Keeping Cells Alive in Microscopy
Abstract
1. Introduction
2. Whole Cell Illumination
2.1. Mechanisms Involved
- -
- Photochemical reactions occur if light is absorbed by photosensitizing molecules, which are able to transfer their excitation energy to adjacent molecules, thus creating radicals or highly reactive singlet oxygen (1O2). These species may cause photo-oxidation with subsequent cell destruction. Absorbing molecules include the coenzymes nicotinamide adenine dinucleotide (NADH, absorption maximum: 350 nm) [8] as well as flavin mono- and dinucletide (FMN/FAD; absorption maxima: 380 nm and 440 nm) [9], and porphyrin related molecules (with an absorption wavelength below 620 nm). However, while free porphyrins have a high quantum yield for creating singlet oxygen [10], this potential is reduced when porphyrins are bound within hemoglobin, myoglobin, or cytochromes. At wavelengths in the far UV, the most important phototoxic effect is produced upon light absorption by DNA (with a maximum of around 260 nm), which creates photo-dimers.
- -
- Photothermal reactions occur upon absorption of radiation and energy conversion to heat. Main absorbers are water molecules (λ ≤ 250 nm, λ ≥ 1100 nm), porphyrin molecules (bound, e.g., within cytochromes, 350 nm ≤ λ ≤ 620 nm), and proteins (λ ≤ 300 nm). Thus, in the wavelength range of 620 nm ≤ λ ≤ 1100 nm (“therapeutic window”), absorption is comparably low, but increases at 400–600 nm, the typical excitation range for light microscopy.
- -
- Opto-mechanical destruction of molecular bonds occurs at photon energies above 4 eV, corresponding to wavelengths below 300 nm. This is the spectral range of far UV or X-ray microscopy. At longer wavelengths, cell or tissue disruption may occur by intensive picosecond or femtosecond laser pulses. The focus on these laser pulses is used for ablation, hole drilling, gene transfection, and other kinds of micro-manipulation reported in Section 3.1. However, in many applications of light microscopy, these effects do not play a major role.
2.2. Microscopy Methods and Their Compatibility with Low Light Exposure
2.2.1. General
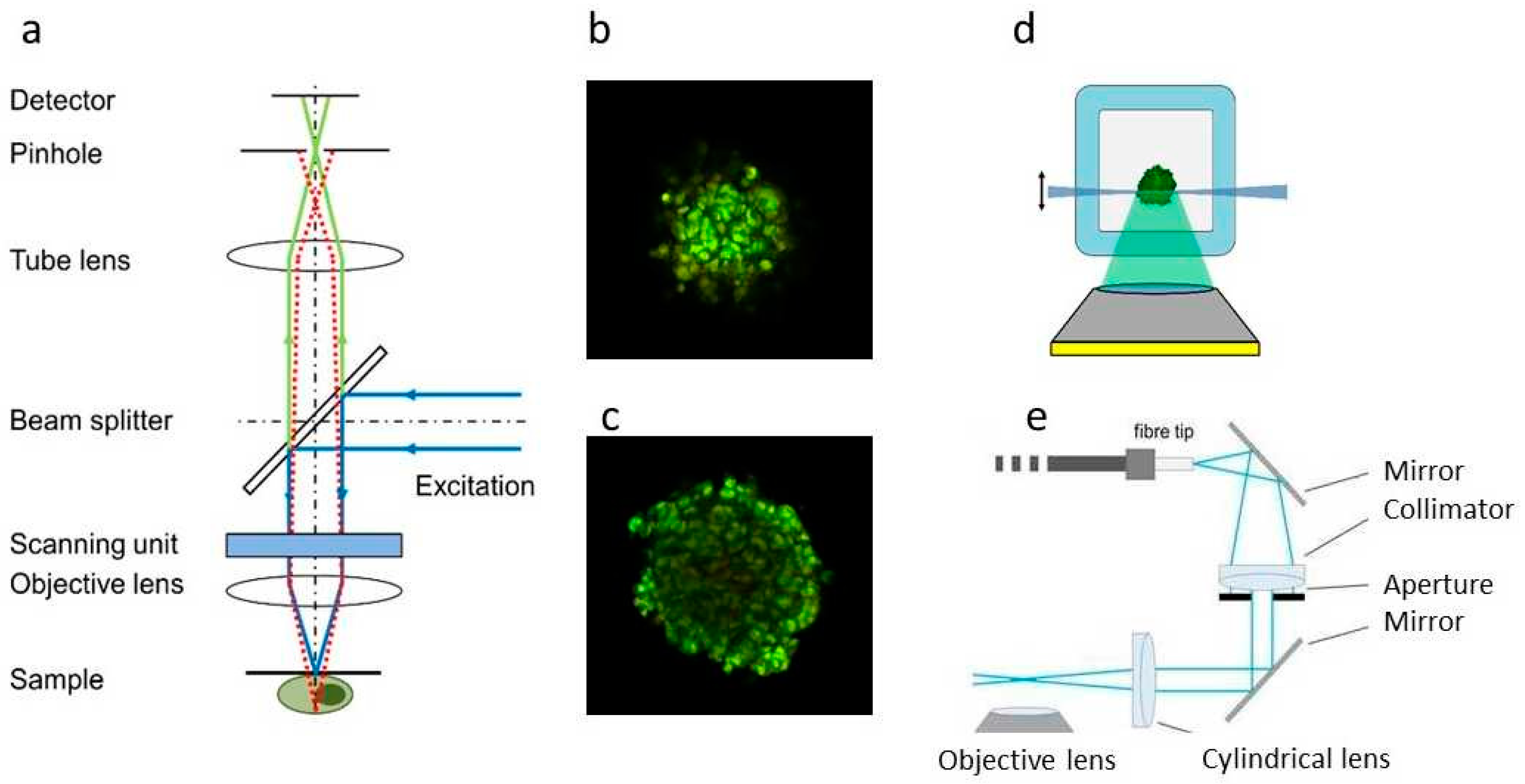
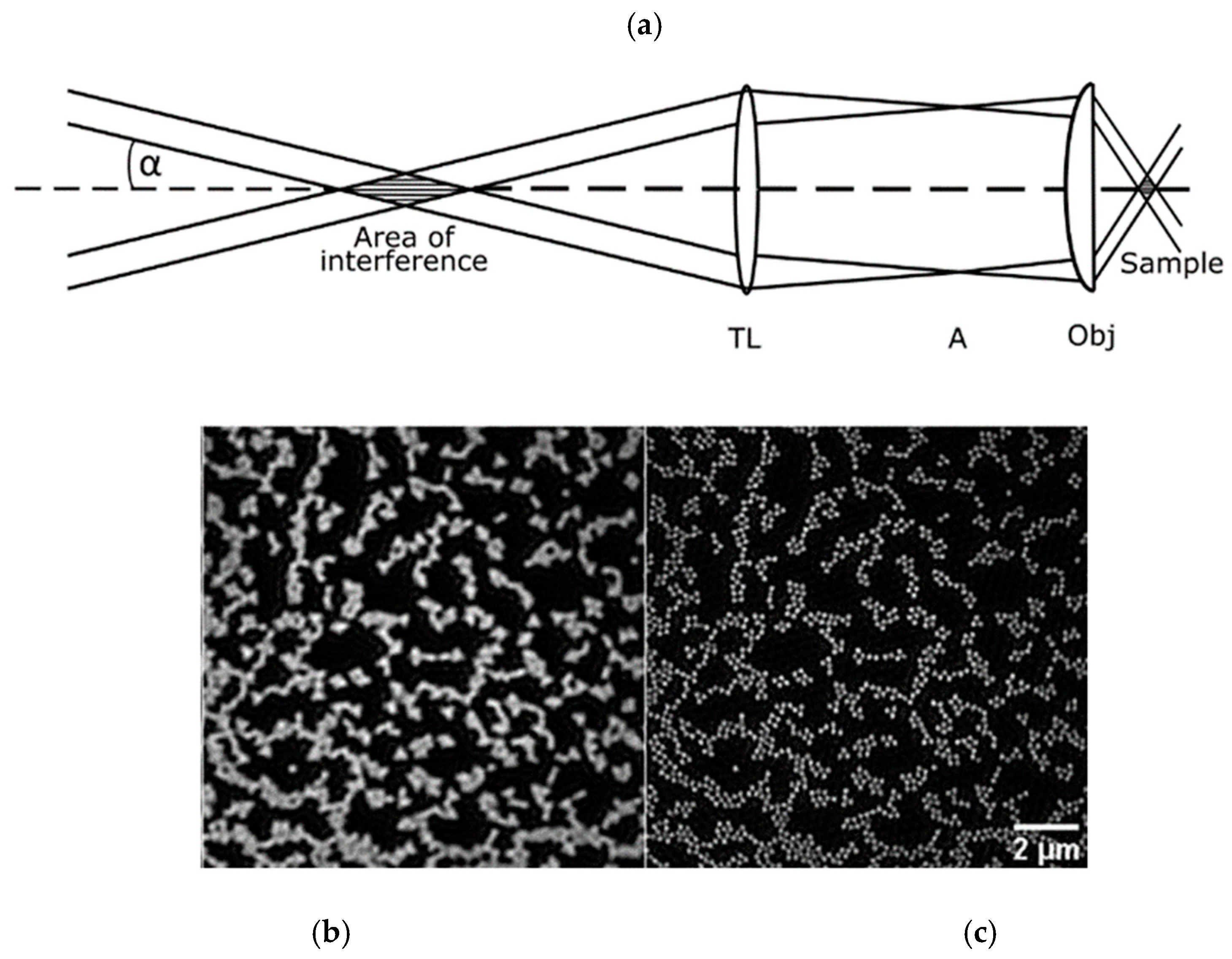
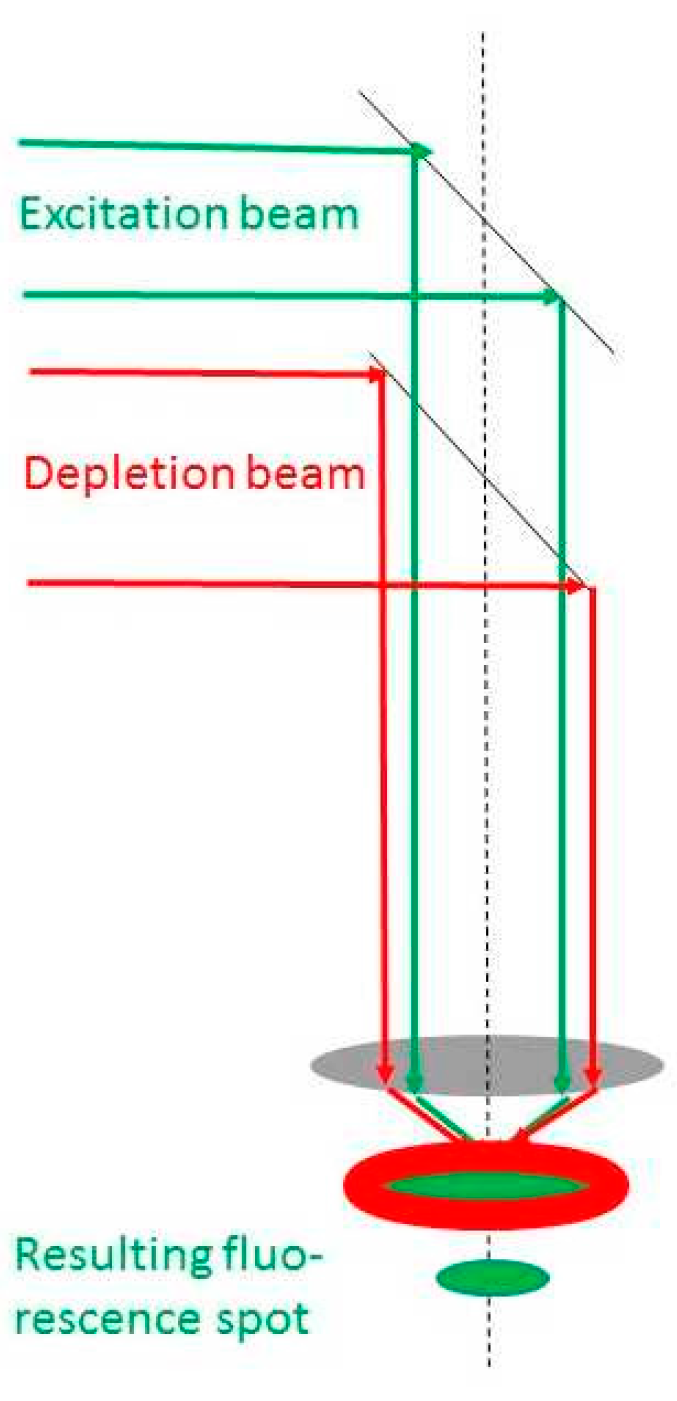
2.2.2. Super-Resolution Microscopy
3. Focused Illumination
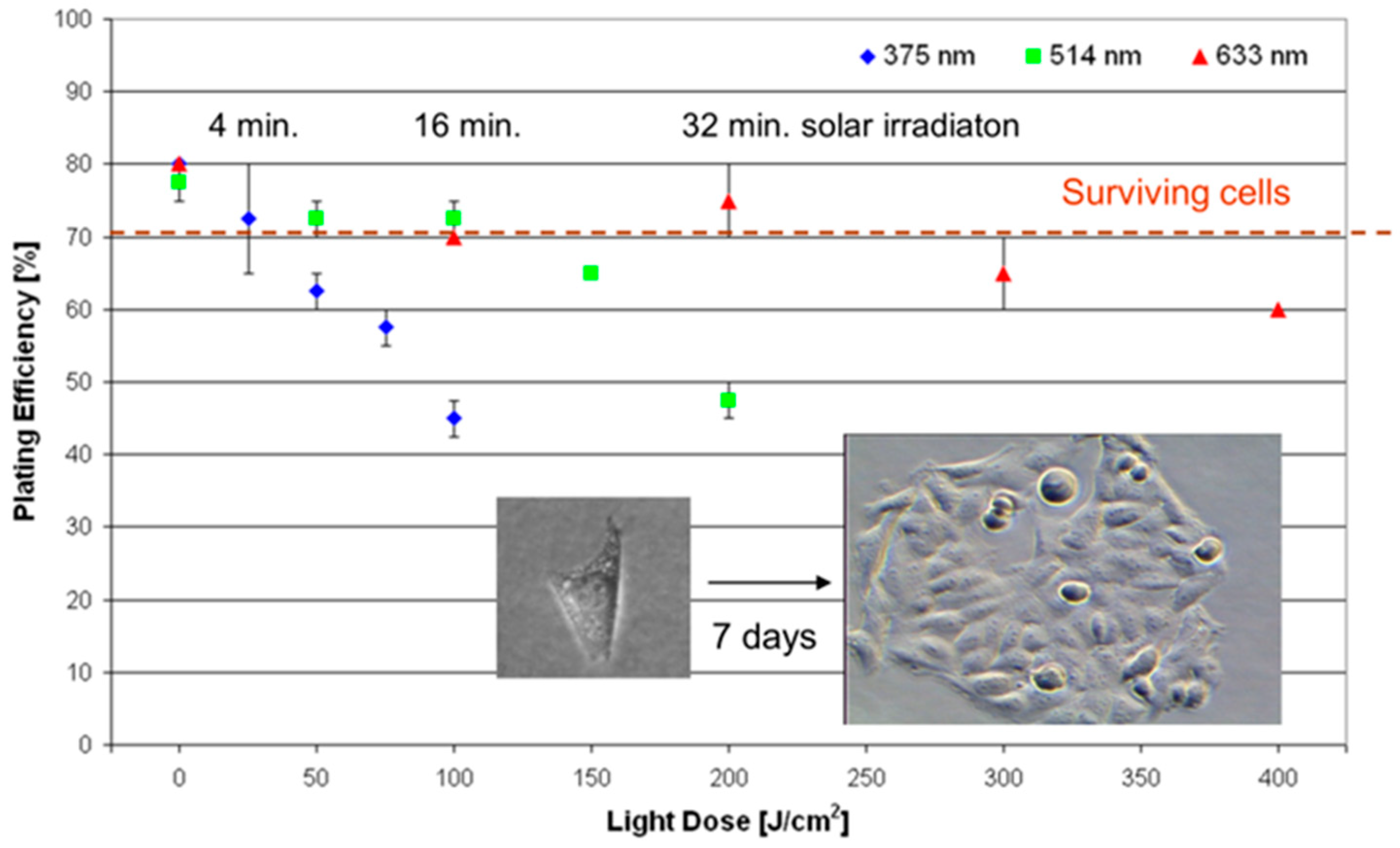
3.1. Visible Irradiation
3.2. UV Irradiation
4. Discussion and Conclusions
- -
- The most sensitive cellular target with respect to cellular viability (in terms of proliferation potential) is the cell nucleus.
- -
- In whole cell irradiation, a profound dependence on the illuminating wavelength exists; while far UV light (absorption maximum of nucleic acids) is highly damaging, the phototoxicity decreases considerably with increasing excitation wavelength.
- -
- Super-Resolution Fluorescence Microscopy, with linear excitation modes (e.g., SIM, MINFLUX, and linear excitation localization) and wavelengths with low photonic energy, is largely compatible with a live cell status, especially if restricted to a small cellular region of interest.
- -
- Live cell applications of Super-Resolution Fluorescence microscopy using non-linear excitation modes (e.g., photoswitching based SMLM; STED) appear to be possible under specific conditions, (such as long excitation wavelengths and small fields of illumination), in particular, for short-term analyses of cellular nanostructures.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cadet, J.; Berger, M.; Douki, T.; Morin, B.; Raoul, S.; Ravanat, J.L.; Spinelli, S. Effects of UV and visible radiation on DNA-final base damage. Biol. Chem. 1997, 378, 1275–1286. [Google Scholar] [PubMed]
- Nakanishi, M.; Niida, H.; Murakami, H.; Shimada, M. DNA damage responses in skin biology—Implications in tumor prevention and aging acceleration. J. Dermatol. Sci. 2009, 56, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Richa; Sinha, R.P.; Häder, D.P. Physiological aspects of UV-excitation of DNA. Top. Curr. Chem. 2015, 356, 203–248. [CrossRef]
- Schneckenburger, H.; Richter, V.; Wagner, M. Live-Cell Optical Microscopy with Limited Light Doses; SPIE Spotlight Series; SPIE: Bellingham, WA, USA, 2018; Volume 42, 38p, ISBN 9781510622593. [Google Scholar]
- Berns, M.W. A history of laser scissors (microbeams). Methods Cell Biol. 2007, 82, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Ashkin, A. Optical trapping and manipulation of neutral particles using lasers. Proc. Natl. Acad. Sci. USA 1997, 94, 4853–4860. [Google Scholar] [CrossRef]
- Greulich, K.O. Micromanipulation by Light in Biology and Medicine: The Laser Microbeam and Optical Tweezers; Birkhäuser: Basel, Switzerland; Boston, MA, USA; Berlin, Germany, 1999. [Google Scholar]
- Scott, T.G.; Spencer, R.G.; Leonard, N.J.; Weber, G. Emission properties of NADH. Studies of fluorescence lifetimes and quantum efficiencies of NADH, AcPyADH and simplified synthetic models. J. Am. Chem. Soc. 1970, 92, 687–695. [Google Scholar] [CrossRef]
- Galland, P.; Senger, H. The role of flavins as photoreceptors. J. Photochem. Photobiol. B 1988, 1, 277–294. [Google Scholar] [CrossRef]
- Ormond, A.B.; Freeman, H.S. Dye Sensitizers for Photodynamic Therapy. Materials 2013, 6, 817–840. [Google Scholar] [CrossRef]
- Schneckenburger, H.; Weber, P.; Wagner, M.; Schickinger, S.; Richter, V.; Bruns, T.; Strauss, W.S.; Wittig, R. Light exposure and cell viability in fluorescence microscopy. J. Microsc. 2012, 245, 311–318. [Google Scholar] [CrossRef]
- Jonkman, J.; Brown, C.M.; Wright, G.D.; Anderson, K.I.; North, A.J. Tutorial: Guidance for quantitative confocal microscopy. Nat. Protoc. 2020, 15, 1585–1611. [Google Scholar] [CrossRef]
- Ehrenberg, M. The Nobel Prize in Chemistry 2014 (Press Release). Available online: http://www.nobelprize.org/nobel_prizes/chemistry/laureates/2014/ (accessed on 1 January 2025).
- Masters, B.R. Selected Papers on Confocal Microscopy; SPIE Milestone Series; SPIE Optical Engineering Press: Bellingham, WA, USA, 1996; Volume MS 131. [Google Scholar]
- Huisken, J.; Swoger, J.; del Bene, F.; Wittbrodt, J.; Stelzer, E.H.K. Optical sectioning deep inside live embryos by SPIM. Science 2004, 305, 1007–1009. [Google Scholar] [CrossRef] [PubMed]
- Santi, P.A. Light sheet fluorescence microscopy: A review. J. Histochem. Cytochem. 2011, 59, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Pampaloni, F.; Chang, B.-J.; Stelzer, E.H.K. Light sheet-based fluorescence microscopy (LSFM) for the quantitative imaging of cells and tissues. Cell Tissue Res. 2015, 360, 129–141. [Google Scholar] [CrossRef] [PubMed]
- Bruns, T.; Schickinger, S.; Schneckenburger, H. Single plane illumination module and micro-capillary approach for a wide-field microscope. J. Vis. Exp. 2014, 15, e51993. [Google Scholar] [CrossRef]
- Abbe, E. Beiträge zur Theorie des Mikroskops und der mikroskopischen Wahrnehmung. Arch. Mikrosk. Anat. 1873, 9, 413–418. [Google Scholar] [CrossRef]
- Rayleigh, L. On the Theory of Optical Images, with Special Reference to the microscope. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1896, 42, 167–195. [Google Scholar] [CrossRef]
- Wu, X.; Hammer, J.A. ZEISS Airyscan: Optimizing Usage for Fast, Gentle, Super-Resolution Imaging. Methods Mol. Biol. 2021, 2304, 111–130. [Google Scholar] [CrossRef]
- Weisshart, K. The Basic Principle of Airyscanning. Carl Zeiss. 2014. Available online: https://pages.zeiss.com/rs/896-XMS-794/images/ZEISS-Microscopy (accessed on 1 January 2025).
- Müller, C.B.; Enderlein, J. Image Scanning Microscopy. Phys. Rev. Lett. 2010, 104, 198101. [Google Scholar] [CrossRef]
- Cremer, C.; Masters, B.R. Resolution enhancement techniques in microscopy. Eur. Phys. J. H 2013, 38, 281–344. [Google Scholar] [CrossRef]
- Sydor, A.M.; Czymmek, K.J.; Puchner, E.M.; Mennella, V. Super-resolution microscopy: From single molecules to supramolecular assemblies. Trends Cell Biol. 2015, 25, 730–748. [Google Scholar] [CrossRef]
- Galbraith, C.G.; Galbraith, J.A. Super-resolution microscopy at a glance. J. Cell Sci. 2011, 124, 1607–1611. [Google Scholar] [CrossRef] [PubMed]
- Toraldo di Francia, G. Resolving power and information. J. Opt. Soc. Am. 1955, 45, 497–499. [Google Scholar] [CrossRef]
- Cox, S. Super-resolution imaging in live cells. Dev. Biol. 2015, 40, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Cremer, C.; Kaufmann, R.; Gunkel, M.; Pres, S.; Weiland, Y.; Müller, P.; Ruckelshausen, T.; Lemmer, P.; Geiger, F.; Degenhard, S.; et al. Superresolution imaging of biological nanostructures by spectral precision distance microscopy. Biotechnol. J. 2011, 6, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Shtengel, G.; Galbraith, J.A.; Galbraith, C.G.; Lippincott-Schwartz, J.; Gillette, J.M.; Manley, S.; Sougrat, R.; Waterman, C.M.; Kanchanawong, P.; Davidson, M.W.; et al. Interferometric fluorescent super-resolution microscopy resolves 3D cellular ultrastructure. Proc. Natl. Acad. Sci. USA 2009, 106, 3125–3130. [Google Scholar] [CrossRef]
- Birk, U. Super-Resolution Microscopy-A Practical Guide; Wiley-VCH: Weinheim, Germany, 2017; ISBN 978-3-527-34133-7. [Google Scholar]
- Heintzmann, R. Answers to fundamental questions in superresolution microscopy. Phil. Trans. R.Soc. A 2021, 379, 20210105. [Google Scholar] [CrossRef]
- Schermelleh, L.; Ferrand, A.; Huser, T.; Eggeling, C.; Sauer, M.; Biehlmaier, O.; Drummen, G.P.C. Super-resolution microscopy demystified. Nat. Cell Biol. 2019, 21, 72–84. [Google Scholar] [CrossRef]
- Fornasiero, E.F.; Opazo, F. Super-resolution imaging for cell biologists: Concepts, applications, current challenges and developments. Bioessays 2015, 37, 436–451. [Google Scholar] [CrossRef]
- Gustafsson, M.G.; Shao, L.; Carlton, P.M.; Wang, C.J.R.; Golubovskaya, I.N.; Cande, W.Z.; Agard, D.A.; Sedat, J.W. Three-Dimensional Resolution Doubling in Wide-Field Fluorescence Microscopy by Structured Illumination. Biophys. J. 2008, 94, 4957–4970. [Google Scholar] [CrossRef]
- Hirvonen, L.M.; Wicker, K.; Mandula, O.; Heintzmann, R. Structured illumination microscopy of a living cell. Eur. Biophys. J. 2009, 38, 807–812. [Google Scholar] [CrossRef]
- Heintzmann, R.; Cremer, C. Lateral modulated excitation microscopy: Improvement of resolution by using a diffraction grating. Proc. SPIE 1999, 3568, 185–196. [Google Scholar] [CrossRef]
- Schock, F.; Best, G.; Celik, N.; Heintzmann, R.; Dithmar, S.; Cremer, C. Structured illumination ophthalmoscope: Super-resolution microscopy on the living human eye. Phil. Trans. R. Soc. A 2022, 380, 20210151. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.R.; Ross, S.T.; Davidson, M.W. Structured illumination microscopy for superresolution. Chem. Phys. Chem. 2014, 15, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Chakrova, N.; Heintzmann, R.; Rieger, B.; Stallinga, S. Studying different illumination patterns for resolution improvement influorescence microscopy. Opt Exp. 2015, 23, 31367–31383. [Google Scholar] [CrossRef]
- Li, D.; Shao, L.; Chen, B.-C.; Zhang, X.; Zhang, M.; Moses, B.; Milkie, D.E.; Beach, J.R.; Hammer, J.A.; Pasham, M.; et al. Extended resolution structured illumination imaging of endocytic and cytoskeletal dynamics. Science 2015, 349, aab3500. [Google Scholar] [CrossRef]
- Gustafsson, M.G.L. Nonlinear structured-illumination microscopy: Wide-field fluorescence imaging with theoretically unlimited resolution. Proc Natl Acad. Sci. USA 2005, 102, 13081–13086. [Google Scholar] [CrossRef]
- Heintzmann, R.; Huser, T. Super-resolution structured illumination microscopy. Chem. Rev. 2017, 117, 13890–13908. [Google Scholar] [CrossRef]
- Gustafsson, M.G.L. Surpassing the lateral resolution limit by a factor of two using structured illumination microscopy. J. Microsc. 2000, 198, 82–87. [Google Scholar] [CrossRef]
- Kraus, F.; Miron, E.; Demmerle, J.; Chitiashvili, T.; Budco, A.; Alle, Q.; Matsuda, A.; Leonhardt, H.; Schermelleh, L.; Markaki, Y. Quantitative 3D structured illumination microscopy of nuclear structures. Nat. Protoc. 2017, 12, 1011–1027. [Google Scholar] [CrossRef]
- Schneckenburger, H. Lasers in Live Cell Microscopy. Int. J. Mol. Sci. 2022, 23, 5015. [Google Scholar] [CrossRef]
- Supekar, O.D.; Sias, A.; Hansen, S.R.; Martinez, G.; Peet, G.C.; Peng, X.; Bright, V.M.; Hughes, E.G.; Restrepo, D.; Shepherd, D.P.; et al. Miniature structured illumination microscope for in vivo 3D imaging of brain structures with optical sectioning. Biomed. Opt. Exp. 2022, 13, 2531. [Google Scholar] [CrossRef] [PubMed]
- Löschberger, A.; Novikau, Y.; Netz, R.; Spindler, M.C.; Benavente, R.; Klein, T.; Kleppe, D.I. Super-Resolution Imaging by Dual iterative Structured Illumination Microscopy, Carl Zeiss. 2017. Available online: https://www.zeiss.com/microscopy/en/products/light-microscopes/super-resolution-microscopes/elyra-7.html? (accessed on 1 January 2025).
- Wang, F.; Ma, Z.; Zhong, Y.; Salazar, F.; Xu, C.; Ren, F.; Qu, L.; Wu, A.M.; Dai, H. In vivo NIR-II structured-illumination light-sheet microscopy. Proc Natl Acad. Sci. USA 2021, 118, e2023888118. [Google Scholar] [CrossRef] [PubMed]
- ISO 15004-2:2007; Ophthalmic Instruments—Fundamental Requirements and Test Methods, Part 2: Light Hazard Protection. International Organization for Standardization: Geneva, Switzerland, 2019. Available online: https://www.iso.org/standard/38952.html (accessed on 1 January 2025).
- Fischer, J.; Otto, T.; Delori, F.; Pace, L.; Staurenghi, G. Scanning Laser Ophthalmoscopy (SLO). In High Resolution Imaging in Microscopy and Ophthalmology. New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Berlin/Heidelberg, Germany, 2019. Available online: https://www.ncbi.nlm.nih.gov/books/NBK554043/ (accessed on 1 January 2025).
- Cremer, C.; Schock, F.; Failla, A.V.; Birk, U. Modulated illumination microscopy: Application perspectives in nuclear nanostructure analysis. J. Microsc. 2024, 296, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Lanni, F.; Bailey, B.; Farkas, D.L.; Taylor, D.L. Excitation field synthesis as a means for obtaining enhanced axial resolution in fluorescence microscopes. Bioimaging 1993, 1, 187–196. [Google Scholar] [CrossRef]
- Frohn, J.; Knapp, H.; Stemmer, A. True optical resolution beyond the Rayleigh limit achieved by standing wave illumination. Proc. Natl. Acad. Sci. USA 2000, 97, 7232–7236. [Google Scholar] [CrossRef]
- Bailey, B.; Farkas, D.; Taylor, D.L.; Lanni, F. Enhancement of axial resolution in fluorescence microscopy by standing-wave excitation. Nature 1993, 366, 44–48. [Google Scholar] [CrossRef]
- Reymann, J.; Baddeley, D.; Gunkel, M.; Lemmer, P.; Stadter, W.; Jegou, T.; Rippe, K.; Cremer, C.; Birk, U. High precision structural analysis of subnuclear complexes in fixed and live cells via spatially modulated illumination (SMI) microscopy. Chromosome Res. 2008, 16, 367–382. [Google Scholar] [CrossRef]
- Reinhardt, S.C.M.; Masullo, L.A.; Baudrexel, I.; Steen, P.R.; Kowalewski, R.; Eklund, A.S.; Strauss, S.; Unterauer, E.M.; Schlichthaerle, T.; Strauss, M.T.; et al. Ångström-resolution fluorescence microscopy. Nature 2023, 617, 711–716. [Google Scholar] [CrossRef]
- Betzig, E.; Patterson, G.H.; Sougrat, R.; Lindwasser, O.W.; Olenych, S.; Bonifacino, J.S.; Davidson, M.W.; Lippincott-Schwartz, J.; Hess, H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science 2006, 313, 1642–1645. [Google Scholar] [CrossRef]
- Rust, M.J.; Bates, M.; Zhuang, X. Sub-diffraction-limit imaging by stochastic optical reconstruction microscopy (STORM). Nat. Methods 2006, 3, 793–796. [Google Scholar] [CrossRef]
- Hess, S.T.; Girirajan, T.P.; Mason, M.D. Ultra-High Resolution Imaging by Fluorescence Photoactivation Localization Microscopy. Biophys. J. 2006, 91, 4258–4272. [Google Scholar] [CrossRef] [PubMed]
- Cox, S.; Rosten, E.; Monypenny, J.; Jovanovic-Talisman, T.; Burnette, D.T.; Lippincott-Schwartz, J.; E Jones, G.; Heintzmann, R. Bayesian localization microscopy reveals nanoscale podosome dynamics. Nat. Methods 2011, 9, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Ha, T. Single-molecule methods leap ahead. Nat. Methods 2014, 11, 1015–1018. [Google Scholar] [CrossRef] [PubMed]
- Cremer, C.; Szczurek, A.; Schock, F.; Gourram, A.; Birk, U. Super-resolution microscopy approaches to nuclear nanostructure imaging. Methods 2017, 123, 11–32. [Google Scholar] [CrossRef]
- Gelléri, M.; Chen, S.-Y.; Hübner, B.; Neumann, J.; Kröger, O.; Sadlo, F.; Imhoff, J.; Hendzel, M.J.; Cremer, M.; Cremer, T.; et al. True-to-scale DNA-density maps correlate with major accessibility differences between active and inactive chromatin. Cell Rep. 2023, 42, 112567. [Google Scholar] [CrossRef]
- Reck-Peterson, S.L.; Derr, N.D.; Stuurman, N. Imaging single molecules using total internal reflection fluorescence microscopy (TIRFM). Cold Spring Harb. Protoc. 2010, 2010, pdb.top73. [Google Scholar] [CrossRef]
- Luo, W.; He, K.; Xia, T.; Fang, X. Single-molecule monitoring in living cells by use of fluorescence microscopy. Anal. Bioanal. Chem. 2013, 405, 43–49. [Google Scholar] [CrossRef]
- Ritter, J.G.; Veith, R.; Veenendaal, A.; Siebrasse, J.P.; Kubitscheck, U. Light Sheet Microscopy for Single Molecule Tracking in Living Tissue. PLoS ONE 2010, 5, e11639. [Google Scholar] [CrossRef]
- Barentine, A.E.; Lin, Y.; Courvan, E.M.; Kidd, P.; Liu, M.; Balduf, L.; Baddeley, D. An integrated platform for high-throughput nanoscopy. Nat. Biotechnol. 2023, 41, 1549–1556. [Google Scholar] [CrossRef]
- Lakadamyali, M. Super-Resolution Microscopy: Going Live and Going Fast. Chem. Phys. Chem. 2014, 15, 630–636. [Google Scholar] [CrossRef]
- Wombacher, R.; Heidbreder, M.; van de Linde, S.; Sheetz, M.P.; Heilemann, M.; Cornish, V.W.; Sauer, M. Live-cell super-resolution imaging with trimethoprim conjugates. Nat. Methods 2010, 7, 717–719. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.-H.; Xia, C.; Zhong, G.; Babcock, H.P.; Vaughan, J.C.; Huang, B.; Wang, X.; Xu, C.; Bi, G.-Q.; Zhuang, X. Super-resolution fluorescence imaging of organelles in live cells with photoswitchable membrane probes. Proc. Natl. Acad. Sci. USA 2012, 109, 13978–13983. [Google Scholar] [CrossRef] [PubMed]
- Zanacchi, F.C.; Lavagnino, Z.; Donnorso, M.P.; Del Bue, A.; Furia, L.; Faretta, M.; Diaspro, A. Live-cell 3D superresolution imaging in thick biological samples. Nat. Methods 2017, 8, 1047–1049. [Google Scholar] [CrossRef] [PubMed]
- Hell, S.W.; Wichmann, J. Breaking the diffraction resolution limit by stimulated emission: Stimulated-emission-depletion fluorescence microscopy. Opt. Lett. 1994, 19, 780–782. [Google Scholar] [CrossRef]
- Wildanger, D.; Medda, R.; Kastrup, L.; Hell, S. A compact STED microscope providing 3D nanoscale resolution. J. Microsc. 2009, 236, 35–43. [Google Scholar] [CrossRef]
- Blom, H.; Widengren, J. STED microscopy—Towards broadened use and scope of applications. Curr. Opin. Chem. Biol. 2014, 20, 127–133. [Google Scholar] [CrossRef]
- Hofmann, M.; Eggeling, C.; Jakobs, S.; Hell, S.W. Breaking the diffraction barrier in fluorescence microscopy at low light intensities by using reversibly photoswitchable proteins. Proc. Natl. Acad. Sci. USA 2005, 102, 17565–17569. [Google Scholar] [CrossRef]
- Hell, S.W. Microscopy and its focal switch. Nat. Methods 2009, 6, 24–32. [Google Scholar] [CrossRef]
- Chmyrov, A.; Keller, J.; Grotjohann, T.; Ratz, M.; D’Este, E.; Jakobs, S.; Eggeling, C.; Hell, S.W. Nanoscopy with more than 100,000 ‘Doughnuts’. Nat. Methods 2013, 10, 737–740. [Google Scholar] [CrossRef]
- Rittweger, E.; Han, K.Y.; Irvine, S.E.; Eggeling, C.; Hell, S.W. STED microscopy reveals crystal colour centres with nanometric resolution. Nat. Photonics 2009, 3, 144147. [Google Scholar] [CrossRef]
- Weber, M.; Leutenegger, M.; Stoldt, S.; Jakobs, S.; Mihaila, T.S.; Butkevich, A.N.; Hell, S.W. MINSTED fluorescence localization and nanoscopy. Nat. Photonics 2021, 15, 361–366. [Google Scholar] [CrossRef] [PubMed]
- Westphal, V.; Rizzoli, S.O.; Lauterbach, M.A.; Kamin, D.; Jahn, R.; Hell, S.W. Video-Rate far-field optical nanoscopy dissects synaptic vesicle movement. Science 2008, 320, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Mai Tai® One Box Ti:Sapphire Ultrafast Lasers. Spectra Physic. 2014. Available online: https://www.spectra-physics.com/en/f/mai-tai-ultrafast-laser (accessed on 1 January 2025).
- Balzarotti, F.; Eilers, Y.; Gwosch, K.C.; Gynnå, A.H.; Westphal, V.; Stefani, F.D.; Elf, J.; Hell, S.W. Nanometer resolution imaging and tracking of fluorescent molecules with minimal photon fluxes. Science 2016, 355, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Gwosch, K.C.; Pape, C.; Balzarotti, J.K.; Hoess, F.; Ellenberg, P.; Ries, J.; Hell, S.W. MINFLUX nanoscopy delivers 3D multicolor nanometer resolution in cells. Nat. Methods 2020, 17, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Sahl, S.J.; Matthias, J.; Inamdar, K.; Weber, M.; Khan, T.A.; Brüser, C.; Jakobs, S.; Becker, S.; Griesinger, C.; Broichhagen, J.; et al. Direct optical measuerement of intramolecular distances with Angström precision. Science 2024, 386, 180–187. [Google Scholar] [CrossRef]
- Huang, B.; Wang, W.; Bates, M.; Zhuang, X. Three-dimensional super-resolution imaging by stochastic optical reconstruction microscopy. Science 2008, 319, 810–813. [Google Scholar] [CrossRef]
- Heilemann, M.; van de Linde, S.; Schüttpelz, M.; Kasper, R.; Seefeldt, B.; Mukherjee, A.; Tinnefeld, P.; Sauer, M. Subdiffraction-resolution fluorescence imaging with conventional fluorescent probes. Angew. Chem. Int. Ed. 2008, 47, 6172–6176. [Google Scholar] [CrossRef]
- Lemmer, P.; Gunkel, M.; Baddeley, D.; Kaufmann, R.; Urich, A.; Weiland, Y.; Reymann, J.; Müller, P.; Hausmann, M.; Cremer, C. SPDM: Light microscopy with single-molecule resolution at the nanoscale. Appl. Phys. B 2008, 93, 1–12. [Google Scholar] [CrossRef]
- Huang, B.; Jones, S.A.; Brandenburg, B.; Zhuang, X. Whole-cell 3D STORM reveals interactions between cellular structures with nanometer-scale resolution. Nat. Methods 2008, 5, 1047–1052. [Google Scholar] [CrossRef]
- Ricci, M.A.; Manzo, C.; Garcıa-Parajo, M.F.; Lakadamyali, M.; Cosma, M.P. Chromatin fibers are formed by heterogeneous groups of nucleosomes in vivo. Cell 2015, 160, 1145–1158. [Google Scholar] [CrossRef]
- Schneckenburger, H. Laser-assisted optoporation of cells and tissues—A mini-review. Biomed. Opt. Express 2019, 10, 2883–2888. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Vu, K.T.; Krishnan, P.; Trang, T.C.; Shin, D.; Kimel, S.; Berns, M.W. Wavelength dependence of cell cloning efficiency after optical trapping. Biophys. J. 1996, 70, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Schneckenburger, H.; Hendinger, A.; Sailer, R.; Gschwend, M.H.; Strauss, W.S.; Bauer, M.; Schütze, K. Cell viability in optical tweezers: High power red laser diode versus Nd:YAG laser. J. Biomed. Opt. 2000, 5, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Greulich, K.O. Manipulation of cells with laser microbeam scissors and optical tweezers: A review. Rep. Prog. Phys. 2017, 80, 026601. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, A.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef]
- Park, S.; Kim, J.; Yoon, S.H. A Review on Quantitative Measurement of Cell Adhesion Strength. J. Nanosci. Nanotechnol. 2016, 16, 4256–4273. [Google Scholar] [CrossRef]
- Arbore, C.; Perego, L.; Sergides, M.; Capitanio, M. Probing force in living cells with optical tweezers: From single-molecule mechanics to cell mechanotransduction. Biophys. Rev. 2019, 11, 765–782. [Google Scholar] [CrossRef]
- Musielak, M. Red blood cell-deformability measurement: Review of techniques. Clin. Hemorheol. Microcirc. 2009, 42, 47–64. [Google Scholar] [CrossRef]
- Priezzhev, A.; Lee, K. Potentialities of laser trapping and manipulation of blood cells in hemorheologic research. Clin. Hemorheol. Microcirc. 2016, 64, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Arai, F.; Ng, C.; Maruyama, H.; Ichikawa, A.; El-Shimy, H.; Fukuda, T. On chip single-cell separation and immobilization using optical tweezers and thermo-sensitive hydrogel. Lab Chip 2005, 5, 1399–1403. [Google Scholar] [CrossRef]
- Wang, X.; Chen, S.; Kong, M.; Wang, Z.; Costa, K.D.; Li, R.A.; Sun, D. Enhanced cell sorting and manipulation with combined optical tweezer and microfluidic chip technologies. Lab Chip 2011, 11, 3656–3662. [Google Scholar] [CrossRef] [PubMed]
- Kaschke, M.; Donnerhacke, K.-H.; Rill, M.S. Optical Devices in Ophthalmology and Optometry: Technology, Design Principles, and Clinical Applications; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef]
- Szczurek, A.T.; Dimitrova, E.; Kelley, J.R.; Blackledge, N.P.; Klose, R.J. The Polycomb system sustains promoters in a deep OFF state by limiting pre-initiation complex formation to counteract transcription. Nat. Cell Biol. 2024, 26, 1700–1711. [Google Scholar] [CrossRef] [PubMed]
- Peker, B.; Rausch, M. Microscopy of Organoids. Breakthrough in live cell microscopy of 3D cellular models. Microsc. Anal. 2020, 34, 15–17. [Google Scholar]
- Cremer, C.; Cremer, T.; Zorn, C.; Schoeller, L. Effects of laser-uv-microirradiation (λ = 257 nm) on proliferation of Chinese hamster cells. Radiat. Res. 1976, 66, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Cremer, C.; Cremer, T.; Fukuda, M.; Nakanishi, K. Detection of Laser-uv-microirradiation induced DNA photolesions by immunofluorescent staining. Hum. Genet. 1980, 54, 107–110. [Google Scholar] [CrossRef]
- Zorn, C.; Cremer, T.; Cremer, C.; Zimmer, J. Laser-uv-micro-irradiation of interphase nuclei and posttreatment with caffeine: A new approach to establish the arrangement of interphase chromosomes. Hum. Genet. 1976, 35, 83–89. [Google Scholar] [CrossRef]
- Cremer, C.; Cremer, T.; Jabbur, G. Laser-uv-microirradiation of Chinese hamster cells: The influence of the distribution of photolesions on unscheduled DNA synthesis. Photochem. Photobiol. 1981, 33, 925–928. [Google Scholar] [CrossRef]
- Lohs-Schardin, M.; Sander, K.; Cremer, C.; Cremer, T.; Zorn, C. Localized ultraviolet laser microbeam irradiation of early Drosophila embryos: Fate maps based on location and frequency of adult defects. Develop. Biol. 1979, 68, 533–545. [Google Scholar] [CrossRef]
- Nüsslein-Volhard, C.H.; Lohs-Schardin, M.; Sander, K.; Cremer, C. A dorso-ventral shift of embryonic primordia in a new maternal-effect mutant of Drosophila. Nature 1980, 283, 474–476. [Google Scholar] [CrossRef]
- Höh, A.E.; Ach, T.; Amberger, R.; Dithmar, S. Lichtexposition bei vitreoretinaler Chirurgie. Ophthalmologe 2008, 105, 898–904. [Google Scholar] [CrossRef]
- Available online: https://en.wikipedia.org/wiki/Antonie_van_Leeuwenhoek (accessed on 1 January 2025).
- Zernike, F. Das Phasenkontrastverfahren bei der mikroskopischen Beobachtung. Phys. Zeitschr. 1935, 36, 848–851. [Google Scholar]
- Campbell, K.H.S.; McWhir, J.; Ritchie, W.A.; Wilmut, I. Sheep cloned by nuclear transfer from a cultured cell line. Nature 1996, 380, 64–66. [Google Scholar] [CrossRef] [PubMed]
- Wilmut, I.; Schnieke, A.E.; McWhir, J.; Kind, A.J.; Campbell, K.H.S. Viable offspring derived from fetal and adult mammalian cells. Nature 1997, 385, 810–813. [Google Scholar] [CrossRef] [PubMed]
- Hoebe, R.A.; Van der Voort, H.T.; Stap, J.; Van Noorden, C.J.; Manders, E.M. Quantitative determination of the reduction of phototoxicity and photobleaching by controlled light exposure microscopy. J. Microsc. 2008, 231 Pt 1, 9–20. [Google Scholar] [CrossRef]
- Zhu, X.; Chen, Q.; Zhao, H.; Yang, Q.; Goudappagouda; Gelléri, M.; Ritz, S.; Ng, D.; Koynov, K.; Parekh, S.H.; et al. Intrinsic Burst-Blinking Nanographenes for Super-Resolution Bioimaging. J. Am. Chem. Soc. 2024, 146, 5195–5203. [Google Scholar] [CrossRef]
| Method | Max. Light Dose [J/cm2] | Irradiance [mW/cm2] | Record. Time [s] | Max. Number of Images |
|---|---|---|---|---|
| Wide-field microscopy | 10 | 100 | 1 | 100 |
| SIM | 10 | 100 | 10 (*) | 10 |
| CLSM | 10 | 100 | 5 | 20 |
| Light Sheet (N layers) | 10 | 100 | 1 | N × 100 |
| Single Molecule Localization | 100 (**) | 50,000 to 1,000,000 | 30 | ≤1 |
| STED | 10−50 (**) | 3,000,000 | 1 | ≤1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schneckenburger, H.; Cremer, C. Keeping Cells Alive in Microscopy. Biophysica 2025, 5, 1. https://doi.org/10.3390/biophysica5010001
Schneckenburger H, Cremer C. Keeping Cells Alive in Microscopy. Biophysica. 2025; 5(1):1. https://doi.org/10.3390/biophysica5010001
Chicago/Turabian StyleSchneckenburger, Herbert, and Christoph Cremer. 2025. "Keeping Cells Alive in Microscopy" Biophysica 5, no. 1: 1. https://doi.org/10.3390/biophysica5010001
APA StyleSchneckenburger, H., & Cremer, C. (2025). Keeping Cells Alive in Microscopy. Biophysica, 5(1), 1. https://doi.org/10.3390/biophysica5010001







