative diseases do not pose an imminent threat to death but entail poor quality of life on account of long-term drug cycles and attract high costs in terms of finance and psychology; the professional care would cost USD 1 trillion worldwide. There is also insufficient medical evidence presently existing to establish that these therapies are able to retard the progression of these disorders. It is also worth noting that, as of yet, no effective therapies have been found [2].
Researchers are leveraging the versatility of 3D printing to develop the understanding of neurodegenerative disorders by integrating it into drug-screening platforms and drug delivery systems for individual patients. Integration of 3D printing, microfabrication technologies, and biosensor technologies facilitates the production of realistic drug-screening environments and microfluidic devices mimicking the complex anatomy and function of the human brain with great precision through 3D printing [3]. Figure 1 shows how these techniques help to manufacture implantable microelectrodes, organ-on-a-chip systems, and other therapeutic devices. Most significantly for neurologic disorders, microfluidic technologies are changing drug delivery technology. The systems allow for the assembly of customized implants and drug delivery devices with maximized therapy impact. Scientists, with the assistance of sophisticated technologies, can prepare customized drug delivery systems suitable to the needs of individual patients and thereby achieve maximal therapy success against diseases like Alzheimer’s and Parkinson’s disease [4].
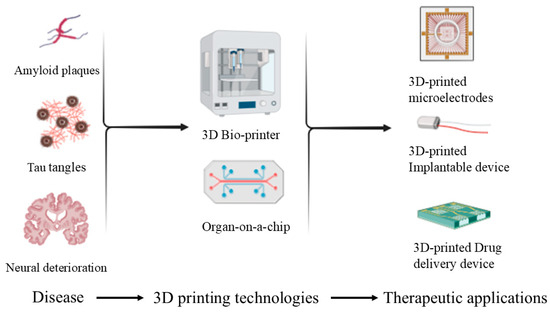
Figure 1.
Schematic depicting key pathological markers in neurodegenerative diseases (amyloid plaques, tau tangles, and neural deterioration) and how 3D printing (e.g., 3D-printed microelectrodes, implantable devices, drug delivery devices, and organ-on-a-chip models) can offer advanced diagnostic and therapeutic strategies.
Three-dimensional printing or additive manufacturing makes objects by adding layers with automatic equipment. It relies on 3D scanning and CAD to create precise models. There are two main ways to create 3D digital models: by hand modelling or sculpting. Three-dimensional modelling is to replicate the appearance and shape of an object as a digital model. The progress of this technique has significantly increased its ability to create complex shapes that are a problem for standard manufacturing techniques. Hence, it provides greater design freedom and efficacy in a diverse range of processes. An STL file is an electronic file that is commonly used in 3D printing and computer-aided design (CAD). STL file is short for Stereolithography and is a greatly used 3D printing technology. Before a 3D model is printed from an STL file, the model must first be prepared using a program named a “slicer”. The model is divided into a succession of thin sections, and a G-code-full-of-instructions file is created from the STL file and submitted to a printer. The 3D printer uses a set of cross-sections to create a model by adding G-code-controlled layers of liquid, powder, or sheet material. Layers are similar to the virtual cross-sections of the CAD model, and the final shape of a model is established by accumulating or fusing together these layers. The main advantage of this process is its potential to create essentially any form or geometric model [5].
Additive manufacturing innovations are making revolution in the process of research and treatment of neurodegenerative diseases. Technologies like digital light processing (DLP) use quick photopolymerization to create highly intricate parts for implantable microelectrodes specifically designed for early electrochemical detection of minute changes in biomarkers of neurodegenerative diseases like Alzheimer’s and Parkinson’s [3]. Its precision holds the promise of making highly sensitive sensors that can react to trace amounts of these vital biomarkers [6]. Stereolithography (SLA) is another vat polymerization technology known for its very high resolution and smooth finish; it is a critical component in the manufacturing of patient-specific anatomical brain models from imaging [7]. These models become an indispensable guide to neurosurgeons preparing for interventions for neurodegenerative diseases giving them a solid model of the intricate neural structures involved in their operations. In addition to this, multiphoton polymerization’s (MPP’s) nanoscale resolution provides the potential to make three-dimensional micro- and nanoscale structures that are already seeing use as neural tissue engineering tools through the formation of highly intricate scaffolds capable of supporting potentially the regeneration of damaged neurons, a goal at the very center of therapeutic strategies intended for such debilitating diseases [8].
Besides vat polymerization, various other additive manufacturing methods are playing important roles in many applications. Powder-based methods like selective laser sintering (SLS) are used to create tailored assistive devices appropriate to the unique needs of individuals with motor disabilities due to diseases like Parkinson’s disease, thus promoting greater autonomy and improved quality of life [9]. For metallic part applications, selective laser melting (SLM) and direct metal laser sintering (DMLS) enable the production of strong and durable metallic implants, including tailored cranial plates and spinal fusion implants, and specialized surgical tools with fine details that improve the precision of neurosurgical interventions [10]. Droplet-based methods, as represented by aerosol jet printing (AJP), are propelling the development of flexible electronic sensors for wearable or implantable devices that can continuously monitor physiology or facilitate targeted drug delivery and stimulation [11]. Binder jet printing (BJP) offers a cost-effective pathway for the fabrication of tailored drug formulations with controlled release properties [12], and poly jet printing (PJP) is superior in the fabrication of highly accurate, multi-material anatomical brain models for surgical planning and patient education [13]. Finally, extrusion-based printing methods, including fused deposition modelling (FDM), offer an easy pathway for the fabrication of large-scale educational brain models and tailored assistive devices, and have the potential to progress in bioprinting neural tissue constructs for vital disease research [14]. Figure 2 shows a flow chart depicting the classification of these 3D printing techniques.
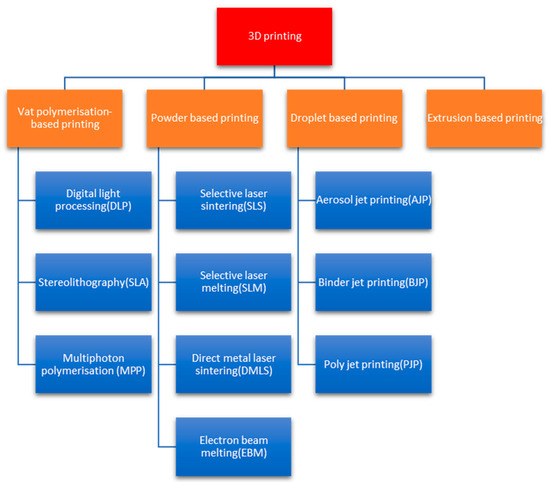
Figure 2.
Three-dimensional printing techniques.
Stereolithography, selective laser sintering, and fused deposition modelling are the most widely used 3D printing techniques [15]. Figure 3a presents the multilayer technique of stereolithography, which was initially developed in the 1970s by a Japanese researcher named Dr. Hideo Kodama, who used UV light to cure photosensitive polymers. In the study, a digital light projector with a computer-driven fabrication stage or a computer-controlled laser beam is used to project a design onto a resin surface through the process of photopolymerization. This causes the resin to stick to a support platform by solidifying it to a certain depth in the pattern [16]. After the procedure is finished, the fabrication bed is taken out, and the object that was created is placed on it. It is then broken free and cleaned using chemicals that contain alcohol. The component is either further cured under intense UV radiation for a brief period or post-treated with additional chemicals, depending on the material used [17]. Figure 3b shows selective laser sintering (SLS), which was developed and patented at the University of Texas in the mid-1980s with support from the Defence Advanced Research Projects Agency (DARPA) by Dr. Carl Deckard and his academic adviser Dr. Joe Beaman. By binding the material at particular locations in space that are determined by a 3D model, the powdered material is fused to produce solid structures using the SLE printing process. Selective laser melting (SLM) uses a similar method, but before the material is sintered, it is fully melted, allowing for a variety of properties. Here, the powder is exposed to a laser, which solidifies it and creates a thin coating of material. The component is constructed layer by layer, bottom to top until it is completed. There is no waste because the remaining powder may be reused [18]. Figure 3c shows fused deposition modelling (FDM), which was developed by S. Scott Crump in the late 1980s. FDM is an additive manufacturing process that builds an actual product directly from a computer-aided design model by layer-by-layer deposition of a feedstock plastic filament material extruded via a nozzle [19]. Materials are delivered to the head of an extrusion nozzle through a coil of wire or thermoplastic filament. The nozzle head not only controls the flow but also warms the material to a specific temperature. Stepper motors are frequently used to move the extrusion head in the z-direction and alter the flow according to the parameters. The head of the mechanism may move in both horizontal and vertical directions, and it is managed by a microprocessor-based computer-aided manufacturing software program. The article highlights upon the advantages of 3D printing technologies in healthcare with the application of 3D bioprinting and analyzes their integration into the management of neurodegenerative disorders.
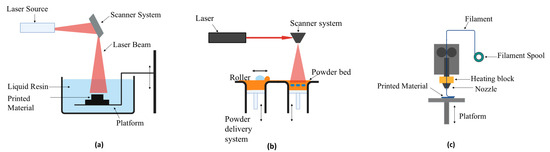
Figure 3.
(a) Fused deposition melting. (b) Stereolithography. (c) Selective laser sintering.
2. Benefits of 3D Printing in Healthcare
By combining many pharmaceuticals into a single solid dosage form, 3D-printed solid formulations can improve patient adherence, speed up deglutition, customize the release profile, and create novel drugs for which there is not a formulation right now. Extended-duration release joint replacement treatment, medical prostheses, and cardiovascular applications are the main uses for 3D-printed implants like stents and other medical devices. Three-dimensional printing has also been used to make locally applied drugs, such as medicated contact lenses, microneedles, and wound dressings [20]. The implantable tissue model is made possible by 3D printing. A few instances include the 3D printing of artificial skin for patients who have burn damage [21] or heart valve replication that modifies the valve’s stiffness by using a combination of biomaterials and cells [22].
In the future, pharmaceutical companies may evaluate the toxicity of new drugs using bioprinted organs rather than animal models [23]. One-of-a-kind implants, surgical tools, and guides may be made with 3D printing. Consequently, prostheses and surgical equipment may be tailored at a lesser cost thanks to additive manufacturing [24]. The benefits of 3D printing in education include the capacity to model various physiologic and pathologic anatomies from a vast photo library, the consistency and dependability of the 3D-printed model, and the ease with which institutions especially those with limited resources can exchange 3D models [25].
With the assistance of medical imaging information, patient-specific anatomical models of the nervous system and the brain can be created, giving researchers and clinicians physical models of the structures in question [26]. This enables improved understanding of the spatial intricacies and structural changes of diseases such as Alzheimer’s and Parkinson’s diseases beyond the confines of standard two-dimensional imaging. Visualization and manipulation of models provide invaluable information regarding the process of disease progression and spatial interactions and make an invaluable contribution to research efforts to clarify disease pathophysiology and improve educational materials for clinicians and patients, thus enabling a more intuitive understanding of subtle but important anatomical changes.
Apart from anatomical models, sophisticated 3D printing techniques using vat polymerization technology are important to the manufacture of sophisticated diagnostic devices [27]. Among such technologies is the fabrication of implantable microelectrodes with uses for the electrochemical detection of certain biomarkers of neurodegenerative disease, like amyloid beta, tau protein, and alpha-synuclein [28]. Such technologies’ precision and resolution enable the fabrication of intricate sensors with enhanced sensitivity and specificity toward their target biomarkers. Moreover, 3D printing enables the fabrication of realistic head phantoms, which are necessary for surgery planning, simulation, and training in the neurosurgery domain [29]. Typically based on patient-specific imaging data, it provides a realistic environment for the simulation of sophisticated surgeries, which allows neurosurgeons to improve their skills and preparedness, thus contributing to improved surgical outcomes for neurodegenerative disease patients.
3. Three-Dimensional Bioprinting
Three-dimensional bioprinting emerges as a creative and practical tool for creating scaffolding among the manufacturing methods for creating 3D models. Printing materials and cells together are referred to as bioprinting [30]. Three-dimensional bioprinting creates 3D models by depositing or solidifying material in successive stages by involving stem cells or cells produced from tissue samples. These cells are held together by a collagen scaffold or binding gel [31]. Then, over the 3D-printed organs and body parts, the patient’s tissue would grow and eventually replace the cells. Furthermore, bioinks facilitate the attachment and proliferation of living cells.
They may print materials like polymer resins, metal, plastic, and rubber, which can then be used by the medical sector to make surgical equipment, implants, and other items [32]. A key distinction between 3D bioprinting and 3D printing is the kind of ink that is ejected from the nozzle. Three-dimensional bioprinting uses bio-based materials in the ink’s manufacturing called biomaterial ink. Biomaterial ink or bioink is frequently used in medical treatments; it can communicate with or react to live cells [33,34]. Bioink needs to be able to sustain biochemistry in addition to providing mechanical strength. Chemical composition, viscosity, elasticity, biodegradability, and biocompatibility are a few characteristics to be considered while selecting bioink, Additionally, the bioink used to create tissues must permit cell division, growth, proliferation, and communication [35].
Furthermore, bioinks facilitate the attachment and proliferation of living cells. Hydrogel biomaterials known as bioinks enable cells to naturally produce their extracellular matrix. Researchers also use bioprinting to make miniature duplicates of human organs [36]. Skin with an intricate and accurate pore structure can now be bioprinted. Moreover, through this method, the medical world can create a bone matrix that perfectly resembles a bone. The research on the anatomy of living cells that can contribute to tissue repair enhances the methodology of 3D bioprinting [37]. This new method consists of piling up layers of living cells for artificially creating tissues. Putting these cells together with biological elements such as collagen, fibrin, and gelatin enables the formation of entire tissues and organs, therefore enhancing the effectiveness of the procedure. These elements will enable scientists to make the bioprinted constructs more functional and realistic, therefore enhancing regenerative medicine and tissue engineering [38].
The technique begins by developing an architectural design based on the basic framework of the aimed tissue or organ [39]. The 3D bioprinting approach is rebuilding the structural model level by level making use of the bioink mixed with resident cells or added cells at the time of the print being completed [40]. Three-dimensional bioprinting will provide a speedier, better value, and more substantial opportunity for drug evaluation and screening during pharmaceutical research. It also makes it possible for new biomedical technologies such as better medication delivery systems and stents that enable simpler vein anastomosis to be developed [41].
Each of the many bioprinting techniques has its benefits and drawbacks. Applied in both 2D and 3D bioprinting, one often used method is the inkjet approach. Using heat and electrical pressures, this technique precisely deposits in a non-contact way individual micro-droplets of bioink onto a substrate. Because of its adaptability, inkjet bioprinting is becoming a preferred method for building intricate biological structures [42]. Compared with other techniques, inkjet bioprinting is quick and inexpensive, and it permits cell survival rates of over 85%. However, it lacks precision and is unsuitable for intricate tissue restoration. Another widely used technique for bioprinting is microextrusion. In microextrusion, bioink is ejected through a micro-nozzle, and the flow of bioink is controlled by mechanical or pneumatic forces. A computer-designed pattern is followed in the continual distribution of the bioink [43]. High viscosity and high cell density bioinks may be used for bioprinting by microextrusion, which is not possible with inkjet technology [44]. Remarkable progress has been made in the production of spinal cord or brain-like structures, as well as in the regeneration of peripheral nerves, an area where 3D bioprinting appears to be especially well adapted [45]. These patient-derived cell constructs have the potential to be useful models of neurodegenerative disorders.
3.1. Advancements and Challenges in 3D Bioprinting for Brain Tissue Engineering and Nerve Regeneration
Brain tissue engineering aims to improve brain tissue function by generating biological substitutes that integrate cells with biomimetic three-dimensional scaffolding. Because they offer cells the structural support they require to function, 3D scaffolds are crucial for efficient nerve regeneration because they enhance host tissue engraftment and the subsequent development of new tissue [32].
The traditional 3D scaffold construction method has shown promise in the treatment of nerve damage. However, there are inherent restrictions on having sufficient control over the scaffold’s internal design and exterior form [33]. In the field of tissue engineering, 3D printing technology is an effective tool for creating tissue and organ designs in order to address this problem. Advancements in recent times have made it possible to generate sophisticated, three-dimensional living tissues by 3D printing of biocompatible materials, cells, and supporting components. Regenerative medicine is using 3D bioprinting to address the lack of transplantable tissues and organs [34]. With precise spatial distribution and biomimetic design, 3D bioprinting can directly create complex tissue scaffolds. Compared with conventional printing methods, 3D bioprinting involves additional challenges such as the selection of materials, types of cells, growth and differentiation agents, and technological challenges related to the sensitivity of living cells and tissue assembly. The application of 3D bioprinting in brain tissue has been the subject of very few investigations. After printing, there was still cell viability in the collagen hydrogel precursor (in liquid form) used as bioink [35]. In a different research, neural stem cells demonstrated vitality following the printing of an artificial neural tissue made of collagen, fibrin gel, vascular endothelial growth factor, and murine neural stem cells [36]. A recent study used a cell culture medium to print retinal glial cells. The very thin layers of neurons or neural stem cells encased in a liquid hydrogel polymer or precursor constitute the bioprinted neural structures in each of the previously described research. When producing brain cells or other tissues, there are not many choices for bioinks [37].
3.2. Bioprinting Neural Tissues Using Stem Cells as a Tool for Screening Drug Targets for Alzheimer’s Disease
Three-dimensional bioprinting has emerged as a promising technology for engineering physiologically relevant neural tissues derived from human-induced pluripotent stem cells (hiPSCs). Recent advancements in bioprinting methods and bioink development have enabled the high-throughput production of hiPSC-derived neural tissues. With microfluidic extrusion-based bioprinting best shown by the Aspect Biosystems RX1 printer, living cells may be integrated with biocompatible materials supporting cell growth and differentiation. This method helps to create complex neural tissue structures that mirror the architecture and cellular composition of the central nervous system (CNS), which therefore includes the brain and the spinal cord. Particularly the creation of functionalized fibrin-based hydrogels including components like laminin, recent developments in bioink design greatly improve the survival and maturation of human-induced pluripotent stem cells (hiPSCs) into mature brain cell types. Moreover, adding morphogen-releasing microspheres into these bioinks lets one to regulate the differentiation of hiPSCs, hence producing tissues very similar to certain CNS areas. Producing physiologically appropriate hiPSC-derived brain tissue models is made possible by these developments in 3D bioprinting and bioink formulation. Offering new directions for study and therapy in neurodegenerative illnesses, such models show promise for uses in disease modelling, drug screening, and even regenerative medicine [38].
Electrospun scaffolds have been proven by researchers to greatly improve the network building and adhesion of neural stem cells. Three-dimensional microtopographic scaffolds were produced in a particular work by Carlson et al. by combining a decellularized brain extracellular matrix (dECM) with electrospun gelatin scaffolds. This new technique provides a better environment for the neural stem cells, hence their growth and development of neural networks. The integration of the dECM with electrospun materials has improved brain tissue engineering, providing a support matrix that is very close to the natural extracellular environment. These scaffolds have been proved to support in situ stem cell neuronal reprogramming and the development of neural networks [39]. Using electrospun PAN, a pure carbon-based polymer, and Jeffamine polymer-infused PAN, a new 3D nanofiber scaffold has been developed. This scaffold design mimics the natural fibrous structure of the extracellular matrix and allows cellular penetration. Both scaffolds are capable of enhancing the survival and proliferation of SH-SY5Y and U-87MG cells. When these cells were treated with PD-mimicking medications, the scaffold ability to support cell survival outperformed that of 2D culture settings [40].
3.3. Biomaterials and Their Advantages
Recent biomaterials advancements have brought into focus fibrin, alginate, and laminin as potential candidates for neural tissue engineering. Fibrin-based hydrogels show excellent biocompatibility, supporting neural cell adhesion, proliferation, and differentiation by mimicking biochemical and mechanical properties similar to soft tissues [41]. Laminin, a crucial ECM component, has also been used to enhance neural regeneration by promoting axonal outgrowth and guiding cell migration within the central nervous system, especially when functionalized with fibrin [42]. Together, these materials meet structural and functional demands for neural damage repair.
PEG has recently emerged as one of the most promising synthetic biomaterials due to its customizability. Upon functionalization with bioactive peptides, PEG supports a variety of neuronal and glial populations and facilitates vascular network formation, which are both critical for sustained tissue viability [43]. Its tunable chemistry allows precise control over degradation rates and mechanical stiffness, enabling researchers to tailor microenvironments that mimic native neural tissues. This adaptability puts PEG in a very basic position to create advanced therapeutic scaffolds and drug-screening platforms.
Both natural and synthetic hydrogels show specific advantages for regenerative use. Natural hydrogels from ECM components remain biologically active and can foster cell signaling as well as integration with the target tissue [44]. Synthetic types reduce the chances of immunogenic reactions by using chemically defined compositions and thus provide more scope for functionalization to accurately model degradation kinetics as well as mechanical properties [45]. It would, therefore, be possible to design hybrid systems that optimize biocompatibility with the strengths of both categories, addressing the complex demands of neural repair and organoid bioprinting.
3.4. Scaffold-Based Cell Culture
Cell culture techniques based on scaffolds have been widely investigated to improve the differentiation of stem cells, growth of neurons, and tissue engineering. As illustrated in Table 1, there are numerous studies that have emphasized different types of biomaterial scaffolds to improve cellular responses.

Table 1.
Different scaffold-based cell culture and their findings.
3.5. Use of 3D Bioprinting to Build 3D Models
The most recent breakthroughs that use this approach to generate the intricate complexity of neural network architecture are summarized in Table 2. The experiments cited in the table outline novel strategies in the regulation of cellular arrangement and differentiation. Other strategies discussed in the table also emphasize bioprinting’s potential in promoting cell viability and functional integration. These studies demonstrate the 3D bioprinting approach’s potential in creating biologically relevant models of the central nervous system. Collectively, the review will be a great base for further research in disease modelling and regenerative medicine.

Table 2.
Different uses of 3D bioprinting to build 3D models.
Bioprinting Neural Tissue
Advances in bioprinting have emerged as a prospective method to create neural tissue models with improved cellular organization and functionality. Table 3 details various forms of bioprinting that have been used to support the viability, differentiation, and network formation of neural cells. The cited articles feature the advancing stereolithography and extrusion bioprinting, which showed their ability in nerve outgrowth, improved neural marker expression, and increased cell proliferation. These findings emphasize the role of bioprinting in developing physiologically relevant neural constructs. The research outlined in the table provides valuable insights into optimizing biomaterial selection and bioprinting conditions for neural tissue engineering applications.

Table 3.
Different approaches for bioprinting neural tissue.
4. Conclusions
Bioprinting as a field of 3D printing goes one step further than customization by adding living cells and biomaterials to print functional tissue and organoids. The scope for generating in vitro models of neural tissue to screen drugs and study disease mechanisms is enormous. Expert opinions underscore the potential to transcend traditional 2D cell culture systems and animal models, which have been demonstrated to insufficiently replicate the complexity of the human brain. It is also important to address the challenges and limitations. For example, identification of appropriate bio-inks for bioprinting neural tissue continues to be an important area of research, and the long-term survival and function of bioprinted scaffolds must be maximized. The ethical considerations of patient-derived cells as well as the potential of creating complex neural models must be approached with care as well. There is an increasing influence of 3D printing and bioprinting technologies on the diagnosis and treatment of neurodegenerative disorders. As demonstrated, the combination of additive manufacturing and biomedical sciences represents not only an incremental advancement but also a paradigm shift with the potential to redefine clinical and therapeutic scenario.
Three-dimensional printing has the potential to transcend the limitations of conventional medical procedures. From the production of patient-specific anatomical models that enhance the precision of surgery to the production of microfluidic devices that mimic the intricate brain environment for drug screening, 3D printing offers a level of customization and control unprecedented in the past. This is particularly relevant in neurodegenerative diseases, where heterogeneity in disease presentation and progression at the individual level necessitates personalized therapy.
In evaluating the various research presented, it is clear that the field is currently rapidly changing. The technological developments are rapid, but their application in the clinic requires interdisciplinary input, rigorous testing, and a willingness to solve the unique problems that neurodegenerative diseases have. Therefore, 3D printing and bioprinting are excellent tools for increasing our understanding and control of neurodegenerative diseases. Through sustained advancements in materials science, bioengineering, and clinical research, we are progressively approaching the personalized and regenerative medicine for these conditions.
5. Challenges and Future Avenues of 3D Printing
The integration of 3D printing in pharmaceutical manufacturing and bioprinting introduces critical challenges related to reproducibility, material suitability, and contamination control. Variations in printed outputs even from identical digital models arise due to inconsistencies in ink composition, printer component wear, slicer software parameters, and environmental factors, necessitating advanced analytical methods to verify drug homogeneity, dosage accuracy, and release profiles [65,66]. Contamination risks further complicate applications involving edible or implantable products, demanding stringent protocols for equipment sterilization and material handling to prevent cross-contamination between batches [67,68]. Additionally, the use of nonpharmaceutical-grade polymers in fused deposition modelling (FDM) raises biocompatibility concerns, while formulation adjustments to optimize printability often alter drug release kinetics, underscoring the need for standardized predictive excipient systems tailored for multifunctional drug production [69,70]. Addressing these challenges requires innovations in printer precision, material science, and quality assurance frameworks to balance cost, scalability, and regulatory compliance.
This limitation impedes the fabrication of complex tissues, particularly neural and vascular networks, which demand high cell density and functional integration. Future advancements on optimizing bioink rheology, nozzle design, and printing parameters to mitigate mechanical trauma also alongside developing dynamic biomimetic scaffolds that support cell proliferation and differentiation are needed. Collaborative efforts between engineers, biologists, and clinicians will be essential to refine resolution, enhance biocompatibility, and enable real-time monitoring during printing, ultimately bridging the gap between laboratory-scale innovation and clinical translation for personalized therapeutics and regenerative medicine.
Author Contributions
A.V., H.S.T. and G.K. made the draft of the review. A.T., R.C., S.R. and S.P. contributed by doing literature searches and preparing a manuscript draft. A.V., S.P., A.C., D.K. and G.K. revised and approved the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data sharing is not applicable to this article as no new data were created or analyzed in this study.
Conflicts of Interest
The authors declare that this study was conducted in the absence of any commercial or financial relationships that can be construed as potential conflicts of interest.
References
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457. [Google Scholar] [CrossRef] [PubMed]
- National Institute of Environmental Health Sciences: Neurodegenerative Diseases. Available online: https://www.niehs.nih.gov/research/supported/health/neurodegenerative (accessed on 11 February 2024).
- Zilinskaite, N.; Shukla, R.P.; Baradoke, A. Use of 3D Printing Techniques to Fabricate Implantable Microelectrodes for Electrochemical Detection of Biomarkers in the Early Diagnosis of Cardiovascular and Neurodegenerative Diseases. ACS Meas. Sci. Au 2023, 3, 315–336. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Yang, Y. Application of 3D Printing in Implantable Medical Devices. BioMed Res. Int. 2021, 2021, 6653967. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Miller, J.; Vezza, J.; Mayster, M.; Raffay, M.; Justice, Q.; Al Tamimi, Z.; Hansotte, G.; Sunkara, L.D.; Bernat, J. Additive Manufacturing: A Comprehensive Review. Sensors 2024, 24, 2668. [Google Scholar] [CrossRef]
- Sun, Y.M.; Wang, Z.Y.; Liang, Y.Y.; Hao, C.W.; Shi, C.H. Digital biomarkers for precision diagnosis and monitoring in Parkinson’s disease. npj Digit. Med. 2024, 7, 218. [Google Scholar] [CrossRef]
- Li, W.; Wang, M.; Ma, H.; Chapa-Villarreal, F.A.; Lobo, A.O.; Zhang, Y.S. Stereolithography apparatus and digital light processing-based 3D bioprinting for tissue fabrication. iScience 2023, 26, 106039. [Google Scholar] [CrossRef]
- Schade, R.; Weiß, T.; Berg, A.; Schnabelrauch, M.; Liefeith, K. Two-photon techniques in tissue engineering. Int. J. Artif. Organs 2010, 33, 219–227. [Google Scholar] [CrossRef]
- Peng, H.; Han, B.; Tong, T.; Jin, X.; Peng, Y.; Guo, M.; Li, B.; Ding, J.; Kong, Q.; Wang, Q. 3D printing processes in precise drug delivery for personalized medicine. Biofabrication 2024, 16, 032001. [Google Scholar] [CrossRef]
- Ma, W.C.; Goh, G.L.; Priyadarshini, B.M.; Yeong, W.Y. 3D printing and 3D-printed electronics: Applications and future trends in smart drug delivery devices. Int. J. Bioprint. 2023, 9, 725. [Google Scholar] [CrossRef]
- Davoodi, E.; Montazerian, H.; Mirhakimi, A.S.; Zhianmanesh, M.; Ibhadode, O.; Shahabad, S.I.; Toyserkani, E. Additively manufactured metallic biomaterials. Bioact. Mater. 2022, 15, 214–249. [Google Scholar] [CrossRef]
- Sen, K.; Mehta, T.; Sansare, S.; Sharifi, L.; Ma, A.W.K.; Chaudhuri, B. Pharmaceutical applications of powder-based binder jet 3D printing process—A review. Adv. Drug Deliv. Rev. 2021, 177, 113943. [Google Scholar] [CrossRef] [PubMed]
- Kurenov, S.N.; Ionita, C.; Sammons, D.; Demmy, T.L. Three-dimensional printing to facilitate anatomic study, device development, simulation, and planning in thoracic surgery. J. Thorac. Cardiovasc. Surg. 2015, 149, 973–979.e1. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, M.H.; Islam, M.A.; Hossain, N.; Al Mahmud, M.Z.; Rayhan, M.T.; Nishi, N.J.; Chowdhury, M.A. Recent advances of additive manufacturing in implant fabrication—A review. Appl. Surf. Sci. Adv. 2023, 18, 100462. [Google Scholar] [CrossRef]
- Cano-Vicent, A.; Tambuwala, M.M.; Hassan, S.S.; Barh, D.; Aljabali, A.A.A.; Birkett, M.; Arjunan, A.; Serrano-Aroca, Á. Fused deposition modelling: Current status, methodology, applications and future prospects. Addit. Manuf. 2021, 47, 102378. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef]
- Rohani Shirvan, A.; Nouri, A.; Wen, C. Structural polymer biomaterials. In Structural Biomaterials Properties, Characteristics and Selection; Woodhead Publishing: Cambridge, UK, 2021; pp. 395–439. [Google Scholar]
- (PDF) A Review Paper on 3D-Printing Aspects and Various Processes Used in the 3D-Printing. Available online: https://www.researchgate.net/publication/350374850_A_Review_paper_on_3D-Printing_Aspects_and_Various_Processes_Used_in_the_3D-Printing (accessed on 24 January 2024).
- Masood, S.H. Advances in Fused Deposition Modeling. Compr. Mater. Process. 2014, 10, 69–91. [Google Scholar]
- Aimar, A.; Palermo, A.; Innocenti, B. The Role of 3D Printing in Medical Applications: A State of the Art. J. Healthc. Eng. 2019, 2019, 5340616. [Google Scholar] [CrossRef]
- He, P.; Zhao, J.; Zhang, J.; Li, B.; Gou, Z.; Gou, M.; Li, X. Bioprinting of skin constructs for wound healing. Burn. Trauma 2018, 6, 5. [Google Scholar] [CrossRef]
- Vukicevic, M.; Mosadegh, B.; Min, J.K.; Little, S.H. Cardiac 3D Printing and its Future Directions. JACC Cardiovasc. Imaging 2017, 10, 171–184. [Google Scholar] [CrossRef]
- Charbe, N.; McCarron, P.A.; Tambuwala, M.M. Three-dimensional bio-printing: A new frontier in oncology research. World J. Clin. Oncol. 2017, 8, 21–36. [Google Scholar] [CrossRef]
- Frame, M.; Huntley, J.S. Rapid prototyping in orthopaedic surgery: A user’s guide. Sci. World J. 2012, 2012, 838575. [Google Scholar] [CrossRef] [PubMed]
- Walker, V. Implementing a 3D printing service in a biomedical library. J. Med. Libr. Assoc. 2017, 105, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Szary, J.; Luis, M.S.; Mikulski, S.; Patel, A.; Schulz, F.; Tretiakow, D.; Kwiatkowska, J. The Role of 3D Printing in Planning ComplexMedical Procedures and Training of Medical Professionals—Cross-Sectional Multispecialty Review. Int. J. Environ. Res. Public Health 2022, 19, 3331. [Google Scholar] [CrossRef] [PubMed]
- Dileep, C.; Jacob, L.; Umer, R.; Butt, H. Review of vat photopolymerization 3D printing of photonic devices. Addit. Manuf. 2024, 86, 104189. [Google Scholar]
- Tsao, H.H.; Huang, C.G.; Wu, Y.R. Detection and assessment of alpha-synuclein in Parkinson disease. Neurochem. Int. 2022, 158, 105358. [Google Scholar] [CrossRef]
- Randazzo, M.; Pisapia, J.; Singh, N.; Thawani, J. 3D printing in neurosurgery: A systematic review. Surg. Neurol. Int. 2016, 7, S801. [Google Scholar]
- Thomas, M.; Willerth, S.M. 3-D bioprinting of neural tissue for applications in cell therapy and drug screening. Front. Bioeng. Biotechnol. 2017, 5, 69. [Google Scholar] [CrossRef]
- Ong, C.S.; Yesantharao, P.; Huang, C.Y.; Mattson, G.; Boktor, J.; Fukunishi, T.; Zhang, H.; Hibino, N. 3D bioprinting using stem cells. Pediatr. Res. 2018, 83, 223–231. [Google Scholar] [CrossRef]
- (PDF) 3D Printing Technology, Material Used for Printing and Its Applications. Available online: https://www.researchgate.net/publication/345763830_3D_Printing_Technology_Material_Used_For_Printing_and_its_Applications (accessed on 10 February 2024).
- Ng, I.C.; Pawijit, P.; Tan, J.; Yu, H. Anatomy and Physiology for Biomaterials Research and Development. Encycl. Biomed. Eng. 2019, 1–3, 225–236. [Google Scholar]
- Smallman, R.E.; Bishop, R.J. Biomaterials, Modern Physical Metallurgy and Materials Engineering; Butterworth-Heinemann: Oxford, UK, 1999; pp. 394–405. [Google Scholar]
- Gungor-Ozkerim, P.S.; Inci, I.; Zhang, Y.S.; Khademhosseini, A.; Dokmeci, M.R. Bioinks for 3D bioprinting: An overview. Biomater. Sci. 2018, 6, 915. [Google Scholar] [CrossRef]
- Murphy, S.V.; De Coppi, P.; Atala, A. Opportunities and challenges of translational 3D bioprinting. Nat. Biomed. Eng. 2019, 4, 370–380. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yang, F.; Zhao, H.; Gao, Q.; Xia, B.; Fu, J. Research on the printability of hydrogels in 3D bioprinting. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Askari, M.; Afzali Naniz, M.; Kouhi, M.; Saberi, A.; Zolfagharian, A.; Bodaghi, M. Recent progress in extrusion 3D bioprinting of hydrogel biomaterials for tissue regeneration: A comprehensive review with focus on advanced fabrication techniques. Biomater. Sci. 2021, 9, 535–573. [Google Scholar] [CrossRef] [PubMed]
- Horvath, L.; Umehara, Y.; Jud, C.; Blank, F.; Petri-Fink, A.; Rothen-Rutishauser, B. Engineering an in vitro air-blood barrier by 3D bioprinting. Sci. Rep. 2015, 5, 7974. [Google Scholar] [CrossRef]
- Matai, I.; Kaur, G.; Seyedsalehi, A.; McClinton, A.; Laurencin, C.T. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020, 226, 119536. [Google Scholar] [CrossRef]
- Arslan-Yildiz, A.; El Assal, R.; Chen, P.; Guven, S.; Inci, F.; Demirci, U. Towards artificial tissue models: Past, present, and future of 3D bioprinting. Biofabrication 2016, 8, 014103. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T. Human microvasculature fabrication using thermal inkjet printing technology. Biomaterials 2009, 30, 6221–6227. [Google Scholar] [CrossRef]
- Li, Y.; Jiang, X.; Li, L.; Chen, Z.N.; Gao, G.; Yao, R.; Sun, W. 3D printing human induced pluripotent stem cells with novel hydroxypropyl chitin bioink: Scalable expansion and uniform aggregation. Biofabrication 2018, 10, 044101. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Joung, D.; Truong, V.; Neitzke, C.C.; Guo, S.Z.; Walsh, P.J.; Monat, J.R.; McAlpine, M.C. 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds. Adv. Funct. Mater. 2018, 28, 1801850. [Google Scholar] [CrossRef]
- Madl, C.M.; LeSavage, B.L.; Dewi, R.E.; Dinh, C.B.; Stowers, R.S.; Khariton, M.; Heilshorn, S.C. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodelling. Nat. Mater. 2017, 16, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, F.; Pozner, T.; Siddiqui, A.; Ceppi, P.; Winner, B.; Rajendiran, M.; O’Connor, K.E. Polyhydroxyphenylvalerate/polycaprolactone nanofibers improve the life-span and mechanoresponse of human IPSC-derived cortical neuronal cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 11, 110832. [Google Scholar] [CrossRef] [PubMed]
- Cantley, W.L.; Du, C.; Lomoio, S.; Depalma, T.; Peirent, E.; Kleinknecht, D.; Hunter, M.; Tang-Schomer, M.D.; Tesco, G.; Kaplan, D.L. Functional and Sustainable 3D Human Neural Network Models from Pluripotent Stem Cells. ACS Biomater. Sci. Eng. 2018, 4, 4278. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.N.; Freitas, B.C.; Qian, H.; Lux, J.; Acab, A.; Trujillo, C.A.; Almutairi, A. Layered hydrogels accelerate iPSC-derived neuronal maturation and reveal migration defects caused by MeCP2 dysfunction. Proc. Natl. Acad. Sci. USA 2016, 113, 3185–3190. [Google Scholar] [CrossRef]
- Karvinen, J.; Joki, T.; Ylä-Outinen, L.; Koivisto, J.T.; Narkilahti, S.; Kellomäki, M. Soft hydrazone crosslinked hyaluronan- and alginate-based hydrogels as 3D supportive matrices for human pluripotent stem cell-derived neuronal cells. React. Funct. Polym. 2018, 124, 29–39. [Google Scholar] [CrossRef]
- Ranjan, V.D.; Qiu, L.; Lee, J.W.L.; Chen, X.; Jang, S.E.; Chai, C.; Zeng, L. A microfiber scaffold-based 3D in vitro human neuronal culture model of Alzheimer’s disease. Biomater. Sci. 2020, 8, 4861–4874. [Google Scholar] [CrossRef]
- Sood, D.; Cairns, D.M.; Dabbi, J.M.; Ramakrishnan, C.; Deisseroth, K.; Black, L.D.; Santaniello, S.; Kaplan, D.L. Functional maturation of human neural stem cells in a 3D bioengineered brain model enriched with fetal brain-derived matrix. Sci. Rep. 2019, 9, 17874. [Google Scholar] [CrossRef]
- Barroca, N.; da Silva, D.M.; Pinto, S.C.; Sousa, J.P.M.; Verstappen, K.; Klymov, A.; Fernández-San-Argimiro, F.J.; Madarieta, I.; Murua, O.; Olalde, B.; et al. Interfacing reduced graphene oxide with an adipose-derived extracellular matrix as a regulating milieu for neural tissue engineering. Biomater. Adv. 2023, 148, 213351. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Wallace, G.G.; Crook, J.M. 3D Bioprinting Human Induced Pluripotent Stem Cell Constructs for In Situ Cell Proliferation and Successive Multilineage Differentiation. Adv. Healthc. Mater. 2017, 6, 1700175. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, H.; Long, X.; Xu, T. Three-dimensional-engineered bioprinted in vitro human neural stem cell self-assembling culture model constructs of Alzheimer’s disease. Bioact. Mater. 2021, 11, 192–205. [Google Scholar] [CrossRef]
- de la Vega, L.; Gómez, D.A.R.; Abelseth, E.; Abelseth, L.; da Silva, V.A.; Willerth, S.M. 3D Bioprinting Human Induced Pluripotent Stem Cell-Derived Neural Tissues Using a Novel Lab-on-a-Printer Technology. Appl. Sci. 2018, 8, 2414. [Google Scholar] [CrossRef]
- Sullivan, M.A.; Lane, S.; Volkerling, A.; Engel, M.; Werry, E.L.; Kassiou, M. Three-dimensional bioprinting of stem cell-derived central nervous system cells enables astrocyte growth, vasculogenesis, and enhances neural differentiation/function. Biotechnol. Bioeng. 2023, 120, 3079–3091. [Google Scholar] [CrossRef] [PubMed]
- Koch, L.; Deiwick, A.; Soriano, J.; Chichkov, B. 344Laser bioprinting of human iPSC-derived neural stem cells and neurons: Effect on cell survival, multipotency, differentiation, and neuronal activity. Int. J. Bioprint 2023, 9, 344–368. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Suri, S.; Han, L.H.; Zhang, W.; Singh, A.; Chen, S.; Schmidt, C.E. Solid freeform fabrication of designer scaffolds of hyaluronic acid for nerve tissue engineering. Biomed. Microdevices 2011, 13, 983–993. [Google Scholar] [CrossRef] [PubMed]
- Lowry Curley, J.; Jennings, S.R.; Moore, M.J. Fabrication of micropatterned hydrogels for neural culture systems using dynamic mask projection photolithography. J. Vis. Exp. 2011, 48, 2636. [Google Scholar] [CrossRef]
- Lee, S.J.; Nowicki, M.; Harris, B.; Zhang, L.G. Fabrication of a Highly Aligned Neural Scaffold via a Table Top Stereolithography 3D Printing and Electrospinning. Tissue Eng. Part A 2017, 23, 491–502. [Google Scholar] [CrossRef]
- Zhu, W.; George, J.K.; Sorger, V.J.; Grace Zhang, L. 3D printing scaffold coupled with low level light therapy for neural tissue regeneration. Biofabrication 2017, 9, 025002. [Google Scholar] [CrossRef]
- Gu, Q.; Tomaskovic-Crook, E.; Lozano, R.; Chen, Y.; Kapsa, R.M.; Zhou, Q.; Wallace, G.G.; Crook, J.M. Functional 3D Neural Mini-Tissues from Printed Gel-Based Bioink and Human Neural Stem Cells. Adv. Healthc. Mater. 2016, 5, 1429–1438. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, D.J.; Kim, H.W.; Park, H.J. Effect of particle size on 3D printing performance of the food-ink system with cellular food materials. J. Food Eng. 2019, 256, 1–8. [Google Scholar] [CrossRef]
- Portoacă, A.I.; Ripeanu, R.G.; Diniță, A.; Tănase, M. Optimization of 3D Printing Parameters for Enhanced Surface Quality and Wear Resistance. Polymers 2023, 15, 3419. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.; Seibi, A.; Jaafar, I.; Amin, A. Study on the Sanitization Efficacy for Safe Use of 3D-Printed Parts for Food and Medical Applications. In Proceedings of the 2023 Intermountain Engineering, Technology and Computing, IETC 2023, Provo, UT, USA, 12–13 May 2023; pp. 220–225. [Google Scholar]
- Hai Alami, A.; Ghani Olabi, A.; Khuri, S.; Aljaghoub, H.; Alasad, S.; Ramadan, M.; Ali Abdelkareem, M. 3D printing in the food industry: Recent progress and role in achieving sustainable development goals. Ain Shams Eng. J. 2024, 15, 102386. [Google Scholar] [CrossRef]
- Melnyk, L.A.; Oyewumi, M.O. Integration of 3D printing technology in pharmaceutical compounding: Progress, prospects, and challenges. Ann. 3D Print. Med. 2021, 4, 100035. [Google Scholar] [CrossRef]
- Solanki, N.G.; Tahsin, M.; Shah, A.V.; Serajuddin, A.T.M. Formulation of 3D Printed Tablet for Rapid Drug Release by Fused Deposition Modeling: Screening Polymers for Drug Release, Drug-Polymer Miscibility and Printability. J. Pharm. Sci. 2018, 107, 390–401. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).